Abstract
Osteoporosis and related fractures are a significant concern for the global community. As the population continues to age, morbidity and mortality from fractures due to low bone mineral density (BMD) will likely continue to increase. Efforts should be made to screen those at risk for osteoporosis, identify and address various risk factors for falls and associated fractures, ensure adequate calcium and vitamin D intake, and institute pharmacological therapy to increase BMD when indicated. Agents which increase BMD and have been shown to decrease fractures, particularly at the hip, should be considered preferentially over those for which only BMD data are available. Drugs which have been shown to decrease the risk of age-related osteoporotic fractures include oral bisphosphonates (alendronate, ibandronate, and risedronate), intranasal calcitonin, estrogen receptor stimulators (eg, estrogen, selective estrogen receptor modulators [raloxifene]), parathyroid hormone (teriparatide), sodium fluoride, and strontium ranelate. Data are beginning to emerge supporting various combination therapies (eg, bisphosphonate plus an estrogen receptor stimulator), though more data are needed to identify combinations which are most effective and confer added fracture protection. In addition, further research is needed to identify ideal regimens in special populations such as nursing home patients and men.
Keywords:
Background
Epidemiology and economic impact
Osteoporosis, defined as a skeletal disorder characterized by decreased bone strength and increased susceptibility of fractures (CitationNIH 2001), is a significant health concern for the international community. Hip fractures, considered to be the most debilitating fragility fracture secondary to osteoporosis, are associated with an approximately 2-fold increased risk of mortality the year following the fracture (CitationCenter et al 1999; CitationEmpana et al 2004). In 1990 it was estimated that approximately 1.3 million hip fractures occurred globally (CitationJohnell and Kanis 2004). These fractures were associated with 740 000 deaths and 1.75 million disability adjusted life-years lost, with the highest rates occurring in established market economies such as North America, Japan, Australia, and Western Europe. In the US alone, it is estimated that over 1.5 million fractures occur annually due to osteoporosis, with over 300 000 occurring at the hip (CitationNOF 2005b).
While hip fractures are considered to be the most debilitating osteoporosis-related facture, other fracture types are common. In the US, it is estimated that wrist fractures occur at almost the same rate as hip fractures with approximately 250 000 annually, and the vertebral fracture rate is more than double that of hip fractures with approximately 700 000/year (CitationNOF 2005b). These fractures lead to numerous adverse sequele include chronic pain, deformity, and disability, decreased quality of life, decreased mobility, increased risk of pressure ulcers, and decreased pulmonary function (CitationNevitt et al 1998; CitationSchlaich et al 1998; CitationLips et al 1999; CitationFink et al 2003; CitationMargolis et al 2003; CitationHallberg et al 2004).
As the global population ages, the prevalence of age-related osteoporosis (ie, postmenopausal osteoporosis, male osteoporosis) and related fractures is likely to increase considerably. In the US, the prevalence of osteoporosis is expected to grow from an estimated 10 million in 2002 to 14 million by 2020 (CitationNOF 2005a). In the EU, the total number of hip fractures is estimated to increase from 414 000 to 972 000 from year 2000 to 2050 (CitationCompston et al 1998). Vertebral fractures are estimated to increase during this time from 23.7 million to 37.3 million.
The economic consequences of osteoporosis with its associated morbidity and mortality due to fractures are staggering. While total world-wide estimates are not readily available, there are data that describe the costs in various countries. In Belgium (population ∼10 million), the total cost of hip fractures in 1996 was almost US$126.2 million per year (CitationReginster et al 1999). The estimated cost of osteoporotic fractures in females greater than 50 years of age using 1997 figures cost the UK £727 million (US$1.23 billion); or an estimated £942 million (US$1.6 billion) including men, assuming the cost of treatment was the same as females (CitationDolan and Torgerson 1998). The annual cost of osteoporotic fractures to the US healthcare system in 2001 was approximately US$17 billion, with a single hip fracture costing approximately US$40 000 (CitationNOF 2004).
Diagnosis and classification
The gold standard for diagnosis of osteoporosis is measurement of bone mineral density (BMD) using central dual-energy x-ray absorptiometry (CitationLewiecki et al 2004). Bone mineral density is defined as the average concentration of mineral per unit of area (g/cm2) measured (CitationCummings et al 2002). This figure is then reported as the number of standard deviations (SD) between the value of an individual and the mean value for a reference population. The World Health Organization (WHO) classification for diagnosis is based on comparison of BMD to a young-adult mean (T-score) for postmenopausal women and men >50 years. Normal is considered a T-score greater than −1.0, osteopenia is a T-score between −1.0 and −2.5, and osteoporosis is a T-score less than −2.5. Severe osteoporosis may be considered when the T-score is less than −2.5 in the presence of one or more fragility fractures (CitationKanis and Gluer 2000). For children, premenopausal women, and men younger than 20 years, Z-scores, which are SD comparisons based on age and gender, should be used instead rather than T-scores (CitationLewiecki et al 2004). Because BMD declines with age, T-scores are lower than Z-scores, particularly for patients with advanced age. For instance, a 70 year old female with a Z-score of −1.0 may have a T-score of −3.0 and thus be considered osteoporotic.
Bone mineral density is a surrogate marker for bone strength and fragility fractures. It is estimated that BMD accounts for approximately 70% of bone strength (CitationNIH 2001) and correlates well with fracture risk. For every 1 SD decline in femoral-neck BMD it is estimated that the risk of an osteoporotic fracture at the hip increases by 2.5-fold (CitationCummings et al 1993; CitationMarshall et al 1996). While BMD is useful to evaluate risk of fracture, it should be noted that a significant portion of bone strength may be derived from other sources (eg, genetics). Thus, while BMD is a useful tool to evaluate risk of osteoporotic fractures, choice of medication used for prevention or treatment should ideally be based on data demonstrating reduction in fractures, recognizing that other factors such as past medical history and adherence may also play a role.
Risk assessment and prevention of fractures
To identify patients at risk of fractures and optimize pharmacotherapy, a thorough risk factor analysis is recommended. Two major areas of risk factor assessment that should be considered, particularly for older individuals, include an assessment of both “bone” and “non-bone” related risks. “Bone-related” risk factors are those factors associated with bone density. “Non-bone” risk factors are variables associated with fracture risk unrelated to bone density, and may increase fracture risk irrespective of osteoporosis diagnosis. However they compound the risk of an osteoporosis end-point in a patient who has decreased bone density.
Traditional risk factor assessment focuses on “bone” related factors. These are variables that increase the risk of fracture by affecting BMD and include physical frame, genetics, lifestyle, hormonal changes, and medication effects (CitationNOF 2004). Prior to obtaining BMD measurements, a full assessment of these “bone” related risk factors should include complete medical history including family history of osteoporosis and fracture and any personal history of fracture as an adult. For women, this should include a gynecological history including history of menses, pregnancies, surgeries, or menopause. Physical exam should reveal weight, height (particularly if height has changed from known adult maximum) and curvature of the spine. Patient interview can reveal diet (including dairy and supplemental calcium/vitamin D intake), exercise, smoking, alcohol use, and current medication use. Tools such as the Osteoporosis Risk Assessment Instrument (ORAI) and the Simple Calculated Osteoporosis Risk Assessment (SCORE) are validated instruments which take into account these “traditional” risk factors (CitationLydick et al 1998; CitationCadarette et al 2000). It has also been reported that the WHO will soon release an instrument to quantify the contribution of various osteoporosis risk factors and calculate an individuals’ anticipated 10-year fracture risk (CitationTucci 2006).
In addition to traditional risk factor assessment, clinicians should be mindful of additional “non-bone” risk factors. While these variables are not directly related to bone density, they can increase risk of fractures by increasing the risk of fall-related injury. Fall-related injuries are a leading cause of morbidity and mortality in older individuals. While falls can cause a range of injuries not limited to fracture, including pain, swelling, bruising, lacerations, sprains, concussions, or subdural hematomas, the combined risk of falls and low BMD puts older individuals at particular risk of functional decline due to fracture. In the US, falls are a significant cause of admission to long term care facilities, and are a leading cause of both injury and death in older individuals (CitationGranek et al 1987; CitationTinetti and Williams 1997).
