Abstract
Frovatriptan is an orally active 5-hydroxytryptamine (5-HT) receptor agonist which binds with high affinity to 5-HT1B and 5-HT1D receptors. Earlier clinical trials demonstrated that frovatriptan 2.5 mg is significantly more effective than placebo in the acute management of migraine and its associated symptoms. More recently, frovatriptan was shown to be effective in the management of menstrual migraine. The incidence of menstrual migraine in subjects receiving frovatriptan 2.5 mg twice daily during the six day perimenstrual period was 41% compared with 67% with placebo. Frovatriptan treatment is generally well tolerated. The most commonly reported adverse effects were dizziness, paresthesia, dry mouth, and fatigue. Pharmacologic studies demonstrated that frovatriptan is cerebroselective. Its selectivity for cerebral vessels lessens the potential for undesirable peripheral effects. Frovatriptan has a terminal deposition half-life of approximately 26 hours, which appears to be independent of age, gender, and renal function. This imparts that frovatriptan may be particularly well suited to patients with prolonged migraines and those who suffer migraine recurrence. Frovatriptan does not alter cytochrome P450 (CYP450) isoenzymes, as such it is unlikely to affect the metabolism of other drugs. No dosage adjustments are necessary based on age, renal, or mild to moderate hepatic impairment. Apart from its efficacy in the acute management of migraine, frovatriptan is an effective agent when used as either acute therapy or as intermittent prophylaxis therapy of menstrual migraines, particularly in women who do not respond to conventional therapies.
Introduction
Migraine is a primary headache disorder which affects approximately 28 million people in the US (CitationLipton et al 2001). Typical symptoms of migraine include unilateral, pulsating pain of moderate to severe intensity, which is often aggravated by routine physical activity. Additionally, patients often report symptoms of nausea, photophobia and/or phonophobia (CitationHCC 1988; CitationRussell et al 1996; CitationMulleners et al 2001). If untreated, attacks typically last 4 to 72 hours (HCC 1998), and often result in disability (CitationLipton and Stewart 1993). The prevalence of migraine varies considerably by age and gender. The disorder is commonly reported in the second and third decades of life (CitationLipton et al 2001), and females are more likely to suffer from migraine attacks (18%) than males (6%) (CitationStewart et al 1994; CitationMarks and Rapoport 1997). This increased prevalence has been linked to menstruation (CitationKing and Herndon 2005). Nonetheless, patients who suffer menstrual migraines may also experience migraine attacks at various times (CitationMacGregor 1997).
Although menstrual migraine was not recognized by the Headache Classification Committee of the International Headache Society (IHS) in the past (CitationHCC 1988), revised version of the IHS (CitationHCS 2004) included “candidate” criteria in the appendix for menstrual related migraine (formerly called menstrual-associated migraine) and pure menstrual migraine (formerly called true menstrual migraine). Though the “candidate” criteria are indicative of insufficient validation for inclusion in the formal classification system, it provides a framework for future research and evaluation of migraines linked to menses.
Irrespective of the cause, migraine patients must deal with quality of life, employment, psychosocial, behavior, and cognitive issues. The economic burden of migraine is substantial, it has been estimated that migraine costs American employers approximately 13 billion dollars a year due to missed work days and impaired work function (CitationHu et al 1999).
Management of migraine continues to evolve and improve as a result of our increased understanding of this disorder. The introduction of serotonin agonists in the early 1990s was a significant advance in the management of migraine due to their established efficacy and favorable adverse effect profile. All agents are approved for the acute management of migraine with and without aura. Second-generation triptans offer an improved pharmacokinetic profile when compared with the previously marketed agents.
The latest triptan released to the global markets, frovatriptan succinate, has shown promise in the management of migraine and is associated with a low incidence of headache recurrence. Frovatriptan has distinctive pharmacokinetic and pharmacologic properties, mainly a long terminal deposition half-life of approximately 26 hours.
This article reviews the pharmacology and pharmacokinetics of frovatriptan and highlights frovatriptan's clinical potential in the management of menstrual migraine.
