Abstract
Zosyn® , also known as Tazocin® for injection, contains piperacillin and tazobactam and was approved by the US Food and Drug Administration in 1993 for the treatment of indicated serious infections. In 1995, United States Pharmacopoeia and European Pharmacopoeias reduced the particulate limit for injectables by 40%, based on general safety concerns. Wyeth attempted to control sporadic batch failures (associated with increased particulate formation) by shortening product expiration dating from 36 to 24 months and optimizing the stopper siliconization process. These modifications did not correct the problem completely. Wyeth reformulated Zosyn by incorporating two stabilizing functional excipients, ethylene diamine tetraacetic acid disodium salt (EDTA disodium) and sodium citrate, which solved the particulate formation problem. These two functional excipients also allowed for the first time Y-site coadministration of reformulated Zosyn product with amikacin and gentamicin at specific doses and concentrations, and with certain diluents, and the use of Ca++ ion-containing Lactated Ringer’s for admixture preparation. Reformulated Zosyn (approved 2005) may provide useful options of drug administration to healthcare professionals to lessen levels of particulates. Supportive data is provided for the expanded compatibility of reformulated Zosyn with different types of Ringer’s solutions used globally and for the Y-site coadministration of amikacin and gentamicin aminoglycosides.
Introduction
Zosyn® background information
Zosyn® , also known as Tazocin® piperacillin/tazobactam (Wyeth Pharmaceuticals Inc., Philadelphia, PA, USA), is an intravenously administered antibiotic which is composed at an 8:1 ratio of piperacillin (a semisynthetic β-lactam) and tazobactam (a β-lactamase inhibitor derived from the penicillin nucleus). The Food and Drug Administration (FDA) approved it for use in the United States in 1993. Zosyn is currently used in hospitals worldwide to treat patients with moderate-to-severe infections caused by piperacillin-resistant, piperacillin/tazobactam-susceptible β-lactamase-producing strains of certain specified microorganisms. Zosyn is indicated for the treatment of moderate to severe hospital-acquired pneumonia, complicated intra-abdominal infections, complicated skin and soft tissue infections (CitationWyeth Prescribing Information [Glass vials] 2007; CitationWyeth Prescribing Information [Galaxy® bags] 2007), and moderate community-acquired pneumonia. Zosyn and Tazocin represent the same product sold by Wyeth in different countries and are used interchangeably in this communication.
History of reformulation of Zosyn
Zosyn, as an injectable antibiotic, is reconstituted and admixed with a variety of diluents before it is administered to a patient. Post-approval, during the 1990’s, Zosyn had sporadic batch failures related to subvisible particulate matter when conducting the usual Particulate Matter Test (United States Pharmacopoeia [USP] <788>), at the new drug application (NDA) limits of 10,000 (particles ≥ 10μM per small volume dose)/1,000 (particles ≥25 μM per small volume dose).
Two key factors identified as responsible for excessive particulates were: (1) small traces of nontoxic silicone oil used to lubricate the elastomeric closures were dispersed in the reconstituted product as an emulsion. The dispersed silicone oil emulsion particles were interpreted by the HIAC particle size analyzer as solid particulate matter, contributing to the out of specification results for sub-visible particulate matter and, (2) subvisible particulate counts increased with the age of the finished product associated with down-shift in the pH of the reconstituted solution of the drug product towards the acidic side.
In response, Wyeth modified its container closure to control silicone lubricant from the rubber stopper insertion process. In addition, the product expiry dating was shortened from 36 to 24 months, as a slight increase occurred in the particulate matter test results in aged product. However, even with these changes, the product still experienced sporadic failures related to particulates. In 1995, USP and European Pharmacopeias adjusted the limits for particulates downwards in part due to ongoing awareness that particulates have the potential to cause morbidity or mortality in patients and because particulate matter had been linked to injection site reactions and thromboembolic events (CitationLonge 1980; CitationFalchuk et al 1985; CitationLehr et al 2002).
