Abstract
Objective
To determine whether purified herbal extract of Salvia miltiorrhiza can improve the amniotic fluid volume in pre-term oligohydramnios by improving uteroplacental circulation.
Methods
Forty-three pregnant women with oligohydramnios received a daily intravenous dose of 30 mL of salvia extract mixed with 5% glucose 500 mL. A control group of 41 women received daily 5% glucose 500 mL only. The amniotic fluid index (AFI) was assessed at least twice a week by ultrasonographists who were blinded to the treatment. Both women and fetuses were monitored closely. The change in AFIwas calculated and compared by paired t test within and between groups. The revised recommendations for improving the quality of reports of parallel group randomized trials were used.
Results
After a mean of 7.2 ± 2.7 days’ therapy, ranging from 3 to 18 days, the AFIincreased significantly from a mean of 4.9 ± 2.3 cm to a mean of 7.12 ± 2.36 cm, by a mean of AFI0.18 ± 0.06 cm/day (paired t = 3.62, p < 0.005). In the control group, the AFIincreased from a mean of 5.1 ± 2.4 cm to a mean of 5.5 ± 3.1 cm after a mean of 6.1 ± 3.3 days’ treatment, ranging from 4 to 15 days. The effect of salvia treatment on AFIin the salvia group was significantly greater than in the control group (p < 0.001). No side effects were observed in treated patients.
Conclusion
Salvia miltiorrhiza is an effective Chinese medicine for the treatment of oligohydramnios.
Introduction
Oligohydramnios, defined as an amniotic fluid index (AFI) of less than the 5th percentile (at term ≤ 5 cm, or at pre-term ≤ 8.0 cm), has an incidence of 8.5% to 15.5% (CitationRutherford et al 1987; CitationSarno et al 1989; CitationMoor et al 1990), and is associated with an increased risk of umbilical cord occlusion, fetal distress in labor, operative deliveries, and stillbirth at term. However, oligohydramnios observed in the second trimester or the early third trimester is particularly ominous, with perinatal mortality rates approaching 80%–90% (CitationBaess et al 1984; CitationMercwe et al 1986). When amniotic fluid is absent, the perinatal loss rate increases to 90% (CitationMoore et al 1989). As shown earlier, oligohydramnios is a result of a decrease in fetal urine production and excretion with intact membranes and without fetal renal anomalies. The cause of the decrease in fetal urine production and excretion is chronic hypoxia because of placental dysfunction, resulting in shunting of fetal blood flow from the kidneys and a decrease in glomerular filtration rate. Therefore, oligohydramnios is usually accompanied by fetal growth restriction.
An effective medical therapy for oligohydramnios is very important for the fetus to grow normally to near term. Though some treatment modalities such as trans-abdominal amniocentesis and maternal hydration have been suggested, none work well or resolve the primary cause.
In China, salvia, a kind of traditional Chinese herbal medicine extracted from Salvia miltiorrhiza, has been used to treat cardiovascular disease and promote microcirculation in microthrombosis for several decades (Zhang et al 2005). In this study, we used salvia to improve maternal blood circulation and enhance placental function, and observed its effect on amniotic fluid volume.
Methods
The study protocol was approved by the local Institute Review Board and the hospital ethics committee. The purified Salvia miltiorrhiza extract (PSME) was obtained from ZDQCB Corporation Ltd (Zhengjiang zhengda Qingchunbao Yao Ye, Zhengjiang, China, Batch No. 0503087, Z33020177).
After the patients had provided written informed consent, they were admitted to hospital and randomly assigned to the study group or the control group by computer at the admission station. The reduced amniotic fluid volume was assessed by the four-quadrant technique, as described previously, and expressed as centimeters (AFIless than 5th percentile) (CitationRutherford et al 1987). Ultrasonography was performed for fetal anatomic study and fetal weight estimation. AFIwas confirmed by a second examiner using the same color Doppler 3-Dimension ultrasonography machine as the first examiner. Patients who were diagnosed with fetal anomalities by ultrosonography (eg, cardiac anomalies, renal cysts), or who were diagnosed with diabetes, were not enrolled in this study.