Pharmacology
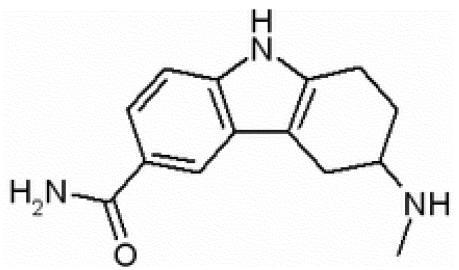
Frovatriptan () is a 5-HT receptor agonist which binds with high affinity to 5-HT1B and 5-HT1D receptors (CitationComer 2002). In vitro, frovatriptan shows moderate affinity for the receptor 5-HT7, which is believed to contribute to its distinctive pharmacologic properties (CitationBrown et al 1998). Frovatriptan demonstrated higher binding affinity than sumatriptan at human 5-HT1B receptor (∼4-fold) and a comparable affinity at human 5-HT1D receptor (CitationComer 2002). Frovatriptan was shown to be the most potent 5-HT1B agonist in vitro −log10 of the concentration causing 50% maximal stimulation (pEC50) of 8.2 as compared with sumatriptan, pEC50 of 7.0 (CitationStewart et al 1999).
Furthermore, frovatriptan was shown to be a full agonist with moderate potency at the 5-HT7 receptor (pEC50: 6.2±0.1) as compared with sumatriptan and naratriptan which were both partial agonists (pEC50 <5.3) (CitationBrown et al 1998).
When compared with sumatriptan, in vitro studies have shown that frovatriptan exhibits higher selectivity for the cerebral vasculature, with minimal effects on the coronary vasculature (CitationParsons et al 1998). The findings are corroborated by various in vivo preclinical studies (CitationComer 2002). Resembling other agents within the class, the pharmacologic effects of frovatriptan can be ascribed to its agonist effects at 5-HT1B/1D receptors on the extracerebral, intracranial blood vessels, which become dilated during migraine attacks, and on nerve terminals in the trigeminal system. It is suggested that activation of these receptors causes vasoconstriction, inhibition of neuropeptide release, and reduced transmission in trigeminal pain pathways (CitationTepper et al 2002).
Pharmacokinetics
As a second-generation triptan, frovatriptan has a distinct pharmacokinetic profile. The mean blood terminal deposition half-life of frovatriptan is approximately 26 hours, the longest among agents within its class, and is not affected by gender, dose, or route of administration (CitationBuchan et al 2002).
The absolute oral bioavailability of Frovatriptan is 22%–30% (CitationBuchan 1998). The mean time to reach peak plasma concentration (Tmax) is approximately 2–3 hours (CitationBuchan et al 1998). Food does not influence the pharmacokinetics of frovatriptan (CitationBuchan et al 2002).
Frovatriptan undergoes hepatic metabolism via CYP 1A2 isoenzyme and is eliminated both by hepatic metabolism and renal excretion. Renal clearance accounts for approximately 40% of total frovatriptan clearance including parent and other minor metabolites. Frovatriptan is poorly protein bound, with total protein binding estimated at 15% (CitationBuchan and Gay-Feutry 2000; CitationBuchan et al 2002).
The pharmacokinetics of frovatriptan were not affected by various degrees of renal impairment (creatinine clearance 16–73ml/min) or mild-to-moderate hepatic impairment (Child-Pugh scores A & B) (CitationCohen et al 1999). Thus, no dosage adjustments are necessary under these conditions. Lastly, it should be noted that the pharmacokinetics and tolerability of frovatriptan were not affected during migraine attacks, and were similar in migraine patients and healthy subjects (CitationBuchan, Ward, Zeig, et al 1999; CitationBuchan et al 2002). summarizes the pharmacokinetic parameters of frovatriptan.
Table 1 Pharmacokinetics of frovatriptan in healthy male and female volunteers (age 18–45 years). Data obtained after a single oral dose of frovatriptan 2.5mg (CitationBuchan et al 2002)
Frovatriptan does not alter CYP450 isoenzymes or monoamine oxidase (MAO); thus drug interactions of frovatriptan are clinically insignificant. Nonetheless, coadministration of frovatriptan and propranolol; fluvoxamine; and combined oral contraceptives resulted in small increases in mean peak plasma concentration (Cmax) and area under the plasma concentration-versus-time curve (AUC) of frovatriptan (CitationBuchan et al 2002). Propranolol resulted in an approximate increase of Cmax and AUC values of approximately 25%. Corresponding values for fluvoxamine were approximately 28% and 39%. Coadministration of frovatriptan with combined oral contraceptives resulted in increased Cmax and AUC of frovatriptan by approximately 40%. Contrarily, coadministration of frovatriptan and ergotamine tartrate resulted in decreases in both Cmax and AUC of frovatriptan by approximately 25% (CitationBuchan, Ward, Oliver, et al 1999). Due to lack of inhibitory or inducing effects on CYP isoenzymes, frovatriptan appears to have a low risk of drug interactions and dosage adjustments are unlikely to be warranted (CitationBuchan et al 2002).
