Abstract
Several options for the treatment of hepatitis B have been licensed in the last years: interferon, pegylated interferon, lamivudine, adefovir, entecavir, and telbivudine. In addition tenofovir has been licensed in the EU and is expected to be licensed in the USA in 2008. The antivirals can be divided into “lamivudine-like” and “adefovir-like”, which clinically differ in their capacity to induce “YMDD” mutants, which are the hallmark of lamivudine resistance. The differing resistance profile makes them good combination partners, even in the absence of synergy in antiviral potency.
Introduction
Hepatitis B virus (HBV) infection affects about 2 billion people, of whom about 350 million display chronic hepatitis B, defined by presence of hepatitis B surface antigen (HBsAg) in the serum (CitationLavanchy 2004). It is estimated that between 235,000 and 328,000 people die annually due to liver cirrhosis and hepatocellular carcinoma, respectively (CitationPerz et al 2006).
Transaminases have for the longest time been the hallmark of liver disease, and patients with normal liver transaminases have been considered to have no significant liver disease. Accordingly, guidelines used to state indication for antiviral therapy only in the presence of elevated liver enzymes (Citationde Franchis et al 2003; CitationLiaw et al 2005; CitationLok and McMahon 2007). This has, however, been challenged, based on recent findings that high viral load seems more important than elevation of liver transaminases (CitationChen et al 2006; CitationIloeje et al 2006).
Furthermore, using an antiviral to reduce HBV viral load has been shown to ameliorate liver disease (CitationLiaw et al 2004), so that it is now recommended to start antiviral therapy, despite normal liver enzymes, when there are other sign of liver disease, such as advanced fibrosis or risk factors to develop hepatocellular carcinoma (CitationCornberg et al 2007).
There are two different ways to inhibit HBV replication, either by using immune modulators such as interferon, pegylated interferon (CitationMarcellin et al 2004; CitationLau et al 2005) or thymosin alfa (CitationYou et al 2006), or with antivirals. Antivirals available at present can then be divided into the “lamivudine-like” (L-nucleosides) and the “adefovir-like” drugs. The “lamivudine-like” and “adefovir-like” drugs differ in their pattern of resistance mutations.
While the “lamivudine-like” antivirals (telbivudine, clevudine, emtricitabin, and even entecavir, though additional mutations are required for full resistance in the latter) share a mutations pattern at position M204 of the YMDD (Tyrosine-Methionine-Aspartate-Aspartate motif in the catalytic domain of the viral polymerase/reverse transcriptase) motif of the HBV-polymerase, the “adefovir-like” antivirals seem to remain fully active against those mutants (CitationYang et al 2005).
The currently licensed and soon to be licensed therapies show differences in achieving viral load below a detectability threshold of 300 to 1000 copies per ml, as indicated in . Additionally, they differ in their selection for viral resistance, which has not yet been reported for interferon, but for all antivirals to different degree.
Figure 1 Efficacy of HBV drugs (48–52 week data). Lai CL, et al. Hepatology 2005; 42:748A (AASLD abstract LB01); CitationLau G, et al. NEJM 2005; 352:2882–2695; CitationChang T-T, et al. NEJM 2006; 354:1000–1010; CitationMarcellin P, et al. NEJM 2003;348:808–816. #CitationHeathcote J et al. AASLD 2007, CitationMarcellin P, et al. AASLD 2007.