While only 5%–10% of falls in the community setting result in fracture and subsequent hospitalization (Citationvan Weel et al 1995), this percentage is magnified by the sheer prevalence of falls. It has been estimated that one third of community dwelling individuals over the age of 65 will fall in one year (CitationTinetti and Williams 1997; CitationCumming 1998). This estimate increases to 50% when considering the population over the age of 85 years.
It may be difficult to elucidate the cause and effect relationship between falls and fractures. While fractures can certainly result from a fall, the fall can likewise be a consequence of a fracture. This can occur when a pathologic fracture of the hip is the precipitating cause. If there is no clear patient history to shed light on circumstances surrounding a fall event, it may be difficult to determine which event, the fall or the fracture, preceded the other. For that reason, both “bone” and “non-bone” risks for fracture should be assessed in all older individuals. Risk factors for falls can be generally categorized as intrinsic or extrinsic as described in (CitationSleeper et al 2000).
Table 1 Intrinsic and extrinsic risk factors for fall-related fractures
A component of both bone and non-bone risk factor assessment that deserves special attention is the drug regimen review including: 1) medications for the prevention or treatment of osteoporosis, 2) medications associated with drug-induced osteoporosis, and 3) medications or environmental factors predisposing to falls. Other areas which may be evaluated include whether a BMD evaluation has been performed, intake of calcium and vitamin D, as well as their respective formulations, doses, and administration regimens. While the presence of an osteoporosis treatment regimen suggests a BMD evaluation has occurred, it should not be assumed that fracture risk has been optimally managed. The presence of pharmacotherapy for an established osteoporosis diagnosis is in fact a surrogate marker that indicates the need for ongoing assessment of both bone and non-bone related risk. Medications associated with drug-induced osteoporosis such as glucocorticoids, chronic heparin, excessive thyroid replacement, immunosuppressants, or anticonvulsants can also be identified (CitationNOF 2004). Concurrent medications may also be important non-bone risk factors for falls. Medications traditionally associated with fall risk include psychoactive medications (eg, antipsychotics, antidepressants, benzodiazepines, sedative hypnotics), opioids, nonsteroidal antiinflammatory drugs (NSAIDs), anticholinergic medications, and cardiovascular medications (CitationKannus et al 2005). Mechanisms by which these agents can cause falls include sedation, dizziness, cognitive impairment, ataxia, blurred vision, agitation, extrapyramidal movements, psychomotor slowing, orthostatic hypotension, increased postural sway, arrhythmias, and delirium. Drug regimen review should reveal not only the presence of a potentially problematic drug, but the daily dosage, frequency and duration of use, and cumulative number of high-risk agents. Optimal management of various comorbidities which may increase the risk of dizziness or instability (eg, prevention of hypoglycemic episodes in patients with diabetes) may decrease the risk for fall-related fractures.
Pharmacological treatment
Pharmacological goals for osteoporosis include halting bone loss, improvement of BMD, and reduction in fragility fractures. Whenever possible, drug therapies that have been shown to decrease fractures, particularly at the hip, should be used as hip fractures have the greatest impact from a patient and societal perspective ().
Figure 1 Fracture reduction data available by agent and site.
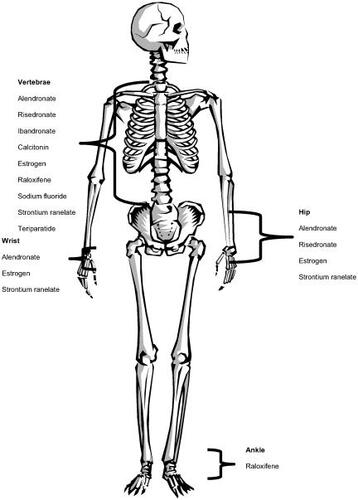
Bone remodeling involves both resorptive and anabolic processes. Drugs that have traditionally been used for the treatment of osteoporosis have largely focused on inhibition of bone resorption. More recently however, medications which stimulate bone formation or that both inhibits resorption and stimulate anabolic processes have been developed or are under investigation.
Calcium and vitamin D
Adequate intake of both calcium and vitamin D are an essential part of osteoporosis prevention, and no treatment regimen should be considered complete without these adjunctive therapies. There are numerous studies that have reported outcomes of supplemental calcium, vitamin D, or both on BMD and fractures ().
Table 2 Clinical trials of calcium and vitamin D
Table 3 Characteristics of various calcium preparations. Data from CitationWickersham and Novak (2002)
Table 4 Approved drug regimens in the US and EU for management of osteoporosis
Calcium
The majority of the body's calcium is stored in bone, and as such, is the primary source when dietary sources are insufficient. For this reason, adequate calcium intake is a first-line defense against bone loss. A variety of calcium regimens have been studied to assess the effects on BMD and fractures (). While not every evaluation of calcium has consistently demonstrated a clear reduction in fracture risk, guidelines recommend 1000–1500 mg elemental calcium daily, whether from diet or from supplements (CitationNIH 1994; CitationNAS 1997; CitationNOF 2004), with higher doses (eg, 1200–1500 mg) for those greater than 50 years or who have low BMD.
Vitamin D
Vitamin D is an essential component that, along with parathyroid hormone (PTH), regulates serum calcium concentrations. Inadequate vitamin D intake may be a greater concern than poor calcium intake, as vitamin D may not be as prevalent in the diet as calcium, and patients may be less aware of vitamin D requirements than calcium. In addition, low exposure to sunlight and poor renal function may also lead to decreased concentrations. Yet supplemental vitamin D, with or without calcium, has been shown to reduce fracture rates and may also have effects on muscle strength and risk of falling. A recent meta-analysis of doubleblinded, randomized controlled trials evaluating various doses of vitamin D found that while lower doses (eg, 400 IU) were not associated with a significant benefit, 700–800 IU daily was associated with 26% reduction in hip fractures (number needed to treat [NNT]=45) and 23% reduction in nonvertebral fracture (NNT=27) when compared with calcium alone or placebo (CitationBischoff-Ferrari et al 2005). Recently, the investigators for the Women's Health Initiative released results of a 7 year follow up of over 30 000 women aged 50–79 (CitationJackson et al 2006). Despite a 1.06% increase in BMD, this study did not show a statistically significant reduction in hip fractures among women who received 1000 mg calcium carbonate and 400 IU vitamin D3, and a 17% increase in risk of renal calculi. However, there are several important points that should be noted about this study. In addition to the use of a daily dose of vitamin D lower than currently advocated, the average T score at baseline was normal (−0.65). Only 3% of subjects for whom bone density was measured at baseline had a T score below −2.5. In addition, it was noted that the reduction in hip fracture risk was statistically significant after non-adherent patients were excluded.
Vitamin D supplementation, particularly with the active form (ie, calcitriol) may be theoretically important for frailer, institutionalized individuals or those with chronic health conditions (eg, PTH disorders, chronic renal failure, and dialysis) due to the inability or reduced capacity to convert vitamin D to the active form. Clinical trials with calcitriol evaluating BMD and fracture outcomes have had mixed results. However, in one trial of 50 women with vertebral fractures, spinal BMD increased 1.94% after 2 years of treatment with calcitriol 0.62 mcg/day plus 1000 mg calcium versus a 3.92% decrease for placebo (p=0.001) (CitationGallagher and Goldgar 1990). In another trial of 622 women with compression fractures, 0.25 mcg calcitriol BID given for 3 years to women with a history of ≤5 fractures decreased the rate of new vertebral fractures compared with 1000 mg calcium alone (4.2 vs 31.0 fractures per 100 patient years, p<0.0001) (CitationTilyard et al 1992). In both of these studies, serum or urine calcium monitoring was necessary to identify subjects requiring dose adjustment due to hypercalcemia or hypercalcuria. Moreover, the true benefit of calcitriol over other vitamin D-containing agents has not been established, and activated vitamin D has not been evaluated against first line therapies such as bisphosphonates. Therefore, further data is needed before the optimal place of calcitriol in osteoporosis therapy is established.
