Abstract
The incidence of invasive fungal infections, especially those due to Aspergillus spp. and Candida spp., continues to increase. Despite advances in medical practice, the associated mortality from these infections continues to be substantial. The echinocandin antifungals provide clinicians with another treatment option for serious fungal infections. These agents possess a completely novel mechanism of action, are relatively well-tolerated, and have a low potential for serious drug–drug interactions. At the present time, the echinocandins are an option for the treatment of infections due Candida spp (such as esophageal candidiasis, invasive candidiasis, and candidemia). In addition, caspofungin is a viable option for the treatment of refractory aspergillosis. Although micafungin is not Food and Drug Administration-approved for this indication, recent data suggests that it may also be effective. Finally, caspofungin- or micafungin-containing combination therapy should be a consideration for the treatment of severe infections due to Aspergillus spp. Although the echinocandins share many common properties, data regarding their differences are emerging at a rapid pace. Anidulafungin exhibits a unique pharmacokinetic profile, and limited cases have shown a potential far activity in isolates with increased minimum inhibitory concentrations to caspofungin and micafungin. Caspofungin appears to have a slightly higher incidence of side effects and potential for drug–drug interactions. This, combined with some evidence of decreasing susceptibility among some strains of Candida, may lessen its future utility. However, one must take these findings in the context of substantially more data and use with caspofungin compared with the other agents. Micafungin appears to be very similar to caspofungin, with very few obvious differences between the two agents.
Introduction
Despite advances in medical practice, the incidence of invasive fungal infections has increased over the past 2 decades, such that Candida species are now the 4th most prevalent causative agent of nosocomial bloodstream infections (CitationWisplinghoff et al 2004), and infections caused by Aspergillus spp. are also increasing rapidly. CitationChandrasekar et al (2001) reported a steady increase in the frequency of clinical isolates of Aspergillus spp. from 1994 to 1999. Presumably, these trends are due to an increasing population of patients at risk for fungal infections, including patients with AIDS, solid organ and hematopoietic stem cell transplant recipients, and other patients at risk for immunosuppression (CitationClark and Hajjen 2002).
Over the past 10 years, a shift has occurred in the species of Candida causing bloodstream infections. According to national intensive care unit (ICU) data (from the National Nosocomial Infections Surveillance [NNIS] system), the percentage of fungal bloodstream isolates (BSI) due to C. albicans significantly decreased from 1989-1999, while the percentage of fungal BSI due to C. glabrata significantly increased (CitationTrick et al 2002). In a recent multicenter observational study, non-albicans species constituted ~50% of bloodstream isolates, while C. glabrata was implicated in 21% of adult bloodstream isolates (CitationClark and Hajjen 2002; CitationPappas et al 2003).
Despite the introduction of newer, more potent antifungal agents, mortality due to fungal infections remains high. The attributable mortality due to nosocomial Candida bloodstream infections at one institution was not significantly different, when compared in two studies conducted 15 years apart (38% in 1983–1986 versus 49% in 1997–2001) (CitationGudlaugsson et al 2003). Meanwhile, mortality associated with invasive Aspergillus approaches 100% in some patient populations, including bone marrow transplant recipients (CitationLin et al 2001; CitationMcNeil et al 2001).
Fortunately, there has been a recent surge in antifungal drug development. Triazole agents with broad spectrums of action have arrived (voriconazole) and several more are in development. Some of these agents have expanded the azole spectrum of action to include fungi of the class Zygomycetes (posaconazole). The liposomal polyenes have combined the efficacy of amphotericin B with a decreased incidence and severity of side effects. Possibly most importantly, compounds with a completely novel mechanism of action have arrived, the echinocandins (caspofungin, micafungin, and anidulafungin).
Pharmacology
Chemistry
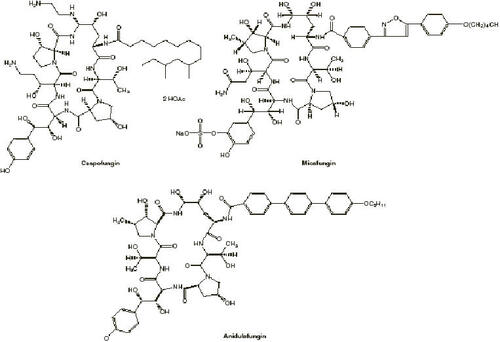
The echinocandins are semisynthetic lipopeptides produced via chemical modification of natural products of fungi: caspofungin from pneumocandin B0 from Glarea lozoyensis, micafungin from the hexapeptide FR901370 from Coleophoma empedra, and anidulafungin from echinocandin B0 from A. nidulans, respectively (CitationCarver 2004; CitationMurdoch and Plosker 2004; CitationCancidas PI 2005).
