Abstract
This article reviews recent research on cannabinoid analgesia via the endocannabinoid system and non-receptor mechanisms, as well as randomized clinical trials employing cannabinoids in pain treatment. Tetrahydrocannabinol (THC, Marinol®) and nabilone (Cesamet®) are currently approved in the United States and other countries, but not for pain indications. Other synthetic cannabinoids, such as ajulemic acid, are in development. Crude herbal cannabis remains illegal in most jurisdictions but is also under investigation. Sativex®, a cannabis derived oromucosal spray containing equal proportions of THC (partial CB1 receptor agonist) and cannabidiol (CBD, a non-euphoriant, anti-inflammatory analgesic with CB1 receptor antagonist and endocannabinoid modulating effects) was approved in Canada in 2005 for treatment of central neuropathic pain in multiple sclerosis, and in 2007 for intractable cancer pain. Numerous randomized clinical trials have demonstrated safety and efficacy for Sativex in central and peripheral neuropathic pain, rheumatoid arthritis and cancer pain. An Investigational New Drug application to conduct advanced clinical trials for cancer pain was approved by the US FDA in January 2006. Cannabinoid analgesics have generally been well tolerated in clinical trials with acceptable adverse event profiles. Their adjunctive addition to the pharmacological armamentarium for treatment of pain shows great promise.
Introduction
Chronic pain represents an emerging public health issue of massive proportions, particularly in view of aging populations in industrialized nations. Associated facts and figures are daunting: In Europe, chronic musculoskeletal pain of a disabling nature affects over one in four elderly people (CitationFrondini et al 2007), while figures from Australia note that older half of older people suffer persistent pain, and up to 80% in nursing home populations (CitationGibson 2007). Responses to an ABC News poll in the USA indicated that 19% of adults (38 million) have chronic pain, and 6% (or 12 million) have utilized cannabis in attempts to treat it (CitationABC News et al 2005).
Particular difficulties face the clinician managing intractable patients afflicted with cancer-associated pain, neuropathic pain, and central pain states (eg, pain associated with multiple sclerosis) that are often inadequately treated with available opiates, antidepressants and anticonvulsant drugs. Physicians are seeking new approaches to treatment of these conditions but many remain concerned about increasing governmental scrutiny of their prescribing practices (CitationFishman 2006), prescription drug abuse or diversion. The entry of cannabinoid medicines to the pharmacopoeia offers a novel approach to the issue of chronic pain management, offering new hope to many, but also stoking the flames of controversy among politicians and the public alike.
This article will attempt to present information concerning cannabinoid mechanisms of analgesia, review randomized clinical trials (RCTs) of available and emerging cannabinoid agents, and address the many thorny issues that have arisen with clinical usage of herbal cannabis itself (“medical marijuana”). An effort will be made to place the issues in context and suggest rational approaches that may mitigate concerns and indicate how standardized pharmaceutical cannabinoids may offer a welcome addition to the pharmacotherapeutic armamentarium in chronic pain treatment.
Cannabinoids and analgesic mechanisms
Cannabinoids are divided into three groups. The first are naturally occurring 21-carbon terpenophenolic compounds found to date solely in plants of the Cannabis genus, currently termed phytocannabinoids (CitationPate 1994). The best known analgesic of these is Δ9-tetrahydrocannabinol (henceforth, THC)(), first isolated and synthesized in 1964 (CitationGaoni and Mechoulam 1964). In plant preparations and whole extracts, its activity is complemented by other “minor” phytocannabinoids such as cannabidiol (CBD) (), cannabis terpenoids and flavonoids, as will be discussed subsequently.
Long before mechanisms of cannabinoid analgesia were understood, structure activity relationships were investigated and a number of synthetic cannabinoids have been developed and utilized in clinical trials, notably nabilone (Cesamet®, Valeant Pharmaceuticals), and ajulemic acid (CT3, IP-751, Indevus Pharmaceuticals) ().
In 1988, the first cannabinoid receptor was identified (CB1) (CitationHowlett et al 1988) and in 1993, a second was described (CB2) (CitationMunro et al 1993). Both are 7-domain G-protein coupled receptors affecting cyclic-AMP, but CB1 is more pervasive throughout the body, with particular predilection to nociceptive areas of the central nervous system and spinal cord (CitationHerkenham et al 1990; CitationHohmann et al 1999), as well as the peripheral nervous system (CitationFox et al 2001; CitationDogrul et al 2003) wherein synergy of activity between peripheral and central cannabinoid receptor function has been demonstrated (CitationDogrul et al 2003). CB2, while commonly reported as confined to lymphoid and immune tissues, is also proving to be an important mediator for suppressing both pain and inflammatory processes (CitationMackie 2006). Following the description of cannabinoid receptors, endogenous ligands for these were discovered: anandamide (arachidonylethanolamide, AEA) in 1992 in porcine brain (CitationDevane et al 1992), and 2-arachidonylglycerol (2-AG) in 1995 in canine gut tissue (CitationMechoulam et al 1995) (). These endocannabinoids both act as retrograde messengers on G-protein coupled receptors, are synthesized on demand, and are especially active on glutamatergic and GABA-ergic synapses. Together, the cannabinoid receptors, their endogenous ligands (“endocannabinoids”) and metabolizing enzymes comprise the endocannabinoid system (ECS) (CitationDi Marzo et al 1998), whose functions have been prosaically termed to be “relax, eat, sleep, forget and protect” (p. 528). The endocannabinoid system parallels and interacts at many points with the other major endogenous pain control systems: endorphin/enkephalin, vanilloid/transient receptor potential (TRPV), and inflammatory. Interestingly, our first knowledge of each pain system has derived from investigation of natural origin analgesic plants, respectively: cannabis (Cannabis sativa, C. indica) (THC, CBD and others), opium poppy (Papaver somniferun) (morphine, codeine), chile peppers (eg, Capsicum annuum, C. frutescens, C. chinense) (capsaicin) and willow bark (Salix spp.) (salicylic acid, leading to acetylsalicylic acid, or aspirin). Interestingly, THC along with AEA and 2-AG, are all partial agonists at the CB1 receptor. Notably, no endocannabinoid has ever been administered to humans, possibly due to issues of patentability and lack of commercial feasibility (Raphael Mechoulam, pers comm 2007). For an excellent comprehensive review of the endocannabinoid system, see CitationPacher et al (2006), while Walker and Huang have provided a key review of antinociceptive effects of cannabinoids in models of acute and persistent pain (CitationWalker and Huang 2002).
A clinical endocannabinoid deficiency has been postulated to be operative in certain treatment-resistant conditions (CitationRusso 2004), and has received recent support in findings that anandamide levels are reduced over controls in migraineurs (Sarchielli et al 2006), that a subset of fibromyalgia patients reported significant decreased pain after THC treatment (CitationSchley et al 2006), and the active role of the ECS in intestinal pain and motility in irritable bowel syndrome (CitationMassa and Monory 2006) wherein anecdotal efficacy of cannabinoid treatments have also been claimed.
