Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most frequent life- threatening, hereditary disease. ADPKD is more common than sickle cell anemia, cystic fibrosis, muscular dystrophy, hemophilia, Down’s syndrome, and Huntington’s disease combined. ADPKD is a multisystemic disorder characterized by the progressive development of renal cysts and marked renal enlargement. Structural and functional renal deterioration occurs in ADPKD patients and is the fourth leading cause of end-stage renal disease (ESRD) in adults. Aside from the renal manifestations, extrarenal structural abnormalities, such as liver cysts, cardiovascular abnormalities, and intracranial aneurysms may lead to morbidity and mortality. Recent studies have identified prognostic factors for progressive renal impairment including gender, race, age, proteinuria, hematuria, hypertension and increased left ventricular mass index (LVMI). Early diagnosis and better understanding of the pathophysiology of the disease provides the opportunity to aggressivly treat hypertension with renin-angiotensin-aldosterone system inhibitors and thereby potentially reduce LVMI, prevent cardiovascular morbidity and mortality and slow progression of the renal disease.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by renal cyst enlargement, resulting in abnormal kidney structure and renal insufficiency. End stage renal disease (ESRD) will be observed in 50% of cases by the fifth decade. ADPKD is the most common life- threatening, hereditary disease, which affects 1:400 to 1:1000 live births (Ecder et al 2006). The incidence of ADPKD is more frequent than sickle cell anemia, cystic fibrosis, muscular dystrophy, hemophilia, Down’s syndrome, and Huntington’s disease combined (PKD Foundation 2000). ADPKD is genetically heterogeneous with separate loci on chromosome 16 (PKD1), accounting for approximately 85% of ADPKD cases, and on chromosome 4 (PKD2), accounting for approximately 15% of patients (CitationKimberling et al 1993).
Numerous renal and extra-renal manifestations have been described for ADPKD. Hypertension, left ventricular hypertrophy, cardiac valvular defects, back and abdominal pain, cerebral aneurysms, and liver cysts are the most important clinical findings. There are several risk factors, which affect the progression of renal disease such as gender, race, age, renal volume, hypertension, proteinuria and hematuria (CitationGabow et al 1992). It has been shown that the disease is more severe in PKD1 patients as compared to PKD2. Hypertension and proteinuria are more pronounced in PKD1 patients (CitationChapman et al 2003; Grantham et al 2006a). In both PKD1 and PKD2, older patients generally have more cysts, indicating that the cystogenesis continues throughout the life of patients. Renal volume is highly associated with hypertension and both have significant impact on renal outcomes (CitationFick-Brosnahan et al 2002;CitationSchrier et al 2003). Recently it has been defined that the rate of cyst growth is comparable in PKD1 and PKD2. However, the number of cysts is greater in PKD1 patients (CitationHarris et al 2006). Male, as compared to female, patients of European descent tend to have more severe disease with faster expansion of cyst volumes. There are some preliminary results indicating that African-Americans exhibit worse disease than ADPKD patients of European descent (CitationGabow et al 1992). Understanding the natural history of the disease, clinical risk factors and targeting the treatable aspects of the disease are the main foci in order to choose the appropriate interventions.
Diagnosis
The diagnosis of ADPKD is usually made by family history and renal imaging. In the case of affected family members gene linkage analysis has been used. However, genetic studies are expensive but may be employed if the diagnosis is uncertain and renal imaging is not informative. A recent study showed that physicians and patients’ awareness of the hereditary basis of ADPKD has led to better family histories and an earlier diagnosis of ADPKD (CitationTaylor et al 2005). Earlier diagnosis could have beneficial effects with interventions to slow renal disease progression and prevent cardiovascular complications in ADPKD patients. In recent years, with the widespread availability of ultrasonography, this modality has been established as the main radiologic technique for diagnosis of ADPKD (CitationElles et al 1994; CitationGabow et al 1997). In one study ultrasonograghy was found to be more reliable in patients older than 30 years and especially in PKD1 (CitationNicolau et al 1999). Diagnostic criteria based on US findings in individuals with positive family history are shown in .
Table 1 Criteria for diagnosing autosomal dominant polycystic kidney disease (ADPKD) by renal ultrasound
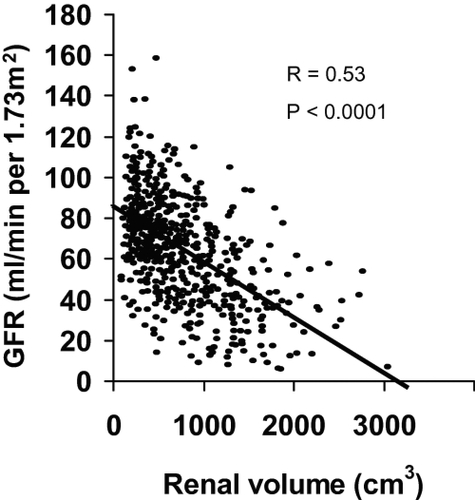
In 2002, Fick et al demonstrated that total kidney volume and renal cyst volumes were strongly correlated with the decline of glomerular filtration rate (GFR) () (CitationFick-Brosnahan et al 2002; CitationChapman et al 2003). A recent national study conducted by the Consortium of Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) provides support for this view (Grantham et al 2006b). They have shown that cystic growth rate is highly variable among ADPKD patients but it is mostly a progressive process during the course of the disease. Patients with larger kidneys have the fastest rates of renal volume enlargement (Grantham et al 2006b). Thus, measurement of renal volume is of clinical relevance. Although ultrasonography is safe, easily performed, available and inexpensive, it is not precise enough to detect short-term disease progression (CitationO’Neill et al 2005). Magnetic resonance imaging (MRI) has been shown to be more sensitive and reliable for measurement of the renal cyst volume. In contrast to ultrasonograghy, MRI is being evaluated as a useful technique to determine the cystic changes over a short time period (CitationChapman et al 2003).
Hypertension
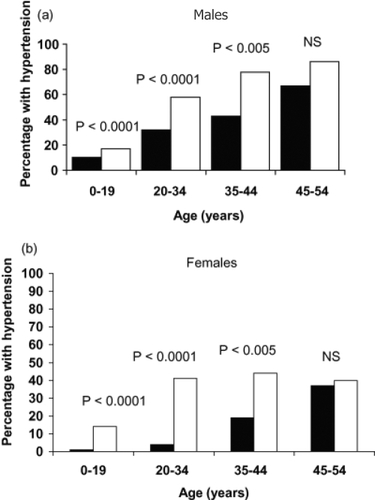
Hypertension is the most frequent complication among ADPKD patients, occurring in approximately 60% of the patients, while their glomelural filtration rate (GFR) is still in the normal range (GFR > 75 mL/min/1.73 m2) (CitationGabow, Chapman et al 1990; CitationChapman et al 1991). Compared to the general population hypertension occurs at a much earlier age in ADPKD patients (CitationKelleher et al 2004). The median age at hypertension diagnosis in ADPKD was found to be 32 years for males and 34 years for females (). The occurrence of hypertension is greater in both male and female ADPKD patients when their affected parents are hypertensive. Moreover, the age of diagnosis of hypertension is also earlier in ADPKD patients with affected hypertensive parents (CitationSchrier et al 2003). Several studies have shown that hypertension in ADPKD patients is associated with increased renal volumes and left ventricular mass index (LVMI), in both adults and children (CitationSedman et al 1987; CitationChapman et al 1997; Gabow et al 2006a). Hypertension and LVMI are important risk factors for cardiovascular disease, which is the most common cause of death in patients with ADPKD (CitationFick et al 1995).
Figure 2 Prevalence of hypertension in autosomal dominant polycystic kidney disease (■) in (a) males and (b) females 1995–2000 vs NHANES IV (□).
