Abstract
In vitro fertilization, popularly referred to as IVF, has captured the attention of the public since its sensational introduction in 1978. Today assisted reproductive technology is available throughout most of the civilized world, and the practice is largely different from that used during the early days. Refinements in laboratory technology and clinical practice have allowed IVF to evolve into a medical procedure that is efficient, safe, readily accessible, and relatively affordable. More than 2 million IVF children have been born to date, and it is likely that continued enhancements will widen its appeal and applicability.
Keywords:
Introduction
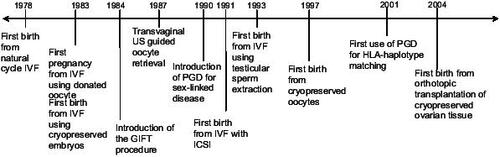
The birth of Louise Brown in 1978 was the culmination of decades of scientific research in reproductive medicine. Since then, an abundance of breakthroughs in both clinical medicine and basic science have allowed increasing numbers of infertile couples the chance to have a baby (). To date, more than 2 million babies have been born worldwide through assisted reproductive technologies (ART). The most recent Centers for Disease Control (CDC) statistics noted that 48 000 babies were born in the US and over 100 000 ART cycles were performed in the year 2003 alone (CitationCDC 2005).
The early days of IVF
Prior to 1978, women without functioning fallopian tubes were largely considered to be sterile by their physicians. At least one patent fallopian tube is necessary for natural fertilization of an oocyte by sperm in vivo. In the past, many women with damaged tubes resorted to reparative surgery, or tuboplasty in hopes of re-establishing a conduit for gametes to transit. Unfortunately, often these surgeries failed.
In the late 1970’s Lesley Brown, a patient with nine years of primary infertility secondary to tubal occlusion, sought the assistance of Patrick Steptoe and Robert Edwards at the Oldham General Hospital in England. At that time, fertilization of oocytes outside the human body, a process known as in vitro fertilization (IVF), was considered entirely experimental and when attempted had only resulted in miscarriages and an unsuccessful pregnancy in the fallopian tube (CitationSteptoe and Edwards 1976). Without using medications to stimulate her ovaries, Lesley Brown underwent laparoscopic egg retrieval, with her single egg fertilized in the laboratory, and later transferred back into the uterus. The embryo transfer resulted in the first live birth from IVF, a daughter Louise Brown, who was born in July 1978 (CitationSteptoe and Edwards 1978).
Following this sentinel and critically important event, Steptoe and Edwards, as well as several other contemporary scientists, not only successfully repeated this clinical achievement but went on to further improve and refine their pioneering efforts. The initial experience with unstimulated cycles by Edwards, Steptoe, and Purdy (CitationEdwards et al 1980) yielded on average 0.7 oocytes per retrieval and an overall pregnancy rate of 6% per initiated cycle (4/65). Stimulated IVF cycles with human menopausal gonadotropin (hMG) prior to laparoscopic egg retrieval was extensively studied at the Jones Intitute (CitationJones et al 1982; CitationGarcia et al 1983a, Citation1983b). Its widespread use led to dramatic improvement in oocyte yield per retrieval and pregnancy rates. Between 1980 and 1983, the use of hMG with IVF resulted in an average recovery of 2.1–2.6 oocytes per retrieval and increasing pregnancy rates of 23.5% per retrieval in 1982 and 30% in 1983 (CitationEdwards and Steptoe 1983).
Premature ovulation due to multi-follicular development became a prevalent problem with the increasing use of hMG for ovulation induction. Approximately 20% of IVF cycles were cancelled due to premature surge of luteinizing hormone (CitationElter and Nelson 2001). Pituitary desensitization by administration of gonadotropin releasing hormone agonist (GnRHa) prior to ovarian stimulation with hMG was first reported in 1984 (CitationPorter et al 1984). Effective suppression of the pituitary gonadotrophes with this protocol decreased the incidence of premature ovulation to about 2% and significantly improved overall pregnancy rates with IVF (CitationElter and Nelson 2001). However, pituitary suppression with GnRHa also contributed to the rising incidence of potentially life threatening ovarian hyperstimulation syndrome (OHSS) in susceptible individuals by permitting more aggressive ovarian stimulation protocols without the limitation of premature ovulation (CitationGolan et al 1988; CitationRizk and Smitz 1992; CitationNugent et al 2000).
Oocyte donation
While advances in early IVF refined the technology for treating women with tubal disease, those with natural or premature ovarian failure had no effective fertility treatments until 1983. In December of that year, a 25 year old patient with secondary amenorrhea and premature ovarian failure became the first person to successfully deliver a pregnancy using a donor egg. Dr. Peter Renou of the Monash IVF group in Australia inseminated a single oocyte, donated by a 29-year-old patient undergoing IVF herself for tubal disease, with the sperm from the recipient’s husband. The embryo was transferred back into the uterus of the recipient and resulted in a healthy full term live-birth (CitationLutjen et al 1984).