The endocannabinoid system is tonically active in control of pain, as demonstrated by the ability of SR141716A (rimonabant), a CB1 antagonist, to produce hyperalgesia upon administration to mice (CitationRichardson et al 1997). As mentioned above, the ECS is active throughout the neuraxis, including integrative functions in the periacqueductal gray (CitationWalker et al 1999a; CitationWalker et al 1999b), and in the ventroposterolateral nucleus of the thalamus, in which cannabinoids proved to be 10-fold more potent than morphine in wide dynamic range neurons mediating pain (CitationMartin et al 1996). The ECS also mediates central stress-induced analgesia (CitationHohmann et al 2005), and is active in nociceptive spinal areas (CitationHohmann et al 1995; CitationRichardson et al 1998a) including mechanisms of wind-up (CitationStrangman and Walker 1999) and N-methyl-D-aspartate (NMDA) receptors (CitationRichardson et al 1998b). It was recently demonstrated that cannabinoid agonists suppress the maintenance of vincristine-induced allodynia through activation of CB1 and CB2 receptors in the spinal cord (CitationRahn et al 2007). The ECS is also active peripherally (CitationRichardson et al 1998c) where CB1 stimulation reduces pain, inflammation and hyperalgesia. These mechanisms were also proven to include mediation of contact dermatitis via CB1 and CB2 with benefits of THC noted systemically and locally on inflammation and itch (CitationKarsak et al 2007). Recent experiments in mice have even suggested the paramount importance of peripheral over central CB1 receptors in nociception of pain (CitationAgarwal et al 2007)
Cannabinoid agonists produce many effects beyond those mediated directly on receptors, including anti-inflammatory effects and interactions with various other neurotransmitter systems (previously reviewed (CitationRusso 2006a). Briefly stated, THC effects in serotonergic systems are widespread, including its ability to decrease 5-hydroxytryptamine (5-HT) release from platelets (CitationVolfe et al 1985), increase its cerebral production and decrease synaptosomal uptake (CitationSpadone 1991). THC may affect many mechanisms of the trigeminovascular system in migraine (CitationAkerman et al 2003; CitationAkerman et al 2004; CitationAkerman et al 2007; CitationRusso 1998; CitationRusso 2001). Dopaminergic blocking actions of THC (CitationMüller-Vahl et al 1999) may also contribute to analgesic benefits.
The glutamatergic system is integral to development and maintenance of neuropathic pain, and is responsible for generating secondary and tertiary hyperalgesia in migraine and fibromyalgia via NMDA mechanisms (CitationNicolodi et al 1998). Thus, it is important to note that cannabinoids presynaptically inhibit glutamate release (CitationShen et al 1996), THC produces 30%–40% reduction in NMDA responses, and THC is a neuroprotective antioxidant (CitationHampson et al 1998). Additionally, cannabinoids reduce hyperalgesia via inhibition of calcitonin gene-related peptide (CitationRichardson et al 1998a). As for Substance P mechanisms, cannabinoids block capsaicin-induced hyperalgesia (CitationLi et al 1999), and THC will do so at sub-psychoactive doses in experimental animals (CitationKo and Woods 1999). Among the noteworthy interactions with opiates and the endorphin/enkephalin system, THC has been shown to stimulate beta-endorphin production (CitationManzanares et al 1998), may allow opiate sparing in clinical application (CitationCichewicz et al 1999), prevents development of tolerance to and withdrawal from opiates (CitationCichewicz and Welch 2003), and rekindles opiate analgesia after a prior dosage has worn off (CitationCichewicz and McCarthy 2003). These are all promising attributes for an adjunctive agent in treatment of clinical chronic pain states.
The anti-inflammatory contributions of THC are also extensive, including inhibition of PGE-2 synthesis (CitationBurstein et al 1973), decreased platelet aggregation (CitationSchaefer et al 1979), and stimulation of lipooxygenase (CitationFimiani et al 1999). THC has twenty times the anti-inflammatory potency of aspirin and twice that of hydrocortisone (CitationEvans 1991), but in contrast to all nonsteroidal anti-inflammatory drugs (NSAIDs), demonstrates no cyclo-oxygenase (COX) inhibition at physiological concentrations (CitationStott et al 2005a).
Cannabidiol, a non-euphoriant phytocannabinoid common in certain strains, shares neuroprotective effects with THC, inhibits glutamate neurotoxicity, and displays antioxidant activity greater than ascorbic acid (vitamin C) or tocopherol (vitamin E) (CitationHampson et al 1998). While THC has no activity at vanilloid receptors, CBD, like AEA, is a TRPV1 agonist that inhibits fatty acid amidohydrolase (FAAH), AEA’s hydrolytic enzyme, and also weakly inhibits AEA reuptake (CitationBisogno et al 2001). These activities reinforce the conception of CBD as an endocannabinoid modulator, the first clinically available (CitationRusso and Guy 2006). CBD additionally affects THC function by inhibiting first pass hepatic metabolism to the possibly more psychoactive 11-hydroxy-THC, prolonging its half-life, and reducing associated intoxication, panic, anxiety and tachycardia (CitationRusso and Guy 2006). Additionally, CBD is able to inhibit tumor necrosis factor-alpha (TNF-α) in its own right in a rodent model of rheumatoid arthritis (CitationMalfait et al 2000). At a time when great concern is accruing in relation to NSAIDs in relation to COX-1 inhibition (gastrointestinal ulcers and bleeding) and COX-2 inhibition (myocardial infarction and cerebrovascular accidents), CBD, like THC, inhibits neither enzyme at pharmacologically relevant doses (CitationStott et al 2005a). A new explanation of inflammatory and analgesic effects of CBD has recently come to light with the discovery that it is able to promote signaling of the adenosine receptor A2A by inhibiting the adenosine transporter (CitationCarrier et al 2006).
Other “minor phytocannabinoids” in cannabis may also contribute relevant activity (CitationMcPartland and Russo 2001). Cannabichromene (CBC) is the third most prevalent cannabinoid in cannabis, and is also anti-inflammatory (CitationWirth et al 1980), and analgesic, if weaker than THC (CitationDavis and Hatoum 1983). Cannabigerol (CBG) displays sub-micromolar affinity for CB1 and CB2 (CitationGauson et al 2007). It also exhibits GABA uptake inhibition to a greater extent than THC or CBD (CitationBanerjee et al 1975), suggesting possible utilization as a muscle relaxant in spasticity. Furthermore, CBG has more potent analgesic, anti-erythema and lipooxygenase blocking activity than THC (CitationEvans 1991), mechanisms that merit further investigation. It requires emphasis that drug stains of North American (CitationElSohly et al 2000; CitationMehmedic et al 2005), and European (CitationKing et al 2005) cannabis display relatively high concentrations of THC, but are virtually lacking in CBD or other phytocannabinoid content.
Cannabis terpenoids also display numerous attributes that may be germane to pain treatment (CitationMcPartland and Russo 2001). Myrcene is analgesic, and such activity, in contrast to cannabinoids, is blocked by naloxone (CitationRao et al 1990), suggesting an opioid-like mechanism. It also blocks inflammation via PGE-2 (CitationLorenzetti et al 1991). The cannabis sesquiterpenoid β-caryophyllene shows increasing promise in this regard. It is anti-inflammatory comparable to phenylbutazone via PGE-1 (CitationBasile et al 1988), but simultaneously acts as a gastric cytoprotective (CitationTambe et al 1996). The analgesic attributes of β-caryophyllene are increasingly credible with the discovery that it is a selective CB2 agonist (CitationGertsch et al 2007), with possibly broad clinical applications. α-Pinene also inhibits PGE-1 (CitationGil et al 1989), while linalool displays local anesthetic effects (CitationRe et al 2000).
Cannabis flavonoids in whole cannabis extracts may also contribute useful activity (CitationMcPartland and Russo 2001). Apigenin inhibits TNF-α (CitationGerritsen et al 1995), a mechanism germane to multiple sclerosis and rheumatoid arthritis. Cannflavin A, a flavone unique to cannabis, inhibits PGE-2 thirty times more potently than aspirin (CitationBarrett et al 1986), but has not been subsequently investigated.
Finally, β-sitosterol, a phytosterol found in cannabis, reduced topical inflammation 65% and chronic edema 41% in skin models (CitationGomez et al 1999).