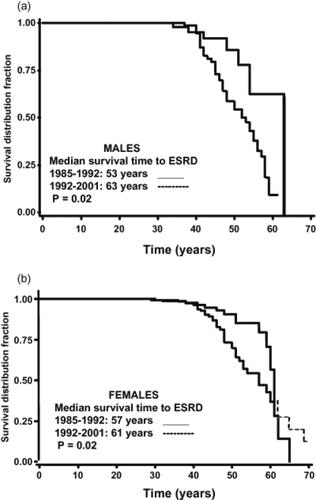
Angiographic studies have demonstrated that cyst enlargement in ADPKD is associated with compression of the adjacent parenchyma and vasculature, with resultant areas of renal ischemia (CitationEttinger et al 1969). Several studies have shown elevated concentrations of renin in both tissue and cyst fluid from ADPKD patients (CitationTorres et al 1992). Further studies have indicated an intrarenal role of RAAS by detecting the angiotensinogen, angiotensin-converting enzyme (ACE), angiotensin II type 1 (AT1) receptor, and angiotensin II within cysts and tubules of ADPKD kidneys (CitationLoghman-Adham et al 2004). These results support activation of the renin-angiotensin-aldosterone system (RAAS) secondary to renal ischemia. The first clue of RAAS activation in ADPKD arose from clinical studies, which demonstrated an increase in the plasma renin activity (PRA) with captopril, an ACE inhibitor, in hypertensive, but not normotensive patients with ADPKD (CitationAnderson et al 1979; CitationTorres et al 1991). Since an increase in blood pressure may decrease PRA, a comparison of the RAAS in patients with essential hypertension and hypertensive ADPKD patients who were matched was undertaken at comparable blood pressure (BP), renal function, age, and sodium intake. These studies demonstrated significantly higher PRA and plasma aldosterone concentrations in the supine and upright positions, as well as after ACE inhibition, with captopril in the ADPKD patients as compared to essential hypertension patients. In addition, after six weeks of ACE inhibition with enalapril, ADPKD hypertensive patients exhibited a significant increase in renal plasma flow due to decrease in renal vascular resistance and consequently a decrease in filtration fraction rate as compared to essential hypertension patients (CitationWatson et al 1992; CitationEcder et al 2001). In a retrospective study, ADPKD hypertensive patients received either ACE inhibitor or diuretics. During a mean follow-up of 5 years, patients who were treated with diuretics without any ACE inhibitors had a greater decrease in GFR and an increase in serum creatinine levels compared with patients who were taking only ACE inhibitors. Although systolic or diastolic blood pressure at baseline or at the end of the survey showed no differences, the diuretic group needed more additional antihypertensive drugs for comparable blood pressure control than the ACE inhibition group (64 vs 21%, p < 0.05) (CitationEcder et al 2001). In an epidemiological paper, it was shown that the risk for male ADPKD male patients entering end stage renal disease (ESRD) is 10 times higher among those with a mean arterial pressure (MAP) greater than 93 mm Hg (>120/80 mm Hg) compare to those patients with a MAP less than 93 mm Hg. A similar analysis estimated that female ADPKD patients with a MAP greater than 93 mm Hg were 2.4 times more likely to progress to ESRD than those patients with MAP of 93 mm Hg or less. Further, when ADPKD patients from 1985–1992 were compared with ADPKD patients from 1992–2001, a later age of ESRD onset was observed in the more recent group in which more aggressive control of hypertension has been emphasized () (CitationSchrier et al 2003).
Left ventricular hypertrophy
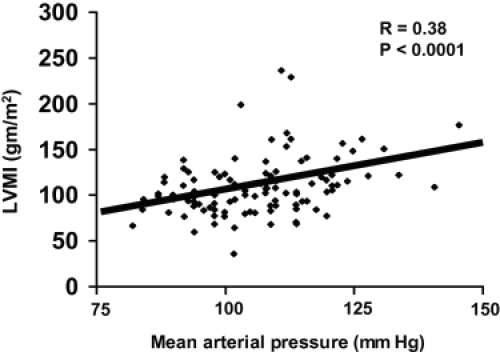
As already noted, in the era of renal replacement therapy with dialysis or transplantation, cardiovascular disease is the leading cause of death in patients with ADPKD (CitationFick et al 1995). One important risk factor is left ventricular hypertrophy (LVH), which exacerbates cardiac morbidity and mortality in patients with other types of disease. Due to early onset of hypertension in ADPKD patients, a significant correlation between LVH and mean arterial pressure may be seen (). Left ventricular mass index (LVMI) is highly related to systolic blood pressure in ADPKD (CitationBardaji et al 1998). In 116 ADPKD patients in their early 40s, LVH was found in 46% of male and 37% in female patients. LVH has also been detected in 23% of ADPKD patients with normal blood pressure (CitationChapman et al 1997; CitationEcder et al 2001). This may be due to the observation that BP may not decrease at night in ADPKD patients, ie, non-dippers.
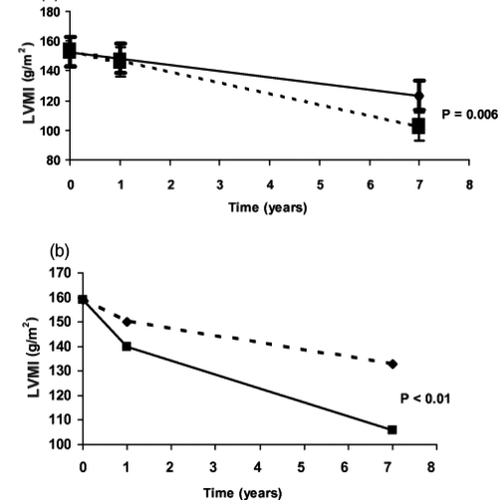
A prospective study demonstrated that ACE inhibition with enalapril controlled BP and reversed LVH in hypertensive ADPKD patients (CitationEcder et al 1999). A recent randomized prospective study on 75 hypertensive ADPKD patients with LVH demonstrated that rigorous BP control (<120/80 mmHg) versus standard (135–140/85–90 mmHg) BP control over a 7-year follow-up period had a significantly greater reversal of LVH. Both standard and rigorous BP control groups had decreased LVH, but LVMI was returned to the normal range in more patients in the rigorous BP control group than in the standard BP group (71% vs. 44%) (). Moreover, patients receiving the ACE inhibitor enalapril had a greater decrease in LVMI as compared to those who received amlodipine, a calcium channel blocker (CitationEcder at al 1999; CitationSchrier et al 2002). Thus, the use of an ACE inhibitor and maintenance of a blood pressure goal at 120/80 mmHg in hypertensive ADPKD patients with LVH should be undertaken (CitationEcder and Schrier 2004). Another study was conducted among 20 hypertensive ADPKD patients compared with an age-and sex-matched essential hypertensive subjects designated as a control group. Both groups had the same 24 hour ambulatory BP and LVMI, although male ADPKD patients had higher LVMI than their matched controls. LVH was detected in 40% of ADPKD patients compared to 30% of the essential hypertensive group. ADPKD patients with LVH did not differ from those without LVH, except that they had higher 24-hour ambulatory BP levels. This study indicates a correlation between LVMI and 24-hour systolic and diastolic blood pressure in ADPKD patients (CitationMartinez et al 2000). ADPKD patients may demonstrate insulin resistance (CitationHjelmesaeth and Hartmann 1999), thus studies have been conducted to determine the prevalence of LVH among these patients. Results showed a significant association between LVMI and insulin resistance independently of other factors such as age, weight, systolic blood pressure and albuminuria (CitationLumiaho et al 2003). The stimulation of angiotensin II (CitationRocchini et al 1990) and the sympathetic nervous system secondary to elevated insulin levels therefore may have a major role in increased LVMI in ADPKD patients (CitationLembo et al 1992).
Figure 5 (a) Effect of rigorous (■) versus standard (◆) lood pressure control to decrease left ventricular mass index in autosomal dominant polycystic kidney disease (ADPKD) patients. (b) Effect of blood pressure control with amlodipine (◆) versus enalapril (■) to decrease left ventricular mass index in ADPKD patients.
In addition to LVH, valvular disorders are common among ADPKD patients. It has been shown that the prevalence of mitral valve prolapse (MVP) is higher in ADPKD patients as compared to general population (26% vs 2%). ADPKD patients also have an increased risk of tricuspid valve prolapse (6%). Mitral and aortic incompetence also has been detected more frequent in ADPKD patients (31 and 8 percent respectively) (CitationHossack et al 1988; CitationTimio et al 1992; CitationMartinez-Vea 1999).