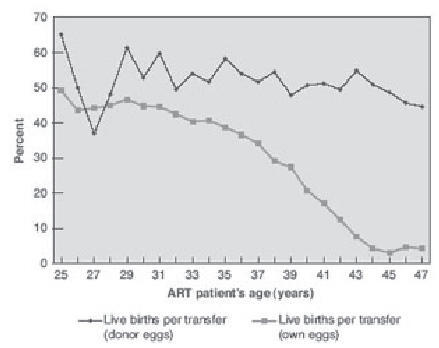
Over the last two decades, the predominant indication for oocyte donation has shifted from women with premature ovarian failure to mostly women of advanced reproductive age. Factors responsible for this trend relate to the changing demographic of the population at large. More women are delaying childbearing to pursue education and careers, marriages are occurring later in life, divorce and remarriage are more common, and effective contraception and available abortion services have eliminated many unintended pregnancies. For the older patient, traditional IVF remains an option, however pregnancy rates decline precipitously after 36 years of age, mostly due to the age associated decline in normal oocytes (). In contrast, pregnancy rates in women using donor oocytes are known to be as high as 50% per embryo transfer in recipients across all age groups (CitationCDC 2005). Indeed, women in their sixties have also given birth with donor oocytes, demonstrating that the postmenopausal uterus maintains the capacity to support pregnancies if provided adequate hormonal support (CitationAntinori et al 1995; CitationPaulson et al 1997). However, oocyte recipients experience increased obstetrical complications such as pregnancy induced hypertension (16%–40%), cesarean section (40%–76%), and gestational diabetes (20%) (CitationSoderstrom Anttila 2001; CitationSheffer-Mimouni et al 2002; CitationPaulson et al 2002).
Figure 2 Live births per transfer for assisted reproductive technology (ART) cycles using fresh embryos from own and donor eggs, by ART patient’s age. Copyright © 2005. Reproduced with permission from National Center for Chronic Disease Prevention and Health Promotion. 2005. 2003 Assisted reproductive technology success rates: National summary and fertility clinic reports.
The success of IVF with donor oocytes not only crossed the traditional boundaries of ART, but also unleashed a barrage of unprecedented social, ethical, and legal concerns. Debates regarding donor anonymity, financial compensation for donor participation, the need for a registry of births from third party reproduction, and age limitation on recipients of donor gametes continue to stir controversy. Despite these unresolved issues, donor IVF remains an integral part of modern ART, and accounts for 11.6% of the IVF cycles performed in the US (CitationCDC 2005).
Embryo cryopreservation
Clinical and laboratory methodology used for ART continued to evolve and improve, and a surplus of embryos in excess to what is used or needed for the initial IVF treatment became increasingly commonplace. During the early days of IVF, options for the patient with supernumerary embryos included discarding them, donating them to another infertile couple, or donating them for use in experimental research. Although cryopreservation of the embryos was an option, the freezing and thawing processes often caused permanent injury to the cells, and most embryos did not survive. This is best reflected in the very low rates of pregnancy seen following the transfer of frozen/thawed embryos throughout the 1980s. Intense efforts to develop various freezing/thawing techniques and cryoprotective agents eventually resulted in the first reported human pregnancy from a frozen embryo in 1983, which unfortunately ended in premature rupture of the membranes and termination of pregnancy at 24 weeks of gestation (CitationTrounson and Mohr 1983).
Despite the initial set back, technology in cryopreservation continued to improve throughout the 1980s, leading to an increase in embryo survival rate and pregnancy rates. During the initial years of experimentation, at best approximately 50% of embryos survived the freeze/thaw process and resulted in a pregnancy rate of 13.4% per embryo transfer procedure, as only 4.6% of the individual thawed embryos implanted (CitationFriedler et al 1988). By 2003, frozen embryo transfers accounted for 21 981 of the 112 872 IVF cycles (17.8%) performed in the US, with an overall live birth rate of 27.0% per embryo transfer procedure.
Refinements in technique: moving away from the operating room
During the mid-1980s, efforts to simplify and improve success rates of assisted reproduction led to the development of gamete intrafallopian transfer (GIFT) where oocytes were laparoscopically retrieved and immediately transferred into fallopian tubes along with sperm (CitationAsch et al 1984). In addition to limiting the number of procedures to one laparoscopy, other theoretical advantages of GIFT included using the natural tubal environment for fertilization, allowing a more appropriate time of entry of the embryos into the uterine cavity, and avoidance of endometrial trauma from a transcervical embryo transfer. During this era, IVF laboratory science was still being developed, and overall success rates were low, ranging from 23.5% to 30% (CitationInge et al 2005). Since surgery was used to harvest gametes, the immediate replacement of the prepared specimens was attractive as it lessened the need for embryo culture and the risk of poor growth over time. However, tubal transfer was not universally applicable since patients with dense pelvic adhesions or tubal occlusion could not be treated, and patients with male factor infertility could not be observed for fertilization success or treated effectively.
Subsequently, the technique of transferring laparoscopically retrieved oocytes fertilized in vitro at the pronuclear stage into the fallopian tube via a second laparoscopy was introduced. This procedure was known as zygote intrafallopian transfer (ZIFT) and it allowed the confirmation of fertilization but maintained some of the theoretical benefits of GIFT (CitationHamori et al 1988). However, the use of two laparoscopies, one for oocyte retrieval and the other for zygote transfer, was a major limitation of this approach.
As the use of assisted reproductive treatments expanded from tubal infertility to include ovulation disorders, male factor infertility and decreased ovarian reserve, the number of ART cycles dramatically increased. The pioneering work reported by Steptoe and Edwards had required a surgical approach using laparoscopy. Subsequent modifications such as GIFT and ZIFT still relied upon laparoscopy. However, the necessity for general anesthesia and its attendant risks, along with the high overhead expense of using operating rooms provided the impetus to develop more efficient nonsurgical oocyte retrieval techniques.