Available cannabinoid analgesic agents and those in development
Very few randomized controlled trials (RCTs) have been conducted using smoked cannabis (CitationCampbell et al 2001) despite many anecdotal claims (CitationGrinspoon and Bakalar 1997). One such study documented slight weight gain in HIV/AIDS subjects with no significant immunological sequelae (CitationAbrams et al 2003). A recent brief trial of smoked cannabis (3.56% THC cigarettes 3 times daily) in HIV-associated neuropathy showed positive results on daily pain, hyperalgesia and 30% pain reduction (vs 15% in placebo) in 50 subjects over a treatment course of only 5 days (CitationAbrams et al 2007) (). This short clinical trial also demonstrated prominent adverse events associated with intoxication. In Canada, 21 subjects with chronic pain sequentially smoked single inhalations of 25 mg of cannabis (0, 2.5, 6.0, 9.5% THC) via a pipe three times a day for 5 days to assess effects on pain (CitationWare et al 2007) with results the authors termed “modest”: no changes were observed in acute neuropathic pain scores, and a very low number of subjects noted 30% pain relief at the end of the study (). Even after political and legal considerations, it remains extremely unlikely that crude cannabis could ever be approved by the FDA as a prescription medicine as outlined in the FDA Botanical Guidance document (CitationFood and Drug Administration 2004; CitationRusso 2006b), due to a lack of rigorous standardization of the drug, an absence of Phase III clinical trials, and pulmonary sequelae (bronchial irritation and cough) associated with smoking (CitationTashkin 2005). Although cannabis vaporizers reduce potentially carcinogenic polyaromatic hydrocarbons, they have not been totally eliminated by this technology (CitationGieringer et al 2004; CitationHazekamp et al 2006).
Table 1 Results RCTs of cannabinoids in treatment of pain syndromes ()
Oral dronabinol (THC) is marketed in synthetic form as Marinol® (Solvay Pharmaceuticals) in various countries, and was approved in the USA for nausea associated with chemotherapy in 1985, and in 1992 for appetite stimulation in HIV/AIDS. Oral dronabinol’s expense, variability of action, and attendant intoxication and dysphoria have limited its adoption by clinicians (CitationCalhoun et al 1998). Two open label studies in France of oral dronabinol for chronic neuropathic pain in 7 subjects (CitationClermont-Gnamien et al 2002) and 8 subjects (CitationAttal et al 2004), respectively, failed to show significant benefit on pain or other parameters, and showed adverse event frequently requiring discontinuation with doses averaging 15–16.6 mg THC. Dronabinol did demonstrate positive results in a clinical trial of multiple sclerosis pain in two measures (CitationSvendsen et al 2004), but negative results in post-operative pain (CitationBuggy et al 2003) (). Another uncontrolled case report in three subjects noted relief of intractable pruritus associated with cholestatic jaundice employing oral dronabinol (CitationNeff et al 2002). Some authors have noted patient preference for whole cannabis preparations over oral THC (CitationJoy et al 1999), and the contribution of other components beyond THC to therapeutic benefits (CitationMcPartland and Russo 2001). Inhaled THC leads to peak plasma concentration within 3–10 minutes, followed by a rapid fall while levels of intoxication are still rising, and with systemic bioavailability of 10%–35% (CitationGrotenhermen 2004). THC absorption orally is slow and erratic with peak serum levels in 45–120 minutes or longer. Systemic bioavailability is also quite low due to rapid hepatic metabolism on first pass to 11-hydroxy-THC. A rectal suppository of THC-hemisuccinate is under investigation (CitationBroom et al 2001), as are transdermal delivery techniques (CitationChallapalli and Stinchcomb 2002). The terminal half-life of THC is quite prolonged due to storage in body lipids (CitationGrotenhermen 2004).
Nabilone (Cesamet) (), is a synthetic dimethylheptyl analogue of THC (British Medical Association 1997) that displays greater potency and prolonged half-life. Serum levels peak in 1–4 hours (CitationLemberger et al 1982). It was also primarily developed as an anti-emetic in chemotherapy, and was recently re-approved for this indication in the USA. Prior case reports have noted analgesic effects in case reports in neuropathic pain (CitationNotcutt et al 1997) and other pain disorders (CitationBerlach et al 2006). Sedation and dysphoria were prominent sequelae. An RCT of nabilone in 41 post-operative subjects actually documented exacerbation of pain scores after thrice daily dosing (CitationBeaulieu 2006) (). An abstract of a study of 82 cancer patients on nabilone claimed improvement in pain levels after varying periods of follow-up compared to patients treated without this agent (CitationMaida 2007). However, 17 subjects dropped out, and the study was neither randomized nor controlled, and therefore is not included in .
Ajulemic acid (CT3, IP-751) (), another synthetic dimethylheptyl analogue, was employed in a Phase II RCT in 21 subjects with improvement in peripheral neuropathic pain (CitationKarst et al 2003) (). Part of its analgesic activity may relate to binding to intracellular peroxisome proliferator-activator receptor gamma (CitationLiu et al 2003). Peak plasma concentrations have generally been attained in 1–2 hours, but with delays up to 4–5 hours is some subjects (CitationKarst et al 2003). Debate surrounds the degree of psychoactivity associated with the drug (CitationDyson et al 2005). Current research is confined to the indication of interstitial cystitis.
Cannador® (IKF-Berlin) is a cannabis extract administered in oral capsules, with differing figures as to THC:CBD ratios (reviewed in (CitationRusso and Guy 2006)), generally approximately 2:1. Two pharmacokinetic studies on possibly related material have been reported (CitationNadulski et al 2005a; CitationNadulski et al 2005b). In a Phase III RCT employing Cannador in spasticity in multiple sclerosis (MS) (CAMS) (CitationZajicek et al 2003) (), no improvement was noted in the Ashworth Scale, but benefit was observed in spasm-associated pain on subjective measures. Both Marinol and Cannador produced reductions in pain scores in long-term follow-up (CitationZajicek et al 2005). Cannador was assayed in postherpetic neuralgia in 65 subjects with no observed benefit (CitationErnst et al 2005) (), and in 30 post-operative pain subjects (CANPOP) without opiates, with slight benefits, but prominent psychoactive sequelae (CitationHoldcroft et al 2006) ().
Sativex® (GW Pharmaceuticals) is an oromucosal whole cannabis-based spray combining a CB1 partial agonist (THC) with a cannabinoid system modulator (CBD), minor cannabinoids and terpenoids plus ethanol and propylene glycol excipients and peppermint flavoring (CitationMcPartland and Russo 2001; CitationRusso and Guy 2006). It was approved by Health Canada in June 2005 for prescription for central neuropathic pain in multiple sclerosis, and in August 2007, it was additionally approved for treatment of cancer pain unresponsive to optimized opioid therapy. Sativex is a highly standardized pharmaceutical product derived from two Cannabis sativa chemovars following Good Agricultural Practice (GAP) (Citationde Meijer 2004), yielding Tetranabinex® (predominantly-THC extract) and Nabidiolex® (predominantly-CBD extract) in a 1:1 ratio. Each 100 μL pump-action oromucosal Sativex spray actuation provides 2.7 mg of THC and 2.5 mg of CBD. Pharmacokinetic data are available, and indicate plasma half lives of 85 minutes for THC, 130 minutes for 11-hydroxy-THC and 100 minutes for CBD (CitationGuy and Robson 2003). Sativex effects commence in 15–40 minutes, an interval that permits symptomatic dose titration. A very favorable adverse event profile has been observed in over 2500 patient years of exposure in over 2000 experimental subjects. Patients most often ascertain an individual stable dosage within 7–10 days that provides therapeutic relief without unwanted psychotropic effects (often in the range of 8–10 sprays per day). In all RCTs, Sativex was adjunctively added to optimal drug regimens in subjects with intractable symptoms, those often termed “untreatable.” Sativex is also available by named patient prescription in the UK and the Catalonia region of Spain. An Investigational New Drug (IND) application to study Sativex in advanced clinical trials in the USA was approved by the FDA in January 2006 in patients with intractable cancer pain.
The clinical trials performed with Sativex have recently been assessed in two independent review articles (CitationBarnes 2006; CitationPérez 2006). In a Phase II clinical trial in 20 patients with neurogenic symptoms (CitationWade et al 2003), Tetranabinex, Nabidiolex, and Sativex were tested in a double-blind RCT vs placebo (). Significant improvement was seen with both Tetranabinex and Sativex on pain (especially neuropathic), but post-hoc analysis showed symptom control was best with Sativex (p < 0.0001), with less intoxication than with THC-predominant extract.