ADPKD in children
ADPKD is usually considered an adult disease. In the 1970s and 1980s cases of early disease in children and even in utero were described (CitationBlyth and Ockenden 1971; CitationKaye and Lewy 1974; CitationBengtsson et al 1975; CitationGarel et al 1983; CitationPretorius et al 1987). Thus, modern imaging techniques, especially renal ultrasonograghy, emerged as the procedure to diagnose ADPKD. In one study approximately 60%–90% of PKD gene carriers under the age of 20 years were recognized by renal ultrasonograghy (CitationSedman et al 1987). Recently, it has been shown that the sensitivity of ultrasonograghy in ADPKD patients younger than 30 years is almost 95% (CitationNicolau et al 1999). In a study on PKD-1 gene mutation carriers, among 0–5-year-old children, renal cysts were detected in 60% of the study group by ultrasound. In children aged 5–18 years, 75%–80% had detectable cysts by renal ultrasound (CitationGabow et al 1997). ADPKD children, in addition to their renal cysts, may present with a variety of clinical findings, ranging from newborns with severe disease including massive renal enlargement, hypertension, oliguria, and pulmonary hypoplasia to the absence of signs or symptoms (CitationMcdonald et al 1999). Unlike adults, the factors in children, which have an impact on the disease progression, are not well understood (CitationFick et al 1994). GFR is not a reliable predictor of disease progression in children (CitationFick et al 1993). Fick et al in an observational study on 312 children described the characteristics, which are associated with faster renal volume growth. Risk factors were renal enlargement in utero or the first year of life, high blood pressure and the presence of more than 10 renal cysts before age 12 years. The rate of renal enlargement may be a relevant marker for disease progression. However, there is considerable variability in renal volumes among affected children. Nevertheless, at any given age, the affected children had significantly larger kidneys as compared to non-affected children (CitationFick et al 2001).
Most ADPKD children have less than 10 cysts before 12 years of age, but children with more than 10 cysts generally have more severe disease. The prevalence of high blood pressure and flank and/or back pain is higher in the children with more cysts and larger kidneys. Polyuria and impaired urinary concentration are also associated with larger kidneys (CitationFick et al 1994). Children with more than 10 cysts, compared to children with less than 10 cysts, had a greater annual increase in renal volumes even though they were younger at their first and last visits. Evaluating the number of renal cysts might be more practical in children than measuring age-adjusted renal volumes. Also children with higher blood pressure exhibited greater renal growth rate. In contrast to adults, in children gender distribution does not seem to have an impact on renal disease progression rate (CitationFick et al 2001).
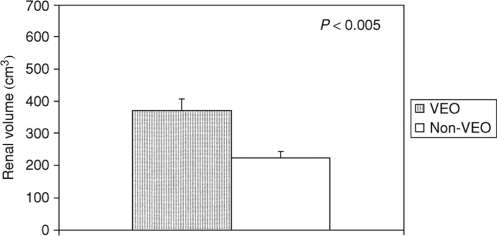
In a recent ADPKD study in children, those cases with very early onset of disease (VEO) (ie, diagnosed before 18 months of life) and children diagnosed between 18 months and 18 years of age (non-VEO) have been evaluated and compared. When adjusted for age, the VEO group had larger renal volumes, more frequent hypertension, and higher serum creatinine, lower creatinine clearance and estimated GFR compared with non-VEO children. LVMI was also greater in VEO children (). Proteinuria, hematuria, and symptoms at diagnosis in both groups were correlated with significantly larger age-adjusted renal volumes (CitationShamshirsaz et al 2005). Interestingly, over 90% of VEO children maintained preserved renal function well into childhood and in general their growth was normal (CitationSedman et al 1987; CitationTaitz et al 1987; CitationFick et al 1993; CitationShamshirsaz et al 2005). However, because of the potential for severe complications such as hypertension and renal insufficiency, these children should be closely and regularly monitored. Due to the significant association between larger kidneys and early progression to ESRD, the beneficial effects of reducing renal growth and LVMI by RAAS blockers was considered important. Such a prospective study is underway at the University of Colorado Health Sciences Center.
Similar to the adult population with ADPKD, there is also a greater prevalence of MVP among the pediatric population (CitationIvy et al 1995). CitationLeier at al (1984) reported that 36% of ADPKD patients have bicuspid aortic valve, but the prevalence of congenital cardiac diseases in adults with ADPKD is unknown.
Proteinuria
The presence of proteinuria may be an indicator of sub-clinical end-organ damage in addition to kidney damage, including the presence of LVH and systemic atherosclerosis. Recent studies have shown significant association between LVMI and proteinuria (CitationLeoncini, Viazzi et al 2002; CitationLeoncini, Sacchi et al 2002; CitationPontremoli et al 2002). Microalbuminuria (30–300 mg/24 h in adults), a sensitive measure of urinary albumin excretion, has been useful in identifying the risk of developing diabetic nephropathy. It has also been positively related to an increased risk of cardiovascular mortality in essential hypertensive patients, especially old, obese, and male patients. In ADPKD patients, proteinuria is considered to be an important predictor of renal disease progression. In a study of 270 ADPKD patients only 18% of cases presented with established proteinuria (>300 mg/day). Interestingly all these patients had hypertension and also had large renal volumes. Renal functional impairment was greater in these patients who also showed a faster tendency for disease progression and an increased incidence of hematuria (CitationChapman et al 1994). Furthermore, 41% of hypertensive ADPKD patients with LVH showed some degree of microalbuminuria (30–300 mg/24 h). These patients with microalbuminuria and LVH had higher MAP (MAP 107 vs 113, p < 0.005) and renal volumes (535 vs 853 cm3, p < 0.01) (CitationEcder et al 2001). A 5-year prospective, randomized study of 24 ADPKD patients was conducted to examine the antialbuminuric effect of amlodipine, a calcium channel blocker, versus enalapril, an ACE inhibitor, on urinary albumin/creatinine ratio. Albumin to creatinine ratio increased significantly in the amlodipine related patients, but was stable in the enalapril-treated ADPKD patients at comparable levels of BP control. (CitationEcder et al 2000) Thus, we can tentatively propose that the sustained antialbuminuric effect of ACE inhibitors may improve the long-term renal and cardiovascular prognosis in ADPKD patients.
Pain
The most frequent complaint in ADPKD patients is pain, occurring in approximately 60% of patients (CitationGabow 1990). Severe abdominal and flank pain is a known frustrating experience among ADPKD patients (Mulutinovic et al 1984; Mulutinovic et al 1990). In a recent study of 171 ADPKD patients, 71.3% reported lower back pain, which was the most common pain pattern in this group. 29.6% of this group had radiculopathy symptoms. Abdominal pain was seen in 61.4% of these patients. The abdominal pain was described as dull pain (49.5%), uncomfortable fullness (42.7%), stabbing pain (40.4%), and cramping (33.0%) (CitationBajwa et al 2004). This pain can occur both acutely or persist on a chronic basis.
Acute pain
Cyst rupture or hemorrhage, infected cysts and renal stones are considered as the most common etiologies of acute pain. (CitationBajwa et al 2001) Generally, urinary tract infections (UTI) are seen in about 60%–70% of adult women and 20% of men with ADPKD (CitationMilutinovic et al 1984, Citation1990; CitationSklar et al 1987). The most frequent clinical presentation is cystitis, which is treated in the same manner as in the general population. Infected renal cysts may result in symptoms of pyelonephritis. Fever and or flank pain are common findings in both pyelonephritis and cyst infection. However, with cyst infection, a low-grade fever in association with localized flank pain is a common pattern. The pain does not radiate and is not relieved by positioning (CitationBajwa et al 2001). Unlike pyelonephritis, urinalysis and cultures may be negative with cyst infection, whereas blood cultures are positive in most of the cases (CitationSklar et al 1987). Although infected cysts may be difficult to diagnose, contrast enhanced CT or MRI and scintigraphy with indium-111 labeling of leukocytes are the best imaging methods (CitationBretan et al 1988; CitationChapman et al 1990). Since highly ionized, water-soluble antibiotics like penicillins and cephalosporins do not readily penetrate the renal cyst cavities, they are not effective in ADPKD. Treatment of infected ADPKD cysts should consist of lipophilic agents such as clindamycin, ciprofloxacin, norfloxacin, and trimethoprim- sulfamethoxazole. In most cases therapy should continued for 6 weeks. (CitationBennett et al 1985) In resistant cases, cyst drainage may be needed (CitationChapman et al 1990). Inadequate treatment of UTI in ADPKD may lead to perinephric abscess with consistent pain and more serious complications needing surgical interventions (CitationSweet and Keane 1979).