Maternal blood samples were drawn for routine baseline laboratory examination, including hemoglobin, hematocrit, platelets, and fibrinogen concentration, for emergency surgery delivery in case of ineffective treatment. Patients were divided into 2 groups: one group of 43 received a daily intravenous injection of 30 mL salvia extract, provided from inter medicine department, mixed with 5% glucose 500 mL over 4–6 hours; a control group 41 received conventional treatment of 5% glucose 500 mL only. In addition, 10 mg of dexamethasone was given once a day for 3 days to those patients in both groups at less than 34 weeks of gestation, to promote fetal lung maturity, in case the treatment was ineffective and the pregnancy terminated. The AFIwas reassessed at least twice a week by an ultrasonographist who was blinded to the treatment. If the AFIincreased to normal levels, the patient was discharged and followed up at clinics until to delivery. If the AFIremained in the abnormal range, the patient treatment was continued until the fetus was viable, or until the pregnancy terminated due to unviable fetus (<24 gestational weeks). If the AFIdecreased sharply, so that fetal life was threatened, or labor began, the pregnancy was concluded by caesarian section. During therapy, fetal well-being was monitored by: (1) fetal heart rate with a Doppler auscultator every 2 hours; (2) fetal body movement counting by the mother 3 times a day for 1 hour each; (3) nonstress test, fetal electrocardiogram, vibro-acoustic stimulation test, and biophysical profile were performed at least twice a week.
If any evidence of harmful effects to the mother or fetus were found during treatment, the therapy was stopped and normal management was used. The study ceased when sufficient data had been collected for statistical analysis. The revised recommendations for improving the quality of reports of parallel group randomized trials were used (CitationMoher et al 2001).
Data analysis
All values were expressed as mean ± SEM. Changes in AFIwere analyzed by paired t test. Statistical significance was set at p < 0.05. Assumptions made at the start of the study were: (1) that the estimated variance of the paired differences was 25%; (2) the certainty that the difference detected was at the 0.05 level and was not because of chance (eg, significance level or α = 0.05); (3) the test power is 0.8 (β = 0.80). Therefore, 16 patients were needed in this study for paired t test; 32 patients were needed for two samples comparison. The government or patients paid for all costs depending on their medicare policy.
Results
Forty-three pregnant women were enrolled in the salvia protocol, and 41 patients in the control group. Mean age of women was 28.5 ± 2.3 years (range 21–39 years) (). Most patients (80/84 = 91%) were primigravida. Mean gestational age was 32.0 ± 3.5 weeks (range 23–36 weeks). At admission, mean AFIwas 4.9 ± 2.3 cm (range 1.0–7.9 cm).
Table 1 The clinical characteristics and the results of treatment with salvia and the control (mean ± SD or CI 95%)
After a mean of 7.2 ± 2.7 days’ therapy, ranging from 3 to 18 days, the AFIincreased significantly to a mean of 7.1 ± 2.4 cm, ranging from 0 to 10.2 cm (paired t = 3.62, p < 0.005). The AFIincreased by a mean of 0.18 ± 0.06 cm/day. Thirteen patients failed to respond to therapy. Among these patients, 6 showed no significant increase in AFI, but maintained the pretreatment level. Six patients showed a decrease in AFIafter treatment, and were delivered by caesarean section. They had viable infants. One of the 13 patients had an inviable fetus and determined of pregnancy at 23 weeks of gestation, due to an extremely low fluid level. The absolute (increased fluid) effective rate of salvia was approximately 70%. The relative (though AFIdid not increase, it was not easy to maintain pretreatment level due to insufficient placenta function) effective rate of salvia was 84%. The patients showed no side effects. There was no drop out due to adverse effects during the clinical trial. The details of the control group are set out in .
Discussion
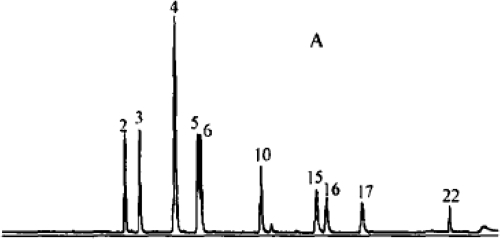
The results of this clinical trial demonstrate that administration of salvia can increase the level of amniotic fluid in general, and improve the fetal survival environment without maternal side effects. The salvia treatment of oligohydramnios has never been reported in English. In China, and other countries, salvia has been used as therapy for cardiovascular disease, chronic renal insufficiency, and intrahepatic cholestasis for at least 30 years (CitationFugh-Berman 2000; Kuang et al 2005; CitationAdams 2006). According to traditional Chinese medicine theory, the decrease in urine volume can be attributed to insufficient blood flow to the kidneys, due to reduced blood volume and blood stasis. Urine volume is an important resource of fluids during pregnancy. Many animal experiments and clinical phase I to phase III trials have demonstrated that salvia can activate blood circulation and remove blood stasis. Salvia includes many active compounds, including magnesium lithospermate A, B, C, D, E, F, G, H, I. Previously published research has shown approximately 25 kinds of effects of these compounds, including magnesium lithospermate, dipotassium lithospermate, sodium rosmarinate and potassium danshensu, dipotassium isolithospermate B and magnesium salvianolate G. The most important of these is magnesium lithospermate B, a tetramer of caffeic acid, which is a most powerful efficacious compound. shows chromatograms of standard mixtures (CitationXu et al 2007). The product here is similar to the standard with 95.0% to 99.9% and good reproducibility and stability (CitationXu et al 2007). They can ameliorate renal ischemia and improve blood circulation and microcirculation in the kidney, thereby enhancing the glomerular filtration rate and increasing urine (CitationYokozawa et al 1989; CitationSun et al 2005).