Improvements in ultrasonography during the 1980s catalyzed the evolution of modern outpatient oocyte retrieval. Using transabdominal ultrasound guidance, various methods of oocyte retrieval included percutaneous (CitationLenz and Lauritsen 1982), transvesical (CitationLenz et al 1981; CitationLenz and Lauritsen 1982), per-urethral (CitationParsons et al 1985), and transvaginal follicle aspiration (CitationDellenbach et al 1985). Further refinements in ultrasound transducers led to the use of transvaginal ultrasound guided transvaginal follicle aspiration (). First reported in 1987, this oocyte retrieval technique quickly became the procedure of choice due to better visualization, finer control, and less patient discomfort compared with other available methods (CitationWikland et al 1987). Obviating the need for laparoscopy decreased the number of personnel, time and procedure expense, reduced the risks of surgery and general anesthesia, and provided greater patient acceptance. IVF cases transitioned from 1–2 hours of hospital-based operating room time to 10–15 minute procedures that can be performed in an office setting.
Figure 3 Schematic of modern outpatient in vitro fertilization setup. Copyright © 2006. Reproduced with permission from Cook Group Incorporated. 2006. Ovum aspiration diagram [online]. Accessed on 2 January 2006. URL: http://www.cookwomenshealth.com/products/infertility/1_01/1_01_01a.html.
![Figure 3 Schematic of modern outpatient in vitro fertilization setup. Copyright © 2006. Reproduced with permission from Cook Group Incorporated. 2006. Ovum aspiration diagram [online]. Accessed on 2 January 2006. URL: http://www.cookwomenshealth.com/products/infertility/1_01/1_01_01a.html.](/cms/asset/a56686a8-224a-4281-8848-c1ab4c384a7c/dtcr_a_24355_f0003_b.jpg)
Comparing techniques for embryo transfer
Multiple studies in the early 1990s comparing IVF, ZIFT, and GIFT often showed conflicting results due to multiple confounding variables inherent to different patient-selection criteria and the wide variation of practice used from clinic to clinic. While some studies showed no differences (CitationTanbo et al 1990; CitationToth et al 1992), others found that GIFT and ZIFT achieved higher pregnancy rates than IVF (CitationDevroey et al 1989; CitationCrosignani et al 1991; CitationMills et al 1992). Nevertheless, potential enhancements in outcomes of GIFT and ZIFT were marginalized by the reliance on invasive surgery, especially as IVF evolved into less expensive and minimally invasive ultrasound guided aspirations. By 1995, Society for Assisted Reproductive Technology (SART) data noted that IVF, GIFT, and ZIFT consisted of 70%, 6%, and 2% of ART cycles respectively, despite pregnancy rates of 22.3%, 28.7%, and 30.3% for each procedure. As the pregnancy rate of IVF improved over the last decade from 22.3% in 1995 to 33% in 2003, the popularity of GIFT and ZIFT diminished further (CitationCDC 2005). In 2003, GIFT and ZIFT were used in only 0.1% and 0.4% of ART cycles, while IVF represented the remaining 99.5% of cases (CitationCDC 2005).
Addressing the needs of infertile men
As IVF became more commonplace in the treatment of female infertility, male infertility remained a limiting factor to overall success. Conventional IVF was much less effective when semen parameters were below the reference values for concentration (oligozoospermia), motility (asthenozoospermia), and morphology (teratozoopermia), resulting in significantly lower fertilization rates and fewer embryos available for transfer. Furthermore, azoospermic males were completely devoid of treatment options.
Several procedures were developed in the late 1980s to improve treatment fertilization problems. The first technique developed was partial zona dissection (PZD) where a small opening was made in the zona pellucida in hopes of facilitating sperm entry into the oolemma. The results of PZD were inconsistent and disappointing (CitationMalter and Cohen 1989). Another technique introduced was subzonal insemination (SUZI) where a few motile sperm were microinjected through the perivitelline space. SUZI achieved an overall 20% fertilization rate but was still too low to be used for routine clinical application (CitationNg et al 1991; CitationVan Steirteghem, Liu, et al 1993; CitationVan Steirteghem, Nagy, et al 1993). Palermo and Van Steirteghem introduced a novel procedure called intracytoplasmic sperm injection (ICSI), where a single spermatozoon was microinjected into the oocyte after passage through the zona pellucida and the membranes of the oocyte (CitationPalermo et al 1992). This procedure achieved fertilization rates of approximately 60%–70% when using ejaculated sperm, a rate significantly greater than SUZI or PZD and equivalent to fertilization rates experienced by men with normal parameters using conventional methods. The first pregnancies using embryos generated by ICSI were reported in 1992 (CitationPalermo et al 1992) and the procedure has been applied increasingly from 11% of IVF cycles in 1995 to 55.6% in 2003 (CitationCDC 2005).
ICSI not only overcame the fertilization barrier presented by oligo-, astheno-, and teratozoospermia, it also created a new possibility for azoospermic men to achieve fertility. While Temple-Smith and colleagues reported the first pregnancy by microepididymal sperm aspiration (MESA) in a patient with secondary obstructive azoospermia in 1985, epididymal sperm motility was generally low and outcomes were poor compared with that of ejaculated sperm (CitationTemple-Smith et al 1985). The advent of ICSI markedly improved fertilization rates with epididymal sperm, making the combination of MESA–ICSI widely-used procedures for patients with congenital bilateral absence of vas deferens or secondary obstructive azoospermia. Subsequently, modified percutaneous sperm aspiration (PESA) performed with needle aspiration of the epididymis through a 1 cm scrotal incision, was developed as an alternative to MESA which required unilateral hemiscrototomy to allow dissection, exploration, and aspiration of the epididymis with an operating microscope (CitationShrivastav et al 1994). Comparatively, PESA is cheaper, more acceptable to patients, has less postoperative morbidity than MESA, but retrieves fewer sperm.
