Abstract
Pancreatic cancer is a largely chemo-resistant disease with a poor prognosis. Despite the adoption of gemcitabine monotherapy as a standard of care, outcomes remain poor. Until recently randomized phase III studies have not demonstrated superiority of various cytotoxic combinations or a number of the newer biologic targeted drugs. The situation has changed with capecitabine and erlotinib, either of which in combination with gemcitabine produces a small increase in survival. Erlotinib is a small molecule tyrosine kinase inhibitor against epidermal growth factor receptor which has an important role in the molecular pathogenesis of pancreatic cancer. In both pre-clinical and early clinical evaluation it has shown anti-tumor activity against pancreatic cancer in combination with gemcitabine. A randomized phase III study in locally advanced and metastatic pancreatic cancer has shown a survival advantage for the combination of gemcitabine plus erlotinib over gemcitabine alone. The rationale for the clinical development of erlotinib in combination with gemcitabine in pancreatic cancer culminating in this randomized trial, together with pharmacologic, toxicity and patient selection considerations form the focus of this review.
Introduction
Pancreatic cancer is an aggressive malignancy, the vast majority of patients presenting with advanced unresectable disease. Despite advances in the development of conventional chemotherapy, notably the establishment of gemcitabine as a standard of care, response rates to therapy are low and survival from the disease is still depressingly poor. Gemcitabine gained favor in the treatment of advanced pancreatic cancer worldwide after CitationBurris et al (1997) reported the results of their randomized controlled trial comparing gemcitabine, a novel nucleoside analogue with bolus 5-fluorouracil (5-FU). This demonstrated significantly improved clinical benefit response rates (24% vs 5%; p=0.0022) and median survival duration (5.65 vs 4.4 months; p=0.0025) for the gemcitabine arm. The one-year survival rate was 19% in the gemcitabine group and only 2% in the 5-FU treatment group. The response rate was 5.4% versus 0% (nonsignificant) and stable disease 39% versus 19% in favour of gemcitabine. Despite the modest but significant increment in survival, gemcitabine was adopted as the standard of care based on the significant improvement in clinical benefit response which is a composite measure of pain (intensity and analgesic requirement), performance status and weight. There are data suggesting that modulating the rate of infusion of gemcitabine may enhance anti-tumor activity with a randomized phase II study of fixed-dose rate gemcitabine given at an infusion rate of 10 mg/m2/min, demonstrating a trend towards improvement in response rate and survival when compared with standard infusion of gemcitabine over thirty minutes (CitationTempero et al 2003).
Gemcitabine has become the reference arm in randomized trials in the treatment of advanced pancreatic cancer to which newer agents have been tested against either alone or in combination, particularly doublets. However, several alternative cytotoxics as part of single agent or combination therapies (usually doublets) have failed to produce superior results over gemcitabine alone (CitationCheverton et al 2004; CitationRichards et al 2004; CitationRocha Lima et al 2004; CitationLouvet et al 2005). Recently a UK randomized phase III study of 533 patients with advanced pancreatic cancer reported significantly improved survival for the combination of gemcitabine plus capecitabine over gemcitabine alone with a median overall survival of 6.0 months versus 7.4 months in favor of the combination arm (hazard ratio [HR] 0.80; 95% confidence interval [CI]: 0.65, 0.98: p=0.026) and 12 month survival of 19% and 26% respectively (CitationCunningham et al 2005). Combination therapy was well tolerated. This represents the first positive phase III study demonstrating superiority of a cytotoxic doublet over gemcitabine monotherapy. These results contrast with a recently reported negative Swiss phase III study of gemcitabine and capecitabine in which the doublet was administered according to a different dosing and schedule to that used in GEMCAP (CitationHerrmann et al 2005).
There is still a clear need for new therapies and the identification of novel therapeutic targets in an attempt to improve on current standards. Within the last decade there have been significant advances in our understanding of the molecular pathogenesis underlying the development and progression of pancreatic cancer. A greater understanding of the interplay between tumor, stroma, and host and of important genetic and epigenetic events has been vital in identifying and developing potential therapeutic interventions with the capacity to disrupt tumor progression. A few randomized studies of gemcitabine versus gemcitabine plus a biologic agent directed at promising novel targets have proved negative (CitationBramhall et al 2001, Citation2002; CitationMoore et al 2003; CitationVan Cutsem et al 2004). The reasons for this are unclear and for some of the candidate targets may reflect that in metastatic pancreatic cancer, and indeed many cancers, cell proliferation is likely to be dependent on more than one genetic lesion such that growth control is non-linear. The biologics have often been most successful when targeting a critical genetic lesion upon which cellular proliferation has become dependent, the notion of oncogene addiction.
The contribution of the epidermal growth factor receptor (EGFR) pathway to oncogenesis has been well documented and therapeutic exploitation of this axis has proved to be a successful strategy in several other tumor types including colorectal and head and neck cancers. There has been considerable interest in targeting the EGFR pathway in advanced pancreatic cancer with agents such as erlotinib currently in the limelight. The application of erlotinib to the treatment of pancreatic cancer will be the focus of this review.