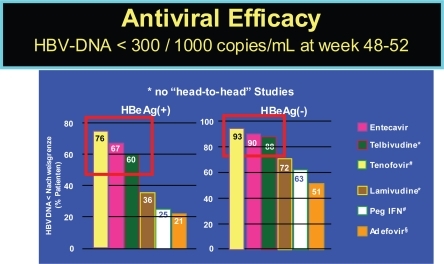
Nonresponse to interferon might be more based on the cellular than on the viral level, as has been demonstrated in case of HCV infection (CitationAus dem Siepen et al 2007). In contrast to interferon, resistance to antivirals can occur frequently, especially in case of insufficient response (CitationHan et al 2007). Resistance after one year ranges from below 1% in adefovir (CitationHadziyannis et al 2003; CitationMarcellin et al 2003) and entecavir (CitationChang et al 2006; CitationLai et al 2006a) to about 32% in lamivudine-treated patients (CitationDienstag et al 1999). Long term data on resistance are scarce. There are no real controlled data for lamivudine, but it is generally presumed to rise to about 70% over 5 years. Likewise, it has been shown that resistance to adefovir emerges with a constant increase from 0%, 3%, 9%, and 18% to 29% within 1, 2, 3, 4, and 5 years, respectively, in HBeAg-negative patients years (CitationHadziyannis et al 2006). In addition, one must be aware that the chance of developing resistance is higher in HBeAg-positive vs. HBeAg-negative patients () (CitationHan et al 2007). For telbivudine reliable 1 and 2 year data are available, while data for entecavir only included subgroups of their patients (CitationColonno et al 2006), thus making final conclusions difficult. The risk for entecavir resistance is significantly increased with underlying lamivudine resistance (CitationColonno et al 2006).
Table 1 Resistance defined by 1 log viral load increase over nadir after one and two years within the GLOBE trial
Adefovir
When adefovir was initially developed for HIV therapy, its development was halted due to nephrotoxicity at the doses of 60 and 120 mg, which would have been required for HIV therapy (CitationKahn et al 1999). Because of its anti-HBV-activity, adefovir was still further evaluated for treatment of HBV, where a 10 mg dose was shown to be sufficient to suppress HBV to similar levels as lamivudine, while in that dose there was no significant nephrotoxicity or other toxicity (CitationIzzedine et al 2004).
A clear strength of the adefovir-like nucleosides is their higher genetic barrier to resistance. Though there are no “head to head” studies, adefovir in the licensed 10 mg dose is at least not more efficient than lamivudine, given in 100 mg. Still the resistance rate is much lower for adefovir, with “zero” after one year (CitationHadziyannis et al 2003; CitationMarcellin et al 2003). However, it has been noted that resistance to adefovir develops more frequently in the presence of underlying lamivudine resistance, if lamivudine is not continued (Citationvan der Poorten et al 2007).
Furthermore, it has been demonstrated that the 30 mg dose of adefovir would be superior than the 10 mg concerning antiviral efficacy (CitationMarcellin et al 2003), which was recently reinforced by a study using 20 mg daily in 5 lamivudine-resistant patients with insufficient response to the 10 mg adefovir dose (CitationHezode et al 2007). Thus, a higher dose of adefovir would be desired, but the 30 mg dose was shown to lead to a significantly more frequent increase in creatinine levels than the 10 mg dose (CitationMarcellin et al 2003).
The viral load reduction achieved by lamivudine is no longer the standard, but instead the viral load reduction achieved by entecevair and telbivudine is preferred, both leading to more than 6 log viral load reduction (CitationChang et al 2006; CitationHan et al 2007). As a result, it would be more beneficial to utilise adefovir-like drugs, with both higher antiviral activity and lower toxicities. Adefovir-like drugs are currently pradefovir (also known as remofovir; almefovir; Hepavir B™) (CitationTillmann 2007), which is a prodrug of adefovir, tenofovir, LB-80380 (also known as ANA 380) and alamifovir (also known as MCC-478) (CitationChan et al 2005).
Pradefovir, a liver-targeted adefovir
Based on the concept of increased levels of adefovir at the main target organ, the liver, with low systemic exposure, pradefovir was developed. Pradefovir (also known as remofovir; almefovir; Hepavir B) is based on adefovir and a specific side chain making it into an inert drug, until activation through cytochrome p450 (CYP3A4). As this compound is abundant in the liver but scarce elsewhere in the body (CitationBerggren et al 2007), it is almost exclusively activated in the liver, thereby high drug levels are achieved in the liver, while the systemic exposure has been shown to be low (CitationLin et al 2004).