The prevalence of nephrolithiasis in ADPKD adult patients ranges from 20% to 36% (CitationTorres et al 1988; CitationGambaro et al 2006). Twenty to 28% of these patients are symptomatic (CitationMilutinovic et al 1990; dimitraovand Simeonov 1994), thus a substantial portion of these ADPKD patients with nephrolithiasis may be asymptomatic (CitationLevine and Grantham 1992). Flank pain, hematuria and UTI are presentations of nephrolithiasis in ADPKD. The most common renal stones in ADPKD are uric acid stones (50%), which is a much higher incidence compared to the general population (CitationLevine and Grantham 1992; CitationDaudon et al 2003). The low prevalence of stones containing calcium, such as calcium oxalate, suggests a minor role of hypercalciuria and hyperoxaluria (CitationTorres et al 1993). Stone formation in ADPKD patients mostly relates to metabolic defects, and/or urinary stasis within structural abnormalities due to cyst compression. Hypocitraturia has been found to be the most important metabolic factor, occurring in approximately 60% and 49% of ADPKD patients with and without stones respectively (CitationTorres et al 1993; CitationGrampsas et al 2000). In the same study CitationGrampsas et al (2000) have reported that the stone-forming ADPKD patients have higher cyst numbers and also larger cysts compared to non-stone formers. These findings support the theory that cyst enlargement with secondary distortion of the renal collecting system contributes to stone formation, probably through the mechanism of urine stasis (CitationTorres et al 1993). Because of the nature of the stone-related urinary tract obstruction and distension, pain is a very common symptom. Short-term administration of non-steroidal anti-inflammatory drugs (NSAIDs) (limited to two to three days) in ADPKD patients suffering from renal colic can be helpful (CitationHetherington and Philp 1986; CitationOsterlinck et al 1990; CitationMcIntire et al 1993). These agents may decrease the inflammation and edema, and thereby facilitate stone passage. Although opioids are still a basic analgesic for renal colic therapy, a large number of studies have demonstrated no differences between the usage of NSAIDs and opioids in the treatment of renal calculi. It has been shown that extracorporeal shock wave lithotripsy (ESWL) and nephrolithotomy may provide favorable results in symptomatic ADPKD patients with renal calculi (CitationAnderson et al 1993; CitationChen et al 1993; CitationTorres et al 1993; CitationDelakas et al 1997).
Renal cyst rupture in ADPKD may present with acute colicky pain, usually associated with gross hematuria (CitationDalgaard 1957). Approximately 42% to greater than 50% of ADPKD patients have experienced at least one episode of gross hematuria (CitationRovitch et al 1976; CitationGabow, Duley and Johnson 1992). In 20% of ADPKD patients gross hematuria was the initial diagnostic clue. UTI is the most frequent cause of gross hematuria, especially among women. Sports, abdominal surgeries, kidney stones and cyst ruptures also should be considered as causative factors for gross hematuria in ADPKD (CitationGabow et al 1992). The mean age of the first episode of gross hematuria in ADPKD is approximately 30 years (CitationGabow et al 1992). It should be noted that earlier onset of bleeding (ie, younger than 30 years), and more frequent episodes may indicate a risk of worsening renal function (CitationJohnson and Gabow 1997). The incidence of gross hematuria is related to both kidney size and hypertension (CitationGabow et al 1992). Usually these episodes are self-limited, lasting for 2–7 days, and are best treated with conservative management. However, in cases of prolonged bleeding accompanied by pain, renal complications including UTI and/or obstruction must be excluded (CitationGabow et al 1992). Pain associated with cyst rupture tends to be more localized. Compression of renal structures due to massive hemorrhage may cause radiating pain to shoulder or abdomen (CitationBajwa et al 2001). In severe cases of gross hematuria prolonged bed rest, hydration and analgesic administration are often needed. Usage of NSAIDs should be limited. Rarely is hematuria so serious and persistent as to necessitate either nephrectomy or renal arterial embolization. Embolization may cause a severe pain, thus a combination of pharmalogical agents (eg, NSAIDs and opioids), surgical interventions, or an epidural anesthesia may be needed (CitationOrdan et al 1983).
Chronic pain
During the prolonged course of the ADPKD, cyst formation and progressive kidney enlargement may cause a dull, chronic pain. Larger renal volumes accompanied by asymmetric, hypertrophic lumbosacral muscle groups tend to be the source of mechanical back pain. Moreover, traction of the renal capsule, related to enlarging cysts, is another cause for chronic pain in ADPKD patients. As expected, severe chronic pain correlates with larger kidneys (CitationMilutinovic et al 1990; CitationBajwa et al 2001). A sequential, approach to chronic pain should be employed. A complete, non-judgmental history taking is the first important step. The optional treatments consist of:
Physical therapy measures (heating pads, ice massage);
Psychobehavioral modification techniques;
Systemic analgesics;
Physical interventions such as:
transcutaneous electrical nerve stimulation (TENS),
autonomic plexus blockade,
neuromodulation by spinal cord stimulation,
neuraxial opioids and local anesthetics,
surgical decompression of kidney and liver cysts, and
hepatectomy (partial) and/or nephrectomy.
(CitationBajwa et al 2001, Citation2004). Initially the least toxic systemic analgesics, eg acetaminophen, should be administered. The recommended dose of acetaminophen in patients with renal disease is 500–650 mg orally every six hours. It can be increased to 1000 mg every four and six hours (CitationHenrich et al 1996). Surgical or laparoscopic deroofing is a conventional procedure as an alternative pain management in the case of kidneys with several large cysts (CitationElashry et al 1996; CitationBarr et al 1998). Sulikowski et al found pain relief after laparoscopic renal decortication in 80% of ADPKD patients after 1 year and 60% after 3 years (CitationSulikowski et al 2006). Recently a pilot study on 15 selected patients with ADPKD using 99% ethanol to decompress renal cysts, showed successful results regarding patient’s pain and disappearance of dominant large cysts (CitationSingh and Mehrotra 2006). In spite of the small group of patients, this procedure should be considered as a safe, cost effective therapeutic option in patients suffering from chronic flank pain.
Liver cysts
Hepatic cysts are the most frequent extra-renal manifestations of ADPKD. The prevalence of liver cysts is between 75 to 90% among ADPKD patients (CitationMilutinovic et al 1980; CitationRunfeld et al 1985; CitationGabow, Johnson et al 1990; CitationD’Agata et al 1994; CitationQue et al 1995). Age is an independent factor on the prevalence of hepatic cysts. In addition to age, deterioration of renal function and gender also are risk factors for liver disease in patients with ADPKD. The occurrence of hepatic cysts in female patients range from 58%–75% while among male subjects the prevalence is from 42 to 62%. It is believed that estrogen has a stimulatory effect on liver parenchyma and increases hepatic cyst volumes without a significant effect on renal volume. Findings of the largest size and number of hepatic cysts in multiparous women and postmenopausal female patients receiving hormone replacement therapy support this hypothesis (CitationGabow et al 1990; CitationSulikowski et al 2006). Patients with more severe renal disease and lower creatinine clearance may present with more liver cyst involvement (CitationGabow, Johnson et al 1990). It is of interest that polycystic liver disease may also occur in the absence of polycystic kidney disease (CitationQue et al 1995).
Hepatomegaly is the common feature of hepatic complications in ADPKD. Most patients often remain asymptomatic; however, abdominal mass, pain, early satiety, nausea, and vomiting may occur as complications of long-standing ADPKD liver disease. It should be noted that liver functional tests are usually normal in ADPKD patients with liver involvement. In symptomatic patients, however, alkaline phosphatase and Gamma-glutamyl transferase (GGT) values may be elevated in 30%–47% and 60%–70%, respectively (CitationQue et al 1995;CitationSherstha et al 1997). Despite the progressive nature of the cystic disease, the hepatic parenchyma retains its normal pattern and function (CitationEverson et al 1988). Abdominal ultrasound and computer-assisted tomography (CT) are recommended methods for radiologic evaluations (CitationLevine et al 1985; CitationEverson et al 1988).