When there are no motile sperm in the ejaculate or in the proximal epididymis, sperm can be extracted directly from testicular tissue by either blind needle puncture or open tissue excision. CitationCraft et al (1993) reported successful ICSI fertilization with testicular sperm in 1993, but no pregnancy occurred. The first successful pregnancy was reported in that year (CitationSchoysman et al 1993). Fertilization rates as high as 70% can be achieved with testicular sperm extraction (TESE) despite only using a few poor-quality sperm. Pregnancies have even been achieved in conditions such as Klinefelter syndrome, generally associated with germ cell atrophy and fibrotic, hyalinized seminiferous tubules (CitationStaessen et al 1996). To date, there have been 39 reports of healthy children born after ICSI procedures with spermatozoa retrieved from nonmosaic Klinefelter patients since the first pregnancy in 1996 (CitationDenschlag et al 2004).
As we enter the second decade using ICSI, debates continue on the implications of altering the process of natural selection. Inheritable causes of male fertility such as constitutional chromosomal aberrations, cystic fibrosis transmembrane conductance regulator gene mutation, or AZF deletion on the Y chromosome may be inadvertently transmitted via ICSI. In addition, potential damage to the cytoplasmic organelles of oocytes inflicted by the procedure may increase the risk of health problems in children. In a case series of 1586 ICSI established pregnancies, prenatal diagnosis showed a significantly higher percentage of de novo sex and autosomal chromosomal abnormalities (2.1%) when sperm concentration was <20 × 106 sperm/ml as compared with 0.24% if the concentration was ≥20 × 106 sperm/ml (CitationBonduelle et al 2002). In addition, CitationHansen et al (2002) found the prevalence of major birth defects in infants conceived after ICSI, as well as IVF, were doubled compared with infants conceived naturally. Furthermore, a multi-center study with a five year follow-up period also showed a significantly increased risk of malformation in children born after ICSI compared with that of naturally conceived children, particularly in the genitourinary system of male children (CitationBonduelle et al 2005). Nevertheless, a meta-analysis comparing birth defects among ICSI-births and standard IVF-births failed to attribute additional risks to the ICSI procedure, above the baseline risk associated with IVF itself (CitationLie et al 2005). Hence, the current available evidence suggests the increased risk of congenital malformation associated with IVF is not attributable to ICSI, per se, and may be related to other factors such as patient characteristics, ovarian stimulation protocols, or embryo culture environment.
Assessment of the developmental outcome of children at 24–28 months of age by Bayley Scale shows no significant differences between the ICSI and IVF group (CitationBonduelle et al 2003) In addition, motor and cognitive development are equivalent among IVF-conceived, ICSI conceived, and naturally conceived children at the age of five (CitationPonjaert-Kristoffersen et al 2005). Nevertheless, there is evidence for increased incidence of otherwise rare and sporadic imprinting disorders such as Beckwith-Wiedeman and Angelman syndrome in ICSI children, possibly due to disruption of methylation in the maternal genome or early embryo by the procedure (CitationSutcliffe et al 1995; CitationStaessen et al 1996; CitationCox et al 2002). Another study also found an increase in the incidence of Beckwith-Wiedman syndrome among children born with ART but independent of ICSI (CitationMaher et al 2003). This implies that the embryo culture condition alone may alter DNA methylation and imprinting, as has been shown by in vitro mouse embryo studies (CitationKhosla et al 2001). Interestingly, CitationLudwig et al (2005) found a similar increase in the incidence of Angelman syndrome between children born to subfertile couples whose time to natural conception was greater than two years and children born to subfertile couples who underwent ART, regardless of ICSI. These findings suggest that imprinting defects and subfertility may have a common cause, and superovulation with ART in this population may further increase the risk of conceiving children with imprinting defect. Due to the overall rarity of these syndromes, large-scale systematic studies are needed to clarify the link between genomic imprinting defects and ART as well as to establish the exact biological basis of any such link.
IVF and preimplantation genetic diagnosis
Prior to 1990, options to prevent transmission of genetic defects were limited to performing chorionic villus sampling or amniocentesis and offering abortion if the fetus were found to be affected. The 3 to 5 day window between oocyte fertilization and embryo transfer provided a new opportunity to delineate which embryos are unaffected by a specific single gene disorder or chromosomal imbalance prior to transfer into the uterus. The first clinical application of this procedure called preimplantation genetic diagnosis (PGD) was used in 1990 to prevent the transmission of two X-linked conditions: adrenoleukodystrophy and X-linked mental retardation (CitationHandyside et al 1990). These embryos were biopsied on day 3 in vitro at the six to ten cell stages by first drilling a hole into the zona pellucida with acid Tyrodes and then aspirating blastomeres with micropipettes for analysis. Male DNA was identified by amplifying a short fragment of a Y-specific repeat sequence utilizing polymerase chain reaction (PCR). Cells not containing the amplification were presumed as female, and the embryos of origin were transferred back into the uterus to prevent transmission of the X-linked diseases. The first application of PGD resulted in two sets of healthy female twins (CitationHandyside et al 1990).