Chemical structures of the currently Food and Drug Administration (FDA)-approved echinocandins are provided in (CitationBoucher et al 2004). Echinocandins are cyclic hexapeptides with N-linked acyl lipid side chains and molecular weights of approximately 1200 (CitationAbruzzo et al 1997; CitationMikamo et al 2000). Early investigation revealed that the position and conformation of this N-linked acyl side chain is crucial to the antifungal activity of the echinocandins. Interestingly, these fatty side chains were also implicated as a cause of hemolysis in early echinocandin compounds; subsequent modifications led to compounds with potent antifungal activity without hemolytic effects (CitationKlein and Li 1999).
Figure 1 Structures of echinocandins. Copyright © 2004. Reproduced with permission from CitationBoucher HW, Groll AH, Chiou CC, et al. 2004. Newer systemic antifungal agents: pharmacokinetics, safety and efficacy. Drugs, 64:1997-2020.
Mechanism of action of echinocandins
The echinocandins are concentration-dependent, noncompetitive inhibitors of 1,3-β- and 1,6-β-D-glucan synthase, an enzyme complex composed of two subunits, encoded by the genes FKS1 and FKS2. Glucan synthase is involved in the synthesis of 1,3-β-D-glucan, a polysaccharide composed of 3 helically-entwined polymers of glucose. Despite substantial work to elucidate the precise location where echinocandins bind to the glucan synthase enzyme complex, this question remains unresolved. Glucan is an essential carbohydrate component of all fungal cell walls, comprising 30%–60% of the fungal cell wall in Candida and Saccharomyces. Changes in the characteristics of the cell wall can lead to osmotic instability and eventual cell lysis. Importantly, human cells do not contain 1,3-β-D-glucan, thus avoiding direct human cell toxicity (CitationKlein and Li 1999; CitationDeresinski and Stevens 2003; CitationCarver 2004; CitationStevens et al 2006).
The proportion of the fungal cell wall composed of glucan varies widely between different species of fungi. 1,3-β-D-glucan is more predominant in the cell walls of Candida and Aspergillus species (especially C. albicans and A. fumigatus) than in yeast forms of dimorphic fungi. Likewise, the cell walls of mycelial forms of Histoplasma capsulatum, Blastomyces dermatitidis, and Paracoccidioides braziliensis contain significant amounts of 1,3-β-D-glucan, while zygomycetes lack this target component. However, these characteristics do not always predict echinocandin activity. For example, the cell wall of Cryptococcus neoformans contains 1,3-β-D-glucan but the echinocandins demonstrate little activity against this pathogen. This suggests that there are likely additional (or alternate) components of the mechanism of action of the echinocandins (CitationFeldmesser et al 2000; CitationMaligie and Selitrennikoff 2005).
In vitro, caspofungin is fungicidal against C. albicans, causing lysis of growing and metabolically active cells (CitationBartizal et al 1997). However, its activity against Aspergillus is more complex: although caspofungin does not reduce the number of colony-forming units (CFU) of A. fumigatus, it causes significant injury, and perhaps lysis, to the hyphal tips of actively growing cells. It is postulated that this may prevent the organism from spreading beyond the initial site of infection. As such, caspofungin is considered fungistatic against Aspergillus, (as are the azole antifungals, although some in vitro work suggests that voriconazole may be fungicidal as well) in contrast to amphotericin B, which is fungicidal (CitationKrishnan et al 2005). The clinical significance of these in vitro differences is not clear (CitationBowman et al 2002). However, a recent in vitro study in which phagocytic cells were combined with micafungin provides a potential (and logical) explanation for the confusing lack of correlation between in vitro and in vivo activity of echinocandins. In vitro combination of micafungin and phagocytes results in greater inhibition of A. fumigatus growth than either micafungin or various phagocytes by themselves (CitationChoi et al 2004). Another study further demonstrated that pretreatment of polymorphonuclear leukocytes (PMNs) with granulocyte-macrophage colony-stimulating-factor (GM-CSF) increased the efficacy of the drug–immune component combination, resulting in synergy (CitationGil-Lamaignere et al 2004).