Targeting the epidermal growth factor pathway in pancreatic cancer
The erbB family of growth tyrosine kinase receptors comprises four structurally homologous members including erbB1, also known as the epidermal growth factor receptor (EGFR), erbB2 (HER2/neu), erbB3 (HER3) and erbB4 (HER4) (CitationYarden and Sliwkowski 2001). ErbB tyrosine kinase receptors are functionally inactive monomers that contain an extracellular ligand binding domain, a single hydrophobic transmembrane domain and an intracellular tyrosine kinase domain. Binding of one of over ten cognate ligands results in receptor homo- or hetero-dimerisation mediated by cysteine rich loops in the ectodomain (CitationOgiso et al 2002). The resulting phosphorylation of tyrosine residues within the adenosine triphosphate (ATP) binding kinase domain activates kinase activity and results in phosphorylation of residues in the regulatory carboxyl terminal tail of the receptor. This leads to the phosphorylation, activation and recruitment of signaling effectors containing SRC homology 2 and phosphotyrosine binding domains that initiate a cascade of downstream signaling events ultimately culminating in gene transcription (CitationMarmor et al 2004).
This complex network of erbB receptors, their associated ligands, and the various signal processing pathways contributes to the intricate regulation of normal cellular processes including proliferation, differentiation, cell motility, and survival. However, dysregulation of the network can lead to aberrant control of cell growth which may potentiate malignant transformation. Of the various components of the network, abnormal signaling through the erbB1 and erbB2 receptors has been most widely studied and implicated in the pathogenesis of several tumor types including pancreatic cancer. The EGFR, in particular, has been selected as a rational target in the treatment of this disease.
EGFR signaling in pancreatic cancer
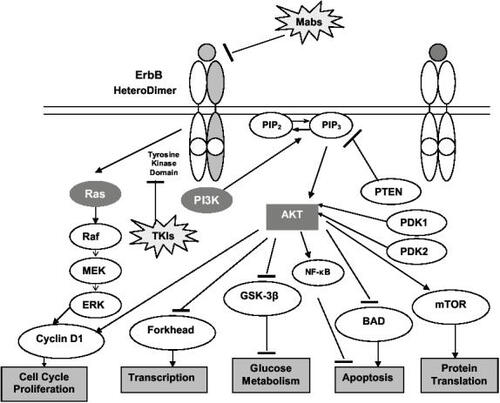
Aberrant signal transduction through EGFR and HER2 influences several processes pertinent to cancer progression including proliferation, resistance to apoptosis, invasion, angiogenesis, and metastasis. Dysregulation of the EGFR signaling pathway may occur through various mechanisms including receptor or ligand overexpression, receptor mutation (as with EGFRvIII which has a truncated extracellular domain and demonstrates constitutive ligand-independent activation) and receptor crosstalk (CitationArteaga 2003). In contrast to receptor homodimerization, EGFR heterodimerization, for instance with HER2, provides a stronger mitogenic stimulus mediated predominantly through the ras-raf mitogen activated protein kinase (MAPK) and AKT-PI3-kinase (phosphatidylinositol 3-kinase) pathways () (CitationMendelsohn and Baselga 2003; CitationMarmor et al 2004). The latter leads to degradation of the inhibitory IKK protein and translocation of the nuclear factor κB (NF-κB) into the nucleus where it activates transcription of genes involved in cell survival and chemoresistance (CitationGuttridge et al 1999; CitationHinz et al 1999). The epidermal growth factor has been shown to activate NF-κB in smooth muscle cells, A431 cells, fibroblasts and estrogen receptor negative, EGF-overexpressing breast cancer cell lines (CitationHabib et al 2001). HER2 has no cognate ligand but acts as a dimerization partner for other members of the erbB family utilizing the same effector signaling pathways and strengthening the mitogenic stimulus through such means as decreasing the rate of receptor downregulation and more efficient coupling to signaling pathways (CitationMarmor et al 2004).
Figure 1 The epidermal growth factor receptor pathway. Signaling through the epidermal growth factor receptor initiates a cascade intracellular cell signaling events which result in proliferation, angiogenesis and cell survival.
Molecular dysfunction of the EGFR signal transduction axis in pancreatic cancer can occur at several levels. A significant proportion of pancreatic cancers demonstrate increased expression of EGFR and its cognate ligand and this has been correlated with enhanced tumor aggressiveness and a worse prognosis (CitationLemoine et al 1992; CitationYamanaka et al 1993). Akt-2, a molecule downstream of PI3 kinase in the EGFR signal cascade is overexpressed in up to 60% of human pancreatic biopsies and the Akt-2 oncogene is amplified in 10%–20% of pancreatic cancer cells (CitationRuggeri et al 1998; CitationSchlieman et al 2003). Akt has demonstrated basal phosphorylation and activation in pancreatic cell lines which may confer resistance to apoptosis (CitationBondar et al 2002; CitationSchlieman et al 2003). Akt activation may in some cases result from loss of the inhibitory effect of PTEN and whilst PTEN is not known to be mutated in pancreatic cancer, loss of function could occur through alternative mechanisms (CitationPerren et al 2000; CitationEbert et al 2002). Further downstream in the signaling cascade constitutive activation of the transcription factor NF-κB has been identified in human pancreatic cells and this may also enhance the drive for cell survival (CitationWang et al 1999). EGFR signaling may also initiate early malignant transformation via activation of the Notch genes which are implicated in the regulation of cellular differentiation during pancreatic organogenesis and their activation can result in an expansion of undifferentiated cells (CitationMiyamoto et al 2003; CitationHeiser and Hebrok 2004).
Modulation of EGFR-mediated signaling therefore provides an attractive approach to the treatment of pancreatic cancer. The two main therapeutic modalities used to target the EGFR are monoclonal antibodies against the extracellular domain and small molecules tyrosine kinase inhibitors (TKIs) that compete at the ATP binding site of the tyrosine kinase domain (). Of the latter, erlotinib is the furthest in clinical development in pancreatic cancer.