In 2001, partially in response to FDA requests regarding another Wyeth intravenous (IV) product and partially because of FDA concerns about Zosyn, Wyeth undertook to ensure that Zosyn met the revised (1995) USP <788> specifications. The revised specifications lowered the acceptable levels of subvisible particulate matter from 10,000 (particles ≥10 μM per small volume dose)/1,000 (particles ≥25 μM per small volume dose) to 6,000 (particles ≥10 μM per small volume dose)/600 (particles ≥25 μM per small volume dose). This corresponds to a required reduction in the acceptable number of 10 μm and 25 μm particle counts by 40%.
To accomplish this, Wyeth conducted due diligence investigations to establish the root cause and discovered that low pH and trace metal ions can be encountered (CitationDesai et al 2007a, Citation2007b) through the use of various commercial diluents. Both factors may increase the rate of particulate matter formation in admixtures prepared from Zosyn and potentially penicillins and other β-lactams. This can cause the products to fail USP <788>. The particulate failures in Zosyn may occur even though the pH of the drug substance is in its approved range of 5.5 to 6.8. Wyeth took corrective action by reformulating Zosyn.
Additional limitations of the original formulation and associated stability problems
In 1993, when Zosyn was introduced, its label instructed that the mixing of Zosyn with an aminoglycoside in vitro can result in substantial inactivation of the aminoglycoside. Chemical degradation of the aminoglycoside (possibly involving microbiologically inactive penicillin-aminoglycoside complexes or metal ion leachables) was observed to result in subpotent doses of the aminoglycoside. Such coadministration was also believed to lead to an unacceptably high particulate load (CitationDesai et al 2007a, Citation2007b). In addition, the label of Zosyn instructed that lactated Ringer’s injection solution (LRS) was not compatible with Zosyn and hence could not be used as a reconstitution or admixture diluent.
Transition metal ions as leachables from exogenous/endogenous sources
Contaminants in a drug product solution are defined as “leachables.” Potentially contaminating components of the drug product’s container system are termed “extractables.” Potential links between these two types of compounds have formed much of the basis for the increased scrutiny associated with revisions to the USP-NF and its General Chapter <788> standards for particulate matter in injectable solutions. CitationJenke (2005) recently proposed a paradigm for the assessment of interaction between these two classes of compounds based upon the origin of and ability to detect extractables in containers, relative to drug substances they may contain. This report cites the example of a regulatory body imposing a requirement of knowledge of such a linkage upon a drug manufacturer, and its potential impact upon a product’s life cycle (CitationJenke 2005).
Reformulation of Zosyn to overcome limitations
CitationDesai and colleagues (2007a, Citation2007b) recently reported that there is significant inter- and intra-batch variability in both pH and zinc ion content in commercially available IV diluents and solutions across different US manufacturers. Healthcare practitioners administering drugs in these diluents are generally not aware of this variability. In addition, since zinc is a common transition metal in drug-container packaging and delivery systems, it can leach into the IV solution from container components as well, and inactivate a drug via catalysis (CitationDesai et al 2007a, Citation2007b).
Commonly used IV diluents may contain small amounts of a variety of exogenous inorganic ions and/or organic substances extracted from the containers and closures into the product during manufacturing or storage. Zinc oxide is used as filler in rubber elastomeric closures of vials, septa at the necks of infusion bags, or in the plungers of infusion syringes. Zinc-based organic substances are also used as polymerization initiators for the polymers used for the construction of admixture bags or infusion line tubing. Zinc and other transition metal ions are known to catalyze chemical degradation of drug molecules (CitationBandoh et al 1991; CitationDesai et al 2007a, Citation2007b).
Degradation pathways of antibiotics may include acid hydrolysis, ß-lactam ring opening (CitationBandoh et al 1991), or epimerization. In the case of ionizable drugs generating anionic species, zinc, calcium, and other metal ions are known to form insoluble solid particulates. In the clinical field, risks associated with the injection of intravenous/intrathecal solutions with particulate contamination are well documented (CitationNath et al 2004).