Biofilms often develop on prosthetic devices (eg, catheters), and Candida species, especially C. albicans and C. parapsilosis, are often implicated as causative agents. Biofilms develop in three phases: an early phase in which fungi attach to the surface of the device; an intermediate phase where blastospores aggregate to produce an extracellular matrix; and a maturation phase, during which fungi are incorporated into the matrix (CitationCocuaud et al 2005). In 96-well biofilm models, echinocandins, unlike azoles, appear to have potent activity against preformed Candida-related biofilms. In addition, caspofungin prevents adherence of C. albicans to epithelial cells, inhibiting the early phase of development (CitationBachmann, VandeWalle, et al 2002; CitationSoustre et al 2004). In silicone elastomer models of biofilm infection, caspofungin and micafungin are effective in killing C. albicans and C. parapsilosis biofilm cells (sometimes >99% of cells, even at concentrations achieved in vivo). Only amphotericin B lipid complex and liposomal amphotericin B express similar activity (CitationKuhn et al 2002). Interestingly, in the 96-well biofilm model, no evidence of synergy or additive effects were observed when fluconazole, amphotericin B, and caspofungin were used in combination against C. albicans biofilms (CitationBachmann et al 2003). These are important developments, since biofilm-related infection and colonization are very difficult to eradicate with azole antifungal therapy. Often, removal of the infected device is required (CitationRamage et al 2002). However, whether these in vitro models of biofilm infection are applicable to the clinical setting is unknown, but merit further investigation for the therapy of biofilm-associated infections.
Spectrum of activity
Comparison of in vitro activity of echinocandins
Minimum inhibitory concentration (MIC) values should be interpreted cautiously as standardized methods to test the susceptibility of echinocandins have yet to be developed. Interpretive breakpoints, which help guide the clinician to make a correlation between in vitro susceptibility and in vivo outcomes, are not yet available and will require validation in clinical trials (CitationCarver 2004). In vitro MIC studies with echinocandins have employed multiple methodologies, including differing inoculae, composition and pH of media, reading times (24 vs 48 hours), determination of MIC (“prominent” vs 80% or 90% inhibition of growth), and growth forms (yeast vs mycelium form). CitationKurtz and colleagues (1994) proposed the use of the minimum effective concentration (MEC) as a new endpoint of morphologic change for testing of caspofungin and other echinocandins. The MEC is defined as the lowest concentration of drug that cases the formation of microcolonies of the organism, or the lowest concentration at which a prominent decrease in turbidity occurs compared with the growth control (CitationKurtz et al 1994, CitationCarver 2004)
As a class, the echinocandins demonstrate excellent activity against Candida spp. All 3 agents display higher MICs for C. parapsilosis, C. lusitaniae, and C. guilliermondii compared with other Candida species (CitationPfaller, Boyken, et al 2005; CitationPfaller et al 2006). However, at this time, little correlation can be made between MICs and clinical outcomes with the echinocandins. In fact, a recent study showed that patients with Candida isolates displaying higher MICs (>2 μg/mL) had superior clinical outcomes compared with those with isolates displaying MICs <1 μg/mL (CitationKartsonis, Killar, et al 2005). A study which attempted to determine whether the in vitro MIC discrepancies between different Candida species (with C. albicans displaying significantly lower MICs than C. parapsilosis and C. guilliermondii) translated to differences in an in vivo murine kidney infection model found conflicting results. The isolate of C. parapsilosis that displayed the highest MIC to caspofungin responded in vivo to the lowest dose (1 mg/kg/day). Conversely, the two isolates with the lowest MICs only responded in vivo to the highest dose (5 mg/kg/day). Predictably, however, the overall reduction of fungal burden was 100-fold less in the models infected with non-albicans isolates (CitationBarchiesi et al 2006). The echinocandins demonstrate excellent in vitro activity against Aspergillus spp. An interesting component of the spectrum of the echinocandins is their in vitro activity against Pneumocystis carinii (P. jerovici) (CitationIto et al 2000; CitationAnonymous 2003; CitationPacetti