Clinical efficacy and tolerability in menstrual migraine
CitationSilberstein et al (2004) sought to evaluate the efficacy, safety, and tolerability of frovatriptan in prophylactic therapy of menstrually-associated migraine (MAM). The primary objective of the study was to determine whether frovatriptan, administered for 6 days beginning 2 days prior to expected onset of MAM, was more effective than placebo. Secondary objectives included the severity and duration of MAM attacks and associated symptoms.
MAM was defined as migraine that occurred between day −2 and day +4 of the onset of menstruation, where the first day of menstruation was defined as day +1. Of the participants, 34% had a history of true menstrual migraine, defined as migraine attacks occurring between day −2 to day +2 of menstruation and did not occur at any other time.
In this multicenter, double-blind, placebo-controlled, three-way crossover trial, patients were randomized to one of six treatment sequences consisting of placebo, frovatriptan 2.5 mg daily, and frovatriptan 2.5 mg twice daily. Participants had to be at least 18 years of age, with >1-year history of MAM, diagnosed with migraine according to IHS criteria and have an attack frequency of at least three of four perimenstrual periods (PMPs) in the previous 12 months. Patients were excluded from the study if they had >3 non-MAM attacks per month, >15 headache days per month, or if they had any contraindication to the use of serotonin-receptor agonists such as uncontrolled hypertension or coronary artery disease. Patients receiving oral contraceptives or migraine prophylactic therapies were eligible to participate in the study if the dosages of these medications had been stable for at least 2 months prior to enrollment in the study.
Patients administered their study medications 2 days before the anticipated MAM-onset date. Medications were administered twice daily at 12-hour intervals for a total of six days. Patients who failed treatment with study medications were permitted to manage their migraines with rescue medications, including acetaminophen-containing products and propionic acid derivatives such as ibuprofen. However, other triptans or ergotamine derivatives were not permitted. Subjects were given diary cards to record efficacy and tolerability. Additionally, patients documented headache severity according to the IHS 4-point rating scale, where 0=absent, 1=mild, 2=moderate, and 3=severe. Functional disability was assessed using the IHS 4-point scoring scale and the presence of migraine-associated symptoms (nausea, vomiting, phonophobia, and photophobia).
Five hundred and forty six subjects comprised the safety population. The intention to treat analysis consisted of 506 subjects, 445 of which were treated and had efficacy data for three PMPs. Both frovatriptan regimens were superior to placebo however, there was a dose-dependent reduction in the incidence of MAM; 52% and 41% in subjects receiving frovatriptan 2.5 mg daily and frovatriptan 2.5 mg twice daily, respectively compared with 67% among placebo recipients (p<0.0001). Similarly, the severity of MAM attacks was in favor of both frovatriptan regimens. The incidence of moderate or severe attacks was 28%, 37%, and 51% for frovatriptan 2.5 mg twice daily, frovatriptan 2.5 mg daily, and placebo, respectively (all p-values <0.0001).
The mean duration of MAM attacks was 31.1 hours in the placebo group compared with 20.3 hours and 16.6 hours in subjects receiving frovatriptan 2.5 mg daily and frovatriptan 2.5 mg twice daily, respectively (p<0.0001). However, the difference between frovatriptan regimens did not reach statistical significance.
The rates of associated MAM symptoms (nausea, vomiting, photophobia, and phonophobia) were favorable for both frovatriptan dose regimens; p<0.001, daily frovatriptan vs placebo; p<0.0001, twice daily frovatriptan vs placebo; and p<0.0001 for both frovatriptan dose regimens in favor of frovatriptan 2.5 mg twice daily.
Lastly, functional disability, the use of rescue medications, and patient satisfaction with study medications were all in favor of frovatriptan treatment groups. Overall, moderate to severe functional impairment was reported in 38% of subjects receiving placebo compared with 18% (p<0.001) and 29% (p<0.01) of subjects receiving frovatriptan twice daily and frovatriptan once daily regimens, respectively.
The use of rescue medications was significantly reduced by both frovatriptan regimens, 34% (p<0.01) and 44% (p<0.001) when compared with placebo, 53%.