The target pH listed on the diluent bags from commercial suppliers can be 3.2 to 6.5 for 5% dextrose injection, and 4.5 to 7.0 for 0.9% sodium chloride injection. The low pH extremes of 3.2 (for 5% dextrose injection) and 4.5 (for 0.9% sodium chloride injection) are considered to be acidic, thus having the potential to accelerate drug degradation, especially in the presence of zinc (CitationDesai et al 2007a, Citation2007b).
Thus, for the therapeutic performance and safety of drug admixtures infused by the IV route, having data of the pH and levels of zinc in commercial diluents from various parenteral manufacturers is crucial. Since this information is not readily available to the health professionals preparing the solution for parenteral administration, the availability of a drug product resilient to pH or trace metal ion levels commonly found in the clinical setting is preferable.
As part of the root cause analysis, the particulates were isolated and confirmed to be dimers of piperacillin by spectroscopic characterization. The chemical pathway for the dimer formation is shown in .
Figure 1 Hydrolysis of piperacillin followed by formation of a piperacillin dimer with low solubility.
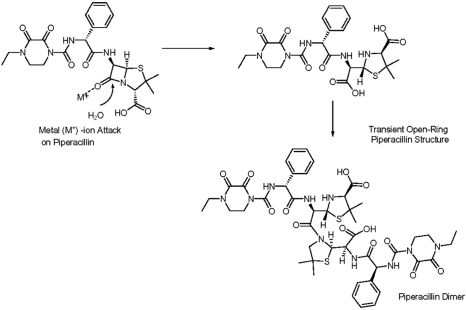
After extensive research, particulate formation in Zosyn was found to be related to the opening of the β-lactam ring of piperacillin, which is accelerated in acidic pH solutions, and is followed by the dimerization of piperacillin molecules that may be further catalyzed by zinc ions.
In summary, the presence of zinc may contribute to incompatibility by: (1) catalysis of drug degradation and potential underdosing, and (2) generation of insoluble solid particulate matter from the dimer formation and/or chelation with ionic species of the drug. Thus, CitationDesai and colleagues (2007a, Citation2007b) concluded that it is highly desirable that all new drug molecules be screened in the early product development phase for susceptibility to catalysis by zinc, and that the drug product be designed from the outset with sufficient robustness to withstand exposure to variable amounts of zinc ion. It is also recommended that USP monographs address the potential issues posed by zinc in the drug manufacturing and dosage form infusion process.
Combination therapy with Zosyn and amikacin/gentamicin
Because of the prevalent clinical need to administer piperacillin/tazobactam in combination with the aminoglycosides amikacin or gentamicin to treat nosocomial pneumonia caused by Pseudomonas aeruginosa as well as conditions caused by other pathogens, the clinician often faces operational challenges: either administer Zosyn and amikacin or gentamicin separately, or through the same intravenous line, typically through a Y-site tube. The latter mode, if possible, would expedite the treatment of the patients in critical care. Mixing of the original formulation of Zosyn with an aminoglycoside in vitro resulted in substantial inactivation of the aminoglycoside by piperacillin. It was postulated that penicillin-aminoglycoside complexes are created that are microbiologically inactive and are of unknown toxicity (CitationBenveniste and Davis 1973; CitationGlew and Pavuk 1983).
Evaluation of reformulated Zosyn to expand compatibility
The compatibility of reformulated Zosyn was evaluated by different physicochemical and spectroscopic methods to show that it can be coadministered as admixture with different types of Ringer’s solutions which was not feasible with the original Zosyn. Also, the feasibility of Y-site coadministration with amikacin or gentamicin aminoglycosides was evaluated as shown in the following sections.
Materials and methods
The samples of reformulated Zosyn from Wyeth, used in the admixture diluent compatibility and Y-site aminoglycoside studies, were manufactured and released as per approved product specifications. Commercial intravenous diluents, amikacin and gentamicin antibiotic products were purchased from US/German or other European countries and were used within expiry dating.