Table 1 Therapeutic approaches to targeting the epidermal growth factor receptor
Pre-clinical evaluation of erlotinib
Erlotinib (Tarceva; Genentech Inc, South San Francisco, CA) is an orally available quinazoline-based small molecule TKI with highly selective activity against the EGFR (in-vitro IC50: 0.02 µmol/L; intact cells IC50: 0.2 µmol/L) (CitationMoyer et al 1997; CitationArteaga 2001). Examples of other small molecule TKIs are provided in and include some of the newer dual or multi-targeted TKIs with activity against more than one member of the erbB family of tyrosine kinase receptors. In pre-clinical evaluation, erlotinib demonstrated anti-tumor activity in a number of tumor xenograft models including those modeling colon, head and neck and non small cell lung cancers (CitationMoyer et al 1997; CitationPollack et al 1999; CitationDesai et al 2002). Erlotinib was shown to inhibit EGFR activation as well as to potentiate gemcitabine-induced apoptosis in pancreatic carcinoma xenografts implanted orthotopically into immunodeficient mice (CitationNg et al 2002). This enhancement of gemcitabine-induced apoptosis by erlotinib provides a reasonable scientific rationale for their combination in clinical evaluation.
Further support for the therapeutic potential of combined EGFR blockade and gemcitabine therapy has been derived from pre-clinical studies of another EGFR inhibitor, the monoclonal antibody C225 (the precursor of cetuximab); in vitro, C225 was shown to exert anti-proliferative effects on BxPC-3 human pancreatic cancer cells (CitationOverholser et al 2000) and in an orthotopic nude mouse model of pancreatic cancer the anti-tumor effects of C225 were potentiated by the coadministration of gemcitabine (CitationBruns et al 2000). Erlotinib has been combined with a number of other cytotoxic agents in several pre-clinical models across tumor types without the demonstration of antagonism and with no clear demonstration of sequence-dependence (CitationAkita and Sliwkowski 2003).
The mechanism of action of erlotinib in terms of the exact effects on downstream receptor signaling or activation and mechanisms of resistance to the drug are still being elucidated. Pharmacodynamic evaluation of erlotinib is crucial to this process and will be discussed later. At a pre-clinical level, one of the studies mentioned earlier demonstrated reduction in phosphorylation of ERK1/2 (extracellular-regulated kinase), part of the ras-Raf transduction effector cascade, in one of the xenograft models with no effect on phospho-PKB (protein kinase B), part of the PI3-kinase/Akt pathway, in either pancreatic xenograft (CitationNg et al 2002).
Other potential cellular consequences of EGFR suppression by erlotinib include the induction of G1 cell cycle arrest possibly mediated through p27KIP1, potentiation of apoptosis by activation of pro-apoptotic molecules such as bax and caspase-8, inhibition of angiogenesis through decreased production of growth factors such as vascular endothelial growth factor (VEGF) and inhibition of invasion and metastasis via inhibition of matrix metalloproteinases (MMPs) (CitationMendelsohn and Baselga 2003). There is a strong body of evidence to suggest EGFR suppression impairs the ability of cells to repair cytotoxic or radiotherapy-induced damage (CitationCiardiello et al 2000; CitationSirotnak et al 2000; CitationMendelsohn and Baselga 2003). This together with observations from pre-clinical studies suggests that the most effective therapeutic application of this particular targeted agent in advanced pancreatic cancer is as part of a combinatorial strategy.
Another important consideration with regards to the mode of action of EGFR inhibitors such as erlotinib is that whilst these drugs have been designed to target the EGFR, it appears that quantification of the target per se in preclinical models may not predict for responsiveness to inhibition. Erlotinib appears to inhibit both high and moderate EGFR expressing tumor cell lines suggesting that expression is not the only determinant of responsiveness to erlotinib (CitationDesai et al 2002). Although variability does exist in EGFR quantification methodology, it is likely that other factors such as receptor mutation, gene amplification, dimerisation partners (such as Her2), receptor cross-talk and alternative pathways for downstream activation may be confounding factors. Taking these into account quantification of EGFR status may not be reflective of dependence of cell survival/proliferation on EGFR signaling which may be a determinant of response to EGFR inhibitors.
Early phase studies: pharmacology and pharmacokinetics
Four initial phase I trials, two of which were conducted in volunteers, established preliminary pharmacokinetic (PK) data for erlotinib (CitationHidalgo et al 2001; CitationHidalgo and Bloedow 2003). These demonstrated rapid absorption with peak plasma concentrations occurring within 4 hours, dose dependent PK between 3 mg to 30 mg but non-linear PK at higher doses, drug accumulation and continuous exposure with daily treatment (CitationHidalgo et al 2001; CitationHidalgo and Bloedow 2003). The phase I study that established the current recommended dosing for erlotinib was performed in patients with advanced, refractory solid tumors and explored a daily dosing of dose-escalated erlotinib according to three different schedules (CitationHidalgo et al 2001). A daily dosing schedule was established as tolerable with PK indicating increased plasma levels of erlotinib with escalating doses and no unexpected drug accumulation. Erlotinib appeared to be well tolerated and diarrhea and acneiform skin rash were defined as the dose-limiting toxicities, dose-limiting diarrhea occurring at the 200 mg/day dose. At a dose of 150mg/day diarrhea appeared to be manageable as was acneiform rash which was seen to occur at all doses of erlotinib. This dose was subsequently recommended as the dose to be taken forward in further clinical evaluation.