Monitoring of serum vitamin D concentrations is intriguing, and has been suggested as a means of clinically identifying individuals in need of additional supplementation (CitationHolick et al 2005). The reported range of normal values may vary by laboratory, but is typically 16–120 ng/ml (CitationWallach 1986). Opinion varies as to the threshold for deficiency, but on average is around 15 ng/ml (CitationLeBoff et al 1999). Various studies have demonstrated that deficiency of vitamin D is associated with decreased BMD and increased fractures (CitationAaron et al 1974; CitationBischoff-Ferrari et al 2004). It is also clear that vitamin D supplementation confers benefit with respect to both BMD and fracture risk (CitationBischoff-Ferrari et al 2005). However, it is not clear if laboratory assessment is routinely necessary or cost effective in all patients. Monitoring may be useful in those individuals for whom severe deficiency or coexisting hyperparathyroidism is suspected. For instance, several studies have demonstrated improvements in elevated PTH concentrations in patients with established vitamin D deficiency who receive supplemental vitamin D (CitationMalabanan et al 1998; CitationNeed et al 2004). However, while studies evaluating the efficacy of vitamin D for osteoporosis often report data regarding subjects’ 25-hydroxyvitamin D levels, not all studies consistently report an association between the change in serum level and the amelioration of fracture risk (CitationLips et al 1996; CitationDawson-Hughes et al 1997; CitationAdams et al 1999; CitationJackson et al 2006). Further, an optimal target vitamin D concentration has not been universally established. Despite this, there is sufficient evidence to recommend vitamin D supplementation as a routine part of osteoporosis prevention and treatment (CitationBischoff-Ferrari et al 2005). Therefore, an individual patient's eligibility for such therapy should not be contingent upon laboratory assessment of deficiency.
Product recommendations
As the quantities and formulations of calcium or vitamin D products have varied from study to study, there is currently no clear consensus which agent or regimen should be recommended to all patients. Based upon preponderance of data from multiple studies, a total daily intake of 1200–1500 mg calcium and 600–800 IU of vitamin D is recommended for those with age-related osteoporosis or who are at risk. As these total daily doses can be achieved by a variety of products, clinicians need to be prepared to answer questions regarding product selection. Patients should be counseled that the recommended daily amount of calcium refers to the elemental form, and this may vary depending upon the form chosen (). Second, there are potential concerns regarding bioavailability of various formulations based upon gastric absorption. It has been suggested that acid-dependent forms of calcium, such as calcium carbonate, are not as efficiently absorbed as acid-independent forms, such as calcium citrate. The clinical significance of this has not been clearly determined but may be worth considering, particularly in patients with age-related changes in gastric pH or who are on acid suppression therapy. Regardless of formulation selected, patients should also be counseled that bioavailability can be maximized by administering calcium supplements in divided doses, limiting intake at a single dosing interval to 500–600 mg, and giving with meals, particularly with calcium carbonate. The administration intervals of calcium supplements should also be timed so as to avoid absorption interactions with other products such as iron salts, fiber laxatives, quinolones, or tetracyclines.
Bisphosphonates
The bisphosphonates are a first line therapy for most patients with age-related osteoporosis. The nitrogen-containing bisphosphonates alendronate, risedronate, and ibandronate, are the most common agents used for treating and preventing age-related osteoporosis. While other bisphosphonates are available (eg, etidronate, pamidronate, tiludronate, and zoledronic acid) for other indications (eg, Paget's disease, hypercalcemia of malignancy, corticosteroid-induced osteoporosis), these agents are not first-line therapy for age-related osteoporosis (CitationLicata 2005).
The nitrogen-containing bisphosphonates increase BMD by inhibiting several key enzymes in the mevalonate pathway (CitationLicata 2005). This leads to increased BMD through several antiresorptive mechanisms including inhibition of osteoclast recruitment, osteoclast activity on the bone, induction of osteoclastic apoptosis, and alteration of the bone or bone mineral to reduce dissolution rate. Through inhibition of bone resorption, the bisphosphonates increase BMD approximately 2%–8% depending upon the agent, dose, and site measured and are effective for both the prevention and treatment of age-related osteoporosis (CitationCummings et al 1998; CitationHarris et al 1999; CitationBlack et al 2000; CitationOrwoll et al 2000; CitationReginster et al 2000; CitationMcClung et al 2001; CitationChesnut et al 2004).
Alendronate is an oral bisphosphonate that has been shown to decrease both vertebral and non-vertebral fractures. In the Fracture Intervention Trials (FIT), the effects of alendronate 5 mg daily for 2 years followed by 10 mg daily for 2 years in women with low BMD and no history of fracture or at least one-vertebral fracture was assessed. In the arm of the trial that included women with at least one prior vertebral fracture, 2027 women were followed to assess the risk of new vertebral and nonvertebral fractures (CitationBlack et al 2000). After an average follow-up of 2.9 years, alendronate increased BMD at the femoral neck 4.1%, total hip 4.7%, and posterior–anterior lumbar spine 6.2% compared with placebo (p<0.001 for all) and decreased the risk of new radiographic fractures by 47% (NNT=14), clinical vertebral fractures 55% (NNT=37), hip fractures 51% (NNT=91), wrist fractures 48% (NNT=53), and any non-vertebral fracture by 20% (NNT=36; p<0.05 for all). In the FIT arm that included women with low BMD but without a prior history of fracture, 4432 women were followed for an average of 4.2 years (CitationCummings et al 1998). At the end of the study, alendronate increased BMD at the femoral neck 4.6%, total hip 5.0%, and lumbar spine 6.6% compared with placebo (p<0.001 for all) and decreased the rate of any clinical fracture by 36% (NNT=15), though this effect was only significant in patients with osteoporosis at baseline (initial femoral neck T-score ≤−2.5). Alendronate decreased the rate of radiographic vertebral fractures overall by 44% (NNT=60).
Vertebral and non-vertebral fracture data is available for risedronate. In the Vertebral Efficacy with Risedronate Therapy in North American (VERT-NA) and Multi-National (VERT-MN) studies, the effects of risedronate 2.5 mg daily and 5 mg daily for 3-years was compared with placebo in postmenopausal women with osteoporosis and prior vertebral fractures (CitationHarris et al 1999; CitationReginster et al 2000). After 3 years, risedronate 5 mg daily decreased the rate of new vertebral fractures similarly in the two studies; 41% (NNT=10) and 49% (NNT=20) in the VERT-NA and VERTMN studies respectively. Nonvertebral fractures were reduced in the VERT-NA study 40% (NNT=31) and 33% (NNT=20; p=0.06) in the VERT-MN trial.
Risedronate has also been studied in patients at very high risk for fracture. In the Hip Intervention Program (HIP) study, the effects of risedronate 2.5 mg or 5 mg daily in 5455 elderly women with osteoporosis (femoral neck T-score <− 4 or <−3 plus a non-skeletal risk for falls) was compared with placebo (CitationMcClung et al 2001). After mean follow-up of 2.3 years, risedronate decreased the overall rate of hip fracture 30% (NNT=90), 40% in women 70–79 years of age (NNT=77), and 60% in those 70–79 years of age with at least 1 vertebral fracture at baseline (NNT=29).