and Gelone 2003). Interestingly, the echinocandins were originally developed in part because of their efficacy against Pneumocystis (CitationAnonymous 2003; CitationDenning 2003; CitationCarver 2004). The echinocandins do not possess activity against the zygomycetes, Fusarium solani, Scedosporium prolificans, Cryptococcus spp, or Trichosporon spp. (CitationZaas and Alexander 2005). Although caspofungin, like other echinocandins, has minimal in vitro activity against the agents of mucormycosis when tested in vitro by standard techniques, the accuracy of current in vitro testing against molds remains unclear. R. oryzae expresses the target enzyme for echinocandins, and in the murine model of disseminated mucormycosis, caspofungin displayed limited activity against R. oryzae and the combination of caspofungin (1 mg/kg/day) plus amphotericin B lipid complex (5 mg/kg/day) was synergistic and resulted in significantly improved survival versus either therapy used alone. Nevertheless, clinical experience with echinocandins for these infections are limited (CitationSpellberg et al 2005). While the echinocandins display activity against the mycelial forms of endemic fungi such as Histoplasma spp., Blastomyces spp., and Coccidioides spp., they display significantly higher MIC values against the yeast forms of these organisms (CitationWiederhold, Graybill, et al 2005). Finally, caspofungin was found to inhibit the growth (via 1,3-β-D-glucan synthase inhibition) of several rare molds, including Alternaria sp., Curvularia sp., Scedosporium apiospermum and prolificans, Acremonium sp., Bipolaris sp., and Trichoderma sp. (CitationKahn et al 2006).
Susceptibility breakpoints have not been determined for the echinocandins. In general, the echinocardins display MIC90 values (MIC required to inhibit growth of 90% of organisms) of ≤2 μg/mL against Candida spp. A review by CitationZaas and Alexander (2005) succinctly compares the MIC values of the echinocandins, and illustrates that in general, anidulafungin displays the lowest MIC values against most Candida spp., followed by micafungin and then caspofungin.
Anidulafungin displays low MICs against strains of C. parapsilosis with high MICs to caspofungin and micafungin and limited cases have shown a potential for activity in isolates with increased MICs to caspofungin and micafungin (CitationGhannoum et al 2005). In addition, recent data reveals that isolates of C. glabrata with elevated MICs to caspofungin (8 μg/mL to 64 μg/mL) still display low MICs to anidulafungin (2 μg/mL to 4 μg/mL) (CitationWiederhold, Graybill, et al 2005). These results are interesting, and suggest that anidulafungin may be more potent against certain resistant Candida isolates when compared with the other echinocandins. However, these results must be corroborated with clinical data due to the lack of correlation of MICs with treatment outcomes. In addition, the presence of human serum decreases the in vitro potency of all the echinocandins and neutralizes the in vitro MIC superiority of micafungin over caspofungin (CitationPark et al 2006).
Resistance to echinocandins
Although spontaneous resistance of C. albicans to echinocandins has been documented in vitro, the specific mechanisms of resistance have not been fully elucidated and prospective worldwide surveillance of clinical Candida isolates has revealed no evidence of emerging caspofungin resistance (CitationKurtz et al 1996; CitationPfaller et al 2006). The molecular target of echinocandins is the FKS1 subunit of glucan synthase; predictably, mutations to this site confer varying degrees of resistance to the echinocandins (CitationDouglas et al 1997). Recently, in vitro analysis of spontaneous mutants of C. albicans has implicated this mechanism as an important determinant of resistance (CitationBalashov et al 2006). In addition, analysis of a C. albicans strain (MIC=8 mg/L) in a patient with recurrent esophagitis revealed a single amino acid substitution (serine-to-proline) at position 645 of the FKS1 gene (CitationMiller et al 2006). However, CitationHakki and colleagues (2006) recently published a case of C. krusei endophthalmitis and oropharyngeal candidiasis in which the isolate displayed reduced susceptibility to all three echinocandins, and was not mediated by a mutation of FKS1. In addition, recent analysis of C. albicans isolates with reduced echinocandin susceptibility found that overexpression of the RER1 (Regulator of Echinocandin Resistance) gene conferred resistance through an unclear pathway (CitationKetko et al 2006).