On a continuous dosing schedule, the steady state was reached in seven days with a half life of approximately 18 hours (CitationHidalgo et al 2001). Further support for the 150 mg/day dosing was provided by the fact that the minimum plasma concentration at this dose exceeded 500 mg/mL, which is the concentration associated with EGFR inhibition and anti-tumor activity in pre-clinical studies (CitationHidalgo et al 2001). Plasma concentrations in this range were seen less frequently with dosing of 50 mg or 100 mg per day.
Erlotinib undergoes hepatic metabolism, 80% occurring via cytochrome CYP3A4 which is commonly involved in the hepatic metabolism of several other drugs thereby increasing the potential for drug-drug interaction (CitationHidalgo and Bloedow 2003). In particular potent 3A4 inhibitors such as ketoconazole, certain other anti-fungals (fluconazole and itraconazole) and antibiotics (ie, erythromycin) may increase exposure to erlotinib whilst potent 3A4 inducers such as rifampicin and some anti-epileptic drugs will reduce exposure (CitationAbbas et al 2003). Caution should be exercised when co-administering these drugs with erlotinib, particularly the CA4 inhibitors, and dose adjustment of the erlotinib may be necessary. The predominant metabolite of erlotinib is OSI-420 which has a similar pharmacokinetic profile to erlotinib, also inhibits EGFR and undergoes biliary excretion (CitationMoyer et al 1997).
Combination of cytotoxics with other agents such as erlotinib is intuitively attractive in terms of the potential for targeting different oncogenic processes, avoiding development of resistance, potential for synergy and the resultant possible enhancement of anti-tumor effect therein. The pre-clinical rationale for combining gemcitabine with erlotinib has already been stated. In phase I evaluation in patients with pancreatic cancer, the combination of gemcitabine administered according to the standard schedule plus erlotinib at doses of 100 mg/day or 150 mg/day was found to be tolerable with no dose-limiting toxicity or significant pharmacokinetic interactions (CitationDragovich et al 2003; CitationPorterfield et al 2004). In another phase I study, the combination of erlotinib with the chemotherapy doublet gemcitabine (according to a standard schedule) and cisplatin in patients with advanced solid tumors was tolerable and did not indicate significant pharmacokinetic interactions (CitationRatain et al 2002). These studies provide support for evaluation of a gemcitabine-based chemotherapy plus erlotinib in the treatment of pancreatic cancer.
Erlotinib in combination with chemotherapy in clinical studies
Initially erlotinib was evaluated as monotherapy in phase II studies across a broad range of tumor types including lung, ovary, colorectal, and head and neck cancer (CitationHerbst 2003; CitationShepherd et al 2004; CitationSoulieres et al 2004) with the demonstration of responses and induction of stable disease in several of these studies. Notably, the demonstration of single agent activity for erlotinib in the treatment of chemo-refractory lung cancer led to regulatory approval for this indication. Toxicities were in line with those seen in earlier phase I evaluation.
In pancreatic cancer, clinical evaluation of erlotinib has been undertaken as part of a combination approach with gemcitabine. The National Canadian Institute of Cancer (NCIC) conducted an international, multicenter randomized placebo controlled phase III trial (PA.3) of gemcitabine versus gemcitabine in combination with erlotinib in advanced pancreatic cancer (CitationMoore et al 2005). 569 patients were randomized to receive either gemcitabine, which was administered at a dose of 1000 mg/m2 over 30 minutes given weekly for seven weeks then for three out of every four weeks thereafter or gemcitabine (same schedule) plus erlotinib. The starting dose of erlotinib in the combination arm was 100 mg/day based on the potential for additive toxicity. After several planned safety reviews, including a review of toxicity by the Drug Safety Monitoring Committee, a smaller final cohort of patients received the higher dose of 150 mg daily. Expression of EGFR was not mandated for trial entry although this information was collected. Patient characteristics were well balanced and approximately 25% of patients in both arms had locally advanced disease.
Overall survival was significantly better in the erlotinib arm compared with the placebo controlled arm with a median survival of 6.37 versus 5.91 months (HR 0.81; 95% CI 0.67, 0.97; p=0.025) and one year survival of 24% versus 17% respectively. Whilst the difference in median survival appears small, it should be noted that the survival curves came closer together for this point estimate and that the HR is probably more representative of the overall difference between the two arms. Similarly, progression-free survival was significantly improved in the combination arm, the medians being 3.75 versus 3.55 months respectively (HR=0.76; 95% CI 0.63, 0.91; p=0.003). Overall response rate did not appear to be different between the combination and placebo controlled arms (8.6% vs 8.0% respectively) although disease control did appear different (57.5% vs 49.2%). Toxicity will be discussed later but skin rash and diarrhea appeared to be increased in the erlotinib arm. Quality of Life assessment was undertaken in the North American centers according to the EORTC QLQ-c30 and indicated that per cycle there was a significant difference in favor of the placebo controlled arm in terms of diarrhea. There were no significant differences for global quality of life scores (CitationMoore et al 2005). Although the survival increment in this study was small, it is now one of two positive randomized studies demonstrating a survival benefit for combining gemcitabine with another agent, importantly a biologic response modifier. The study also led to regulatory approval for the combination of gemcitabine and erlotinib in advanced pancreatic cancer in the US in November 2005. Given that the majority of patients had been treated with the 100 mg dose in the trial, this has been recommended as the combination dose.