Ibandronate, an oral bisphosphonate with an extended dosing interval, is the newest agent in this class to be approved for both the prevention and treatment of postmenopausal osteoporosis. In the oral ibandronate osteoporosis vertebral fracture trial in North America and Europe (BONE) study, ibandronate 2.5 mg daily or 20 mg every other day for 12 doses every 3 months was compared with placebo in 2946 osteoporotic women with 1–4 prior vertebral fractures (CitationChesnut et al 2004). After 3 years, vertebral fractures were significantly reduced 62% (NNT=20) and 50% (NNT=21) respectfully for the daily and intermittent doses compared with placebo. Ibandronate did not decrease clinical non-vertebral factures or hip fractures. However, a possible reason for this lack of benefit was the relatively high femoral neck T-score of patients at baseline (mean T-score −2.0). Post hoc analyses of patients at greater risk for hip fracture (ie, femoral-neck T-scores <−3.0) demonstrated that daily ibandronate was effective in decreasing nonvertebral fractures by 69%.
Traditionally, oral bisphosphonates had been given on a daily basis. More recently however, intermittent regimens (eg, once weekly with alendronate and risedronate and once monthly with ibandronate) have been developed in an attempt to increase patient adherence. It is important to note that fracture data are not available for these intermittent regimens, though studies are available that have demonstrated equivalent effects on BMD compared with daily dosing (CitationBrown et al 2002; CitationRizzoli et al 2002; CitationMiller et al 2005). However, while fracture data are not specifically available for these intermittent regimens, data are beginning to emerge that suggest these regimens do increase patient adherence. In an administrative claims study of 30 health plans, once weekly bisphosphonate use was associated with greater medication persistence than once daily users (69% vs 58%, p<0.001) and had higher rates of retention on treatment (44% vs 32%) at one year of use (CitationCramer et al 2005). However, it is important to note that long-term adherence (eg, 1 year) was still very poor with these intermittent regimens. Thus, continued research on modalities to increase patient adherence to osteoporosis therapies is warranted.
Calcitonin
Calcitonin is a 32-amino acid peptide that can be obtained from numerous animals, including a human form through recombinant technology. However, salmon calcitonin is the most potent and therefore most frequently used isolate (CitationZaidi et al 2002). The primary effect of calcitonin is to decrease bone resorption by inhibiting the function of mature osteoclasts. Calcitonin works via both cAMP and calciumdependent pathways to accomplish this effect (CitationZaidi et al 2002).
There are data supporting the efficacy of nasal calcitonin in reducing vertebral fractures, however non-vertebral fracture data is lacking. The prevent recurrence of osteoporotic fractures (PROOF) study consisted of 1255 postmenopausal women with pre-existing vertebral fractures who were randomized to 200 IU of intranasal salmon calcitonin or placebo (CitationChesnut et al 2000). Patients were followed for a total of five years to assess incidence of new vertebral fractures. The calcitonin group had a 33% reduction in the risk of vertebral fractures (NNT=33) compared with placebo.
In addition to antiresorptive properties, calcitonin has also been shown to be effective for managing the pain associated with acute vertebral compression fractures (CitationLyritis et al 1991). Thus, this agent may be useful in patients who have experienced a recent osteoporosis-related vertebral fracture. However, there are limitations with use of this agent. First, data regarding antifracture efficacy is significantly less when compared with other agents used for osteoporosis. Secondly, data are available that suggest the antiresorptive effects of calcitonin may diminish significantly with time. Between 19–26 months, a plateau effect has been demonstrated, which is likely due to down-regulation of calcitonin receptors (CitationGruber et al 1984). Thus, due to the availability of agents with more favorable fracture data, particularly when used long-term, calcitonin should not be a first line agent in the treatment of osteoporosis.
Estrogen receptor stimulators
Stimulation of estrogen receptors is another method by which BMD may be increased. Agents which stimulate estrogen receptors include estrogen (various forms), selective estrogen receptor modulators (SERMs), and tibolone. Estrogen receptors are located in various tissues throughout the body, including breast, uterine, intestinal, and bone tissues. In bone tissue estrogen receptors are located on osteoblasts and osteoclasts, and stimulation of these receptors by estrogen decreases osteoclast activity, thereby decreasing bone resorption. In addition, estrogen is also associated with peripheral inhibition of PTH, increased intestinal absorption of calcium, and decreased renal excretion of calcium (CitationNotelovitz 1997).
Estrogen
Estrogen, with or without a progesterone, has beneficial effects on surrogate markers of bone turnover and on fracture risk, and has been used extensively for the prevention of osteoporosis. There is evidence that estrogen increases BMD at the hip, lumbar spine, and peripheral body sites (CitationWells et al 2002). However, there has been relatively less data with respect to fracture outcomes. In 2 meta-analyses of 13 and 22 trials, estrogen was associated with an overall 13% reduction in vertebral fracture and a 27% reduction in non-vertebral fractures respectfully (CitationTorgerson and Bell-Syer 2001a, Citation2001b).
Two large, randomized, placebo controlled trials, the Heart and Estrogen/Progestin Replacement Study (HERS) and the Women's Health Initiative (WHI), evaluated the combination of estrogen plus progestin (CitationHulley et al 1998; CitationWGWHII 2002; CitationWHISC 2004). The HERS and its open label follow-up HERS II (CitationCauley, Black, et al 2001) did not report a reduction in fractures for hip, wrist, vertebral or total fractures (hazard ratio [HR] 1.04, 95%CI 0.87–1.25). The WHI was a much larger study evaluating 0.625 mg conjugated equine estrogen (CEE) plus 2.5 mg medroxyprogesterone acetate versus placebo (CitationWGWHII 2002). The estrogen-only arm evaluated 0.625 mg daily CCE or placebo in women with a hysterectomy (CitationWHISC 2004). In the estrogen plus progesterone arm, there was a 33% reduction (NNT=345) in the risk of hip fracture (CitationCauley et al 2003). The reduction in hip fracture risk was greater for those individuals receiving estrogen plus progesterone who also received at least 1200 mg of daily calcium at baseline. Estrogen plus progesterone was also associated with a 36% reduction in vertebral fractures (NNT=387), and a 30% reduction in lower arm/wrist fractures (NNT=125). The results of the estrogen-only arm likewise decreased the risk of hip and vertebral fractures slightly (NNT=216 and NNT=225 respectively).
Despite the benefit of hormone replacement therapy for osteoporosis, there are many concerns with chronic use. The estrogen plus progesterone arm of the WHI was stopped after an average follow-up of 5.2 years due to increased risk of breast cancer (number needed to harm [NNH]=237). Safety analysis also revealed a slight increase in risk of coronary heart disease events (NNH=237), pulmonary embolism (NNH=227), stroke (NNH=225), and deep vein thrombosis (NNH=141). Similar results were reported in the estrogen-only arm of the WHI, which was stopped after an average follow-up of 6.8 years. While there did not appear to be an increased risk of breast cancer or pulmonary events, there was no benefit reported for the primary outcome of coronary heart disease events (CHD) and an increased risk of stroke (NNH=125) and deep vein thrombosis (NNH=220).
Based on findings from the WHI, HERS, and other metaanalyses of estrogen or estrogen plus progestin, the 2005 US Preventive Services Task Force (USPSTF) recommended against routine use of estrogen with or without a progestin for the prevention of chronic conditions in older women (CitationUSPSTF 2005). While citing good evidence for increased BMD, and fair to good evidence for reducing fracture risk, the task force concluded that the risk of breast cancer, venous thromboembolism, CHD, stroke, and cholecystitis “likely to exceed the chronic disease prevention benefits in most women.” After the completion of the estrogen-only arm of the WHI, the task force updated its recommendations to recommend against the routine use of unopposed estrogen for the treatment of chronic conditions in women who have had a hysterectomy. As with estrogen and progestin therapy, they cited good evidence for osteoporosis benefit, but fair evidence for risks associated with thromboembolism, stroke, lower cognitive function, and dementia. For women with an intact uterus, unopposed estrogen is associated with an increased risk of endometrial cancer (CitationGrady et al 1995).