In a Phase II double-blind crossover study of intractable chronic pain (CitationNotcutt et al 2004) in 24 subjects, visual analogue scales (VAS) were 5.9 for placebo, 5.45 for Nabidiolex, 4.63 for Tetranabinex and 4.4 for Sativex extracts (p < 0.001). Sativex produced best results for pain in MS subjects (p < 0.0042) ().
In a Phase III study of pain associated due to brachial plexus avulsion (N = 48) (CitationBerman et al 2004), fairly comparable benefits were noted in Box Scale-11 pain scores with Tetranabinex and Sativex extracts ().
In a controlled double-blind RCT of central neuropathic pain, 66 MS subjects showed mean Numerical Rating Scale (NRS) analgesia favoring Sativex over placebo (CitationRog et al 2005) ().
In a Phase III double-blind, placebo-controlled trial (N = 125) of peripheral neuropathic pain with allodynia (CitationNurmikko et al 2007), Sativex produced highly statistically significant improvements in pain levels, dynamic and punctate allodynia ().
In a SAFEX study of Phase III double-blind RCT in 160 subjects with various symptoms of MS (CitationWade et al 2004), 137 patients elected to continue on Sativex after the initial study (CitationWade et al 2006). Rapid declines were noted in the first twelve weeks in pain VAS (N = 47) with slower sustained improvements for more than one year. During that time, there was no escalation of dose indicating an absence of tolerance to the preparation. Similarly, no withdrawal effects were noted in a subset of patients who voluntarily stopped the medicine abruptly. Upon resumption, benefits resumed at the prior established dosages.
In a Phase II double-blind, randomized, placebo-controlled, 5-week study of 56 rheumatoid arthritis patients with Sativex (CitationBlake et al 2006), employed nocturnal treatment only to a maximum of 6 sprays per evening (16.2 mg THC + 15 mg CBD). In the final treatment week, morning pain on movement, morning pain at rest, DAS-28 measure of disease activity, and SF-MPQ pain at present all favored Sativex over placebo ().
Results of a Phase III study (N = 177) comparing Sativex, THC-predominant extract and placebo in intractable pain due to cancer unresponsive to opiates (CitationJohnson and Potts 2005) demonstrated that Sativex produced highly statistically significant improvements in analgesia (), while the THC-predominant extract failed to produce statistical demarcation from placebo, suggesting the presence of CBD in the Sativex preparation was crucial to attain significant pain relief.
In a study of spinal injury pain, NRS of pain were not statistically different from placebo, probably due to the short duration of the trial, but secondary endpoints were clearly positive (). Finally, in an RCT of intractable lower urinary tract symptoms in MS, accompanying pain in affected patients was prominently alleviated ().
Highly statistically significant improvements have been observed in sleep parameters in virtually all RCTs performed with Sativex in chronic pain conditions leading to reduced “symptomatic insomnia” due to symptom reduction rather than sedative effects (Russo et al 2007).
Common adverse events (AE) of Sativex acutely in RCTs have included complaints of bad taste, oral stinging, dry mouth, dizziness, nausea or fatigue, but do not generally necessitate discontinuation, and prove less common over time. While there have been no head-to-head comparative RCTs of Sativex with other cannabinoid agents, certain contrasts can be drawn. Sativex (CitationRog et al 2005) and Marinol (CitationSvendsen et al 2004) have both been examined in treatment of central neuropathic pain in MS, with comparable results (). However, adverse events were comparable or greater with Marinol than with Sativex employing THC dosages some 2.5 times higher due to the presence of accompanying CBD (CitationRusso 2006b; CitationRusso and Guy 2006).
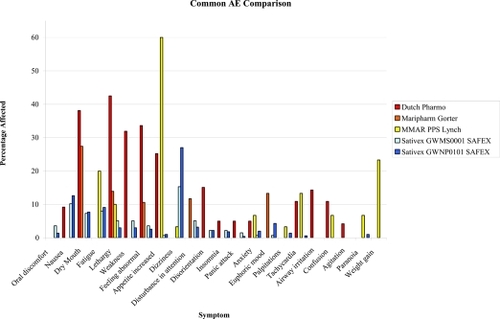
Similarly, while Sativex and smoked cannabis have not been employed in the same clinical trial, comparisons of side effect profiles can be made on the basis of SAFEX studies of Sativex for over a year and up to several years in MS and other types of neuropathic pain (CitationRusso 2006b; CitationWade et al 2006), and government-approved research programs employing standardized herbal cannabis from Canada for chronic pain (CitationLynch et al 2006) and the Netherlands for general conditions (CitationJanse et al 2004; CitationGorter et al 2005) over a period of several months or more. As is evident in Figure 2 (), all adverse events are more frequently reported with herbal cannabis, except for nausea and dizziness, both early and usually transiently reported with Sativex (see (CitationRusso 2006b) for additional discussion).
Figure 2 Comparison of adverse events (AE) encountered with long term therapeutic use of herbal cannabis in the Netherlands (CitationJanse et al 2004; CitationGorter et al 2005) and Canada (CitationLynch et al 2006), vs that observed in safety-extension (SAFEX) studies of Sativex oromucosal spray (CitationRusso 2006; CitationWade et al 2006).
Practical issues with cannabinoid medicines
Phytocannabinoids are lipid soluble with slow and erratic oral absorption. While cannabis users claim that the smoking of cannabis allows easy dose titration as a function of rapid onset, high serum levels in a short interval inevitably result. This quick onset is desirable for recreational purposes, wherein intoxication is the ultimate goal, but aside from paroxysmal disorders (eg, episodic trigeminal neuralgia or cluster headache attack), such rapid onset of activity is not usually necessary for therapeutic purposes in chronic pain states. As more thoroughly reviewed elsewhere (CitationRusso 2006b), cannabis smoking produces peak levels of serum THC above 140 ng/mL (CitationGrotenhermen 2003; CitationHuestis et al 1992), while comparable amounts of THC in Sativex administered oromucosally remained below 2 ng/mL (CitationGuy and Robson 2003).
The vast majority of subjects in Sativex clinical trials do not experience psychotropic effects outside of initial dose titration intervals () and most often report subjective intoxication levels on visual analogue scales that are indistinguishable from placebo, in the single digits out of 100 (CitationWade et al 2006). Thus, it is now longer tenable to claim that psychoactive effects are a necessary prerequisite to symptom relief in the therapeutic setting with a standardized intermediate onset cannabis-based preparation. Intoxication has remained a persistent issue in Marinol usage (CitationCalhoun et al 1998), in contrast.
Recent controversies have arisen in relation to non-steroidal anti-inflammatory drugs (NSAID), with concerns that COX-1 agents may provoke gastrointestinal ulceration and bleeding, and COX-2 drugs may increase incidents of myocardial infarction and cerebrovascular accidents (CitationFitzgerald 2004; CitationTopol 2004). In contrast, neither THC nor CBD produce significant COX inhibition at normal dosage levels (CitationStott et al 2005a).
Frequent questions have been raised as to whether psychoactive drugs may be adequately blinded (masked) in randomized clinical trials. Internal review and outside analysis have confirmed that blinding in Sativex spasticity studies has been effective (CitationClark and Altman 2006; CitationWright 2005). Sativex and its placebo are prepared to appear identical in taste and color. About half of clinical trial subjects reported previous cannabis exposure, but results of two studies (CitationRog et al 2005; CitationNurmikko et al 2007) support the fact that cannabis-experienced and naïve patients were identical in observed efficacy and adverse event reporting
Great public concern attends recreational cannabis usage and risks of dependency. The addictive potential of a drug is assessed on the basis of five elements: intoxication, reinforcement, tolerance, withdrawal and dependency. Drug abuse liability (DAL) is also assessed by examining a drug's rates of abuse and diversion. US Congress placed cannabis in Schedule I of the Controlled Substances Act in 1970, with drugs categorized as addictive, dangerous, possessing severe abuse potential and no recognized medical value. Marinol was placed in Schedule II, the category for drugs with high abuse potential and liability to produce dependency, but certain recognized medical uses, after its FDA approval in 1985. Marinol was reassigned to Schedule III in 1999, a category denoting a lesser potential for abuse or lower dependency risk after documentation that little abuse or diversion (CitationCalhoun et al 1998) had occurred. Nabilone was placed and has remained in Schedule II since 1985.