Frovatriptan regimens provided higher rates of patient satisfaction when compared with placebo (p<0.0001), with the twice daily regimen being more superior to the once daily regimen. 86%, 80%, and 66% of patients rated their treatment as “excellent’, “good” or “fair” in the frovatriptan twice daily regimen, once daily regimen, and placebo, respectively.
In view of safety, adverse effects were consistent with those reported in other trials and most frequently consisted of headache, nausea, dizziness, nasopharyngitis, and dysmenorrhea. Adverse effects reported in the frovatriptan groups were comparable with those reported by placebo recipients. Nonetheless, nausea, dizziness, and pharyngitis were more commonly reported among frovatriptan recipients and were reported more frequently in the frovatriptan twice daily regimen. Nausea was reported in 3.4% of subjects receiving placebo as compared with 4.8% (p=0.091) and 6.8% (p=0.028) in subjects receiving once daily and twice daily frovatriptan regimens. Other reports of adverse effects were numerically higher in both frovatriptan regimens when compared with placebo albeit did not reach statistical significance.
A total of 21 subjects withdrew from the study, 17 in the frovatriptan groups and 4 in placebo. The most common reasons for withdrawal were nausea and dizziness in the frovatriptan groups and abnormal electrocardiogram (EKG) findings in placebo.
Overall, the authors concluded that frovatriptan is effective in reducing the incidence of menstrually-associated migraines, with the twice daily regimen being more effective. Frovatriptan treatment was superior to placebo and reduced severity, duration, and the need for rescue medications.
In a six-month open-label design, the efficacy of frovatriptan 2.5 mg in the abortive management of migraine attacks was assessed both during and outside of menstrual attacks (CitationMacGregor and Keywood 2000). Menstrually-associated migraine was defined as occurring between three days prior to and four days post onset of menstruation. Data was reported from 151 menstrual subjects for 2439 migraine attacks, 659 of which occurred during a menstrual period. Albeit the time to achieve relief was longer in menstrual attacks; 5.5 hours vs 3.6 hours, migraine relief within 24 hours was achieved for both menstrual and non-menstrual attacks, 82% and 87%, respectively. Of note, the majority of attacks (∼40%) were managed by a single dose of frovatriptan 2.5mg. The authors concluded that frovatriptan is effective in the acute management of menstrual migraines.
Discussion
Migraine is a common headache disorder that disproportionately affects women.
Approximately half of all women affected by migraine report that they are more likely to experience migraines in association with menstrual periods (CitationEdelson 1985; CitationJohannes et al 1995; CitationStewart et al 2000). Unlike other types of migraine, the pathophysiologic bases of menstrual migraine are related to neuroendocrine changes associated with the menstrual cycle, particularly the fluctuations of estrogen levels (CitationSilberstein and Merriam 1991). Management of menstrual migraines is challenging as the attacks are reported to be of longer duration and are more refractory to therapies (CitationMassiou 1999; CitationGranella et al 2004).
In addition to hormonal manipulations, menstrual migraines can be managed with nonsteroidal antiinflammatory drugs and/or triptans given acutely or as prophylactic therapies. Various triptans have been evaluated in the acute management of menstrual migraines. Two randomized controlled trials of sumatriptan and zolmitriptan support their use in the acute management of menstrual migraine (CitationFacchinetti et al 1995; CitationLoder et al 2004). In both trials, oral zolmitriptan and subcutaneous sumatriptan exhibited efficacy and well tolerability in the acute treatment of menstrual migraines.
The role of triptans in the prophylactic therapy of menstrual migraines emerged in the late 1990s. In total, four triptans including sumatriptan, frovatriptan, naratriptan, and zolmitriptan were evaluated against placebo (CitationKarageorgiou et al 2001; CitationNewman et al 2001; CitationBrandes et al 2002; CitationBrandes, Savani, Alderton, et al 2003; CitationBrandes, Savani, Kwong, et al 2003; CitationDiamond et al 2003; CitationMannix et al 2003; CitationNett et al 2003; CitationSinger and Schim 2003; CitationSmith and Nett 2003; CitationTepper and Freitag 2003; CitationTuckman et al 2005).
The majority of the studies were reported in abstract form and are limited by a number of factors including but not limited to open-label design and suboptimal number of participants.