Acute hepatic complications are cyst infection and cyst hemorrhage. Right abdominal pain accompanied with fever in ADPKD patients could be result either from right kidney or liver cyst infection (CitationTelenti et al 1990). Infected cysts account for 3% of morbidity and 2% of mortality in ADPKD patients (CitationGrunfeld et al 1985). The incidence of bacteremia is high with infected liver cysts and is detected in about 63% of cases with positive blood cultures. The causative agents are usually Gram-negative organisms such as E.coli (CitationGrunfeld et al 1985; CitationKwok and McDougall 1996), and infected liver cysts should be diagnosed and treated aggressively. In order to identify the microorganisms, blood and/or cyst fluid cultures should be employed. In contrast to renal cyst infections, both antibiotic therapy and percutaneous cyst drainage under ultrasound or CT guidance should be considered in infected liver cysts. The antibiotics administered should be capable of penetration into liver cysts, such as ciprofloxacin or amikacin. Additional studies are necessary to evaluate newer treatment methods including hepatic artery infusion of antibiotics (CitationMatoba et al 2004). Cyst hemorrhage may mimic the symptoms of an infected liver. This is a rare complication, which can be diagnosed best by MRI.
Massively enlarged liver cysts may displace adjacent organs, including the gut, diaphragm, and abdominal wall, and are responsible for early satiety, abdominal hernias, uterine prolapse and dyspnea. Pain is the most disabling complication of chronic hepatic disease. Jaundice related to biliary compression by cysts has been reported. Portal hypertension may occur secondary to portal vein compression, but this is uncommon among ADPKD patients with liver cysts. Both jaundice and portal vein compression may need surgical decompression of cysts (CitationHoward et al 1976; CitationSimonetti et al 1993; CitationDmitrewski et al 1996; CitationMisra et al 1999). Ascites and variceal bleeding are uncommon in the ADPKD patient with liver cysts, but when present investigation to rule out vascular thrombosis is indicated (CitationChauveau et al 1997). Huge liver cysts may obstruct hepatic venous outflow and cause the Budd-Chiari syndrome (CitationTorres et al 1994). Several cases have been reported following bilateral nephrectomy in ADPKD patients (CitationTorres et al 1994; CitationDioniso et al 1995; CitationDionisio et al 1997). Sclerotherapy with alcohol, resection or fenestration of the hepatic cyst wall may be helpful with hepatic venous occlusion caused by solitary enlarged cysts. In a study on 35 patients with hepatic cysts, the recurrence rate with these treatments after a 1-year follow up was 17% (CitationSimonetti et al 1993; Citationvan Sonnenberg et al 1994). Cyst aspiration and sclerosants to treat multiple cysts results in more failures with higher symptomatic recurrence risk (CitationSimonetti et al 1993; Citationvan Sonnenberg et al 1994). In these subjects stenting procedures may reestablish hepatic venous patency and abolish the pressure gradient. Generally, however, liver transplantation must be considered (CitationYang et al 2004). As mentioned above, pain in ADPKD patients with liver cysts can become both physically and psychologically incapacitating. In most cases pharmacological agents are ineffective. In the presence of extreme disabling symptoms such as malnutrition and/or intolerable pain, surgical approaches should be employed (CitationBajwa et al 2001). Cyst fenestration and combined liver resection-fenestration are two different surgical strategies. Both laparatomy and laparoscopic fenestration have been performed. These techniques are more beneficial in superficial and large cysts. Because of adverse effects and particularly the higher incidence of recurrent symptoms with the cyst fenestration technique, combined resection-fenestration seems to be the best method to reduce/relieve pain in ADPKD patents with cystic liver involvement (CitationNewman et al 1990; CitationKabbej et al 1996; CitationYang et al 2004).
Intracranial aneurysms
Intracranial aneurysms (ICAs) and rupture of intracranial aneurysms (RICAs) are critical extra-renal manifestations of ADPKD. In the general population the estimated prevalence of ICAs derived from autopsy studies is 1%–5%. In contrast ICAs are seen in approximately 4%–11.7% of ADPKD patients (Chapman et al 1992, Citation1997; CitationRinkel et al 1998; CitationThe International Study of Unruptured Intracranial Aneurysms Investigators 1998; CitationWiebers et al 2003). More than 90% of these aneurysms are less than 10 mm and over 70% less than 6 mm in diameter (CitationSchievink 1997; CitationThe International Study of Unruptured Intracranial Aneurysms Investigators 1998). Familial clustering of aneurysms has been reported both in the general population and in ADPKD patients (CitationHutson et al 1993; CitationSchievink 1997; CitationBelz et al 2001). In the general population, the incidence of subarachnoid hemorrhage (SAH) resulting from a ruptured ICA is about 0.05% per year (CitationSchievink 1997; CitationWijdicks et al 2005). The risk of SAH is approximately fivefold higher in ADPKD patients (CitationChievink et al 1992). It has been proven that previous SAH and size are of the most important factors determining the occurrence of an aneurysm rupture (CitationThe International Study of Unruptured Intracranial Aneurysms Investigators 1998; CitationBrennan and Schwartz 2000; CitationJuvela et al 2000). The International Study of Unruptured Intracranial Aneurysms (ISUIA) that included 1449 asymptomatic UIA patients has described that the risk of rupture in patients without a prior SAH is higher in the cases with aneurysms larger than 10 mm. Also, it has shown that patients with a history of bleeding aneurysm were 10 times more at risk of hemorrhage (CitationThe International Study of Unruptured Intracranial Aneurysms Investigators 1998). The peak incidence of SAH in the general population is between 55 and 60 years (CitationGreenberg 2000). Aneurysmal bleeding in ADPKD patients tends to occur at a younger age with a mean age of 35–45 years (CitationChauveau et al 1994; CitationChapman et al 1993). RICAs are responsible for approximately 80% of SAH’s. It has been shown that the mortality rate within 30 days after bleeding is almost 45% (CitationJohnston et al 1998). A high proportion of individuals surviving a SAH may experience serious disabilities. 4%–7% of deaths in patients with ADPKD are due to RICAs (CitationChapman et al 1997). An important concern in ADPKD patients with prior RICA is the recurrence of SAH. One study showed that 25% of ADPKD patients with a previous ICA developed a new ICA over a mean period of 11.4 years (CitationBelz et al 2003). The increased frequency of ICA in ADPKD and the potential life-threatening complications has led to the screening of patients. A recent prospective 10-year follow-up study, conducted at the University of Colorado Health Sciences Center, demonstrated that among patients with ADPKD who had a previous negative imaging study, only a very small percentage (2.6%) had a new finding after a mean of 9.8 year follow-up (CitationSchrier et al 2004). These findings have been verified by another study on patients with small-unruptured ICAs (<7 mm in diameter) detected by presymptomatic screening. The risk of growth and development of new aneurysms in these patients who underwent an imaging follow-up within 81 months and clinical follow-up of 92 months were low (CitationGibbs et al 2004). Therefore, which patients should be screened, remains controversial. Screening in the following sub group of patients is highly recommended: patients with positive family history of aneurysm or patients with a previous SAH, patients with high-risk occupations eg, pilots, patients with elective up-coming surgeries that might place the aneurysms at a higher risk of rupture and those who request screening. As mentioned above, SAH occurs earlier in ADPKD patients, thus the screening program for this group should start at a young age. Twenty years of age has been recommended for screening in high-risk patients (CitationChauvau et al 1994; CitationHughes and Becker 2003). Severe headache is the most prominent clinical presentation of ICAs. Many patients describe the headache as the “worst headache of their life” (CitationWijdicks et al 2005).
CT angiography (CTA) after a venous injection of a contrast agent, magnetic resonance angiography (MRA), and angiography by direct intra-arterial catheterization (catheter angiography) are the preferred imaging techniques for screening ICAs. Non-invasive procedures (CTA and MRA) are recommended as the first approach. Sensitivity and specificity is considerably higher in MRA rather than CT (sensitivity, 0.69–0.99; specificity, 1.00) (CitationWhite et al 2001; CitationKouskours et al 2004).