The degree to which a drug is reinforcing is determined partly by the by the rate of its delivery to the brain (CitationSamaha and Robinson 2005). Sativex has effect onset in 15–40 minutes, peaking in a few hours, quite a bit slower than drugs of high abuse potential. It has been claimed that inclusion of CBD diminishes psychoactive effects of THC, and may lower potential drug abuse liability of the preparation (see CitationRusso (2006b)) for discussion). Prior studies from Sativex clinical trials do not support the presence reinforcement or euphoria as problems in administration (CitationWade et al 2006).
Certain facets of acute cannabinoid exposure, including tachycardia, hypothermia, orthostatic hypotension, dry mouth, ocular injection, intraocular pressure decreases, etc. are subject to rapid tachyphylaxis upon continued administration (CitationJones et al 1976). No dose tolerance to the therapeutic effects of Sativex has been observed in clinical trials in over 1500 patient-years of administration. Additionally, therapeutic efficacy has been sustained for several years in a wide variety of symptoms; SAFEX studies in MS and peripheral neuropathic pain, confirm that Sativex doses remain stable or even decreased after prolonged usage (CitationWade et al 2006), with maintenance of therapeutic benefit and even continued improvement.
Debate continues as to the existence of a clinically significant cannabis withdrawal syndrome with proponents (CitationBudney et al 2004), and questioners (CitationSmith 2002). While withdrawal effects have been reported in recreational cannabis smokers (CitationSolowij et al 2002), 24 volunteers with MS who abruptly stopped Sativex after more than a year of continuous usage displayed no withdrawal symptoms meeting Budney’s criteria. While symptoms recurred after 7–10 days of abstinence from Sativex, prior levels of symptom control were readily re-established upon re-titration of the agent (CitationWade et al 2006).
Overall, Sativex appears to pose less risk of dependency than smoked cannabis based on its slower onset, lower dosage utilized in therapy, almost total absence of intoxication in regular usage, and minimal withdrawal symptomatology even after chronic administration. No known abuse or diversion incidents have been reported with Sativex to date (as of November 2007). Sativex is expected to be placed in Schedule IV of the Misuse of Drugs Act in the United Kingdom once approved.
Cognitive effects of cannabis have been reviewed (CitationRusso et al 2002; CitationFride and Russo 2006), but less study has occurred in therapeutic contexts. Effects of chronic heavy recreational cannabis usage on memory abate without sequelae after a few weeks of abstinence (CitationPope et al 2001). Studies of components of the Halstead-Reitan battery with Sativex in neuropathic pain with allodynia have revealed no changes vs placebo (CitationNurmikko et al 2007), and in central neuropathic pain in MS (CitationRog et al 2005), 4 of 5 tests showed no significant differences. While the Selective Reminding Test did not change significantly on Sativex, placebo patients displayed unexpected improvement.
Slight improvements were observed in Hospital Anxiety and Depression Scales depression and anxiety scores were noted with Sativex in MS patients with central neuropathic pain (CitationRog et al 2005), although not quite statistically significant. No long-term mood disorders have been associated with Sativex administration.
Debate continues with regard to the relationship between cannabis usage and schizophrenia (reviewed (CitationFride and Russo 2006)). An etiological relationship is not supported by epidemiological data (CitationDegenhardt et al 2003), but if present, should bear relation to dose and length of high exposure. It is likely that lower serum levels of Sativex in therapeutic usage, in conjunction with anti-psychotic properties of CBD (CitationZuardi and Guimaraes 1997), would minimize risks. Children and adolescents have been excluded from Sativex RCTs to date. SAFEX studies of Sativex have yielded few incidents of thought disorder, paranoia or related complaints.
Adverse effects of cannabinoids on immune function have been observed in experimental animals at doses 50–100 times the psychoactive level (CitationCabral 2001). In four patients using herbal cannabis therapeutically for over 20 years, no abnormalities were observed in leukocyte, CD4 or CD8 cell counts (CitationRusso et al 2002). Investigation of MS patients on Cannador revealed no major immune changes (CitationKatona et al 2005), and similarly, none occurred with smoked cannabis in a short-term study of HIV patients (CitationAbrams et al 2003). Hematological measures have been normal in all Sativex RCTs without clinical signs of immune dysfunction.
Concerns are frequently noted with new drug-drug interactions, but few have resulted in Sativex RCTs despite its adjunctive use with opiates, many other psychoactive analgesic, antidepressant and anticonvulsant drugs (CitationRusso 2006a), possibly due to CBD ability to counteract sedative effects of THC (CitationNicholson et al 2004). No effects of THC extract, CBD extract or Sativex were observed in a study of effects on the hepatic cytochrome P450 complex (CitationStott et al 2005b). On additional study, at 314 ng/ml cannabinoid concentration, Sativex and components produced no significant induction on human CYP450 (CitationStott et al 2007). Thus, Sativex should be safe to use in conjunction with other drugs metabolized via this pathway.
The Marinol patient monograph cautions that patients should not drive, operate machinery or engage in hazardous activities until accustomed to the drug’s effects (http://www.solvaypharmaceuticals-us.com/static/wma/pdf/1/3/1/9/Marinol5000124ERev52003.pdf). The Sativex product monograph in Canada (http://www.bayerhealth.ca/display.cfm?Object_ID=272&Article_ID=121&expandMenu_ID=53&prevSubItem=5_52) suggests that patients taking it should not drive automobiles. Given that THC is the most active component affecting such abilities, and the low serum levels produced in Sativex therapy (vide supra), it would be logical that that patients may be able to safely engage in such activities after early dose titration and according to individual circumstances, much as suggested for oral dronabinol. This is particularly the case in view of a report by an expert panel (CitationGrotenhermen et al 2005) that comprehensively analyzed cannabinoids and driving. It suggested scientific standards such as roadside sobriety tests, and THC serum levels of 7–10 ng/mL or less, as reasonable approaches to determine relative impairment. No studies have demonstrated significant problems in relation to cannabis affecting driving skills at plasma levels below 5 ng/mL of THC. Prior studies document that 4 rapid oromucosal sprays of Sativex (greater than the average single dose employed in therapy) produced serum levels well below this threshold (CitationRusso 2006b). Sativex is now well established as a cannabinoid agent with minimal psychotropic effect.
Cannabinoids may offer significant “side benefits” beyond analgesia. These include anti-emetic effects, well established with THC, but additionally demonstrated for CBD (CitationPertwee 2005), the ability of THC and CBD to produce apoptosis in malignant cells and inhibit cancer-induced angiogenesis (CitationKogan 2005; CitationLigresti et al 2006), as well as the neuroprotective antioxidant properties of the two substances (CitationHampson et al 1998), and improvements in symptomatic insomnia (Russo et al 2007).
The degree to which cannabinoid analgesics will be adopted into adjunctive pain management practices currently remains to be determined. Data on Sativex use in Canada for the last reported 6-month period (January-July 2007) indicated that 81% of prescriptions issued for patients in that interval were refills (data on file, from Brogan Inc Rx Dynamics), thus indicating in some degree an acceptance of, and a desire to, continue such treatment. Given their multi-modality effects upon various nociceptive pathways, their adjunctive side benefits, the efficacy and safety profiles to date of specific preparations in advanced clinical trials, and the complementary mechanisms and advantages of their combination with opioid therapy, the future for cannabinoid therapeutics appears very bright, indeed.