Dependence on CYP3A4, however, makes pradefovir prone to interaction with other drugs that also require, induce or inhibit CYP3A4. In line with the need for further metabolization, the AUC variation was higher on prade-fovir compared to adefovir (CitationLee et al 2006). The antiviral activity in that phase II study was more pronounced on pradefovir doses of 10 mg and higher. The open-label phase II trials compared pradefovir (5, 10, 20, or 30 mg) with 10 mg adefovir in chronically HBV-infected patients, who were treated for 24 to 48 weeks (CitationLee et al 2006, CitationLin et al 2006) This study included 47, 49, 48, 48, and 50 patients into the pradefovir 5, 10, 20 and 30 mg arm and the 10 mg adefovir arm respectively. After 24 and 48 weeks, a viral reduction was described to be 3.39, 4.22, 4.33, and 5.02 log copies per ml for the pradefovir mesylate dose of 5, 10, 20, and 30 mg, respectively, compared with 3.66 with 10 mg adefovir dipivoxil after 24 weeks (CitationLee et al 2006) and 4.09, 4.84, 4.89, 5.54 log copies per ml for the pradefovir mesylate dose of 5, 10, 20, and 30 mg, respectively, compared to 4.19 on 10 mg adefovir dipivoxil after 48 weeks (CitationLin et al 2006). However, pradefovir was recently put on hold concerning its further development based on increased tumor incidence in animal studies (CitationTillmann 2007). At present, it is unclear whether this is a class effect of “adefovir-like” substances, the high doses of adefovir in the liver, or related to the prodrug delivery used here.
Tenofovir
Tenofovir as adefovir was developed for HIV therapy and has proven to have lower renal toxicity and good activity, actually making it currently one of the top nucleoside reverse transcriptase inhibitor backbones in therapies for HIV (CitationDe Clercq 2007). Nonetheless, renal toxicity is still a concern in relation to tenofovir, though not quite proven to be causally associated (CitationRöling et al 2006). It has been demonstrated in some case series that tenofovir, at least in the dose of 245 mg tenofovir dipivoxil equivalent to 300 mg tenofovir dipivoxil fumarate equivalent to 136 mg tenofovir as given for HIV, is superior to the 10 mg dose of adefovir (Citationvan Bömmel et al 2004), which has been reinforced by reactivation after switching from tenofovir to adefovir (Citationvan Bömmel and Berg 2005; CitationDel Poggio et al 2007) and improved suppression after switching from adefovir to tenofovir (Citationvan Bömmel et al 2006). A trend towards better response has also been seen in a placebo controlled trial of HIV infected patients (CitationPeters et al 2006).
There are two controlled trials currently underway comparing tenofovir to adefovir, which have only been presented as press releases (CitationGilead 2007a, Citation2007b) and abstract form. The one year data have been presented at the 2007 AASLD (American Association for the Study of the Liver) annual meeting. According to these press releases and the presentations (CitationHeathcote et al 2007; CitationMarcellin et al 2007), Tenofovir is significantly more potent than adefovir. Tenofovir achieved the highest rates in undetectability of HBV-DNA observed in any drug trial related to hepatitis B. However, the comparison to the other currently marketed drug is difficult given that the viral load of the enrolled patients was in average about 1 log lower than in the other trials. It might well be that the antiviral efficacy is superior to that of the other currently marketed drugs. However, to finally asses this, a head to head study would be required, but unlikely to take place. However, in addition to its efficacy, no emergence of viral breakthrough has been observed on tenofovir. In addition, HBsAg loss, otherwise mostly only seen in relation to interferon-based therapies has been observed in about 3% after 48 weeks and 4% of tenofovir-treated patients after 69 weeks of therapy (CitationHeathcote et al 2008). However, no loss of HBsAg has been observed in HBeAg-negative patients on tenofovir.
After tenofovir’s licensure for HBV therapy, it is probable that adefovir’s only indication will be the HIV-positive patient needing HBV-treatment, but not yet requiring anti-HIV therapy (CitationThio and Locarnini 2007).
A mutation, rtA194T, has been reported to emerge on tenofovir (CitationSheldon et al 2005), but it did not lead to a real viral load increase, thus awaiting further confirmation.