Converse to the echinocandins, azole antifungals block synthesis of lanosterol demethylase, which is encoded by ERG11. Alteration in this gene may confer decreased susceptibility to azoles. Upregulation of multidrug efflux pumps coded for by multidrug resistance (MDR) or Candida drug resistance (CDR) genes may also confer decreased azole activity. Theoretically, caspofungin activity should not be affected by these mechanisms of azole resistance. Indeed, CitationBachmann, Patterson, and colleagues (2002) demonstrated that caspofungin was highly active against 32 isolates of both fluconazole susceptible- and resistant-C. albicans, including those possessing alterations of the ERG11 and/or CDR/MDR genes. However, overexpression of CDR2 efflux can result in increased MICs to echinocandins (CitationShuetzer-Muehlbauer et al 2003). In C. glabrata, upregulation of CDR1 pumps does not confer reduced susceptibility to caspofungin (CitationKatiyar et al 2005). Finally, CitationPaderu and colleagues (2004) have shown that a high-affinity, saturable, facilitated-diffusion transporter mediates caspofungin entry into C. albicans. Disruption of this system represents a potential mechanism of resistance.
Resistance mechanisms have also been elucidated for several other fungal organisms. Overexpression of Sbe2p, a Golgi protein which is involved in cell wall construction, may confer resistance to caspofungin in Saccharomyces cerevisiae (CitationOsherov et al 2002). In vitro resistance to A. fumigatus has been documented, with one strain demonstrating a target-site mutation which conferred low-level resistance. Another strain, deemed resistant, in fact demonstrated an “Eagle-like” effect (described below) (CitationGardiner et al 2005). Finally, as mentioned previously, echinocandins do not display in vitro or in vivo activity against Cryptococcus neoformans. CitationMaligie and colleagues (2005) attempted to determine if the mechanism of this lack of effect was due to 1,3-β-D-glucan synthase resistance to echinocandins. Surprisingly, the authors found that the synthase enzyme was sensitive to caspofungin, although less so than for C. albicans. As such, the mechanism of Cryptococcus neoformans resistance to echinocandins is still unknown (CitationMaligie and Selitrennikoff 2005).
Several case reports have documented echinocandinresistant Candida spp. in the clinical setting. An AIDS patient with thrush and esophagitis (due to C. albicans) refractory to therapy with fluconazole and amphotericin B lipid complex initially responded to caspofungin monotherapy, but when therapy was discontinued, the infection returned. Subsequent therapy with caspofungin eventually failed. Serial isolates demonstrated caspofungin MICs of 0.25 mg/mL and >64 mg/mL in the first and final isolates, respectively. Although the utility of MIC values with caspofungin have been questioned, this case nevertheless presents an intriguing picture (CitationHernandez et al 2004). In a second case, a patient with prosthetic valve endocarditis due to C. parapsilosis was initially treated with amphotericin B (0.7 mg/kg/day) + flucytosine for 7 days; therapy was then changed to caspofungin + intravenous fluconazole. The initial fungal isolate displayed low MICs to caspofungin and anidulafungin (2 μg/mL and 1 μg/mL, respectively), but a high MIC (8 mg/mL) to micafungin. After 6 weeks of therapy and sterile blood cultures, the patient was discharged on chronic, suppressive therapy with oral fluconazole. The patient was re-admitted 3 months later with C. parapsilosis which was resistant to fluconazole and voriconazole, had high MICs to caspofungin and micafungin (>16 mg/mL), yet retained activity to anidulafungin and amphotericin B. This case raises many questions about echinocandin resistance that remain unanswered. Especially intriguing is whether resistance to certain echinocandins confer cross-resistance to others or to the entire class, and whether azole resistance is mediated via mechanisms that may result in reduced susceptibility to echinocandins (CitationMoudgal et al 2005). In addition, this case, combined with newer data, raises the question of whether anidulafungin has superior activity against C. parapsilosis. In a recent study, researchers examined several caspofungin-resistant isolates of C. parapsilosis from patients in a burn unit. The investigators found that certain isolates displayed MICs of 64 μg/mL to caspofungin and micafungin, yet displayed MICs of 1–2 μg/mL to anidulafungin. In addition, treatment with caspofungin did not affect the cellular structure, while treatment with anidulafungin caused cell lysis (CitationGhannoum et al 2005).