None of the triptans is currently approved for the management of menstrual migraines. Additionally, there are no head-to-head trials to evaluate the efficacy of various triptans in the management of menstrual migraines. Promising aspects of frovatriptan include its favorable pharmacologic and pharmacokinetic profiles. Frovatriptan offers functional selectivity for cerebral over coronary arteries. This effect is thought to lessen the potential for deleterious peripheral adverse effects. Nonetheless, such effect has not been clinically substantiated. Frovatriptan has a terminal deposition half-life of approximately 26 hours, which implies that frovatriptan may provide additional benefits in patients with migraines of long duration and those who experience headache recurrence. Collectively, its long half-life and potential benefits in headache recurrence make frovatriptan a valuable agent for prophylaxis and acute management of menstrual migraines.
Using frovatriptan in the prophylaxis of menstrual migraines is unlikely to be shown cost-effective, particularly if used as first-line therapy. Patients who respond to traditional pharmacologic agents such as nonsteroidal antiinflammatory drugs should be continued on these therapies. Additionally, menstrual migraines should be managed with acute therapies prior to attempting prophylactic therapy with triptans. Nonetheless, patients who experience intractable and prolonged migraine attacks that are not amenable to acute therapies may attain additional benefits from prophylactic therapy with frovatriptan.
Conclusions
Frovatriptan is a potent, 5-HT1B/1D receptor agonist that demonstrated safety and efficacy in the management of migraine. Its efficacy, long duration of action and good tolerability profiles show considerable potential in acute and prophylactic management of menstrual migraines. Future studies will further elucidate the role of frovatriptan in the management of menstrual migraine.
References
- BrandesJSmithTPowersCLong-term safety, tolerability and efficacy of naratriptan 1mg BID in the prophylactic treatment of menstrually associated migraine [abstract]Headache200242451
- BrandesJSavaniNKwongJWPatient satisfaction with oral naratriptan for intermittent prophylaxis of menstrually associated migraine: results form a double-blind, placebo-controlled, parallel group study [abstract]Cephalagia200323705
- BrandesJSavaniNAldertonCNaratriptan vs. placebo for intermittent prophylaxis for menstrually associated migraine (MAM): analysis of migraine-free days [abstract]Cephalagia200323705
- BrownAMHoMThomasDRFunctional effects of frovatriptan (VML 251), sumatriptan and naratriptan on human recombinant 5-HT1 and 5-HT7 receptors [abstract]Headache199838376
- BuchanPThe pharmacokinetics of frovatriptan (VML 251/SB 209509), a potent selective 5-HT1B/1D agonist, following single dose administration by oral and intravenous routes to healthy male and female volunteersFunc Neurol199813177
- BuchanPKeywoodCWardCPharmacokinetics of frovatriptan (VML 251/SB 209509) in healthy young and elderly male and female subjects [poster]199812th Migraine Trust International Symposium1–4 Sept 1998London, UK
- BuchanPWardCOliverSDLack of clinically significant interactions between frovatriptan and ergotamine [abstract]Cephalagia199919364
- BuchanPWardCZeigSFrovatriptan pharmacokinetics are unaffected during a migraine attack [abstract]Cephalagia199919365
- BuchanPGay-FeutryCIn vitro metabolism of frovatriptan [poster]Eur J Neurol20007S38110809919
- BuchanPKeywoodCWadeAClinical pharmacokinetics of frovatriptanHeadache200242Suppl 2S546212028321
- CohenAFPostJSacksSPharmacokinetics of frovatriptan in patients with renal impairment [abstract]Cephalagia199919365
- ComerMBPharmacology of the selective 5-HT1B/1D agonist frovatriptanHeadache200242Supp 24753
- DiamondMAuroraSAmesMNaratriptan for intermittent prophylaxis for menstrually associated migraine: an analysis of migraine-free days [abstract]Headache2003405489
- EdelsonRNMenstrual migraine and other hormonal aspects of migraineHeadache19852537693908402
- FacchinettiFBonellieGKangasniemiPThe efficacy and safety of subcutaneous sumatriptan in the acute treatment of menstrual migraine. The Sumatriptan Menstrual Migraine Study GroupObstet Gynecol199586911167501338
- GranellaFSancesGAllaisGCharacteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centresCephalagia20042470716