Alamifovir
Alamifovir, also known as MCC-478 or LY582563, is a adenosine nucleotide analogue, which has activity against both HIV and HBV. In a phase I study 66 HBV-DNA positive patients were randomized to alamifovir or placebo in a 3:1 ratio within each of 7 dosing groups: 2.5 mg BID, 5 mg BID, 10 mg BID, 2.5 mg QD, 5 mg QD, 10 mg QD, and 20 mg QD. That study showed no difference between once daily and twice daily dosing (). In this small study, there were also no significant differences between the groups except towards placebo. At present, no new data have been presented within the last two years.
Table 2 Viral load reduction on alamifovir
LB-80380
LB-80380 is also known as ANA 380, and is a 9-[1-(Ph osphonomethoxycyclopropyl) methyl] guanine (PMCG). Even though it is frequently regarded as a “adefovir-like” nucleotide, it is considered, at least by some, as a representative of a novel class of phosphonate nucleosides that blocks HBV replication with an excellent potency (EC50 of 0.5 μM) in HepG2 2.2.15 cells (CitationChoi et al 2004). LB-80380 did not show any cytotoxicity in several human cell lines in concentrations up to 2000 times of that required for an EC50 for HBV inhibition. It seems not to inhibit HIV replication, at least at 30 μM, which is 60 times the dose required for HBV. This, however, requires further evaluation in light of the unexpected activity of entecavir against HIV (CitationMcMahon et al 2007). Furthermore, in its oral form, PMCDG dipivoxil, excellent efficacy was achieved in HBV infected woodchucks at 5 mg/kg/d (CitationChoi et al 2004).
In vivo LB-80380 has been also tested in naïve and lamivudine refractory patients (CitationYuen et al 2006). When doses of 30, 60, 120, and 240 mg were given once daily for 4 weeks, a mean maximum viral load reduction of 3.05, 4.20, 3.67, and 3.68 log10 copies/ml was achieved for the 30, 60, 120, and 240 mg doses, respectively, in naïve patients (CitationYuen et al 2006). Slightly lower reduction was achieved in lamivudine refractory patients with 2.1, 2.5, 2.6, 3.0, and 3.0 log10 as well as 2.8, 3.2, 3.9, and 3.7 log10 after 4 and 12 weeks, respectively in the 30, 60, 90, 150, and 240 mg dose groups (CitationLai et al 2006b).
Conclusions
In summary, the “adefovir-like” drugs best used in practice are adefovir in the HIV-infected patient in need of anti-HBV therapy, while not yet needing anti-HIV therapy (CitationThio and Locarnini 2007). In all other patients, I would presume teno-fovir to take over, where adefovir is currently used given its lower toxicity and higher activity. Whether any of the other “adefovir-like” drugs will make it to the market remains to be seen. It is probable that all could be well combined with lamivudine, which will soon be off-patent. Thus, it might be a cheap but potentially very active addition to any “adefovir-like” drug, given their different resistance profile. However, in the case of tenofovir, this is not required, given its existence in combination with the lamivudine-like drug emtricitabine.
Disclosure
The author reports no conflicts of interest.
References
- Aus dem SiepenMOniangue-NdzaCWieseM2007Interferon-alpha and ribavirin resistance of Huh7 cells transfected with HCV subgenomic repliconVirus Res1251091317254660
- BerggrenSGallCWollnitzN2007Gene and protein expression of P-glycoprotein, MRP1, MRP2, and CYP3A4 in the small and large human intestineMol Pharm4252717263554
- ChanCAbu-RaddadEGolorG2005Clinical pharmacokinetics of alamifovir and its metabolitesAntimicrob Agents Chemother4918132215855501
- ChangTTGishRGde ManR2006A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis BN Engl J Med35410011016525137
- ChenCJYangHISuJ2006REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA levelJAMA295657316391218
- ChoiJRChoDGRohKY2004A novel class of phosphonate nucleosides. 9-[(1-phosphonomethoxycyclopropyl)methyl]guanine as a potent and selective anti-HBV agentJ Med Chem472864915139764