These results are in contrast to those observed in two randomized studies of chemotherapy doublets plus or minus erlotinib in over 1000 patients each (TRIBUTE and TALENT) performed in chemonaive lung cancer patients (CitationGatzemeier et al 2004; CitationHerbst, Johnson, et al 2005). Importantly, presence of EGFR mutations in the tyrosine kinase domain and other molecular aberrations in lung cancer patients are associated with response/resistance to EGFR TKIs (CitationLynch et al 2004; CitationEberhard et al 2005) and therefore the lack of selection for potential responders may have diluted any therapeutic effect of erlotinib in these large studies. EGFR mutations have not been found in pancreatic cancers and this is therefore unlikely to be a molecular marker that could potentially facilitate better selection of patients for erlotinib therapy. This will be discussed in more detail in later.
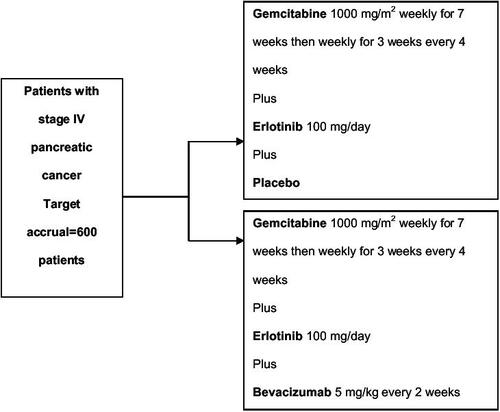
Combination of erlotinib with an effective cytotoxic doublet such as gemcitabine plus capecitabine would appear to be an attractive therapeutic option. Furthermore combination of erlotinib with another biologic agent such as the anti-VEGF agent bevacizumab, also appears attractive. In colon and gastric cancer cell lines, combined blockade of EGFR and VEGF resulted in significant antitumor responses which were greater than suppression of one pathway alone (CitationCiardiello et al 2000; CitationJung et al 2002). In a phase I/II study in lung cancer, the combination of erlotinib and bevacizumab was tolerable, had no pharmacokinetic interaction and importantly resulted in responses in the absence of cytotoxic treatment (CitationHerbst, Prager, et al 2005). Currently, there is an international placebo controlled phase III study underway comparing the combination of gemcitabine plus erlotinib according to the PA.3 schedule versus gemcitabine, erlotinib plus bevacizumab in patients with metastatic pancreatic cancer (), the results of which should prove very interesting. The erlotinib dose being used is 100 mg/day and the study is powered to demonstrate a survival difference between the arms.
At the Royal Marsden Hospital, UK, a phase I/II study (TARGET) is exploring the combination of gemcitabine, capecitabine (according to the GEMCAP schedule) plus erlotinib and bevacizumab in locally advanced and metastatic pancreatic cancer. It is likely that these multi-agent, multi-targeted approaches will be increasingly investigated in the treatment of advanced pancreatic cancer. In addition studies of alternative EGFR inhibitors in the first-line treatment of advanced pancreatic cancer are in progress with the randomised phase III Southwest Oncology Group (SWOG) trial of gemcitabine versus gemcitabine plus cetuximab having recently completed accrual. In a randomized phase II study performed by the University of Chicago, patients with advanced pancreatic cancer are randomized between gemcitabine, bevacizumab and erlotinib or gemcitabine, bevacizumab and cetuximab which will facilitate selection of a future comparator arm for phase III evaluation.
Toxicities associated with erlotinib
Skin rash
A class effect of EGFR inhibitors such as erlotinib and indeed the monoclonal antibodies is the development of a skin rash characterized by clusters of monomorphic pustular lesions and which is often likened to an acneiform eruption. The distribution of the rash is usually limited to the face and upper torso and although grading in the Common Toxicity Criteria version 2.0 categorizes rash according to percentage of body distribution, the latest version, 3.0, perhaps more appropriately allows grading of acneiform rash according to the need for medical intervention, although even the latest classification is not ideal.
Other dermatological manifestations of EGFR inhibition include dry skin, pruritis, erythema and nail, hair and eyelash changes (CitationPerez-Soler and Saltz 2005). The onset of rash is usually within the first two weeks of therapy and often improves despite further continuous dosing (CitationHidalgo et al 2001). Erlotinib-induced rash also appears to be dose-dependent (CitationHidalgo et al 2001) in line with the dose-dependence demonstrated for another EGFR TKI in clinical use, gefitinib (CitationPerez-Soler and Saltz 2005). The rash is usually well tolerated but when associated with symptoms such as itching or supra-added infection can be very problematic. Furthermore, the cosmetic appearance of the rash can be unacceptable to the patient and impact on their quality of life.
In the initial phase I studies biopsy of affected areas revealed neutrophil infiltration of dermal tissues, particularly in relation to the infundibular portion of the hair follicle, and thinning of the stratum corneum layer of the epidermis (CitationHidalgo et al 2001; CitationMalik et al 2003). The etiology of this skin reaction is not entirely clear but may relate to disruption of EGFR signaling in the dermis. EGFR is expressed in normal keratinocytes, skin fibroblasts, and in the outer route sheath of the hair follicle and is likely to have a physiological role in maintenance of the epidermis and skin/hair development (CitationKing et al 1990; CitationHansen et al 1997). Alternatively, EGFR inhibition may induce a local or systemic inflammatory skin reaction (CitationPerez-Soler and Saltz 2005).
Pharmacogenomics may play a role in the inter-patient variability of rash development with a recent study suggesting the number of germline polymorphic CA dinucleotide repeats in intron 1 of the EGFR gene may be associated with response to EGFR inhibition and rash; cell lines (mainly head and neck) demonstrating fewer than 36 CA repeats were more sensitive to inhibition by erlotinib than those with greater than or equal to 36 CA repeats (CitationAmador et al 2004). In the same study, a smaller number of CA repeats appeared to correlate with development of skin rash in patients with colorectal cancer (n=19) treated with gefitinib. These results are of interest and importantly indicate a potential mechanism for the association of rash with outcome which will be discussed further, but do require validation in larger studies.