Figure 1 Liquid chromatography – mass spectrometry: Chromatograms of standard mixtures (CitationXu et al 2007).
In pregnant women with oligohydramnios, plasma viscosity and coagulation parameters were significantly higher before onset (CitationBattaglia et al 1995). In the oligohydramnios placenta, there exist platelet and fibrin aggregates and thrombosis of the fetal vessels, suggesting that a hypercoagulative state and hyperfibrinolysis might affect the microcirculation and result in microthrombis, obliteration or sclerosis in the small arteries and arteries of the tertiary villi of the placental cotyledon (CitationRedline et al 1995). Salvia treatment can regulate capillary tension and improve hemorrheological properties, by affecting the activity of fibrinolysis and suppressing platelet aggregation, reducing blood viscosity and improving microcirculation. In this way, the fetus can gain sufficient blood from the placenta and excrete urine normally, leading to a gradual increase in amniotic fluid. As we know, fetal urine production is the major source of amniotic fluid at the second and third trimesters. However, there is no effective treatment for a low fluid level, if the placenta has been severely changed pathologically, such as by extreme fibrin formation, calcification, infarction or embolism.
In conclusion, salvia therapy oligohydramnios is a primary study. The potential benefits and its detailed mechanisms need further in-depth study at multiple centers, based on large samples and case-controlled design. This treatment is an exciting finding for clinical practice.
Disclosure
The authors declare there are no conflicts of interest.
References
- AdamsJDWangRYangJPreclinical and clinical examinations of Salvia miltiorrhiza and its tanshinones in ischemic conditionsChin Med2006166394
- BarssVABenacerrafBRFrigolettoFDSecond trimester oligohydramnios, a predictor of poor fetal outcomeObstet Gynecol198464608136387555
- BattagliaCArtiniPGBallestriMHemodynamic, hematological and hemorrheological evaluation of post-term pregnancyActa Obstet Gynecol Scand199574336407778423
- Fugh-BermanAHerbs and dietary supplements in the prevention and treatment of cardiovascular diseasePrev Cardiol20003243211834913
- MercweLJBrownLGFetal outcome with oligohydramnios in the second trimesterObstet Gynecol19866784093703407
- MoherDSchulzKFAltmanDGThe revised CONSORT statement for reporting randomized trialsBMC Medical Research Methodology20011210.1186/1471-2288-1-211336663
- MooreTRCayleJEThe amniotic fluid index in normal human pregnancyAm J Obstet Gynecol19901621168732187347
- MooreTRLongoJLeopoldGThe reliability and predictive volume of an amniotic fluid scoring system in severe second trimester oligohydramniosObstet Gynecol198973739152649820
- RedlineRWPappinAFetal thrombotic vasculopathy: the clinical significance of extensive avascular villiHum Pathol1995268057821920
- RutherfordSEJeffreyPPSmithCVThe four-quadrant assessment of amniotic fluid volume: an adjunct to antepartum fetal heart rate testingObstet Gynecol19877035363306497
- SarnoPAAhnOMBrarSHIntrapartum Doppler velocimetry, amniotic fluid volume, and fetal heart rate as predictors of subsequent fetal distressAm J Obstet Gynecol19891611508142690625
- SunJHuangSHTan BennyK-HEffects of purified herbal extract of salvia miltiorrhiza on ischemic rat myocardium after acute myocardial infarctionLife Science200576284960
- XuMLiuACuiYComparative study on HPLC fingerprints of Danshenin Xiangdan injections from different manufacturersChin J Nat Med200751206
- YokozawaTChungHYOuraHIsolation of a renal function-facilitating constituent from the Oriental drug, salviae miltiorrhizae radixJap J Nephrology19893110919