While the Task Force recommendations provide general guidance on the use of estrogen with or without a progestin, there are several clinical considerations that should be evaluated when weighing the risks and benefits of hormone replacement therapy (HRT). The Task Force concedes that while the risks of HRT are statistically significant, the absolute risk is small. For women aged 50–79 years, treatment of 10 000 women with HRT for 1 year might result in 8 additional cases of breast cancer, 8 additional strokes, 8 additional pulmonary emboli, and 7 CHD events, while resulting in five fewer hip fractures. A woman at high risk of fracture with a low risk of breast cancer or cardiovascular disease may consider the benefits of estrogen more compelling.
In contrast, HRT has not demonstrated superiority with respect to increased BMD or reductions in fracture risk compared with bisphosphonates in patients with osteoporosis. Thus, it should generally not be considered first line therapy for osteoporosis in bisphosphonate-eligible individuals, irrespective of the risk-benefit controversies previously described.
Emerging questions that remain unanswered about estrogen therapy include the roles of either low-dose or transdermal formulations in osteoporosis prevention or treatment. These forms of estrogen therapy were not evaluated in the large randomized trials upon which much of the current risk-benefit analysis is based, and are therefore not formally associated with the same risks as traditionally dosed HRT. While there are data demonstrating the efficacy of these forms in maintaining or improving bone density (CitationLindsay et al 2002; CitationEttinger et al 2004), conclusive evidence of reduced fracture risk is lacking.
Selective estrogen receptor modulators (SERMs)
For women who are not candidates for bisphosphonate therapy and in whom the risk of estrogen outweighs the potential benefits, other options may include selective estrogen receptor modulators (SERMs). The mechanism of action for these agents is to act as an estrogen receptor agonist on bone and uterine tissues, but as an antagonist in breast tissues (CitationRiggs and Hartmann 2003). Therefore, SERMs have a similar effect on bone density as estrogen, but carry a different risk profile.
Raloxifene is the most commonly used SERM for prevention and treatment of osteoporosis, and is associated with a 2%–3% increase in BMD (CitationDelmas et al 1997). Raloxifene has also demonstrated benefits on vertebral fractures, though data supporting a fracture benefit at non-vertebral sites is generally lacking. In the 36 month Multiple Outcomes of Raloxifene Evaluation (MORE) study, raloxifene 60 mg daily given for 3 years was associated at 30% (NNT=29) reduction in vertebral fractures (CitationEttinger et al 1999). The risk of breast cancer may be as much as 72% lower with raloxifene compared with placebo (CitationCauley, Norton, et al 2001). Because raloxifene is not associated with some of the traditional side effects of HRT such as breast tenderness and vaginal bleeding (CitationEttinger et al 1999), it is not necessarily an alternative for those women wishing to mitigate some of the cardiovascular risks of HRT, such as deep vein thromboembolism, which is increased approximately 1.7–3-fold compared with placebo (CitationEttinger et al 1999; CitationMartino et al 2005). It also does not provide the symptom relief from hot flushes associated with menopause for which some women may still elect short term HRT, and may actually be associated with an increased incidence (CitationMartino et al 2005).
There is interest in new SERMs for osteoporosis. Agents such as lasofoxifene, ospemifene, and bazedoxifene, as well as other novel SERM are in development. Most are primarily being evaluated for breast cancer prevention, however data describing their effects on bone density are emerging (CitationLasofoxifene 2005; CitationRonkin et al 2005; CitationWurz et al 2005).
Tibolone
Another agent active at the estrogen receptor is tibolone. Tibolone is a prodrug that must be converted to active metabolites 3α-hydroxytibolone, 3β-hydroxytibolone, and a Δ4-isomer, and is available in several countries, though not the US. Tibolone is classified as a non-estrogen synthetic steroid which may have estrogenic, progestogenic, or androgenic effects. In osteoporosis, the effect on trabecular bone density is thought to be related to activity at the estrogen receptor (CitationRubin and Bilezikian 2003).
Tibolone is used for the treatment of postmenopausal symptoms, and has been evaluated for the prevention and treatment of osteoporosis. The effects of 1.25–2.5 mg daily have been studied for improvements in surrogate markers of bone turnover and bone density improvements at the hip and lumbar spine. Increases in bone density have ranged from 3.6% to 7.2% at the lumbar spine and 2.6% to 4.6% at the femoral neck, though data on fracture reduction is currently lacking (CitationThiebaud et al 1998; CitationPavlov et al 1999; CitationRymer et al 2001; CitationRoux et al 2002).
Clear comparisons between tibolone and estrogen replacement are not available. In one study, the magnitude of BMD increase was greater with tibolone than with conjugated equine estrogen/medroxyprogesterone after 3 years (CitationThiebaud et al 1998). However, in another study the BMD improvements with tibolone were not superior to estradiol and norethindrone after 2 years (CitationRoux et al 2002). These authors did report though, that the incidence of side effects such as vaginal bleeding and breast tenderness was lower in the tibolone group. Thus, tibolone may be an alternative to estrogen in postmenopausal women for the amelioration of menopausal symptoms, osteoporosis prevention and possibly for osteoporosis treatment. However, considering the lack of fracture data, this agent should not be used first line for osteoporosis prevention or treatment, particularly in bisphosphonate-eligible women.
Strontium ranelate
Strontium ranelate is a di-strontium salt that has been shown to act as both a bone-forming and antiresorptive agent (CitationMarie 2005). A proposed mechanism for increased bone formation is via stimulation of replication of osteoprogenitor cells and collagen. In addition, strontium has been shown to inhibit osteoclast activity and differentiation in vitro (CitationReginster, Sarlet, et al 2005). It is currently approved in Europe for the treatment of postmenopausal osteoporosis, though it is not yet available in the US.
The effects of strontium ranelate on BMD and fractures have been studied. The strontium ranelate for treatment of osteoporosis (STRATOS) trial evaluated the effects of 0.5 g, 1 g, or 2 g strontium ranelate compared with placebo over 3 years in 353 postmenopausal women with at least one previous vertebral fracture and a lumbar T-score <−2.4 (CitationMeunier et al 2002). Lumbar BMD increased in a dosedependent manner from a mean annual slope of 1.4% with 0.5 g/day to 3.0% with 2 g/day (p<0.01 for both doses vs placebo). In a phase 3 study, 1649 postmenopausal women with osteoporosis and at least one prior vertebral fracture were given strontium ranelate 2 g/day or placebo and followed for three years (CitationMeunier et al 2004). The relative risk of new vertebral fractures decreased 41% compared with placebo (NNT=9). In the Treatment of Peripheral Osteoporosis Study (TROPOS), strontium ranelate 2 g/day or placebo was given to 5091 postmenopausal women with osteoporosis (CitationReginster, Seeman, et al 2005). After a mean follow up of 3 years, nonvertebral fractures were reduced 16% (NNT=59) and major fragility fractures by 19% (NNT=59) with strontium therapy versus placebo. Although strontium ranelate has been shown to be beneficial in postmenopausal osteoporosis, it is not generally considered a first line agent at this time due to less clinical experience and fracture data compared with bisphosphonates.
Sodium fluoride
Although sodium fluoride has been available for several years, it is not generally considered a routine therapy for age-related osteoporosis. While sodium fluoride has been shown to increase BMD by stimulating osteoblastics (CitationFarley et al 1983), concerns have been raised regarding strength and quality of new bone formed as well as tolerability. In a 4-year, prospective study of 202 postmenopausal women with osteoporosis and vertebral fractures who were randomized to either 75 mg/day of sodium fluoride or placebo, the incidence of nonvertebral fractures increased 3-fold compared with placebo (CitationRiggs et al 1990). The postulated mechanism for this increase in fracture is overstimulation of bone formation by sodium fluoride, leading to excessive production of weaker bone. In addition, sodium fluoride has been associated with other adverse events such as gastrointestinal disturbances likely as a result of conversion of sodium fluoride to hydrofluoric acid in the stomach, and lower extremity pain possibly due to excessive bone remodeling (CitationRubin et al 2001).