Following this, PCR-based tests amplifying DNA fragments with causative mutations specific for single gene disorders such as α-1 antitrypsin deficiency and cystic fibrosis allowed identification of unaffected embryos and resulted in normal children (CitationVerlinsky et al 1990; CitationHandyside et al 1992). Biopsies of polar bodies during meiosis rather than blastomeres during the cleavage stage were used in some of these cases. Although this approach may be less disruptive to the embryo, it only allowed analyses of maternal DNA and hence had limited clinical applications. In the last 15 years, the number of inherited disorders diagnosed at the preimplantation stage has expanded to over 40 diseases, and the advent of multiplex-PCR which simultaneously amplifies several DNA fragments in a single reaction greatly improved the accuracy of the analyses (CitationKuliev et al 1998).
In additional to single gene mutations, chromosomal screening represents another application of PGD, in an attempt to decrease the risk of transferring aneuploid embryos. Initial techniques to identify X- and Y-chromosomes using PCR was quickly supplanted by fluorescence in situ hybridization (FISH) due to its speed and capacity to identify multiple chromosomes simultaneously (CitationDelhanty et al 1993; CitationMunne et al 1993; CitationGriffin et al 1994).
Today, FISH remains the primary method for routine aneuploidy screening but has expanded up to 9 chromosomes per embryo-X, Y, 13, 15, 16, 17, 18, 21, and 22. Aneuploidy screening (AS) is currently offered to various patient groups including women of advanced reproductive age, carriers of chromosomal rearrangements, and patients with history of recurrent pregnancy losses or unexplained repeated IVF failures (CitationMunne and Wells 2002).
Evidence supporting the benefit of aneuploidy screening by PGD in the abovementioned patient groups remains equivocal. While some studies indicate that PGD–AS increases the embryo implantation rate and reduces the miscarriage rate (CitationGianaroli et al 2002; CitationMunne et al 2003), a prospective randomized controlled trial comparing the outcome after blastocyst transfer combined with aneuploidy screening with PGD using fluorescence in situ hybridization (FISH) for the chromosomes X, Y, 13, 16, 18, 21, and 22 in women with advanced reproductive age (≥37 years old) with a control group without PGD–AS showed no improvement in implantation or pregnancy rate (CitationStaessen et al 2004). A recent meta-analysis also failed to show significant increases in pregnancy rates using PGD for aneuploidy screening (CitationTwisk et al 2006). More prospective randomized studies are needed to determine the efficiency of PGD–AS.
Expanding applications beyond infertility
HLA-typing for stem cell donation
Recent indications for PGD have expanded to genetic disorders that have a late onset such as Alzheimer’s disease (CitationVerlinsky et al 2002), Huntington disease (CitationSermon et al 1998), and cancer predisposition (CitationAo et al 1998). Another new application is human leukocyte antigen (HLA) matching first used to cure a child with Fanconi’s anemia by transplanting stem cells derived from the umbilical cord blood of an unaffected sibling originating from an embryo analyzed by PGD and known to have matching HLA typing but without Fanconi’s anemia mutation (CitationVerlinsky et al 2001). Since then, similar approaches to obtain stem cell from siblings for transplantation in couples with an affected offspring have been used for acute lymphoblastic leukemia, thalasemia, and Wiscott-Aldrich (CitationKahraman et al 2004). These cases precipitated intense media interest and will continue to fuel ethical debates concerning “designer babies” produced for the purpose of benefiting the health and wellbeing of another.
Fertility preservation for cancer patients
Contemporary investigative efforts continue to focus on providing even more couples the opportunity to have healthy children. One such group is women diagnosed with cancer who often sustain partial or total loss of their fertility following cancer therapy. While embryo cryopreservation prior to initiating potentially gonadotoxic treatment is the most reliable method to preserve fertility, absence of a male partner or desire not to use donor sperm often precludes this approach. In such cases, oocyte cryopreservation may provide an alternative. To date, there have been approximately 100 pregnancies and 50 live-births from cryopreserved oocytes since it was first reported twenty years ago (CitationVan der Elst 2003). The large size, high water content, and chromosomal arrangement along the meiotic spindle render metaphase-II oocytes extremely vulnerable to the intracellular ice formation during the freezing or thawing process. In addition, hardening of zona pellucida can interfere with normal fertilization process. Currently, each thawed oocyte has a mean survival rate of 47%, fertilization rate of 52%, but pregnancy rate of only 1.52% (CitationSonmezer et al 2004) based on 21 publications. Nevertheless, recent experimentation with different rates of freezing/thawing, modification of cryoprotectant from dimethylsulphoxide to 1,2-propanediol, and the addition of fertilization using ICSI has culminated in greater efficiency as reported by CitationFosas et al (2003) who achieved a 90% survival rate, 75% fertilization rate, and 50% pregnancy rate.
In terms of outcome, the incidence of chromosomal abnormalities in human embryos obtained from cryopreserved oocytes is similar to that of control embryos using FISH (CitationCobo et al 2001). In addition, limited data based on the outcomes of 17 children resulting from cryopreserved oocytes showed no increased incidence of abnormal karyotype, preterm delivery, low birth weight, birth defects, intellectual or developmental deficits by the age of three (CitationWinslow et al 2001).
Other methods to circumvent oocyte damage caused by the freeze/thaw process include vitrification and cryopreservation of germinal vesicles. Vitrification uses high concentration of cryoprotectants to solidify the cell into a glass-like state without the formation of ice. Based on a small study, post-thaw survival rates and pregnancy rate of this approach were 68.8% and 21.4%, respectively, and resulted in six livebirths (CitationYoon et al 2003). The other alternative of freezing oocytes at the germinal vesicle stage allows higher survival rates than those frozen at the metaphase II stage (CitationBoiso et al 2002). However, germinal vesicles need in vitro maturation (IVM) to become mature oocytes, and the inefficiency of current IVM protocol nullifies the improvement in survival rate, leading to equivalent final yield of mature oocytes as compared with that of freezing metaphase II oocytes. At this time, both nascent protocols are continuing to evolve and lack long term outcome data.