- ColonnoRJRoseREPokornowskiK2006Assessment at three years shows high barrier to resistance is maintained in entecavir-treated nucleoside naïve patients while resistance emergence increases over time in lamivudine refractory patientsHepatology4422930
- CornbergMProtzerUDollingerMM2007Prophylaxis, diagnosis and therapy of hepatitis-B-virus-(HBV) infection: upgrade of the guideline. AWMF-Register 021/011Z Gastroenterol451287328
- De ClercqE2007Anti-HIV drugsVerh K Acad Geneeskd Belg698110417550060
- de FranchisRHadengueALanGEASL Jury2003EASL International Consensus Conference on Hepatitis B. 13–14 September, 2002. Geneva, Switzerland. Consensus statementJ Hepatol3932512821036
- Del PoggioPZaccanelliMOggionniM2007Low-dose tenofovir is more potent than adefovir and is effective in controlling HBV vire-mia in chronic HBeAg-negative hepatitis BWorld J Gastroenterol134096917696228
- DienstagJLSchiffERWrightTL1999Lamivudine as initial treatment for chronic hepatitis B in the United StatesN Engl J Med34112566310528035
- Gilead2007aPhase III Study evaluating Gilead’s Viread® for the treatment of chronic hepatitis B virus meets primary endpoint [online]. Accessed on May 28, 2008. URL:http://www.gilead.com/wt/sec/pr_1012569
- Gilead2007bSecond Phase III Study evaluating Gilead’s Viread® for the treatment of chronic hepatitis B virus meets primary endpoint [online]. Accessed on May 28, 2008. URL:http://www.gilead.com/wt/sec/pr_1018988
- HadziyannisSJTassopoulosNCHeathcoteEJ2003Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis BN Engl J Med348800712606734
- HadziyannisSJTassopoulosNCHeathcoteEJ2005Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis BN Engl J Med35226738115987916
- HadziyannisSJTassopoulosNCHeathcoteEJ2006Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 yearsGastroenterology13117435117087951
- HanSLaiCGaneEJ2007Telbivudine globe trial at year two: efficacy, safety, and predictors of outcome in patients with chronic hepatitis BGastroenterology132765
- HeathcoteEJGaneEDe ManRA Randomized, Double-Blind, Comparison of Tenofovir DF (TDF) Versus Adefovir Dipivoxil (ADV) for the Treatment of HBeAg Positive Chronic Hepatitis B (CHB): Study GS-US-174-0103Hepatology2007Suppl.1231A
- HeathcoteEJGeorgeJGordonSTenofovir Disoproxil Fumarate (TDF) for the treatment of HBeAg-positive chronic hepatitis B: Week 72 TDF data and week 24 Adefvoir Dipivoxil switch data (Study 103)J Hepatol2008Suppl.2S32
- HezodeCChevaliezSBouvier-AliasM2007Efficacy and safety of adefovir dipivoxil 20 mg daily in HBeAg-positive patients with lamivudine-resistant hepatitis B virus and a suboptimal virological response to adefovir dipivoxil 10 mg dailyJ Hepatol46791617321635
- IloejeUHYangHISuJRisk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) Study Group. 2006. Predicting cirrhosis risk based on the level of circulating hepatitis B viral loadGastroenterology1306788616530509
- IzzedineHHulotJSLaunay-VacherV2004Renal safety of adefovir dipivoxil in patients with chronic hepatitis B: two double-blind, randomized, placebo-controlled studiesKidney Int661153815327411
- JacquardACBrunelleMNPichoudC2006In vitro characterization of the anti-hepatitis B virus activity and cross-resistance profile of 2′,3′-dide-oxy-3′-fluoroguanosineAntimicrob Agents Chemother509556116495257
- KahnJLagakosSWulfsohnM1999Efficacy and safety of adefovir dipivoxil with antiretroviral therapy: a randomized controlled trialJAMA2423051210612317
- LaiCLShouvalDLokAS2006aEntecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis BN Engl J Med35410112016525138
- LaiCLHanKHYoonSK2006bPhase II, multicentre, dose-escalating study of LB8030 (ANA380) in hepatitis B patients with lamivudine resistant YMDD mutant HBVJ Hepatol445
- LauGKPiratvisuthTLuoKX2005Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis BN Engl J Med35226829515987917