The optimal management of intact or ruptured aneurysms has not been well understood. In general, observation, craniotomy with clipping, or endovascular occlusions with the use of detachable coils (coiling) are treatment strategies. It has been shown that orally administered nimodipine (60 mg every four hours for 21 days) after SAH will improve the outcomes (CitationBarker and Ogilvy 1996). Incidentally discovered small aneurysms (<10 mm) need not to be treated. Close observation and regular periodic surveillances are necessary in these subjects. Although previously it was believed that clipping compared to coiling resulted in a lower recurrence rate (CitationDavid et al 1999), recent studies have demonstrated that the use of coils tend to have a lower inpatient mortality rate, shorter hospital stay, and lower costs (CitationJohnston et al 1999, Citation2000, Citation2001).
References
- AndersonRJMillerPDLinasSLRole of the renin-angiotensin system in hypertension of polycystic kidney diseaseMineral Electrolyte Metab1979213741
- AndersonGADegrootDLawsonRKPolycystic renal diseaseUrol199342358648212435
- BajwaZHGuptaSWarfieldCAPain management in polycystic kidney diseaseKidney Int20016016314411703580
- BajwaZHSialKAMalikABPain patterns in patients with polycystic kidney diseaseKidney Int2004661561915458452
- BardajiAMatinez-VeaAGutierrezCLeft ventricular mass and diastolic function in normotensive young adults with autosomal dominant polycystic kidney diseaseAm J Kidney Dis19983297059856512
- BarkerFGIIOgilvyCSEfficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysisJ Neurosurg199684405148609551
- BarryJTeichmanJMHulbertJCRole and long term results of laparoscopic decortication in solitary cystic and autosomal dominant polycystic kidney diseaseJ Urol199815970259474129
- BelzMMHughesRLKaehnyWDFamilial clustering of ruptured intracranial aneurysms in autosomal dominant polycystic kidney diseaseAm J Kidney Dis20018770611576880
- BelzMMFick-BrosnahanGMHughesRLRecurrence of intracranial aneurysms in autosomal-dominant polycystic kidney diseaseKidney Int20036318243012675859
- BengtssonUHedmanLSvalanderCAdult type of polycystic kidney disease in a new-born childActa Med Scand1975197447501155219
- BennettWMElzingaLWPulliamJPCyst fluid antibiotic concentration in autosomal dominant polycystic kidney diseaseAm J Kidney Dis1985640044073019
- BlythHOckendenBGPolycystic disease of kidney and liver presenting in childhoodJ Med Genet19718257845097134
- BrennanJWSchwartzMLUnruptured intracranial aneurysms: appraisal of the literature and suggested recommendations for surgery, using evidence-based medicine criteriaNeurosurgery20004713597211126907
- Bretan PNJrPriceDCMcClureRDLocalization of abscess in adult polycystic kidney by indium-111 leukocyte scanUrology198832169713400143
- ChapmanABThickmanDGabowPAPercutaneous cyst puncture in the treatment of cyst infection in autosomal dominant polycystic kidney diseaseAm J Kidney Dis19901625252399917
- ChapmanABSchrierRWPathogenesis of hypertension in autosomal dominant polycystic kidney diseaseSemin Nephrol199111653601767138
- ChapmanABRubinsteinDHughesRIntracranial aneurysms in autosomal dominant polycystic kidney diseaseN Engl J Med1991327916201513348
- ChapmanABJohnsonAMGabowPAIntracranial aneurysms in patients with autosomal dominant polycystic kidney disease: how to diagnose and who to screenAm J Kidney Dis199322526318213791
- ChapmanABJohnsonAMGabowPAOvert proteinuria and microalbuminuria in autosomal dominant polycystic kidney diseaseJ Am Soc Nephrol199451349547894001
- ChapmanABJohnsonAMRainguetSLeft ventricular hypertrophy in autosomal dominant polycystic kidney diseaseJ Am Soc Nephrol19978129279259356
- ChapmanABGuay-WoodfordLMGranthamJJRenal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohortKidney Int20036410354512911554
- ChauveauDPirsonYVerellen-DumoulinCIntracranial aneurysms in autosomal dominant polycystic kidney diseaseKidney Int199445114068007584
- ChauveauDGrunfeldJPDurandFAscites in a polycystic patientNephrol Dial Transplant199712228309027810
- ChenWCLeeYHHuangJKExperience using extracorporeal shock-wave lithotripsy to treat urinary calculi in problem kidneysUrol Int1993513288333089
- ChievinkWITorresVEPiepgrasDGSaccular intracranial aneurysms in autosomal dominant polycystic kidney diseaseJ Am Soc Nephrol1992388931391712
- D’AgataIDJonasMMPerez-AtaydeARCombined cystic disease of the liver and kidneySemin Liver Dis199414215287939783
- DalgaardDZBilateral polycystic disease of the kidneys. A follow up of 284 patients and their familiesActa Med Scand19573281233
- DaudonMCohen-SolalFLacourBLithiases et anomalies des voies urinaires: la composition des calculs est-elle indépendante de l’anomalie anatomique? [Urinary stones and urinary tract abnormalities. Is the stone composition independent of the anatomical abnormality?Prog Urol2003131320915000307
- DavidCAVishtehAGSpetzlerRFLate angiographic follow-up review of surgically treated aneurysmsJ Neurosurg19999139640110470813
- DelakasDDaskalopoulosGCranidisAExtracorporeal shockwave lithotripsy for urinary calculi in autosomal dominant polycystic kidney diseaseJ Endourol199711167709181443
- DimitrakovDSimeonovSStudies on nephrolithiasis in patients with autosomal dominant polycystic kidney diseaseFolia Med1994362730
- DionisioPCaramelloEBergiaRBudd-Chiari syndrome following pretransplantation nephrectomy in an ADPKD patient with liver cystsContrib Nephrol199511516368585908
- DionisioPSessaAConteFBudd-Chiari syndrome following pretransplant mononephrectomy in an autosomal dominant polycystic kidney disease patient with liver cystsNephron199775109119031284
- DmitrewskiJOlliffSBuckelsJAObstructive jaundice associated with polycystic liver diseaseHPB Surg199610117209184868
- EcderTEdelsteinCLChapmanABReversal of left ventricular hypertrophy with angiotensin converting enzyme inhibition in hypertensive patients with autosomal dominant polycystic kidney diseaseNephrol Dial Transplant19991411131610344347
- EcderTChapmanABBrosnahanGMEffect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney diseaseA J Kidney Dis20003542732
- EcderTSchrierRWHypertension in autosomal-dominant polycystic kidney disease: early occurrence and unique aspectsJ Am Soc Nephrol20011219420011134267
- EcderTEdelsteinCLFick-BrosnahanGDiuretics versus angiotensin-converting enzyme inhibitors in autosomal dominant polycystic kidney diseaseAm J Nephrol2001219810311359016
- EcderTSchrierRWHypertension and left ventricular hypertrophy in autosomal dominant polycystic kidney diseaseExpert Review of Cardiovascular Therapy200414779072
- EcderTFick-BrosnahanGSchrierRWSchrierRWPolycystic kidney diseaseDiseases of the kidney and urinary tract8Philadelphia, PALippincott Williams and Wilkins
- ElashryOMNakadaSYWolfJSJrLaparoscopy for adult polycystic kidney disease: A promising alternativeAm J Kidney Dis199627224338659498
- EllesRGHodgkinsonKAMallickNPDiagnosis of adult polycystic kidney disease by genetic markers and ultrasonograghy imaging in a voluntary family registerJ Med Genet199431115208182715
- EttingerAKahnPCWiseHMJrThe importance of selective renal angiography in the diagnosis of polycystic diseaseJ Urol1969102156615796888
- EversonGTScherzingerABerger-LeffNPolycystic liver disease: Quantitation of parenchymal and cyst volumes from computed tomography images and clinical correlates of hepatic cystsHepatology198881627343192176
- FickGMJohnsonAMStrainJDCharacteristics of very early onset autosomal dominant polycystic kidney diseaseJ Am Soc Nephrol199331863708338917