Despite the fact cryopreservation of oocytes obviates the immediate need for sperm, the inability to delay initiation of chemotherapy or the presence of estrogen sensitive malignancies precludes the use of ovarian stimulation and hence oocyte cryopreservation in many clinical scenarios. Ovarian tissue cryopreservation, achieved by biopsying and cryopreserving ovarian cortex containing primordial follicles, followed by thawing and transplanting the autograft after completion of cancer treatment offers a potential solution in those circumstances. Transplantation can be either orthotopic (in close proximity to the infundibulo-pelvic ligament) or heterotopic (ie, forearm or abdomen). To date, transient restoration of endocrine function has been reported with both approaches. Embryo transfer resulting from stimulation of cryopreserved ovarian heterotopically grafted beneath the abdominal skin did not result in pregnancy. CitationDonnez et al (2004) reported restoration of endocrine function and a live birth following orthotopic transplantation of cryopreserved ovarian tissue in a woman who became amenorrheic after being treated for Hodgkin’s lymphoma. However, some critics challenge the validity of the pregnancy arising from the cryopreserved ovarian tissue since the patient had not undergone oophorectomy. Nonetheless, these encouraging findings offer new hope to women diagnosed with cancer who desire fertility and ovarian preservation options.
Conclusion
In the three decades following the birth of Louise Brown, innovations in ART have overcome numerous seemingly insurmountable barriers to allow couples the chance to have families. Significant developments in the first decade led to greater efficiency and expanded accessibility of in vitro fertilization to the general public. In the ensuing decade, innovation and refinements in technology led to the introduction of ICSI, MESA, and TESE which provided effective treatment for male infertility. More recently, the advent of PGD provided couples with sex-linked diseases and numerous genetic disorders the ability to have children free of the condition. Efforts continue to focus on potential ways to increase the success of ART using PGD for aneuploidy screening. Finally, improving the efficiency of oocyte cryopreservation and of ovarian tissue transplantation promises to provide options to women who must delay childbearing.
Despite these major technological advances achieved by ART in the last three decades, intense efforts must be devoted to follow the long term impact of its technologies as the oldest child conceived with IVF is only twenty-seven-years-old. Furthermore, many technologies such as ICSI, in vitro maturation, oocyte cryopreservation and vitrification, and PGD have only a smattering of data on developmental outcomes. Heightened awareness of potential health risks secondary to ovulation induction medications, in vitro culture conditions, and oocyte/embryo manipulations is paramount to the continuous surveillance of rare complications of ART that may only manifest over time. Periodic meta-analysis to pool well-conducted studies limited by sample size and statistical power may help uncover true associations between ART and infrequent diseases. Diligent maintenance of national ART birth registries and expanded access to international data will further enhance the investigations on IVF-associated birth defects and imprinting disorders.
In summary, few fields of medicine have enjoyed the popular growth and sustained improvements witnessed by physicians and their patients with infertility. However, there is increasing evidence that ART-conceived children may be at greater risk of perinatal complications than naturally conceived children and that knowledge on long-term health effects of ART is incomplete. Hence, all clinicians and researchers involved in the care of these patients must maintain a heightened awareness of these potential issues. As ART approaches its third decade, new and existing technologies must be used responsibly to help infertile couples achieve their goals without compromising the principle of ‘first, do no harm’.
References
- AntinoriSVersaciCPanciCFetal and maternal morbidity and mortality in menopausal women aged 45–63 yearsHum Reprod19951046497769080
- AoAWellsDHandysideAHPreimplantation genetic diagnosis of inherited cancer: familial adenomatous polyposis coliJ Assist Reprod Genet19981514049547690
- AschRHEllsworthLRBalmacedaJPPregnancy after translaparoscopic gamete intrafallopian transferLancet19842103456149412
- BoisoIMartiMSantaloJA confocal microscopy analysis of the spindle and chromosome configurations of human oocytes cryopreserved at the germinal vesicle and metaphase II stageHum Reprod20021718859112093855
- BonduelleMPonjaertISteirteghemAVDevelopmental outcome at 2 years of age for children born after ICSI compared with children born after IVFHum Reprod2003183425012571172
- BonduelleMVan AsscheEJorisHPrenatal testing in ICSI pregnancies: incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parametersHum Reprod20021726001412351536
- BonduelleMWennerholmUBLoftAA multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conceptionHum Reprod2005204131915576393
- [CDC] National Center for Chronic Disease Prevention and Health Promotion2003 Assisted reproductive technology success rates: National summary and fertility clinic reports [online]2005 Accessed on 5 December 2005. URL: http://www.cdc.gov/ART/ART2003/PDF/ART2003.pdf