For the simulated Y-site administration study, reformulated Zosyn was admixed in a (high dose, low dose) crossover with two different gentamicin drug products in a (high dose, low dose) matrix design. The crossover pairings of reformulated Zosyn and gentamicin were based on the dose extremes expected in clinical use and were derived from the label dosing instructions listed on the package insert for each drug product.
The subvisible particulate (10 and 25 μm) counts were measured by the HIAC light obscuration method described in USP<788> and equivalent EU pharmacopeia 5.0 (2004) 2.9.19 sections.
Typical HPLC assay methods were used for potency determinations of amikacin, piperacillin, and tazobactam in the presence of reformulated Zosyn components.
For gentamicin potency determinations in the presence of reformulated Zosyn components, it was necessary to design a customized nuclear magnetic resonance (NMR) method. For the NMR study, it was necessary to remove the water from the commercial gentamicin product by lyophilization. The simulated Y-site samples were prepared by dissolving lyophilized gentamicin and reformulated Zosyn (lyophilized powder) in D2O in accordance with the concentration matrix described in .
Table 1 Combination matrix for reformulated Tazocin® plus gentamicin (reconstituted and diluted in D2O) at clinically relevant bracketed ranges of concentrations
Initially, the NMR method by Professor Holzgrabe (CitationWinters 2005) was evaluated to quantify gentamicin potency in the presence of reformulated Zosyn as a function of time. The NMR method appears to be applicable only to gentamicin drug product as-is, with a low ambient pH of about 4 to 4.6. Upon the addition of reformulated Zosyn, the final pH of the simulated Y-site solution with gentamicin is raised to about 6, so the ionic environment of the anomeric protons and the –N-methyl protons is changed, and hence the NMR spectrum is totally different than that of gentamicin drug product alone as observed by the Holzgrabe group.
For the customized NMR method, two groups of proton resonances of gentamicin were isolated from other proton resonances and were explored for the quantification of gentamicin. One is from the anomeric protons at δH 5.84 (doublet) and δH 5.80 (multiplet). The other is from the N-methyl group at δH 2.85 (singlet). Although the anomeric proton resonances were well separated, due to the relatively low concentration of gentamicin used in the clinical admixtures, the observed signal-to-noise ratio for the anomeric protons was very low. The N-methyl proton resonance peaks (with very good separation from the other peaks, and a peak intensity significantly higher than the anomeric proton peaks) were considered more appropriate to provide quantification of clinically relevant parenteral admixtures with low levels of gentamicin in the presence of very high levels of reformulated Zosyn.
NMR experiments were performed at room temperature on a Bruker DRX-500 NMR spectrometer, operating at 500.13 MHz (1H), equipped with TOPSPIN software (Version 1.3). 112 scans were collected into 64 k data points giving a digital resolution of 0.16 Hz per point. The spectral width was 10330 Hz, the transmitter offset at 6.17 ppm, and the flip angle was 90°. Using an acquisition time of 3.17 s and an additional delay of 1 s, the pulse repetition period was about 4.17 s. Samples were measured in D2O at 298 K. Each 1H NMR data point for gentamicin represents cumulative scans collected over ten minutes.
Hydroquinone monoethyl ether was used as an internal standard due to the absence of chemical interactions with gentamicin and all components of reformulated Zosyn. Also, the associated peaks of the hydroquinone monoethyl ether protons do not overlap or cause interference with the N-methyl protons of gentamicin that were used for quantification. This customized NMR method provides a kinetic snapshot at 10-minute intervals over a period of 1 hour for gentamicin strength in the presence of reformulated Zosyn.