- FickGMDuleyITJohnsonAMThe spectrum of autosomal dominant polycystic kidney disease in childrenJ Am Soc Nephrol199441654608011974
- FickGMJohnsonAMHammondWSCauses of death in autosomal dominant polycystic kidney diseaseJ Am Soc Nephrol199552048567579053
- FickGMTranZVJohnsonAMProgression of autosomal-dominant polycystic kidney disease in childrenKidney Int20015916546211318935
- Fick-BrosnahanGMBelzMMMcFannKKRelationship between renal volume growth and renal function in autosomal dominant polycystic kidney disease: a longitudinal studyAm J Kidney Dis20023911273412046022
- GabowPAAutosomal dominant polycystic kidney disease – more than a renal diseaseAm J Kidney Dis199016403132239929
- GabowPADuleyIJohnsonAMClinical profiles of gross hematuria in autosomal dominant polycystic kidney diseaseAm J Kidney Dis19922014031496966
- GabowPAChapmanABJohnsonAMRenal structure and hypertension in autosomal dominant polycystic kidney diseaseKidney Int1990381177802074659
- GabowPAJohnsonAMKaehnyWDRisk factors for the development of hepatic cysts in autosomal dominant polycystic kidney diseaseHepatology199011103372365280
- GabowPAKimberlingWJStrainJDUtility of ultrasonograghy in the diagnosis of autosomal dominant polycystic kidney disease in childrenJ Am Soc Nephrol19978105109013454
- GabowPAJohnsonAMKaehnyWDFactors affecting the progression of renal disease in autosomal-dominant polycystic kidney diseaseKidney Int1992411311191614046
- GambaroGFabrisAPuliattaDLithiasis in cystic kidney disease and malformations of the urinary tractUrol Res2006341027 Epub 2006 Jan 1416416113
- GarelLSauvegrainJFiliatraultDDominant polycystic disease of the kidney in a newborn child. Report of one caseAnn Radiol (Paris)19832618366881878
- GibbsGFHustonJ3QianQFollow-up of intracranial aneurysms in autosomal-dominant polycystic kidney diseaseKidney Int2004651621715086900
- GrampsasSAChandhokePSFanJAnatomic and metabolic risk factors for nephrolithiasis in patients with autosomal dominant polycystic kidney diseaseAm J Kidney Dis20003653710873872
- GranthamJJTorresVEChapmanABVolume progression in polycystic kidney diseaseN Engl J Med200635421223016707749
- GranthamJJTorresVEChapmanABVolume progression in polycystic kidney diseaseN Engl J Med200635421223016707749
- GreenbergMSGreenbergMSSAH and aneurysmsHandbook of neurosurgery20005New YorkThieme Medical754803
- GrunfeldJPAlbouzeGJungersPLiver changes and complications in adult polycystic kidney diseaseAdv Nephrol Necker Hosp1985141202983516
- HarrisPCBaeKTRossettiSCyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney diseaseJ Am Soc Nephrol200617301319 Epub 2006 Oct 1117035604
- HenrichWLAgodoaLEBarrettBAnalgesics and the kidney: summary and recommendations to the Scientific Advisory Board of the National Kidney Foundation from an Ad Hoc Committee of the National Kidney FoundationAm J Kidney Dis19962716258546133
- HetheringtonJWPhilpMHDiclofenac sodium versus pethidine in acute renal colicBr Med J198629223783081085
- HjelmesaethJHartmannAInsulin resistance in patients with adult polycystic kidney diseaseNephrol Dial Transplant1999142521210528694
- HossackKFLeddyCLJohnsonAMEchocardiographic findings in autosomal dominant polycystic kidney diseaseN Engl J Med1988319907123419455
- HowardRJHansonRFDelaneyJPJaundice associated with polycystic liver disease. Relief by surgical decompression of the cystsArch Surg197611181617938229
- HughesPDBeckerGJScreening for intracranial aneurysms in autosomal dominant polycystic kidney diseaseNephrology (Carlton)2003841637015012716
- HutsonJTorresVESullivanPPValue of magnetic resonance angiography for the detection of intracranial aneurysms in autosomal dominant polycystic kidney diseaseJ Am Soc Nephrol19933187178338918
- IvyDDShafferEMJohnsonAMCardiovascular abnormalities in children with autosomal dominant polycystic kidney diseaseJ Am Soc Nephrol19955203267579051
- JohnsonAMGabowPAIdentification of patients with autosomal dominant polycystic kidney disease at highest risk for endstage renal diseaseJ Am Soc Nephrol19978156079335384
- JohnstonSCDudleyRAGressDRSurgical and endovascular treatment of unruptured cerebral aneurysms at university hospitalsNeurology199952179980510371526
- JohnstonSCSelvinSGressDRThe burden, trends, and demographics of mortality from subarachnoid hemorrhageNeurology1998501413189595997
- JohnstonSCWilsonCBHalbachVVEndovascular and surgical treatment of unruptured cerebral aneurysms: comparison of risksAnn Neurol200048111910894211
- JohnstonSCZhaoSDudleyRATreatment of unruptured cerebral aneurysms in CaliforniaStroke20013259760511239174
- JonesDBArterial and glomerular lesions associated with severe hypertension. Light and electron microscopic studiesLab Invest197431303134412080
- JuvelaSPorrasMPoussaKNatural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm ruptureJ Neurosurg2000933798710969934
- KabbejMSauvanetAChauveauDLaparoscopic fenestration in polycystic liver diseaseBr J Surg19968316977019038542
- KayeCLewyPRCongenital appearance of adult-type (autosomal dominant) polycystic kidney diseaseJ Pediatr197485807104419701
- KelleherCLMcFannKKJohnsonAMCharacteristics of hypertension in young adults with autosomal dominant polycystic kidney diseaseAm J Hypertens20041710293415533729
- KimberlingWJKumarSGabowPAAutosomal dominant polycystic kidney disease: Localization of the second gene to chromosome 4q13-q23Genomics199318467728307555
- KouskourasCCharitantiAGiavroglouCIntracranial aneurysms: evaluation using CTA and MRA: correlation with DSA and intraoperative findingsNeuroradiology2004468425015448952
- KwokCGMcDougallIRPersistent fever in a patient with polycystic kidney and liver diseases and bilateral hip prosthesesJ Nucl Med199637206258970535
- LeierCVBakerPBKilmanJWCardiovascular abnormalities associated with adult polycystic kidney diseaseAnn Intern Med198410068386231874
- LemboGNapoliRCapaldoBAbnormal sympathetic over activity evoked by insulin in the skeletal muscle of patients with essential hypertensioJ Clin Invest1992902491634611
- LeonciniGSacchiGRaveraMMicroalbuminuria is an integrated marker of sub-clinical organ damage in primaryhypertensionJ Hum Hypertens2002167989 Review11850764
- LeonciniGViazziFMicroalbuminuria identifies overall cardiovascular risk in essential hypertension: an artificial neural network-based approachJ Hypertens20022013152112131528
- LevineECookLTGranthamJJLiver cysts in autosomal-dominant polycystic kidney disease: clinical and computed tomographic studyAJR Am J Roentgenol1985145229333875218
- LevineEGranthamJJCalcified renal stones and cyst calcifications in autosomal dominant polycystic kidney disease: clinical and CT study in 84 patientsAJR Am J Roentgenol199215977811609726
- Loghman-AdhamMSotoCEInagamiTThe intrarenal rennin-angiotensin system in autosomal dominant polycystic kidney diseaseAm J Physiol Renal Physiol2004287F7758815187005
- LumiahoAPihlajamakiJHartikainenJInsulin resistance is related to left ventricular hypertrophy in patients with polycystic kidney disease type 1Am J Kidney Dis20034112192412776274
- Martinez-VeaABardajiAGutierrezCEchocardiographic evaluation in patients with autosomal dominant polycystic kidney disease and end-stage renal diseaseAm J Kidney Dis1999342647210430973
- Martinez-VeaAValeroFABardajiA Left ventricular hypertrophy in hypertensive patients with autosomal dominant polycystic kidney disease: influence of blood pressure and humoral and neurohormonal factorsAm J Nephrol20002019320010878400
- MatobaMTonamiHKuginukiMIntermittent hepatic artery antibiotic infusion therapy for pyogenic hepatic abscessActa Radiol200445131715164773