- CoboARubioCGerliSUse of fluorescence in situ hybridization to assess the chromosomal status of embryos obtained from cryopreserved oocytesFertil Steril2001753546011172839
- Cook Group IncorporatedOvum aspiration diagram [online]2006 Accessed on 2 January 2006. URL: http://www.cookwomenshealth.com/products/infertility/1_01/1_01_01a.html
- CoxGFBurgerJLipVIntracytoplasmic sperm injection may increase the risk of imprinting defectsAm J Hum Genet200271162412016591
- CraftIBennettVNicholsonNFertilising ability of testicular spermatozoaLancet19933428648104288
- CrosignaniPGWaltersDESolianiAThe ESHRE multicentre trial on the treatment of unexplained infertility: a preliminary report. European Society of Human Reproduction and EmbryologyHum Reprod1991695381761665
- DelhantyJDGriffinDKHandysideAHDetection of aneuploidy and chromosomal mosaicism in human embryos during preimplantation sex determination by fluorescent in situ hybridisation, (FISH)Hum Mol Genet19932118358401499
- DellenbachPNisandIMoreauLTransvaginal sonographically controlled follicle puncture for oocyte retrievalFertil Steril198544656623932103
- DenschlagDTempferCKunzeMAssisted reproductive techniques in patients with Klinefelter syndrome: a critical reviewFertil Steril200482775915482743
- DevroeyPStaessenCCamusMZygote intrafallopian transfer as a successful treatment for unexplained infertilityFertil Steril19895224692666176
- DonnezJDolmansMMDemylleDLivebirth after orthotopic transplantation of cryopreserved ovarian tissueLancet36414051015488215
- EdwardsRGSteptoePCCurrent status of in-vitro fertilisation and implantation of human embryosLancet19832126596139617
- EdwardsRGSteptoePCPurdyJMEstablishing full-term human pregnancies using cleaving embryos grown in vitroBr J Obstet Gynaecol198087737566775685
- ElterKNelsonLRUse of third generation gonadotropin-releasing hormone antagonists in in vitro fertilization-embryo transfer: a reviewObstet Gynecol Surv2001565768811524623
- FosasNMarinaFTorresPJThe births of five Spanish babies from cryopreserved donated oocytesHum Reprod20031814172112832365
- FriedlerSGiudiceLCLambEJCryopreservation of embryos and ovaFertil Steril198849743643282929
- GarciaJEJonesGSAcostaAAHuman menopausal gonadotropin/human chorionic gonadotropin follicular maturation for oocyte aspiration: phase I, 1981Fertil Steril1983a39167736217993
- GarciaJEJonesGSAcostaAAHuman menopausal gonadotropin/human chorionic gonadotropin follicular maturation for oocyte aspiration: phase II, 1981Fertil Steril1983b3917496401635
- GianaroliLMagliMCFerrarettiAPThe role of preimplantation diagnosis for aneuploidiesReprod Biomed Online20024Suppl 331612470562
- GolanARon-ElRHermanAOvarian hyperstimulation syndrome following D-Trp-6 luteinizing hormone-releasing hormone microcapsules and menotropin for in vitro fertilizationFertil Steril198850912162974429
- GriffinDKHandysideAHHarperJCClinical experience with preimplantation diagnosis of sex by dual fluorescent in situ hybridizationJ Assist Reprod Genet199411132437827442
- HamoriMStuckensenJARumpfDZygote intrafallopian transfer (ZIFT): evaluation of 42 casesFertil Steril198850519213410103
- HandysideAHKontogianniEHHardyKPregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplificationNature1990344768702330030
- HandysideAHLeskoJGTarinJJBirth of a normal girl after in vitro fertilization and preimplantation diagnostic testing for cystic fibrosisN Engl J Med199232790591381054
- HansenMKurinczukJJBowerCThe risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilizationN Engl J Med20023467253011882727
- IngeGBBrinsdenPRElderKTOocyte number per live birth in IVF: were Steptoe and Edwards less wasteful?Hum Reprod2005205889215689347
- JonesHWJrJonesGSAndrewsMCThe program for in vitro fertilization at NorfolkFertil Steril19823814217095165
- KahramanSKarlikayaGSertyelSClinical aspects of preimplantation genetic diagnosis for single gene disorders combined with HLA typingReprod Biomed Online200495293215588472
- KhoslaSDeanWBrownDCulture of preimplantation mouse embryos affects fetal development and the expression of imprinted genesBiol Reprod2001649182611207209
- KulievARechitskySVerlinskyOPreimplantation diagnosis of thalassemiasJ Assist Reprod Genet199815219259604751
- LenzSLauritsenJGUltrasonically guided percutaneous aspiration of human follicles under local anesthesia: a new method of collecting oocytes for in vitro fertilizationFertil Steril19823867377141008
- LenzSLauritsenJGKjellowMCollection of human oocytes for in vitro fertilisation by ultrasonically guided follicular punctureLancet19811116346112519
- LieRTLyngstadassAOrstavikKHBirth defects in children conceived by ICSI compared with children conceived by other IVF-methods; a meta-analysisInt J Epidemiol20053469670115561745
- LudwigMKatalinicAGrossSIncreased prevalence of imprinting defects in patients with Angelman syndrome born to subfertile couplesJ Med Genet2005422899115805153
- LutjenPTrounsonALeetonJThe establishment and maintenance of pregnancy using in vitro fertilization and embryo donation in a patient with primary ovarian failureNature198430717456690997
- MaherERBruetonLABowdinSCBeckwith-Wiedemann syndrome and assisted reproduction technology (ART)J Med Genet20034062412525545
- MalterHECohenJPartial zona dissection of the human oocyte: a nontraumatic method using micromanipulation to assist zona pellucida penetrationFertil Steril198951139482910709