The therapeutic management of EGFR inhibitor-induced skin rash has generated significant debate and there are currently no evidence-based guidelines for treatment. A variety of dermatological interventions have been employed including topical and systemic antibiotics, retinoids, corticosteroids and emollients with varying degrees of success (CitationPerez-Soler and Saltz 2005; CitationSegaert and Van Cutsem 2005). Certainly where there is clear evidence of supra-added infection, antibiotics may be beneficial. Otherwise, experience with all these agents is largely anecdotal and there is a clear need for systematic evaluation of interventions within controlled trials.
Other toxicities
Other commonly observed toxicities in the phase I studies and the Canadian phase III study were diarrhea, headache, nausea, vomiting, fatigue and mucositis (CitationHidalgo et al 2001; CitationDragovich et al 2003; CitationPorterfield et al 2004; CitationMoore et al 2005). demonstrates the toxicities seen in the NCIC study. Apart from skin rash, the rate of grade 3/4 diarrhea was different between the arms being 6% in the gemcitabine/erlotinib arm and 2% in the gemcitabine/placebo arm. Erlotinib-induced diarrhea should be managed aggressively with medical assessment, admission and the use of antidiarrheal agents and parenteral fluids as necessary. Both gemcitabine and erlotinib can cause interstitial fibrosis (pneumonitis) and in the NCIC study this occurred in 2% of patients receiving combination therapy compared with <1% who received gemcitabine alone. The overall toxicity profile of erlotinib in combination with gemcitabine, however, appears to be tolerable.
Table 2 Toxicities associated with the combination of erlotinib and gemcitabine in the NCIC Phase III study
Selection of patients for therapy
The issue of patient selection with targeted agents is important in terms of optimizing the chance of response to therapy, limiting exposure to drugs that do add additional toxicity and to help rationalize resources. As discussed earlier, skin rash may be a surrogate marker of outcome to EGFR inhibitors such as erlotinib. Although this association has been less consistent with other EGFR TKIs such as gefitinib (CitationPerez-Soler and Saltz 2005) it appears to be stronger with erlotinib as demonstrated in a number of trials, predominantly in lung cancer (CitationGatzmeier et al 2004; CitationHerbst, Johnson, et al 2005; CitationPerez-Soler and Saltz 2005). Not only the presence but the intensity of skin rash appeared to correlate with survival in these trials. Certainly in the NCIC Phase III study in pancreatic cancer, sub-group analysis also suggested an association with skin rash and survival; median survival for patients with grade 0 skin rash (n=79) was 5.29 months, with grade 1 skin rash (n=108) was 5.75 months and with grade 3 skin rash (n=103) was 10.51 months (CitationMoore et al 2005). The difference in survival between patients who developed grade 2 rash compared with grade 0/1 was statistically significant (p<0.0001). However, titration of erlotinib to skin rash in an attempt to optimize efficacy is likely to be difficult given that diarrhea has been demonstrated as the main dose-limiting toxicity. A dose-to-rash study is currently ongoing with erlotinib in lung cancer to explore this issue further (CitationMita et al 2005).
Several pharmacodynamic studies have been undertaken, often using skin as a surrogate tissue for response, in order to identify molecular aberrations in the EGFR pathway that may indicate response or resistance to therapy (CitationAlbanell et al 2002; CitationLoRusso 2003; CitationMalik et al 2003). However, whilst these often reveal interesting hypothesis-generating observations, none has identified a marker that has immediate clinical utility (CitationAbanell et al 2002; CitationMalik et al 2003). Furthermore, molecular changes occurring in response to EGFR suppression in the skin may not mirror changes in the tumor for a number of reasons including differences in EGFR dimerization partners and the presence of somatic mutations (CitationBaselga 2003; CitationLaux et al 2006).
Across studies and tumor types, there has consistently been a lack of association between EGFR expression, as judged by immunohistochemistry, and response to therapy in line with the pre-clinical observations (CitationDesai et al 2002; CitationHerbst 2003; CitationHortobagyi and Sauter 2003). In the NCIC study in pancreatic cancer, EGFR expression did not correlate with survival by treatment arm with the cut-off for EGFR positive tumors being 10% membranous staining (CitationMoore et al 2005). Selection for erlotinib therapy in pancreatic cancer should not be made on the basis of EGFR expression by immunohistochemistry. At the present time, there are no validated predictive markers that allow rationalization of erlotinib use in pancreatic cancer but work in this area is ongoing.
Conclusion
Erlotinb in combination with gemcitabine has recently been shown to be superior to gemcitabine monotherapy with a very modest improvement in survival. There are now two positive randomized phase III trials which have demonstrated a survival advantage for gemcitabine-based combination therapy, one incorporating a cytotoxic agent (CitationCunningham et al 2005) and the other, a biologic agent directed against the EGFR pathway (CitationMoore et al 2005). Both represent important, albeit small, steps in improving outcome from this aggressive malignancy and the latter importantly validating an approach that has been rationally selected based on the perceived importance of one of the pathophysiological mechanisms underlying the disease. Both could be considered standard treatment options for advanced pancreatic cancer. However, quality of life and pharmacoeconomic considerations are likely to dictate the uptake of either approach and the latter are yet to be undertaken. Ongoing and future studies in advanced pancreatic cancer are building on combinatorial approaches and the next generation of trials are incorporating biologic agents with conventional cytotoxics and additionally combining biologic agents. This multi-targeted approach may ultimately prove more effective in treating what is effectively a chemo-resistant disease. Given the efficacy of erlotinib demonstrated in the Canadian trial, erlotinib or alternative EGFR inhibitors such as cetuximab, are likely to be part of this strategy.