Due to previously noted adverse effects, systemic sodium fluoride has not gained wide use or acceptance. However, more recent data in which sodium fluoride is given on an intermittent basis and in sustained release form may change this agent's place in therapy. Sustained-release sodium fluoride given on an intermittent basis has been shown to decrease vertebral fractures and increase BMD (CitationRubin et al 2001). In a randomized, double-blind, placebo-controlled trial of 85 postmenopausal women with 1 or more vertebral compression fractures, 25 mg sustained-release sodium fluoride given twice daily in 3, 12-week cycles with a 2-month drug-free period in between was compared with placebo. After a mean follow up of 42 months, BMD in L2–L4 increased by 5.4% compared with placebo and decreased vertebral fractures decreased by 68% (NNT=8). Thus, while intermittent therapy with sustained release sodium fluoride may be a viable treatment option for osteoporosis, it is currently not recommended as a first or second line therapy due to lack of definitive, long-term data.
Teriparatide (recombinant parathyroid hormone 1–34)
Parathyroid hormone may be anabolic or catabolic in the skeleton depending on the mode of administration. When PTH is given as a once-daily injection, its primary affect is to increase bone mass (CitationHodsman et al 2005). The mechanism by which PTH is thought to stimulate bone production is by increasing the remodeling rate. PTH increases the amount of new bone that is laid down in each remodeling unit, which leads to a net gain in bone mass. In addition, it is also thought that PTH initially uncouples formation from resorption and is able to directly stimulate bone formation independent of resorption (CitationHodsman et al 2005).
The effects of PTH 1–34 (teriparatide) on BMD and fractures have been studied. In a placebo controlled study of 1637 postmenopausal women with at least 1 prior vertebral fracture, teriparatide 20 mcg and 40 mcg given as a once-daily subcutaneous injection was compared with placebo (CitationNeer et al 2001). After a median follow-up of 21 months, teriparatide increased BMD by 9% and 13% in the lumbar spine and 3 and 6% at the femoral neck compared with placebo for the 20 mcg and 40 mcg groups respectively. This increase in BMD lead to a significant decreases in both vertebral fractures (65% and 69%) and nonvertebral fractures (53% and 54%) for the PTH groups respectively.
While teriparatide does increase BMD and has been shown to be effective in reducing the risk of fractures, there are significant limitations to its use. First, it must be given once daily as an injection. Thus, patient acceptance and adherence to therapy may be significantly diminished, particularly when compared with an intermittent oral bisphosphonate regimen. In addition, the extremely high cost of this agent is also a limitation. Teriparatide is approximately US$17.50/day (€15/day) for the 20 mcg/day dose (CitationSelby 2004).
Another limitation of teriparatide may be the slight increased risk of osteosarcoma. This risk is postulated based on the induction of osteosarcoma in a rat model. Teriparatide therapy is not recommended for more than a two year period and should not be used in patients with an increased risk of malignancies of the bone (CitationHodsman et al 2005). Thus, although teriparatide has been shown to be effective for osteoporosis, it should generally be reserved for patients who are unable to tolerate a bisphosphonate and who have severe/established osteoporosis.
Combination therapy
Use of combination therapies that have different mechanisms of action is a potential treatment option for those at high risk for osteoporotic fracture. Data are available that demonstrate an approximate 1% additional increase in BMD with combination of a bisphosphonate and estrogen or raloxifene versus a bisphosphonate alone (CitationBone et al 2000; CitationHarris et al 2001; CitationJohnell et al 2002; CitationGreenspan et al 2003). However, there are some theoretical concerns that over-suppression of bone formation may occur with utilization of two or more antiresorptives, thus potentially increasing the risk for fractures.
The combination of a bisphosphonate with PTH has had mixed results. In two randomized, controlled trials, the combination of alendronate with PTH failed to increase BMD in men and women (CitationBlack et al 2003; CitationFinkelstein et al 2003). Alendronate attenuation of PTH-induced stimulation of bone formation was postulated as a potential etiology for this lack of benefit. However, in the continuation study by CitationBlack et al (2005), patients who were assigned alendronate for 1 year after receiving 1-year of PTH, BMD at the lumbar spine increased significantly more in the combination group (12.1% vs 4.1%). Thus, although fracture data are not available for the combination of PTH followed by a bisphosphonate, this may be a reasonable intervention.
Treatment in special populations
As many as 85% of American nursing home residents suffer from osteoporosis (CitationZimmerman et al 1999). Comorbidities, inadequate nutrition, multiple medications, immobility, difficulty transferring, disabilities, and fragility all contribute to a much higher fracture rate among nursing home dwelling elderly as compared with community dwelling elderly. Osteoporosis treatment and prevention of related fractures among nursing home residents involves multiple perspectives including fall prevention, adequate nutrition, strength and balance training, and frequent medication review. Environmental modifications to nursing home floors, doors, and hallways are often the best method to reduce fall rates and therefore fractures.
Unfortunately, the majority of nursing home residents with osteoporosis receive inadequate drug therapy. Calcium and vitamin D supplements are prescribed for approximately 60% of nursing home residents with osteoporosis and only 25% of residents with hip fractures (CitationKamel 2004). Bisphosphonate use is also low among nursing home residents with documented hip fractures. The pharmacologic foundation for osteoporosis treatment in elderly nursing home residents should include adequate calcium and vitamin D supplements and bisphosphonate therapy, taking into account individual patient considerations such as swallowing function, mobility/position, and esophageal function.
Osteoporosis is increasingly recognized as a serious men's health issue with 25%–30% of hip fractures occurring in men (CitationElliott et al 2004). One in four men over aged 50 will experience an osteoporosis-related fracture in their life time (CitationNOF 2005b). Male nursing home residents have a 5–10-fold increase in fracture risk compared with males residing in the community. Men experience higher morbidity and mortality after hip fracture with one year mortality post hip fracture approximately double in men when compared with women (CitationAmin and Felson 2001). After sustaining a hip fracture, up to 50% of men require institutional care. Thus, fall prevention is an important concern for both community dwelling men and nursing home male residents. Aged-related osteoporosis in men may be exacerbated by lifestyle habits including low physical activity, low body weight, excessive alcohol intake, and smoking. The pharmacologic foundation for osteoporosis treatment in men, as well as women, includes adequate calcium and vitamin D supplementation. First line therapy includes oral bisphosphonates (eg, alendronate). Teriparatide is indicated for men with idiopathic or hypogonadism-related (associated low levels of estradiol and testosterone) osteoporosis who are at high risk of fracture (CitationOrwoll et al 2003; CitationTeriparatide 2003). Public relations campaigns of the National toward men to help patients and practitioners understand that both genders are susceptible to the devastating effects of age-related bone loss.
Conclusions
Osteoporosis is a significant problem for the global community. With the aging of the population, osteoporosis and related fractures will likely continue to increase. Various interventions are available to decrease the morbidity, mortality, and economic impact of age-related osteoporosis. This includes identification of risk factors for low BMD and falls, screening patients at risk, and instituting pharmacological therapy for those with documented osteoporosis. All patients should receive adequate calcium and vitamin D. Medications which have proven to decrease factures should be used preferentially over medications for which there is only positive BMD data. Further research is needed to determine if combination therapies have additive effects on fracture reduction. In addition, attention should be paid to patients who may be at significant risk of age-related osteoporosis and related fractures, but are often neglected, such as men and those in the nursing homes.
Acknowledgements
We would like to thank Janet Schwartzenberg M.D. for her valuable insight in the preparation of this manuscript.