Finally, an interesting “Eagle-like” effect has been observed in vitro with caspofungin, whereby higher drug concentrations do not result in a greater degree of killing when compared with lower drug concentrations. When exposed to therapeutic concentrations of caspofungin, growth of C. albicans ceases. Paradoxically, exposure to supratherapeutic concentrations of caspofungin results in significant growth. This can occur either dramatically, in which the number of colonies is too numerous to count, or more subtly, where seemingly only a small number of cells are able to survive (the so-called “mini-paradoxical effect”). However, increasing the concentration even further results in cessation of growth, suggesting that the resistance can eventually be overcome. Investigators hypothesize that the phenomenon may be due to selection of derepressed resistance mechanisms, which require high concentrations of drug to “turn-on.” Interestingly, when isolates which illustrated this effect with caspofungin were tested against micafungin and anidulafungin, the “Eagle-like” effect was not observed (CitationStevens et al 2004, Citation2005). Subsequent studies support the hypothesis that when C. albicans is exposed to high caspofungin concentrations, genes encoding chitin, a key cell wall polymer which is not targeted by caspofungin, are rapidly and transiently induced to compensate for the decreased synthesis of β-1,3- and β-1,6-glucan. Interestingly, it appears that the presence of a calcineurin inhibitor such as cyclosporine, inhibits this effect (CitationWiederhold, Kontoyiannis, et al 2005; CitationStevens et al 2006).
In in vivo models, the “Eagle-like” effect was observed in a rabbit model of invasive pulmonary aspergillosis, where caspofungin doses ≥3 mg/kg/day were associated with poorer survival (CitationPetraitiene et al 2002). In a murine model of invasive pulmonary aspergillosis, a concentration-dependent reduction in mean pulmonary fungal burden was observed following single intraperitoneal doses of caspofungin of 0.25 mg/kg, 1.0 mg/kg, and 4.0 mg/kg. When mice were treated for 96 hours with the same dosages fractionated into 3 different dosing intervals (every 6, 24, or 48 hours), a concentration-dependent reduction in mean pulmonary fungal burden was observed in the 1mg/kg dosage-fractionation group, with significantly lower mean pulmonary fungal burden in mice dosed every 48 hours versus every 6 hours. However, a paradoxical increase in pulmonary fungal burden was observed in the highest dosage-fractionation group (CitationWiederhold et al 2004). However, recent studies offer conflicting results. Addition of mouse serum to an in vitro model eliminated the paradoxical effect (CitationAbruzzo et al 2005). In a study assessing whether the in vitro effect observed with several isolates could be duplicated in an in vivo mouse model, no paradoxical effect was observed following administration of doses ranging from 0.01 mg/kg to 20 mg/kg, no paradoxical effect was observed. However, a type of “cap effect” was seen in certain isolates, whereby increasing the dose higher than 0.5 mg/kg did not result in increased killing (CitationClemons et al 2006).
At this time, many questions remain regarding the mechanisms of resistance to the echinocandin antifungals. Fortunately, the echinocandins have proven to be worthy options in the treatment of azole-resistant Candida infections (further highlighted in clinical studies presented below), and clinical resistance remains a rare occurrence. However, clinical examples do raise interesting questions regarding cross-resistance among the echinocandins, and the potential role of anidulafungin in resistant isolates. Again, however, it is important to stress that differences based on echinocandin MICs should be interpreted with caution. Finally, it is unknown whether the interesting “Eagle-like” effect observed in some in vitro models translates into a meaningful clinical effect as human studies have not been performed. However, supratherapeutic plasma concentrations utilized in in vitro studies have been observed in pharmacokinetic studies in healthy volunteers, and may be expected to occur in patients if larger doses of echinocandins are employed in future studies.