References
- AbbasRFettnerSRiekMA drug-drug interaction study to evaluate the effect of rifampicin on the pharmacokinetics of the EGF receptor tyrosine kinase inhibitor, erlotinib, in healthy subjects [abstract]Proc Am Soc Clin Oncol200322548
- AkitaRWSliwkowskiMXPreclinical studies with erlotinib (Tarceva)Semin Oncol2003303 Suppl 7152412840797
- AlbanellJRojoFAverbuchSPharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD-1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibitionJ Clin Oncol2002201102411773160
- AmadorMLOppenheimerDPereaSAn epidermal growth factor receptor intron 1 polymorphism mediates response to epidermal growth factor receptor inhibitorsCancer Res20046491394315604284
- ArteagaCLThe epidermal growth factor receptor: from mutant oncogene in nonhuman cancers to therapeutic target in human neoplasiaJ Clin Oncol20011918 Suppl32S-40S
- ArteagaCTargeting HER1/EGFR: a molecular approach to cancer therapySemin Oncol2003303 Suppl 7314
- BaselgaJSkin as a surrogate tissue for pharmacodynamic end points: is it deep enough?Clin Cancer Res2003923899012855608
- BondarVMSweeney-GotschBAndreeffMInhibition of the phosphatidylinositol 3'-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivoMol Cancer Ther200219899712481421
- BramhallSRRosemurgyABrownPDMarimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trialJ Clin Oncol20011934475511481349
- BramhallSRSchulzJNemunaitisJA double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancerBr J Cancer200287161712107836
- BrunsCJHarbisonMTDavisDWEpidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanismsClin Cancer Res2000619364810815919
- BurrisHAIIIMooreMJAndersenJImprovements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trialJ Clin Oncol1997152403139196156
- ChevertonPFriessHAndrasCPhase III results of exatecan (DX-8951f) versus gemcitabine in chemotherapy-naive patients with advanced pancreatic cancer [abstract]Proc Am Soc Clin Oncol2004234005
- CiardielloFBiancoRDamianoVAntiangiogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cellsClin Cancer Res2000637394710999768
- CiardielloFCaputoRBiancoRAntitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitorClin Cancer Res2000620536310815932
- CunninghamDChauIStockenDPhase III randomised comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancerEur J Cancer2005312
- DesaiBHigginsBSmithMAntitumour activity of the EGFR/TK inhibitor Tarceva (erlotinib, OSI-774) tumour models [abstract]Eur J Cancer200238203
- DragovichTPatnaikARowinskyEKA phase I B trial of gemcitabine and erlotinib HCL in patients with advanced pancreatic adenocarcinoma and other potentially responsive malignancies [abstract]Proc Am Soc Clin Oncol200322223
- EberhardDAJohnsonBEAmlerLCMutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinibJ Clin Oncol2005235900916043828
- EbertMPFeiGSchandlLReduced PTEN expression in the pancreas overexpressing transforming growth factor-beta 1Br J Cancer2002862576211870516
- GatzemeierUPluzanskaASzczesnaAResults of a phase III trial of erlotinib (OSI-774) combined with cisplatin and gemcitabine (GC) chemotherapy in advanced non-small cell lung cancer (NSCLC) [abstract]J Clin Oncol20042214S7010
- GuttridgeDCAlbaneseCReutherJYNF-KappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1Mol Cell Biol19991957859910409765
- HabibAAChatterjeeSParkSKThe epidermal growth factor receptor engages receptor interacting protein and nuclear factor-kappa B (NF-Kappa B)-inducing kinase to activate NF-kappa B. Identification of a novel receptor-tyrosine kinase signalosomeJ Biol Chem200127688657411116146
- HansenLAAlexanderNHoganMEGenetically null mice reveal a central role for epidermal growth factor receptor in the differentiation of the hair follicle and normal hair developmentAm J Pathol19971501959759176390
- HeiserPWHebrokMDevelopment and cancer: lessons learned in the pancreasCell Cycle20043270214726662
- HerbstRSErlotinib (Tarceva): an update on the clinical trial programSemin Oncol2003303 Suppl 7344612840799
- HerbstRSJohnsonDHMininbergEPhase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancerJ Clin Oncol20052325445515753462
- HerbstRSPragerDHermannRTRIBUTE: a Phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancerJ Clin Oncol2005235892916043829
- HerrmannRBodokyGRuhstallerTGemcitabine (G) plus Capecitabine (C) versus G alone in locally advanced or metastatic pancreatic cancer. A randomized phase III study of the Swiss Group for Clinical Cancer Research (SAKK) and the Central European Cooperative Oncology Group (CECOG) [abstract]J Clin Oncol20052316S4010
- HidalgoMBloedowDPharmacokinetics and pharmacodynamics: maximizing the clinical potential of erlotinib (Tarceva)Semin Oncol2003303 Suppl 7253312840798
- HidalgoMSiuLLNemunaitisJPhase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignanciesJ Clin Oncol20011932677911432895
- HinzMKrappmannDEichtenANF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transitionMol Cell Biol1999192690810082535
- HortobagyiGNSauterGChallenges and opportunities for erlotinib (Tarceva): what does the future hold?Semin Oncol2003303 Suppl 7475312840800
- JungYDMansfieldPFAkagiMEffects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse modelEur J Cancer20023811334012008203
- KingLEJrGatesREStoscheckCMThe EGF/TGF alpha receptor in skinJ Invest Dermatol1990946 Suppl164S-70S