Results and discussion
Improved clinical utility of the reformulated version of Zosyn
In 2005 (USP <788> 2007), the FDA approved the new formulation of Zosyn that complies with USP<788> particulate specifications and has an expanded compatibility profile with the aminoglycosides, amikacin and gentamicin. The modifications to the formulation consisted of the addition of the disodium salt of ethylene diamine tetraacetic acid (disodium EDTA; edetate disodium dihydrate), which acts as a metal-chelating agent, and sodium citrate, which acts as a buffer. These modifications lessen the possibility of particulate matter accumulation during storage of the solution form of Zosyn or the development of particulate matter upon reconstitution of Zosyn lyophilized powder with commonly used diluents. In addition, reformulated Zosyn can be administered simultaneously, either with amikacin or gentamicin, via Y-site infusion at specific doses and concentrations, and with certain diluents (CitationWyeth Prescribing Information [Glass vials] 2007a; CitationWyeth Prescribing Information [Galaxy® bags] 2007b).
Also, unlike original Zosyn, reformulated Zosyn has been shown () to be compatible with LRS or Hartmann’s solution (European version of LRS) for dilution. Healthcare practitioners sometimes prefer to use LRS or Hartmann’s solution.
Table 2 Summary of drug potency results for admixtures of reformulated Zosyn® in various diluents and stored at room temperature for up to 24 hours
Allowance of Y-site coadministration of reformulated Zosyn with aminoglycosides
The inactivation of aminoglycosides in the presence of penicillin-class drugs containing β-lactam rings has been recognized (CitationBenveniste and Davis 1973; CitationGlew and Pavuk 1983). However, amikacin and gentamicin have been shown to be compatible in vitro with reformulated Zosyn containing disodium EDTA supplied in vials or bulk pharmacy containers in certain diluents at specific doses and concentrations for a simultaneous Y-site infusion.
Simulated Y-site administration studies by Wyeth were conducted for reformulated Zosyn with amikacin or gentamicin in the concentration ranges and clinical doses described in the labels of Zosyn and each of the aminoglycosides. The simulated studies mimicked Y-site coadministration for Zosyn-amikacin systems and evaluated potency and degradation products by HPLC analyses ( and ). Reformulated Zosyn was shown to be compatible for simultaneous administration via a Y-site intravenous tube with amikacin in the concentration ranges of 2.25 g reformulated Zosyn/150 mL to 4.5 g/50 mL for Zosyn and 1.75 mg/mL to 7.5 mg/mL for amikacin in sterile water for injection, USP and 0.9% sodium chloride injection, USP, 5% dextrose in water for injection, USP and lactated Ringer’s injection, USP (). Degradation products and related compounds in the solution were quantified in a further study, which confirmed the first study’s conclusions, and showed that reformulated Zosyn (piperacillin/tazobactam) is compatible for Y-site coadministration with amikacin or gentamicin. US-sourced amikacin and gentamicin, evaluated here, represent the composition ranges of the products prescribed globally.
Table 3 Summary of drug potency results for simulated Y-site coadministration of reformulated Zosyn® with amikacin in compound sodium lactate intravenous infusion BP (Hartmann’s solution) at room temperature
Table 4 Degradation products of reformulated Zosyn® drug components in the presence of amikacin up to 4 hours at room temperature in compound sodium lactate intravenous infusion BP (Hartmann’s solution)
Table 5 Simulated Y-site coadministration of reformulated Zosyn® with amikacin in different admixture diluents (Potency of antibiotics at 4 hours)
The study designs used for Y-site compatibility and admixture stability are based on simulated Y-site injection testing procedures reported by CitationChoi and colleagues (1994) and by CitationTrissel and Martinez (1994a).