Pharmacokinetics of echinocandins
As a class, the echinocandins possess many pharmacokinetic similarities, including low oral bioavailability, high protein binding, and relatively low CSF and urine concentrations of parent drug (). Since urine concentrations of echinocandins (or active metabolites) are minimal, their clinical utility in treating urinary infections may be poor. One study reviewed the response of 12 patients with Candida urinary tract infections enrolled in 2 caspofungin trials. The number of patients who responded favorably (11/12) appears impressive. However, the limitations of the study (retrospective, small patient numbers), compounded by the difficulty in assessing true drug effect (since many fungal urinary tract infections resolve without therapy), limit the clinical utility of this data (CitationKartsonis et al 2003). All echinocandins display linear pharmacokinetics following administration of intravenous dosages, and are degraded primarily by the liver (also in the adrenals and spleen) by hydrolysis and N-acetylation (CitationDenning 2003). Following initial distribution, echinocandins are taken up by red blood cells (micafungin) and the liver (caspofungin and micafungin) where they undergo slow degradation to mainly inactive metabolites, although two uncommon metabolites of micafungin possess antifungal activity. Degradation products are excreted slowly over many days, primarily via the bile (CitationDenning 2003). Fecal recovery data with radiolabeled micafungin in healthy subjects, demonstrate that approximately 40% of a 28mg dose is eliminated as parent drug and metabolites in the bile. In an interesting report of the successful treatment of a patient with candidal cholangitis with caspofungin, the biliary concentration of caspofungin was ~30% that of serum (CitationGoicoechea et al 2004). This finding is not surprising considering the evidence that caspofungin utilizes the OATP-1B1 transporter, which also transports bile, rifampin, and cyclosporine (CitationSandhu et al 2005). Although it is not known whether micafungin or anidulafungin also utilizes this pathway, it appears unlikely since they interact less with cyclosporine than does caspofungin (Citationvan Burik et al 2004; CitationDowell et al 2005). None of the echinocandins serve as substrates, inducers, or inhibitors of cytochrome P450 enzymes, or the P-glycoprotein transport system (CitationDenning 2003).
Table 1 Pharmacokinetic parameters of echinocandins in adult subjects (CitationDenning 2003; CitationDeresinski and Stevens 2003;CitationWiederhold and Lewis 2003; CitationCarver 2004; CitationMurdoch and Plosker 2004; CitationRaasch 2004; CitationZaas and Alexander 2005)
Echinocandins are available only as parenteral formulations, are not dialyzable, and do not require dosage adjustment in patients with renal insufficiency. They have minimal cerebrospinal fluid (CSF) penetration, largely due to their high protein binding and large molecular weights, although the clinical relevance of these findings may be disputed, given that several other antifungal agents (amphotericin B and itraconazole) are effective for the treatment of fungal meningitis despite low CSF concentrations (CitationDenning 2003). However, one case report documents failure of caspofungin in the treatment of Candida endopthalmitis, presumably due to undetectable intravitreous concentrations of caspofungin. The patient was subsequently cured after therapy with 5 mg/kg/day amphotericin B lipid complex (CitationGauthier et al 2005).
Interesting disparities do exist between the agents, however. Among the echinocandins, anidulafungin is unique in being eliminated almost exclusively by slow chemical degradation rather than undergoing hepatic metabolism. Anidulafungin has a lower maximum concentration (Cmax) and degree of protein binding, and much longer half-life and larger volume of distribution than the other two agents (CitationRaasch 2004).
Pharmacokinetics of echinocandins in special populations
Renal insufficiency
As mentioned above, the echinocandins do not require dosage adjustment in patients with renal insufficiency.
Hepatic insufficiency
The echinocandins have been studied in patients with varying degrees of hepatic dysfunction. The area under the concentration-time curve (AUC) of caspofungin is significantly increased in patients with moderate (Child-Pugh 7–9) hepatic insufficiency (CitationStone et al 2001; CitationCancidas PI 2005). By contrast, the AUC of micafungin is decreased in patients with moderate insufficiency; this is likely to be due to an increased volume of distribution and lower protein binding in these populations (CitationHebert, Smith, et al 2005; CitationMycamine PI 2005). Anidulafungin concentrations were not increased in subjects with mild (Child-Pugh 5–6), moderate, or severe (Child-Pugh >9) hepatic insufficiency. Though a slight decrease in AUC was observed in patients with severe hepatic insufficiency, it was within the range of population estimates noted for healthy subjects (CitationEraxis PI 2006).