- LauxIJainASinghSEpidermal growth factor receptor dimerization status determines skin toxicity to HER-kinase targeted therapiesBr J Cancer200694859216306877
- LemoineNRHughesCMBartonCMThe epidermal growth factor receptor in human pancreatic cancerJ Pathol19921667121538276
- LoRussoPMPhase I Studies of ZD1839 in patients with common solid tumorsSemin Oncol2003301 Suppl 121912644981
- LouvetCLabiancaRHammelPGemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD Phase III TrialJ Clin Oncol20052335091615908661
- LynchTJBellDWSordellaRActivating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinibN Engl J Med200435021293915118073
- MalikSNSiuLLRowinskyEKPharmacodynamic evaluation of the epidermal growth factor receptor inhibitor OSI-774 in human epidermis of cancer patientsClin Cancer Res2003924788612855621
- MarmorMDSkariaKBYardenYSignal transduction and oncogenesis by ErbB/HER ReceptorsInt J Radiat Oncol Biol Phys2004589031314967450
- MendelsohnJBaselgaJStatus of epidermal growth factor receptor antagonists in the biology and treatment of cancerJ Clin Oncol20032127879912860957
- MitaCASchwartzGMitaMMA pilot, pharmacokinetic (PK), and pharmacodynamic (PD) study to determine the feasibility of intrapatient dose escalation to tolerable rash and the activity of maximal doses of erlotinib (E) in previously treated patients with advanced non-small cell lung cancer (NSCLC) [abstract]J Clin Oncol20052316S3045
- MiyamotoYMaitraAGhoshBNotch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesisCancer Cell200335657612842085
- MooreMJHammJDanceyJComparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12–9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a Phase III Trial of the National Cancer Institute of Canada Clinical Trials GroupJ Clin Oncol200321329630212947065
- MooreMJGoldsteinDHammJErlotinib plus gemcitabine compared to gemcitabine alone in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) [abstract]J Clin Oncol2005236S115545667
- MoyerJDBarbacciEGIwataKKInduction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinaseCancer Res1997574838489354447
- NgSSTsaoMSNickleeTEffects of the epidermal growth factor receptor inhibitor OSI-774, tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinomaMol Cancer Ther200217778312492110
- OgisoHIshitaniRNurekiOCrystal structure of the complex of human epidermal growth factor and receptor extracellular domainsCell20021107758712297050
- OverholserJPPrewettMCHooperATEpidermal growth factor receptor blockade by antibody IMC-C225 inhibits growth of a human pancreatic carcinoma xenograft in nude miceCancer200089748210897003
- Perez-SolerRSaltzLCutaneous adverse effects With HER1/EGFR-targeted agents: is there a silver lining?J Clin Oncol20052352354616051966
- PerrenAKomminothPSaremaslaniPMutation and expression analyses reveal differential subcellular compartmentalization of PTEN in endocrine pancreatic tumors compared to normal islet cellsAm J Pathol2000157109710311021813
- PollackVASavageDMBakerDAInhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic miceJ Pharmacol Exp Ther19992917394810525095
- PorterfieldBWDragovichTPatnaikAErlotinib + gemcitabine in patients with unresectable pancreatic carcinoma: Results from a phase IB trial [abstract]J Clin Oncol20042214S4110
- RatainMJGeorgeCMJanischLPhase I trial of erlotinib (OSI-774) in combination with gemcitabine (G) and cisplatin (P) in patients with advanced solid tumors [abstract]Proc Am Soc Clin Oncol2002212115
- RichardsDAKindlerHLOettleHA randomized phase III study comparing gemcitabine plus pemetrexed versus gemcitabine in patients with locally advanced or metastatic pancreatic cancer [abstract]Proc Am Soc Clin Oncol2004234007
- Rocha LimaCMGreenMRRotcheRIrinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rateClin Oncol200422377683
- RuggeriBAHuangLWoodMAmplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomasMol Carcinog1998218169496907
- SchliemanMGFahyBNRamsamoojRIncidence, mechanism and prognostic value of activated AKT in pancreas cancerBr J Cancer20038921101514647146
- SegaertSVan CutsemEClinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitorsAnn Oncol20051614253316012181
- ShepherdFAPereiraJCiuleanuTEA randomized placebo-controlled trial of erlotinib in patients with advanced non-small cell lung cancer (NSCLC) following failure of 1st line or 2nd line chemotherapy. A National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) trial [abstract]J Clin Oncol20042214S7022
- SirotnakFMZakowskiMFMillerVAEfficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinaseClin Cancer Res2000648859211156248
- SoulieresDSenzerNNVokesEEMulticenter Phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neckJ Clin Oncol200422778514701768
- TemperoMPlunkettWRuiz Van HaperenVRandomized Phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinomaJ Clin Oncol2003213402812885837
- Van CutsemEvan de VeldeHKarasekPPhase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancerJ Clin Oncol2004221430815084616
- WangWAbbruzzeseJLEvansDBThe nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cellsClin Cancer Res19995119279918209
- YamanakaYFriessHKobrinMSOverexpression of HER2/neu oncogene in human pancreatic carcinomaHum Pathol1993241127348104858
- YardenYSliwkowskiMXUntangling the ErbB signalling networkNat Rev Mol Cell Biol200121273711252954