References
- AaronJEGallagherJCAndersonJFrequency of osteomalacia and osteoporosis in fractures of the proximal femurLancet19741229334130245
- AdamsJSKantorovichVWuCResolution of vitamin D insufficiency in osteopenic patients results in rapid recovery of bone mineral densityJ Clin Endocrinol Metab19998427293010443668
- AminSFelsonDTOsteoporosis in menRheum Dis Clin North Am200127194711285995
- Bischoff-FerrariHADietrichTOravEJPositive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adultsAm J Med2004116634915093761
- Bischoff-FerrariHAWillettWCWongJBFracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trialsJAMA200529322576415886381
- BlackDMBilezikianJPEnsrudKEOne year of alendronate after one year of parathyroid hormone (1-84) for osteoporosisN Engl J Med20053535556516093464
- BlackDMGreenspanSLEnsrudKEThe effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosisN Engl J Med200334912071514500804
- BlackDMThompsonDEBauerDCFracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research GroupJ Clin Endocrinol Metab20008541182411095442
- BoneHGGreenspanSLMcKeeverCAlendronate and estrogen effects in postmenopausal women with low bone mineral densityJ Clin Endocrinol Metab200085720610690882
- BrownJPKendlerDLMcClungMRThe efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosisCalcif Tissue Int2002711031112085156
- CadaretteSMJaglalSBKreigerNDevelopment and validation of the Osteoporosis Risk Assessment Instrument to facilitate selection of women for bone densitometryCMAJ200016212899410813010
- CauleyJABlackDMBarrett-ConnorEEffects of hormone replacement therapy on clinical fractures and height loss: The Heart and Estrogen/Progestin Replacement Study (HERS)Am J Med20011104425011331055
- CauleyJANortonLLippmanMEContinued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluationBreast Cancer Res Treat2001651253411261828
- CauleyJARobbinsJChenZEffects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trialJAMA200329017293814519707
- CenterJRNguyenTVSchneiderDMortality after all major types of osteoporotic fracture in men and women: an observational studyLancet19993538788210093980
- ChapuyMCArlotMEDuboeufFVitamin D3 and calcium to prevent hip fractures in the elderly womenN Engl J Med19923271637421331788
- ChesnutCH3rdSilvermanSAndrianoKA randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study GroupAm J Med20001092677610996576
- ChesnutICSkagAChristiansenCEffects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosisJ Bone Miner Res2004191241915231010
- CompstonJEPapapoulosSEBlanchardFReport on osteoporosis in the European Community: current status and recommendations for the future. Working Party from European Union Member StatesOsteoporos Int19988531410326056
- CramerJAAmonkarMMHebbornACompliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosisCurr Med Res Opin20052114536016197664
- CummingRGEpidemiology of medication-related falls and fractures in the elderlyDrugs Aging19981243539467686
- CummingsSRBatesDBlackDMClinical use of bone densitometry: scientific reviewJAMA200228818899712377088
- CummingsSRBlackDMNevittMCBone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research GroupLancet19933417258093403
- CummingsSRBlackDMThompsonDEEffect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention TrialJAMA19982802077829875874
- Dawson-HughesBHarrisSSKrallEAEffect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or olderN Engl J Med199733767069278463
- DelmasPDBjarnasonNHMitlakBHEffects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal womenN Engl J Med1997337164179385122
- DolanPTorgersonDJThe cost of treating osteoporotic fractures in the United Kingdom female populationOsteoporos Int199886111710326069
- ElliottMEDrinkaPJKrausePOsteoporosis assessment strategies for male nursing home residentsMaturitas2004482253315207888
- EmpanaJPDargent-MolinaPBreartGEffect of hip fracture on mortality in elderly women: the EPIDOS prospective studyJ Am Geriatr Soc2004526859015086646
- EttingerBBlackDMMitlakBHReduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) InvestigatorsJAMA19992826374510517716
- EttingerBEnsrudKEWallaceREffects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trialObstet Gynecol20041044435115339752
- FarleyJRWergedalJEBaylinkDJFluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cellsScience198322233026623079
- FinkHAEnsrudKENelsonDBDisability after clinical fracture in postmenopausal women with low bone density: the fracture intervention trial (FIT)Osteoporos Int200314697612577187
- FinkelsteinJSHayesAHunzelmanJLThe effects of parathyroid hormone, alendronate, or both in men with osteoporosisN Engl J Med200334912162614500805
- GallagherJCGoldgarDTreatment of postmenopausal osteoporosis with high doses of synthetic calcitriol. A randomized controlled studyAnn Intern Med1990113649552221645
- GradyDGebretsadikTKerlikowskeKHormone replacement therapy and endometrial cancer risk: a meta-analysisObstet Gynecol199585304137824251
- GranekEBakerSPAbbeyHMedications and diagnoses in relation to falls in a long-term care facilityJ Am Geriatr Soc198735503113571802
- GrantAMAvenellACampbellMKOral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trialLancet20053651621815885294
- GreenspanSLResnickNMParkerRACombination therapy with hormone replacement and alendronate for prevention of bone loss in elderly women: a randomized controlled trialJAMA200328925253312759324
- GruberHEIveyJLBaylinkDJLong-term calcitonin therapy in postmenopausal osteoporosisMetabolism1984332953036423929
- HallbergIRosenqvistAMKartousLHealth-related quality of life after osteoporotic fracturesOsteoporos Int2004158344115045468
- HarrisSTEriksenEFDavidsonMEffect of combined risedronate and hormone replacement therapies on bone mineral density in postmenopausal womenJ Clin Endocrinol Metab2001861890711344179
- HarrisSTWattsNBGenantHKEffects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study GroupJAMA199928213445210527181
- HodsmanABBauerDCDempsterDParathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its useEndocr Rev20052668870315769903
- HolickMFSirisESBinkleyNPrevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapyJ Clin Endocrinol Metab20059032152415797954
- HulleySGradyDBushTRandomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research GroupJAMA1998280605139718051
- JacksonRDLa CroixAZGassMCalcium plus vitamin D supplementation and the risk of fracturesN Engl J Med20063546698316481635
- JohnellOKanisJAAn estimate of the worldwide prevalence, mortality and disability associated with hip fractureOsteoporos Int20041589790215490120
- JohnellOScheeleWHLuYAdditive effects of raloxifene and alendronate on bone density and biochemical markers of bone remodeling in postmenopausal women with osteoporosisJ Clin Endocrinol Metab2002879859211889149
- KamelHKUnderutilization of calcium and vitamin D supplements in an academic long-term care facilityJ Am Med Dir Assoc200459810014984620
- KanisJAGluerCCAn update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis FoundationOsteoporos Int20001119220210824234
- KannusPSievanenHPalvanenMPrevention of falls and consequent injuries in elderly peopleLancet200536618859316310556
- [Lasofoxifene]Lasofoxifene: CP 336156, CP-336156Drugs R D20056566015801869
- Le BoffMSKohlmeierLHurwitzSOccult vitamin D deficiency in postmenopausal US women with acute hip fractureJAMA199928115051110227320
- LewieckiEMWattsNBMcClungMROfficial positions of the international society for clinical densitometryJ Clin Endocrinol Metab2004893651515292281
- LicataAADiscovery, clinical development, and therapeutic uses of bisphosphonatesAnn Pharmacother2005396687715755793
- LindsayRGallagherJCKleerekoperMEffect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal womenJAMA200228726687612020302
- LipsPCooperCAgnusdeiDQuality of life in patients with vertebral fractures: validation of the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO). Working Party for Quality of Life of the European Foundation for OsteoporosisOsteoporos Int1999101506010501796
- LipsPGraafmansWCOomsMEVitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trialAnn Intern Med199612440068554248