- [HCC] Headache Classification Committee of the International Headache SocietyClassification and diagnostic criteria for headache disorders, cranial neuralgias and facial painCephalagia19888S7196
- [HCS] Headache Classification Subcommittee of the International Headache SocietyThe international classification of headache disordersCephalagia2004242nd edSupp 11169
- HuXHMarksonLELiptonRBBurden of migraine in the United States: disability and economic costsArch Intern Med19991598131810219926
- JohannesCBLinetMSStewartWFRelationship of headache to phase of the menstrual cycle among young women: a daily diary studyNeurology1995451076827783866
- KarageorgiouCEHatzidakiGRobotisGThe role of naratriptan as a prophylaxis to the menstrual migraine: a pilot comparative to naproxen study [abstract]Cephalagia200121P2
- KingDSHerndonKCDiPiroJTHeadache disordersPharmacotherapy20056th edNew YorkMcGraw Hill110521
- LiptonRBDiamondSReedMMigraine diagnosis and treatment: results from the American migraine study IIHeadache200141638411554951
- LiptonRBStewartWFMigraine in the United States: A review of epidemiology and health care useNeurol199343S3610
- LoderESilbersteinSDAbu-ShakraSEfficacy and tolerability of oral zolmitriptan in menstrually associated migraine: a randomized, prospective, parallel-group, double-blind, placebo-controlled studyHeadache2004441203014756849
- MacGregorEAMenstruation, sex hormones, and migraineNeurol Clin199715125419058401
- MacGregorEAKeywoodCFrovatriptan is effective in menstrually associated migraine [abstract]Cephalagia200020345
- MannixLBrandesJShackelfordSNaratriptan for intermittent prophylaxis for menstrually associated migraine: a review of efficacy and tolerability [abstract]Headache20034058710940098
- MarksDRRapoportAMDiagnosis of migraineSemin Neurol19971730369474709
- MassiouHIs menstrually associated migraine difficult to treat?Cephalagia199919Suppl 241318
- MullenersWMAuroraSKChronicleEPSelf-reported photophobic symptoms in migraineurs and controls are reliable and predict diagnostic category accuratelyHeadache20014131911168601
- NettRMannixLKLandySA randomized, double-blind, placebo-controlled, parallel-group evaluation of oral naratriptan 1 mg twice daily as prophylactic treatment for menstrually associated migraine [abstract]Neurology200360Suppl 1A95
- NewmanLMannixLKLandySNaratriptan as short-term prophylaxis of menstrually associated migraine: a randomized, double-blind, placebo-controlled studyHeadache2001412485611264684
- ParsonsAARavalPSmithSEffects of the novel high-affinity 5-HT1B/1D-receptor ligand frovatriptan in human isolated basilar and coronary arteriesJ Cardiovasc Pharmacol19983222049700983
- RussellMBRasmussenBKFengerKMigraine without aura and migraine with aura are distinct clinical entities: a study of four hundred and eighty-four male and female migraineurs from the general populationCephalagia19961623945
- SilbersteinSDMerriamGREstrogens, progestins, and headacheNeurology199141786932046918
- SilbersteinSDElkindAHSchreiberCA randomized trial of frovatriptan for the intermittent prevention of menstrual migraineNeurology200463261915277618
- SingerRSchimJFrovatriptan for prophylaxis of menstrually associated migraine: efficacy and tolerability in patients using oral contraceptives compared with nonusers [abstract]Headache200343587
- SmithTRNettRFrovatriptan is well tolerated during repeated use for intermittent prevention of menstrually associated migraine [abstract]Headache200343586
- StewartMNapierCMKatugampolaSDThe binding affinity and functional activity of eletriptan and other 5-HT 1B/1D agonists at the human recombinant 5-HT1B and 5-HT1D receptors [abstract]Br J Pharmacol199912793
- StewartWFShechterARasmussenBKMigraine prevalence. A review of population-based studiesNeurology1994446 Suppl 4S17238008222
- StewartWFLiptonRBCheeEMenstrual cycle and headache in a population sample of migraineursNeurology20005515172311094107
- TepperSJRapoportAMSheftellFDMechanisms of action of the 5-HT1B/1D receptor agonistsArch Neurol2002591084812117355
- TepperSFreitagFIntermittent use of frovatriptan for prevention of menstrually associated migraine is effective and does not cause rebound migraine in the postdosing period [abstract]Headache200343585
- TuckmanMHeeAEmeribeUOral zolmitriptan 2.5mg demonstrates high efficacy and good tolerability in the prophylactic treatment of menstrual migraine headaches [abstract]Headache200545771