A follow-up simulated Y-site compatibility study, conducted by using an innovative NMR method, is supplementary to the previous study using an LC-MS method. The current study differs from the previous study in that it tested the simulated Y-site mixtures of reformulated Tazocin and gentamicin at shorter time intervals of 0, 10, 20, 30, 40, 50, and 60 minutes, while the previous study tested these mixtures at 0, 1, 2, and 4 hours. This change is to collect data at clinically realistic shorter time intervals, because the 4-hour testing time point represents an exaggerated contact time for the Y-site drug compatibility testing. In actual clinical practice, when two drugs are infused through a Y-site the maximum estimated contact time (prior to entering the blood stream) is short, often in the range of 15 minutes and not extending more than 60 minutes (CitationTrissel 1994b; CitationLeissing 1989). In this experiment to simulate Y-site administration, reformulated Tazocin was admixed in a (high dose, low dose) crossover with two gentamicin drug products sourced from Germany in a (high dose, low dose) matrix design, as shown in . The crossover pairings of reformulated Tazocin and gentamicin were based on the dose extremes expected in clinical use and were derived from the label dosing instructions listed on the package insert for each drug product.
Simulated Y-site stability results
The kinetic snapshot at every ten minutes over a period of 0 to 60 minutes of the mixed solutions analyzed by evaluating the –N-methyl proton resonance of the gentamicin molecule in a noninvasive manner at room temperature shows the following:
For both gentamicin products in all four combinations with reformulated Tazocin, the potency values of gentamicin at ten minutes are maintained at greater than 98% of the initial. As described earlier, the typical time for gentamicin and reformulated Tazocin to remain in contact during the actual Y-site co-administration in a clinical setting is about 15 minutes.
At 30 minutes and 60 minutes, better than 97% and 96% of the initial potency of gentamicin is maintained, respectively, even for the worst-case scenario of a solution mixture of “high” reformulated Tazocin and “low” gentamicin during a simulated Y-site administration. Based on the aminoglycoside and β-lactam interaction chemistry, the highest degradation of gentamicin was expected for this combination.
As expected, for the “high” gentamicin combination with “low” or “high” reformulated Tazocin, the gentamicin strength at 60 minutes was found to be greater than 98%.
Representative data for Y-site compatibility at clinically relevant concentration ranges of reformulated Tazocin combined with another German gentamicin commercial drug product (Ratiopharm®) are provided in . Data for the Rebofacin gentamicin product (not shown here) were found to be similar.
Table 6 Reformulated Tazocin® : Simulated Y-site compatibility by NMR for Ratiopharm® German gentamicin drug product
Particulate matter evaluation for reformulated Zosyn – amikacin or gentamicin (simulated Y-site study)
The subvisible particle counts for 10 μm and 25 μm size were determined using the HIAC method. The particulate counts at 0 and 4 hours for simulated Y-site mixtures of reformulated Zosyn and gentamicin or amikacin in admixtures of common commercial diluents are provided in and . The concentrations of reformulated Zosyn and gentamicin or amikacin were chosen to simulate clinically relevant dilutions in the admixture infused. The particulate counts up to the 4 hour test period for the reformulated Zosyn – amikacin system and reformulated Zosyn – gentamicin system were well within the current USP and EU Pharmacopeia specifications. Reformulated Zosyn was shown to be compatible for coadministration via a Y-site intravenous tube with gentamicin under the concentration ranges of 2.25 g reformulated Zosyn/150 mL to 4.5 g/100 mL for Zosyn and 0.7 mg/mL to 3.32 mg/mL for gentamicin in the 0.9% sodium chloride injection USP coadministration of Zosyn with 0.9% sodium chloride.
Table 7 Summary of subvisible particulate counts by HIAC for simulated Y-site coadministration of reformulated Zosyn® and amikacin
Table 8 Summary of subvisible particulate counts by HIAC for simulated Y-site coadministration of reformulated Zosyn® and gentamicin
Conclusions
In summary, the product quality enhancement provides expanded flexibility for the administration of reformulated Zosyn under variable clinical use conditions:
Reformulated Zosyn complies with USP-NF 30 <788>, European and Asia Pacific Pharmacopoeia specifications for particulate matter in injections under all clinical use conditions because it is tolerant to variability in pH and metal ion concentrations of commercial solutions used in the clinical setting. The variabilities of actual pH and metal ion concentrations in commercial IV solutions and diluents are unknown to the pharmacist and nurse end-user.