References
- ABC News, USA Today, Stanford Medical Center PollBroad experience with pain sparks search for relief [online]2005 URL: http://abcnews.go.com/images/Politics/979a1TheFightAgainstPain.pdf
- AbramsDIHiltonJFLeiserRJShort-term effects of cannabinoids in patients with HIV-1 infection. A randomized, placbo-controlled clinical trialAnn Intern Med20031392586612965981
- AbramsDIJayCAShadeSBCannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trialNeurology2007685152117296917
- AgarwalNPacherPTegederICannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptorsNat Neurosci200710870917558404
- AkermanSHollandPRGoadsbyPJCannabinoid (CB1) receptor activation inhibits trigeminovascular neuronsJ Pharmacol Exp Ther2007320647117018694
- AkermanSKaubeHGoadsbyPJAnandamide is able to inhibit trigeminal neurons using an in vivo model of trigeminovascular-mediated nociceptionJ Pharmacol Exp Ther2003309 566314718591
- AkermanSKaubeHGoadsbyPJAnandamide acts as a vasodilator of dural blood vessels in vivo by activating TRPV1 receptorsBr J Pharmacol200414213546015277315
- AttalNBrasseurLGuirimandDAre oral cannabinoids safe and effective in refractory neuropathic pain?Eur J Pain20048173714987627
- BanerjeeSPSnyderSHMechoulamRCannabinoids: influence on neurotransmitter uptake in rat brain synaptosomesJ Pharmacol Exp Ther19751947481168349
- BarnesMPSativex: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic painExpert Opin Pharmacother200676071516553576
- BarrettMLScuttAMEvansFJCannflavin A and B, prenylated flavones from Cannabis sativa LExperientia19864245233754224
- BasileACSertieJAFreitasPCAnti-inflammatory activity of oleoresin from Brazilian CopaiferaJ Ethnopharmacol19882210193352280
- BeaulieuPEffects of nabilone, a synthetic cannabinoid, on postoperative pain: [Les effets de la nabilone, un cannabinoide synthetique, sur la douleur postoperatoire]Can J Anaesth2006537697516873343
- BerlachDMShirYWareMAExperience with the synthetic cannabinoid nabilone in chronic noncancer painPain Med2006725916533193
- BermanJSSymondsCBirchREfficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trialPain200411229930615561385
- BisognoTHanusLDe PetrocellisLMolecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamideBr J Pharmacol20011348455211606325
- BlakeDRRobsonPHoMJubbRWPreliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritisRheumatology (Oxford)20064550216282192
- British Medical AssociationTherapeutic uses of cannabis1997AmsterdamHarwood Academic Publishers142
- BroomSLSufkaKJElsohlyMAAnalgesic and reinforcing proerties of delta9-THC-hemisuccinate in adjuvant-arthritic ratsJournal of Cannabis Therapeutics2001117182
- BudneyAJHughesJRMooreBAReview of the validity and significance of cannabis withdrawal syndromeAm J Psychiatry200416119677715514394
- BuggyDJToogoodLMaricSLack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative painPain20031061697214581124
- BursteinSLevinEVaranelliCProstaglandins and cannabis. II. Inhibition of biosynthesis by the naturally occurring cannabinoidsBiochem Pharmacol1973222905104202579
- CabralGRussoEBGrotenhermenFImmune systemCannabis and cannabinoids: Pharmacology, toxicology and therapeutic potential2001Binghamton, NYHaworth Press27987
- CalhounSRGallowayGPSmithDEAbuse potential of dronabinol (Marinol)J Psychoactive Drugs199830187969692381
- CampbellFATramberMRCarrollDAre cannabinoids an effective and safe option in the management of pain? A qualitative systematic reviewBMJ20013231611440920
- CarrierEJAuchampachJAHillardCJInhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppressionProc Natl Acad Sci USA2006103789590016672367
- ChallapalliPVStinchcombALIn vitro experiment optimization for measuring tetrahydrocannabinol skin permeationInt J Pharm20022413293912100860
- CichewiczDLMartinZLSmithFLEnhancement of mu opioid antinociception by oral delta9-tetrahydrocannabinol: Dose-response analysis and receptor identificationJ Pharmacol Exp Ther19992898596710215664
- CichewiczDLMcCarthyEAAntinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administrationJ Pharmacol Exp Ther20033041010512604676
- CichewiczDLWelchSPModulation of oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral Delta 9-tetrahydrocannabinolJ Pharmacol Exp Ther2003305812712606610
- ClarkPAltmanDAssessment of blinding in Phase III Sativex spasticity studies2006GW Pharmaceuticals56
- Clermont-GnamienSAtlaniSAttalNUtilisation thérapeutique du delta-9-tétrahydrocannabinol (dronabinol) dans les douleurs neuropathiques réfractaires. [The therapeutic use of D9-tetrahydrocannabinol (dronabinol) in refractory neuropathic pain]Presse Med20023139 Pt 11840512496714
- DavisWMHatoumNSNeurobehavioral actions of cannabichromene and interactions with delta 9-tetrahydrocannabinolGen Pharmacol198314247526301931
- de MeijerEGuyGWWhittleBARobsonPThe breeding of cannabis cultivars for pharmaceutical end usesMedicinal uses of cannabis and cannabinoids2004LondonPharmaceutical Press5570
- DegenhardtLHallWLynskeyMTesting hypotheses about the relationship between cannabis use and psychosisDrug Alcohol Depend200371374812821204
- DevaneWAHanusLBreuerAIsolation and structure of a brain constituent that binds to the cannabinoid receptorScience1992258194691470919
- Di MarzoVMelckDBisognoTEndocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory actionTrends Neurosci19982152189881850
- DogrulAGulHAkarATopical cannabinoid antinociception: synergy with spinal sitesPain200310511614499415
- DysonAPeacockMChenAAntihyperalgesic properties of the cannabinoid CT-3 in chronic neuropathic and inflammatory pain states in the ratPain20051161293715936883
- ElSohlyMARossSAMehmedicZPotency trends of delta9-THC and other cannabinoids in confiscated marijuana from 1980–1997J Forensic Sci200045243010641915
- ErnstGDenkeCReifMStandardized cannabis extract in the treatment of postherpetic neuralgia: a randomized, double-blind, placebo-controlled cross-over study2005International Association for Cannabis as Medicine2005 September 9Leiden, Netherlands
- EvansFJCannabinoids: The separation of central from peripheral effects on a structural basisPlanta Med199157S607
- FimianiCLibertyTAquirreAJOpiate, cannabinoid, and eicosanoid signaling converges on common intracellular pathways nitric oxide couplingProstaglandins Other Lipid Mediat199957233410367294
- FishmanSMPain and politics: DEA, Congress, and the courts, oh my!Pain Med2006787816533207
- FitzgeraldGACoxibs and cardiovascular diseaseN Engl J Med200435117091115470192
- Food and Drug AdministrationServices UDoHaHGuidance for industry: Botanical drug products2004US Government48
- FoxAKesinglandAGentryCThe role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic painPain2001929110011323130
- FrideERussoEBOnaiviESugiuraTDi MarzoVNeuropsychiatry: Schizophrenia, depression, and anxietyEndocannabinoids: The brain and body’s marijuana and beyond2006Boca Raton, FLTaylor and Francis37182
- FrondiniCLanfranchiGMinardiMAffective, behavior and cognitive disorders in the elderly with chronic musculoskelatal pain: the impact on an aging populationArch Gerontol Geriatr200744Suppl 11677117317450
- GaoniYMechoulamRIsolation, structure and partial synthesis of an active constituent of hashishJ Am Chem Soc19648616467
- GausonLAStevensonLAThomasACannabigerol behaves as a partial agonist at both CB1 and CB2 receptors17th Annual Symposium on the Cannabinoids2007Saint-Sauveur, Quebec, CanadaInternational Cannabinoid Research Society206
- GerritsenMECarleyWWRangesGEFlavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expressionAm J Pathol1995147278927543732