- LavanchyD2004Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measuresJ Viral Hepat119710714996343
- LeeKSLimSGChuangWL2006Safety, tolerability and antiviral activity of pradefovir mesylate in patients with chronic hepatitis B virus infection: 48-week analysis of a phase 2 studyJ Hepatol44274
- LiawYFLeungNGuanRAsian-Pacific Consensus Update Working Party on Chronic Hepatitis B2005Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 updateLiver Int254728915910483
- LiawYFSungJJChowWCCirrhosis Asian Lamivudine Multicentre Study Group2004Lamivudine for patients with chronic hepatitis B and advanced liver diseaseN Engl J Med35115213115470215
- LinCXuCYehLT2006Pharmacokinetics and pharmacodynamics of pradefovir mesylate, a liver-targeting pro-drug of PMEA, in HBV patientsJ Hepatol4416
- LinCCYehLTVitarellaD2004Remofovir mesylate: A prodrug of PMEA with improved liver-targeting and safety in rats and monkeysAntivir Chem Chemother153071715646644
- LokASMcMahonBJ2007Chronic hepatitis BHepatology455073917256718
- MarcellinPChangTTLimSG2003Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis BN Engl J Med3488081612606735
- MarcellinPLauGKBoninoF2004Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis BN Engl J Med35112061715371578
- MarcellinPButiMKrastevZA Randomized, Double-Blind, Comparison of Tenofovir DF (TDF) versus Adefovir Dipivoxil (ADV) for the Treatment of HBeAg-Negative Chronic Hepatitis B (CHB): Study GS-US-174-0102Hepatology2007Suppl.1231A
- MarcellinPJacobsonIHabersetzerFTenofovir Disoproxil Fumarate (TDF) for the treatment of HBeAg-negative chronic hepatitis B: Week 72 TDF data and week 24 Adefvoir Dipivoxil switch data (Study 102)J Hepatol2008Suppl.2S26
- McMahonMAJilekBLBrennanTP2007The HBV drug entecavir – effects on HIV-1 replication and resistanceN Engl J Med35626142117582071
- PerzJFArmstrongGLFarringtonLA2006The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwideJ Hepatol455293816879891
- PetersMGAndersenJLynchP2006Randomized controlled study of tenofovir and adefovir in chronic hepatitis B virus and HIV infection: ACTG A5127Hepatology441110617058225
- RölingJSchmidHFischerederM2006HIV-associated renal diseases and highly active antiretroviral therapy-induced nephropathyClin Infect Dis4214889516619164
- SheldonJCaminoNRodesB2005Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovirAntivir Ther107273416218172
- SoonDKLoweSLTengCH2004Safety and efficacy of alamifovir in patients with chronic hepatitis B virus infectionJ Hepatol41852815519660
- ThioCLLocarniniS2007Treatment of HIV/HBV coinfection: clinical and virologic issuesAIDS Rev9405317474312
- TillmannHL2007Drug evaluation: Pradefovir, a liver-targeted prodrug of adefovir against HBV infectionCurr Opin Investig Drugs868290
- van BommelFBergT2005Reactivation of viral replication after replacement of tenofovir by adefovirHepatology422394015962310
- van BommelFWunscheTMaussS2004Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infectionHepatology401421515565615
- van BommelFZollnerBSarrazinC2006Tenofovir for patients with lamivudine-resistant hepatitis B virus (HBV) infection and high HBV DNA level during adefovir therapyHepatology443182516871563
- van der PoortenDPrakosoEKhooTL2007Combination adefovir-lamivudine prevents emergence of adefovir resistance in lamivudine-resistant hepatitis BJ Gastroenterol Hepatol221500517683493
- YangHQiXSabogalAMillerMXiongSDelaneyWE4th2005Cross-resistance testing of next-generation nucleoside and nucleotide analogues against lamivudine-resistant HBVAntivir Ther106253316152756
- YouJZhuangLChengHY2006Efficacy of thymosin alpha-1 and interferon alpha in treatment of chronic viral hepatitis B: a randomized controlled studyWorld J Gastroenterol1267152117075991
- YuenMFKimJKimCR2006A randomized placebo-controlled, dose-finding study of oral LB80380 in HBeAg-positive patients with chronic hepatitis BAntivir Ther119778317302367