- LydickECookKTurpinJDevelopment and validation of a simple questionnaire to facilitate identification of women likely to have low bone densityAm J Manag Care19984374810179905
- LyritisGPTsakalakosNMagiasisBAnalgesic effect of salmon calcitonin in osteoporotic vertebral fractures: a double-blind placebo-controlled clinical studyCalcif Tissue Int199149369721818759
- MalabananAVeronikisIEHolickMFRedefining vitamin D insufficiencyLancet199835180569519960
- MargolisDJKnaussJBilkerWMedical conditions as risk factors for pressure ulcers in an outpatient settingAge Ageing2003322596412720610
- MariePJStrontium as therapy for osteoporosisCurr Opin Pharmacol20055633616183330
- MarshallDJohnellOWedelHMeta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fracturesBMJ1996312125498634613
- MartinoSDischDDowsettSASafety assessment of raloxifene over eight years in a clinical trial settingCurr Med Res Opin20052114415216197663
- Mc ClungMRGeusensPMillerPDEffect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study GroupN Engl J Med20013443334011172164
- MeunierPJRouxCSeemanEThe effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosisN Engl J Med20043504596814749454
- MeunierPJSlosmanDODelmasPDStrontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis—a 2-year randomized placebo controlled trialJ Clin Endocrinol Metab2002872060611994341
- MillerPDMc ClungMRMacoveiLMonthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE studyJ Bone Miner Res20052013152216007327
- [NAS] National Academy of Sciences. Institute of Medicine, Food, and Nutrition BoardDietary reference intake tables: vitamins [online]1997 Accessed on 22 December 2005. URL: http://www.iom.edu/Object.File/Master/7/296/0.pdf
- NeedAGO’LoughlinPDMorrisHAThe effects of age and other variables on serum parathyroid hormone in postmenopausal women attending an osteoporosis centerJ Clin Endocrinol Metab2004891646915070925
- NeerRMArnaudCDZanchettaJREffect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosisN Engl J Med200134414344111346808
- NevittMCEttingerBBlackDMThe association of radiographically detected vertebral fractures with back pain and function: a prospective studyAnn Intern Med19981287938009599190
- [NIH] NIH Consensus conferenceOptimal calcium intake. NIH Consensus Development Panel on Optimal Calcium IntakeJAMA1994272194287990248
- [NIH] NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and TherapyOsteoporosis prevention, diagnosis, and therapyJAMA20012857859511176917
- [NOF] National Osteoporosis FoundationPhysician's guide to prevention and treatment of osteoporosis [online]2004 Accessed on 23 March 2005 URL: http://www.nof.org/physguide/index.htm
- [NOF] National Osteoporosis FoundationAmerica's bone health: the state of osteoporosis and low bone mass [online]2005a Accessed on 16 November 2005. URL: http://www.nof.org/advocacy/prevalence/index.htm
- [NOF] National Osteoporosis FoundationOsteoporosis: fast facts [online]2005b Accessed on 15 November 2005. URL: http://www.nof.org/osteoporosis/diseasefacts.htm
- NotelovitzMEstrogen therapy and osteoporosis: principles & practiceAm J Med Sci19973132129001160
- OrwollEEttingerMWeissSAlendronate for the treatment of osteoporosis in menN Engl J Med20003436041010979796
- OrwollESScheeleWHPaulSThe effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosisJ Bone Miner Res20031891712510800
- PavlovPWGinsburgJKicovicPMDouble-blind, placebo-controlled study of the effects of tibolone on bone mineral density in postmenopausal osteoporotic women with and without previous fracturesGynecol Endocrinol199913230710533157
- ReginsterJMinneHWSorensenOHRandomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study GroupOsteoporos Int200011839110663363
- ReginsterJYGilletPBen SedrineWDirect costs of hip fractures in patients over 60 years of age in BelgiumPharmacoeconomics1999155071410537967
- ReginsterJYSarletNLejeuneEStrontium ranelate: a new treatment for postmenopausal osteoporosis with a dual mode of actionCurr Osteoporos Rep2005330416036099
- ReginsterJYSeemanEDe VernejoulMCStrontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) StudyJ Clin Endocrinol Metab20059028162215728210
- RiggsBLHartmannLCSelective estrogen-receptor modulators – mechanisms of action and application to clinical practiceN Engl J Med20033486182912584371
- RiggsBLHodgsonSFO’FallonWMEffect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosisN Engl J Med199032280292407957
- RizzoliRGreenspanSLBoneG 3rdTwo-year results of once-weekly administration of alendronate 70mg for the treatment of postmenopausal osteoporosisJ Bone Miner Res20021719889612412806
- RonkinSNorthingtonRBaracatEEndometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal womenObstet Gynecol2005105139740415932835
- RouxCPelissierCFechtenbaumJRandomized, double-masked, 2-year comparison of tibolone with 17beta-estradiol and norethindrone acetate in preventing postmenopausal bone lossOsteoporos Int200213241811991445
- RubinCDPakCYAdams-HuetBSustained-release sodium fluoride in the treatment of the elderly with established osteoporosisArch Intern Med200116123253311606148
- RubinMRBilezikianJPNew anabolic therapies in osteoporosisEndocrinol Metab Clin North Am20033228530712699304
- RymerJRobinsonJFogelmanIEffects of 8 years of treatment with tibolone 2.5mg daily on postmenopausal bone lossOsteoporos Int2001124788311446564
- SchlaichCMinneHWBrucknerTReduced pulmonary function in patients with spinal osteoporotic fracturesOsteoporos Int1998826179797911
- SelbyPPostmenopausal osteoporosisCurr Osteoporos Rep20042101616036090
- SheaBWellsGCranneyAMeta-analyses of therapies for postmenopausal osteoporosis. VII. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosisEndocr Rev200223552912202470
- SleeperRBBondCRojas-FernandezCPsychotropic drugs and falls: New evidence pertaining to SSRI'sPharmacotherapy2000203081710730686
- [Teriparatide]Teriparatide (forteo) for osteoporosisMed Lett Drugs Ther20034591012571538
- ThiebaudDBiglerJMRenteriaSA 3-year study of prevention of postmenopausal bone loss: conjugated equine estrogens plus medroxyprogesterone acetate versus tiboloneClimacteric199812021011907945
- TilyardMWSpearsGFThomsonJTreatment of postmenopausal osteoporosis with calcitriol or calciumN Engl J Med1992326357621729617
- TinettiMEWilliamsCSFalls, injuries due to falls, and the risk of admission to a nursing homeN Engl J Med19973371279849345078
- TorgersonDJBell-SyerSEHormone replacement therapy and prevention of nonvertebral fractures: a meta-analysis of randomized trialsJAMA2001a2852891711401611
- TorgersonDJBell-SyerSEHormone replacement therapy and prevention of vertebral fractures: a meta-analysis of randomised trialsBMC Musculoskelet Disord2001b2711716794
- TucciJRImportance of early diagnosis and treatment of osteoporosis to prevent fracturesAm J Manag Care200612S1819016686587
- [USPSTF] US Preventive Services Task ForceHormone therapy for the prevention of chronic conditions in postmenopausal women: recommendations from the US Preventive Services Task ForceAnn Intern Med20051428556015897536
- van WeelCVermeulenHvan den BoschWFalls, a community care perspectiveLancet19953451549517791442
- WallachJInterpretation of diagnostic testsA synopsis of laboratory medicine1986Boston, MALittle, Brown & Co
- WellsGTugwellPSheaBMeta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal womenEndocr Rev2002235293912202468
- [WGWHII] Writing Group for the Women's Health Initiative InvestigatorsRisks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative Randomized Controlled TrialJAMA20022883213312117397
- [WHISC] Women's Health Initiative Steering CommitteeEffects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative Randomized Controlled TrialJAMA200429117011215082697
- WickershamRMNovakKKNutrients and nutritional agentsDrug facts and comparisons2002St. Louis, MOWolters Kluwer Health, Inc.267
- WurzGTReadKCMarchisano-KarpmanCOspemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar miceJ Steroid Biochem Mol Biol2005972304016153821
- ZaidiMMoongaBSAbeECalcitonin and bone formation: a knockout full of surprisesJ Clin Invest200211017697112488426
- ZimmermanSIGirmanCJBuieVCThe prevalence of osteoporosis in nursing home residentsOsteoporos Int19999151710367043