- GertschJRadunerSLeontiMScreening of plant extracts for new CB2-selective agonists revewals new players in Cannabis sativa17th Annual Symposium on the Cannabinoids2007Saint-Sauveur, Quebec, CanadaInternational Cannabinoid Research Society213
- GibsonSJIASP global year against pain in older persons: highlighting the current status and future perspectives in geriatric painExpert Rev Neurother200776273517563246
- GieringerDSt LaurentJGoodrichSCannabis vaporizer combines efficient delivery of THC with effective suppression of pyrolytic compoundsJournal of Cannabis Therapeutics20044727
- GilMLJimenezJOceteMAComparative study of different essential oils of Bupleurum gibraltaricum LamarckPharmazie19894428472772005
- GomezMASaenzMTGarciaMDStudy of the topical anti-inflammatory activity of Achillea ageratum on chronic and acute inflammation modelsZ Naturforsch [C]19995493741
- GorterRWButoracMCobianEPMedical use of cannabis in the NetherlandsNeurology200564917915753439
- GrinspoonLBakalarJBMarihuana, the forbidden medicine1997New HavenYale University Pressxv296
- GrotenhermenFPharmacokinetics and pharmacodynamics of cannabinoidsClin Pharmacokinet2003423276012648025
- GrotenhermenFCannabinoids for therapeutic use: designing systems to increase efficacy and reliabilityAmerican Journal of Drug Delivery2004222940
- GrotenhermenFLesonGBerghausGDeveloping science-based per se limits for driving under the influence of cannabis (DUIC)Findings and recommendations by an expert panel2005Hürth, GermanyNova- Institut49
- GuyGWRobsonPA Phase I, double blind, three-way crossover study to assess the pharmacokinetic profile of cannabis based medicine extract (CBME) administered sublingually in variant cannabinoid ratios in normal healthy male volunteers (GWPK02125)Journal of Cannabis Therapeutics2003312152
- HampsonAJGrimaldiMAxelrodJCannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidantsProc Natl Acad Sci USA1998958268739653176
- HazekampARuhaakRZuurmanLEvaluation of a vaporizing device (Volcano) for the pulmonary administration of tetrahydrocannabinolJ Pharm Sci20069513081716637053
- HerkenhamMLynnABLittleMDCannabinoid receptor localization in brainProc Natl Acad Sci USA199087193262308954
- HohmannAGBrileyEMHerkenhamMPre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cordBrain Res1999822172510082879
- HohmannAGMartinWJTsouKInhibition of noxious stimulus-evoked activity of spinal cord dorsal horn neurons by the cannabinoid WIN 55,212-2Life Sci199556211187776839
- HohmannAGSuplitaRLBoltonNMAn endocannabinoid mechanism for stress-induced analgesiaNature200543511081215973410
- HoldcroftAMazeMDoreCA multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain managementAnesthesiology20061041040616645457
- HowlettACJohnsonMRMelvinLSNonclassical cannabinoid analgetics inhibit adenylate cyclase: development of a cannabinoid receptor modelMol Pharmacol1988332973023352594
- HuestisMAHenningfieldJEConeEJBlood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuanaJ Anal Toxicol199216276821338215
- JanseAFCBreekveldt-PostmaNSErkensJAMedicinal gebruik van cannabis.: PHARMO Instituut [Institute for Drug Outcomes Research]200451
- JohnsonJRPottsRCannabis-based medicines in the treatment of cancer pain: a randomised, double-blind, parallel group, placebo controlled, comparative study of the efficacy, safety and tolerability of Sativex and Tetranabinex in patients with cancer-related pain20053Edinburgh, Scotland 8–11
- JonesRTBenowitzNBachmanJClinical studies of cannabis tolerance and dependenceAnn N Y Acad Sci197628222139798533
- JoyJEWatsonSJBensonJAJrMarijuana and medicine: Assessing the science base1999Washington, DCInstitute of Medicine
- KarsakMGaffalEDateRAttenuation of allergic contact dermatitis through the endocannabinoid systemScience20073161494717556587
- KarstMSalimKBursteinSAnalgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trialJAMA200329017576214519710
- KatonaSKaminskiESandersHCannabinoid influence on cytokine profile in multiple sclerosisClin Exp Immunol2005140580515932522
- KingLACarpentierCGriffithsPCannabis potency in EuropeAddiction2005100884615954994
- KoMCWoodsJHLocal administration of delta9-tetrahydrocannabinol attenuates capsaicin-induced thermal nociception in rhesus monkeys: a peripheral cannabinoid actionPsychopharmacology (Berl)1999143322610353438
- KoganNMCannabinoids and cancerMini Rev Med Chem200559415216250836
- LembergerLRubinAWolenRPharmacokinetics, metabolism and drug-abuse potential of nabiloneCancer Treat Rev19829Suppl B17236299550
- LiJDaughtersRSBullisCThe cannabinoid receptor agonist WIN 55,212-2 mesylate blocks the development of hyperalgesia produced by capsaicin in ratsPain199981253310353490
- LigrestiAMorielloASStarowiczKAntitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinomaJ Pharmacol Exp Ther200631813758716728591
- LiuJLiHBursteinSHZurierRBActivation and binding of peroxisome proliferator-activated receptor gamma by synthetic cannabinoid ajulemic acidMol Pharmacol2003639839212695526
- LorenzettiBBSouzaGESartiSJMyrcene mimics the peripheral analgesic activity of lemongrass teaJ Ethnopharmacol1991344381753786
- LynchMEYoungJClarkAJA case series of patients using medicinal marihuana for management of chronic pain under the Canadian Marihuana Medical Access RegulationsJ Pain Symptom Manage20063249750117085276
- MackieKCannabinoid receptors as therapeutic targetsAnn Rev Pharmacol Toxicol2006461012216402900
- MaidaVThe synthetic cannabinoid nabilone improves pain and symptom management in cancer patietnsBreast Cancer Res Treat20071031212
- MalfaitAMGallilyRSumariwallaPFThe nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritisProc Natl Acad Sci USA2000979561610920191
- ManzanaresJCorcheroJRomeroJChronic administration of cannabinoids regulates proenkephalin mRNA levels in selected regions of the rat brainBrain Res Mol Brain Res199855126329645967
- MartinWJHohmannAGWalkerJMSuppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by a cannabinoid agonist: Correlation between electrophysiological and antinociceptive effectsJ Neurosci1996166601118815936
- MassaFMonoryKEndocannabinoids and the gastrointestinal tractJ Endocrinol Invest2006293 Suppl475716751708
- McPartlandJMRussoEBCannabis and cannabis extracts: Greater than the sum of their parts?Journal of Cannabis Therapeutics2001110332
- MechoulamRBen-ShabatSHanusLIdentification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptorsBiochem Pharmacol19955083907605349
- MehmedicZMartinJFosterSDelta-9-THC and other cannabinoids content of confiscated marijuana: potency trends, 1993-2003International Association of Cannabis as Medicine2005 September 10Leiden, Netherlands
- Müller-VahlKRSchneiderUKolbeHTreatment of Tourette’s syndrome with delta-9-tetrahydrocannabinolAm J Psychiatry1999156495
- MunroSThomasKLAbu-ShaarMMolecular characterization of a peripheral receptor for cannabinoidsNature19933656157689702
- NadulskiTPragstFWeinbergGRandomized double-blind placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extractTher Drug Monit2005a2779981016306858
- NadulskiTSporkertFSchnelleMSimultaneous and sensitive analysis of THC, 11-OH-THC, THC-COOH, CBD, and CBN by GC-MS in plasma after oral application of small doses of THC and cannabis extractJ Anal Toxicol2005b29782916356335
- NeffGWO’BrienCBReddyKRPreliminary observation with dronabinol in patients with intractable pruritus secondary to cholestatic liver diseaseAm J Gastroenterol2002972117912190187