- MillsMSEddowesHACahillDJA prospective controlled study of in-vitro fertilization, gamete intra-fallopian transfer and intrauterine insemination combined with superovulationHum Reprod1992749041522191
- MunneSSandalinasMEscuderoTImproved implantation after preimplantation genetic diagnosis of aneuploidyReprod Biomed Online2003791712930584
- MunneSWeierHUSteinJA fast and efficient method for simultaneous X and Y in situ hybridization of human blastomeresJ Assist Reprod Genet19931082908499685
- MunneSWellsDPreimplantation genetic diagnosisCurr Opin Obstet Gynecol2002142394412032378
- NgSCBongsoARatnamSSMicroinjection of human oocytes: a technique for severe oligoasthenoteratozoospermiaFertil Steril1991561117231743331
- NugentDVandekerckhovePHughesEGonadotrophin therapy for ovulation induction in subfertility associated with polycystic ovary syndromeCochrane Database Syst Rev20004CD00041011034687
- PalermoGJorisHDevroeyPPregnancies after intracytoplasmic injection of single spermatozoon into an oocyteLancet199234017181351601
- ParsonsJRiddleABookerMOocyte retrieval for in-vitro fertilisation by ultrasonically guided needle aspiration via the urethraLancet19851107672860290
- PaulsonRJBoostanfarRSaadatPPregnancy in the sixth decade of life: obstetric outcomes in women of advanced reproductive ageJAMA200228823202312425710
- PaulsonRJThorntonMHFrancisMMSuccessful pregnancy in a 63-year-old womanFertil Steril199767949519130906
- Ponjaert-KristoffersenIBonduelleMBarnesJInternational collaborative study of intracytoplasmic sperm injection-conceived, in vitro fertilization-conceived, and naturally conceived 5-year-old child outcomes: cognitive and motor assessmentsPediatrics2005115e283915741353
- PorterRNSmithWCraftILInduction of ovulation for in-vitro fertilisation using buserelin and gonadotropinsLancet19842128456150318
- RizkBSmitzJOvarian hyperstimulation syndrome after superovulation using GnRH agonists for IVF and related proceduresHum Reprod1992732071587936
- SchoysmanRVanderzwalmenPNijsMPregnancy after fertilisation with human testicular spermatozoaLancet199334212377901551
- SermonKGoossensVSenecaSPreimplantation diagnosis for Huntington’s disease (HD): clinical application and analysis of the HD expansion in affected embryosPrenat Diagn1998181427369949443
- Sheffer-MimouniGMashiachSDorJFactors influencing the obstetric and perinatal outcome after oocyte donationHum Reprod20021726364012351541
- ShrivastavPNadkarniPWensvoortSPercutaneous epididymal sperm aspiration for obstructive azoospermiaHum Reprod199492058617868674
- Soderstrom AnttilaVPregnancy and child outcome after oocyte donationHum Reprod Update20017283211212070
- SonmezerMOktayKFertility preservation in female patientsHum Reprod Update2004102516615140872
- StaessenCCoonenEVan AsscheEPreimplantation diagnosis for X and Y normality in embryos from three Klinefelter patientsHum Reprod199611165038921110
- StaessenCPlatteauPVan AsscheEComparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trialHum Reprod20041928495815471934
- SteptoePCEdwardsRGReimplantation of a human embryo with subsequent tubal pregnancyLancet19761880258146
- SteptoePCEdwardsRGBirth after the reimplantation of a human embryoLancet1978236679723
- SutcliffeAGD’SouzaSWCadmanJOutcome in children from cryopreserved embryosArch Dis Child19957229037763057
- TanboTDalePOAbyholmTAssisted fertilization in infertile women with patent fallopian tubes. A comparison of in-vitro fertilization, gamete intra-fallopian transfer and tubal embryo stage transferHum Reprod19905266702351708
- Temple-SmithPDSouthwickGJYatesCAHuman pregnancy by in vitro fertilization (IVF) using sperm aspirated from the epididymisJ In Vitro Fert Embryo Transf1985211922
- TothTLOehningerSTonerJPEmbryo transfer to the uterus or the fallopian tube after in vitro fertilization yields similar resultsFertil Steril1992571110131572482
- TrounsonAMohrLHuman pregnancy following cryopreservation, thawing and transfer of an eight-cell embryoNature198330570796633637
- TwiskMMastenbroekSvan WelyMPreimplantation genetic screening for abnormal number of chromosomes (aneuploidies) in in vitro fertilisation or intracytoplasmic sperm injectionCochrane Database Syst Rev20061 CD005291
- Van der ElstJOocyte freezing: here to stay?Hum Reprod Update200394637014640378
- Van SteirteghemACLiuJJorisHHigher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cyclesHum Reprod199381055608408486
- Van SteirteghemACNagyZJorisHHigh fertilization and implantation rates after intracytoplasmic sperm injectionHum Reprod19938106168408487
- VerlinskyYGinsbergNLifchezAAnalysis of the first polar body: preconception genetic diagnosisHum Reprod1990582692266156
- VerlinskyYRechitskySSchoolcraftWPreimplantation diagnosis for Fanconi anemia combined with HLA matchingJAMA20012853130311427142
- VerlinskyYRechitskySVerlinskyOPreimplantation diagnosis for early-onset Alzheimer disease caused by V717L mutationJAMA200228710182111866650
- WiklandMEnkLHammarbergKUse of a vaginal transducer for oocyte retrieval in an IVF/ET programJ Clin Ultrasound198715245513134424
- WinslowKLYangDBlohmPLOocyte cryopreservation/a three year follow up of sixteen births [abstract]Fertil Steril200176Suppl 1S1201
- YoonTKKimTJParkSELive births after vitrification of oocytes in a stimulated in vitro fertilization-embryo transfer programFertil Steril2003791323612798878