The product maintains chemical and physical stability under the conditions encountered in the clinical field of use where commercial diluents, such as 5% dextrose solution with potential variables of pH and leachable metal ions, are used for admixture preparation for parenteral administration. Regardless of the pH or zinc content of an admixture diluent or IV solution, reformulated Zosyn will maintain potency and lessen the level of particulates infused into patients.
The product is compatible with calcium-containing Ringer’s solutions (as lactate or acetate) or Hartmann’s solution.
The reformulated product provides the capability of simultaneous Y-site coadministration of amikacin (with 0.9% NaCl or 5% dextrose) and gentamicin (with 0.9% NaCl), without compromising either drug’s potency, and provides useful options for the administration of Zosyn, especially for the treatment of nosocomially-acquired pneumonia.
Based on the recent stability data, the expiration dating of reformulated Zosyn has been extended to 36 months from its previously reduced dating of 24 months.
References
- BandohKKatohMMutoYMetal induced degradation of β-lactamsChemotherapy1991393158
- BenvenisteRDavisJStructure activity relationships among the aminoglycoside antibiotics: role of hydroxyl and amino groupsAntimicrob Agents Chemother1973440294598613
- ChoiJSBurmJPJheeSSStability of piperacillin sodium-tazobactam sodium and ranitidine hydrochloride in 0.9% sodium chloride injection during simulated Y-site administrationAm J Hosp Pharm199451227367801988
- DesaiNRVencl-JoncicMKoczoneKScreening of Zinc in commercial intravenous diluents commonly used for reconstitution and admixing of injectable dosage forms, and flushing of administration devices [poster]Am Pharm Assoc Ann Meet (Atlanta, GA)2007a167
- DesaiNRShahSMKoczoneKZinc content of commercial diluents widely used in drug admixtures prepared for intravenous infusionInt J Pharma Compound2007b114226432
- European PharmacopeiaParticulate contamination: Subvisible particles20042535 5.0(2004).2.9.19
- FalchukKHPetersonLMcNealJMicroparticulate induced phlebitis: Its prevention by in-line filtrationN Eng J Med19853127882
- GlewRHPavukRStability of gentamicin, tobramicin and amikacin in combination with four β-lactam antibioticsAntimicrob Agents Chemother19832447476651276
- JenkeDRLinking extractables and leachables in container/closure applicationsDA J Pharm Sci Technol20055926581
- LehrH-ABrunnerJRangoonwalaRParticulate matter contamination of intravenous antibiotics aggravates loss of functional capillary density in post ischemic striated muscleAm J Respir Crit Care Med20021655142011850345
- LeissingNCStoryKOZaskeDInline fluid dynamics in piggyback and manifold drug delivery systemsAm J Hosp Pharm19894689972712034
- LongeRLParticulate contamination in selected parenteral drugsCan Anesthesic Soc J198027624
- NathNMcNealEObenhuberDParticulate contaminants of intravenous medication and the limits set by USP General Chapter <788>Pharmacopeial Forum200430227280
- TrisselLAMartinezJFCompatibility of piperacillin sodium plus tazobactam with selected drugs during simulated Y-site injectionAm J Hosp Pharm1994a5167288203388
- TrisselLAHandbook of Injectable Drugs1994b5Bethesda, MD, USAAmerican Society of Health-System Pharmacists®XVIII
- [USP] United States PharmacopeiaUSP 30, Supplement 2. <788>, Particulate matter in injections2007
- WinterWDeubnerRHolzgrabeUMultivariate analysis of nuclear magnetic resonance data-characterization of critical drug substance quality of gentamicin sulfateJ Pharm Biomed Anal200538833916087045
- Wyeth PharmaceuticalsZosyn® (Piperacillin/Tazobactam For Injection USP [Glass Vials]) United States Prescribing Information2007a
- Wyeth PharmaceuticalsZosyn® (Piperacillin/Tazobactam For Injection USP [Galaxy® Bags]) United States Prescribing Information2007b