- NicholsonANTurnerCStoneBMEffect of delta-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adultsJ Clin Psychopharmacol2004243051315118485
- NicolodiMVolpeARSicuteriFFibromyalgia and headache. Failure of serotonergic analgesia and N-methyl-D-aspartate-mediated neuronal plasticity: Their common cluesCephalalgia199818Suppl 214149533670
- NotcuttWPriceMChapmanGClinical experience with nabilone for chronic painPharmaceutical Sciences199735515
- NotcuttWPriceMMillerRInitial experiences with medicinal extracts of cannabis for chronic pain: results from 34 “N of 1” studiesAnaesthesia2004594405215096238
- NurmikkoTJSerpellMGHoggartBSativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trialPain2007In press
- PacherPBatkaiSKunosGThe endocannabinoid system as an emerging target of pharmacotherapyPharmacol Rev20065838946216968947
- PateDChemical ecology of cannabisJournal of the International Hemp Association19942327
- PérezJCombined cannabinoid therapy via na oromucosal sprayDrugs Today (Barc)20064249550116969427
- PertweeRGCannabidiol as a potential medicine. In: Mechoulam R ed. Cannabinoids as therapeutics2005Basel, SwitzerlandBirkhäuser Verlag4765
- PopeHGJrGruberAJHudsonJINeuropsychological performance in long-term cannabis usersArch Gen Psychiatry2001589091511576028
- RahnEJMakriyannisAHohmannAGActivation of cannabinoid CB(1) and CB(2) receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in ratsBr J Pharmacol2007113
- RaoVSMenezesAMVianaGSEffect of myrcene on nociception in miceJ Pharm Pharmacol19904287781983154
- ReLBarocciSSonninoSLinalool modifies the nicotinic receptor-ion channel kinetics at the mouse neuromuscular junctionPharmacol Res2000421778210887049
- RichardsonJDAanonsenLHargreavesKMSR 141716A, a cannabinoid receptor antagonist, produces hyperalgesia in untreated miceEur J Pharmacol1997319R349042616
- RichardsonJDAanonsenLHargreavesKMAntihyperalgesic effects of spinal cannabinoidsEur J Pharmacol1998a345145539600630
- RichardsonJDAanonsenLHargreavesKMHypoactivity of the spinal cannabinoid system results in NMDA-dependent hyperalgesiaJ Neurosci1998b1845179412521
- RichardsonJDKiloSHargreavesKMCannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptorsPain1998c7511199539680
- RogDJNurmikoTFriedeTRandomized controlled trial of cannabis based medicine in central neuropathic pain due to multiple sclerosisNeurology2005658121916186518
- RussoECannabis for migraine treatment: The once and future prescription? An historical and scientific reviewPain199876389696453
- RussoEBHemp for headache: An in-depth historical and scientific review of cannabis in migraine treatmentJournal of Cannabis Therapeutics200112192
- RussoEBClinical endocannabinoid deficiency (CECD): Can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions?Neuroendocrinol Lett20042531915159679
- RussoEBColeBEBoswellMThe role of cannabis and cannabinoids in pain managementWeiner’s Pain Management: A Practical Guide for Clinicians2006a7Boca Raton, FLCRC Press82344
- RussoEBSchatmanMEThe solution to the medicinal cannabis problemEthical issues in chronic pain management2006bBoca Raton, FLTaylor and Francis165194
- RussoEBGuyGWA tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiolMed Hypotheses2006662344616209908
- RussoEBGuyGWRobsonPJCannabis, pain and sleep: lessons from therapeutic clinical trials of Sativex® cannabis based medicineChem Biodivers2007a417294317712817
- RussoEBMathreMLByrneAChronic cannabis use in the Compassionate Investigational New Drug Program: An examination of benefits and adverse effects of legal clinical cannabisJournal of Cannabis Therapeutics20022357
- SamahaANRobinsonTEWhy does the rapid delivery of drugs to the brain promote addiction?Trends Pharmacol Sci20052682715681025
- SarchielliPPiniLACoppolaFEndocannabinoids in chronic migraine: CSF findings suggest a system failureNeuropsychopharmacology20073213849017119542
- SchaeferCFBrackettDJGunnCGDecreased platelet aggregation following marihuana smoking in manJ Okla State Med Assoc1979724356521861
- SchleyMLeglerASkoppGDelta-9-THC based monotherapy in fibromyalgia patients on experimentally induced pain, axon reflex flare, and pain reliefCurr Med Res Opin20062212697616834825
- ShenMPiserTMSeyboldVSCannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal culturesJ Neurosci1996164322348699243
- SmithNTA review of the published literature into cannabis withdrawal symptoms in human usersAddiction2002976213212084124
- SolowijNStephensRSRoffmanRACognitive functioning of long-term heavy cannabis users seeking treatmentJAMA200228711233111879109
- SpadoneCNeurophysiologie du cannabis [Neurophysiology of cannabis]Encephale19911717221688273
- StottCGAyerakwaLWrightSLack of human cytochrome P450 induction by Sativex17th Annual Symposium on the Cannabinoids2007Saint-Sauveur, Quebec, CanadaInternational Cannabinoid Research Society211
- StottCGGuyGWWrightSThe effects of cannabis extracts Tetranabinex and Nabidiolex on human cyclo-oxygenase (COX) activity2005aInternational Cannabinoid Research SocietyJune 2005Clearwater, FL
- StottCGGuyGWWrightSThe effects of cannabis extracts Tetranabinex and Nabidiolex on human cytochrome P450-mediated metabolism2005bInternational Cannabinoid Research AssociationJune 27 2005Clearwater, FL163
- StrangmanNMWalkerJMCannabinoid WIN 55,212-2 inhibits the activity-dependent facilitation of spinal nociceptive responsesJ Neurophysiol199982472710400973
- SvendsenKBJensenTSBachFWDoes the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trialBMJ200432925315258006
- TambeYTsujiuchiHHondaGGastric cytoprotection of the non-steroidal anti-inflammatory sesquiterpene, beta-caryophyllenePlanta Med199662469709005452
- TashkinDPSmoked marijuana as a cause of lung injuryMonaldi Arch Chest Dis2005639310016128224
- TopolEJFailing the public health ’ rofecoxib, Merck, and the FDAN Engl J Med20043511707915470193
- VolfeZDvilanskyANathanICannabinoids block release of serotonin from platelets induced by plasma from migraine patientsInt J Clin Pharmacol Res1985524362997048
- WadeDTMakelaPRobsonPDo cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patientsMult Scler2004104344115327042
- WadeDTMakelaPMHouseHLong-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosisMult Scler2006126394517086911
- WadeDTRobsonPHouseHA preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptomsClin Rehabil2003171826
- WalkerJMHohmannAGMartinWJThe neurobiology of cannabinoid analgesiaLife Sci1999a656657310462067
- WalkerJMHuangSMStrangmanNMPain modulation by the release of the endogenous cannabinoid anandamideProc Nat Acad Sci USA1999b961219820310518599
- WalkerJMHuangSMCannabinoid analgesiaPharmacol Ther2002951273512182960
- WareMWangWShapiroSSmoked cannabis for chronic neuropathic pain: results of a pilot study17th Annual Symposium on the Cannabinoids2007Saint-Sauveur, Quebec, CanadaInternational Cannabinoid Research Societyp31
- WirthPWWatsonESElSohlyMAnti-inflammatory properties of cannabichromeneLife Sci198026199157401911
- WrightSGWMS001 and GWMS0106: maintenance of blinding2005LondonGW Pharmaceuticals8
- ZajicekJFoxPSandersHCannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trialLancet200336215172614615106
- ZajicekJPSandersHPWrightDECannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow upJ Neurol Neurosurg Psychiatry2005761664916291891
- ZuardiAWGuimaraesFSMathreMLCannabidiol as an anxiolytic and antipsychoticCannabis in medical practice: a legal, historical and pharmacological overview of the therapeutic use of marijuana1997Jefferson, NCMcFarland13341