- McdonaldRAWatkinsSLAvnerEDBarrattTMAvnerEDHarmonWPolycystic kidney disease in Pediatric Nephrology19994Baltimore, LippincottWilliams and Wilkins45974
- McIntireSCRubensteinRCGartnerJCAcute flank pain and reversible renal dysfunction associated with nonsteroidal anti-inflammatory drug usePediatrics199392459638361805
- MilutinovicJFialkowPJRuddTGLiver cysts in patients with autosomal dominant polycystic kidney diseaseAm J Med19806874147377224
- MilutinovicJFialkowPJAgodoaLYAutosomal dominant polycystic kidney disease: Symptoms and clinical findingsQ J Med198453511226240069
- MilutinovicJFialkowPJAgodoaLYClinical manifestations of autosomal dominant polycystic kidney disease in patients older than 50 yearsAm J Kidney Dis199015237432137671
- MisraALoyalkaPAlvaFPortal hypertension due to extensive hepatic cysts in autosomal dominant polycystic kidney diseaseSouth Med J199992626710372860
- NewmanKDTorresVERakelaJTreatment of highly symptomatic polycystic liver disease. Preliminary experience with a combined hepatic resection-fenestration procedureAnn Surg19902123072363601
- NicolauCTorraRBadenasCAutosomal dominant polycystic kidney disease types 1 and 2: assessment of US sensitivity for diagnosisRadiology1999213273610540671
- O’NeillWCRobbinMLBaeKTSonographic assessment of the severity and progression of autosomal dominant polycystic kidney disease: the Consortium of Renal Imaging Studies in Polycystic Kidney Disease (CRISP)Am J Kidney Dis20054610586416310571
- OosterlinckWPhilipNHCharigCA double blind single dose comparison of intramuscular ketorolac, tromethamine and pethidine in the treatment of renal colicJ Clin Pharmacol199030336412341581
- OrdanGHBabcockNCMocnikJJPain control following renal infarction/ablation using continuous epidural combined anesthesia/analgesiaJ Urol198313086126632091
- PKD FoundationPolycystic Kidney DiseaseThe Most Common Life-Threatening Genetic Disease2000Kansas CityPolycystic Kidney Research Foundation
- PontremoliRLeonciniGRaveraMMicroalbuminuria, cardiovascular, and renal risk in primary hypertensionJ Am Soc Nephrol200213S1697212466308
- PretoriusDHLeeMEManco-JohnsonMLDiagnosis of autosomal dominant polycystic kidney disease in utero and in the young infantJ Ultrasound Med19876249553295293
- QueFNagorneyDMGrossJBJrLiver resection and cyst fenestration in the treatment of severe polycystic liver diseaseGastroenterology1995108487947835591
- RinkelGJEDjibutiMAlgraAPrevalence and risk of rupture of intracranial aneurysms: a systemic reviewStroke19982925169445359
- RocchiniAPMooreheadCDeRemerSHyperinsulinemia and the aldosterone and pressor responses to angiotensin IIHypertension19901586162190930
- RovitchLLermanPHDrabkinJRuptured renal cysts in polycystic diseaseUrol197676011246770
- RunfeldJPAlbouzeGJungersPLiver changes and complications in adult polycystic kidney diseaseAdv Nephrol Necker Hosp141202983516
- SchievinkWIIntracranial aneurysmsN Engl J Med19973362840 Erratum, N Engl J Med, 336: 12678970938
- SchrierRWMcFannKJohnsonACardiac and renal effects of standard versus rigorous blood pressure control in autosomal dominant polycystic kidney disease: Results of a seven-year prospective randomized studyJ Am Soc Nephrol2002131733912089368
- SchrierRWMcFannKKJohnsonAMEpidemiological study of kidney survival in autosomal dominant polycystic kidney diseaseKidney Int2003636788512631134
- SchrierRWJohnsonAMMcFannKThe role of parental hypertension in the frequency and age of onset of hypertension in offspring with autosomal dominant polycystic kidney diseaseKidney Int2003641792914531813
- SchrierRWBelzMMJohnsonAMRepeat imaging for intracranial aneurysms in patients with autosomal dominant polycystic kidney disease with initially negative studies: a prospective ten-year follow-upJ Am Soc Nephrol2004151023815034105
- SedmanABellPManco-JohnsonMAutosomal dominant polycystic kidney disease in childhood: a longitudinal studyKidney Int198731100053295357
- ShamshirsazAABekheirniaMRKamgarMAutosomal dominant polycystic kidney disease in infancy and childhood: Progression and outcomeKidney Int20056822182416221221
- ShersthaRMcKinleyCRussPPostmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney diseaseHepatology199726128269362373
- SimonettiGProfiliSSergiacomiGLPercutaneous treatment of hepatic cysts by aspiration and sclerotherapyCardiovasc Intervent Radiol1993168148485748
- SinghIMehrotraGSelective ablation of symptomatic dominant renal cysts using 99% ethanol in adult polycystic kidney diseaseUrology2006684827 discussion 487–817010722
- SklarAHCaruanaRJLamersJERenal infections in patients with adult polycystic kidney diseaseAm J Kidney Dis1987108183300296
- SulikowskiTKaminskiMRozanskiJLaparoscopic removal of renal cysts in patients with ADPKD as an alternative method of treatment and patient preparation for kidney transplantation: preliminary resultsTransplant Proc20063823716504654
- SweetRKeaneWFPerinephric abscess in patients with polycystic kidney disease undergoing chronic hemodialysisNephron19792323740481659
- TaitzLSBrownCBBlankCEScreening for polycystic kidney disease: importance of clinical presentation in the newbornArch Dis Child1987624593545099
- TaylorMJohnsonAMTisonMEarlier diagnosis of autosomal dominant polycystic kidney disease: importance of family history and implications for cardiovascular and renal complicationsAm J Kidney Dis2005464152316129202
- TelentiATorresVEGrossJBJrHepatic cyst infection in autosomal dominant polycystic kidney diseaseMayo Clin Proc199065933422198396
- The International Study of Unruptured Intracranial Aneurysms InvestigatorsUnruptured intracranial aneurysms – risk of rupture and risks of surgical interventionN Engl J Med19983391725339867550
- TimioMMonarcaCPedeSThe spectrum of cardiovascular abnormalities in autosomal dominant polycystic kidney disease: a 10-year follow-up in a five-generation kindredClin Nephrol199237245511606775
- TorresVEDonovanKAScicliGSynthesis of renin by tubulocystic epithelium in autosomal-dominant polycystic kidney diseaseKidney Int199242364731405319
- TorresVEEricksonSBSmithLHThe association of nephrolithiasis and autosomal dominant polycystic kidney diseaseAm J Kidney Dis198811318253354568
- TorresVERastogiSKingBFHepatic venous outflow obstruction in autosomal dominant polycystic kidney diseaseJ Am Soc Nephrol199451186927873728
- TorresVEWilsonDMBurnettJCEffect of inhibition of converting on renal hemodynamics and sodium enzyme management in polycystic kidney diseaseMayo Clin Proc1991661010171921483
- TorresVEWilsonDMHatteryRRRenal stone disease in autosomal dominant polycystic kidney diseaseAm J Kidney Dis199322513198213789
- van SonnenbergEWroblickaJTD’AgostinoHBSymptomatic hepatic cysts: Percutaneous drainage and sclerosisRadiology1994190387928284385
- WanSKCochlinDLSonographic and computed tomographic features of polycystic disease of the liverGastrointest Radiol199015310122210203
- WatsonMLMacnicolAMAllanPLEffects of angiotensin converting enzyme inhibition in adult polycystic kidney diseaseKidney Int199241206101317477
- WhitePMTeasdaleEMWardlawJMIntracranial aneurysms: CT angiography and MR angiography for detection prospective blinded comparison in a large patient cohortRadiology20012197394911376263
- WiebersDOWhisnantJPHustonJIIIUnruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatmentLancet20033621031012867109
- WijdicksEFKallmesDFMannoEMSubarachnoid hemorrhage: neurointensive care and aneurysm repairMayo Clin Proc200580550915819296
- YangGSLiQGLuJHCombined hepatic resection with fenestration for highly symptomatic polycystic liver disease: A report on seven patientsWorld J Gastroenterol200410259860115300916