Thus, it is suggested that the maintenance dose of caspofungin be decreased from 50 mg to 35 mg daily in patients with moderate hepatic insufficiency (CitationCancidas PI 2005). In patients receiving micafungin, dosage adjustments are not recommended for patients with moderate hepatic dysfunction (CitationMycamine PI 2005). Dosage adjustments are not suggested for patients with mild, moderate, or severe hepatic dysfunction who are receiving anidulafungin (CitationEraxis PI 2006). Presently, as there is limited experience with caspofungin and micafungin in patients with severe hepatic insufficiency, recommendations for dosage adjustments cannot be made at this time (CitationCancidas PI 2005; CitationMycamine PI 2005).
Pediatrics
Caspofungin
Limited information is available regarding the use of caspofungin in pediatric patients. The pharmacokinetics of caspofungin in 39 children (2–11 years old) and adolescents (12–17 years old) with neutropenia were compared with those of adults who had received 50 mg or 70 mg daily for mucosal candidiasis. After multiple doses, weight-based dosing (1 mg/kg/day) in children (only two adolescents were studied in the 1 mg/kg/day group) resulted in significantly lower plasma concentrations compared with those achieved in adults. However, in both children and adolescents, dosing based on body surface area (50 mg/m2/day) resulted in an area under the plasma concentration-time curve at steady state (AUCss) similar to those achieved in adults receiving 50mg daily (CitationWalsh et al 2005). In addition, a recent study evaluating caspofungin therapy in 6 neonates with invasive candidiasis revealed that doses of 2 mg/kg/day (or 25 mg/m2/day) resulted in similar plasma concentrations as those in adults receiving 50 mg daily (CitationOdio et al 2005).
Micafungin
In a Phase I, sequential group dose-escalation study in pediatric patients, a 1.3- to 1.5-fold increase in the clearance of micafungin was noted in patients 2–8 years of age. As such, they recommended that a dosage of 1.5 times that of the adult dosage be utilized in this population (CitationSeibel et al 2005).
Anidulafungin
The pharmacokinetics of anidulafungin after daily doses were investigated in immunocompromised pediatric (2–11 years) and adolescent (12–17 years) patients with neutropenia. Steady state plasma concentrations were achieved on the first day after administration of the loading dose (twice the maintenance dose). Cmax and AUCss increased in a dose proportional manner. Concentrations and exposures following administration of maintenance doses of 0.75 mg/kg/day and 1.5 mg/kg/day were similar to those observed in adults following maintenance doses of 50 mg/day and 100 mg/day, respectively (CitationEraxis PI 2006).
Nursing mothers
Caspofungin, micafungin, and anidulafungin can be found in the milk of lactating, drug-treated rats; it is not known whether they are excreted in human milk. Caution should be exercised when echinocandins are administered to a nursing woman (CitationMycamine PI 2005; CitationCancidas PI 2005; CitationEraxis PI 2006).
Pregnancy
The echinocandins are all categorized as Pregnancy Category C. Anidulafungin and caspofungin cross the placental barrier in rats and are detected in fetal plasma. There are no adequate and well-controlled studies in pregnant women; thus, echinocandins should be used only if the potential benefit justifies the risk to the fetus (CitationMycamine PI 2005; CitationCancidas PI 2005; CitationEraxis PI 2006).
Geriatric use
Dosage adjustments are not required for geriatric patients receiving echinocandins (CitationMycamine PI 2005; CitationCancidas PI 2005; CitationEraxis PI 2006). In clinical studies of micafungin, a total of 186 subjects were 65 years of age and older, and 41 subjects were 75 years of age and older. The exposure and disposition of a 50mg micafungin dose administered as a single 1 hour infusion to 10 healthy subjects aged 66–78 years were not significantly different from those in 10 healthy subjects aged 20–24 years. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
Plasma concentrations of caspofungin in healthy older men and women (≥65 years of age) were increased slightly (approximately 28% in AUC) compared with young healthy men. A similar effect of age on pharmacokinetics was seen in patients with candidemia or other Candida infections (intra-abdominal abscesses, peritonitis, or pleural space infections). No dose adjustment is recommended for the elderly; however, greater sensitivity of some older individuals cannot be ruled out.
In population pharmacokinetic analyses of anidulafungin, the median clearance differed slightly between the elderly group (patients ≥65, median clearance (CL)=1.07 L/h) and the nonelderly group (patients <65, median CL=1.22 L/h); however, the range of clearance was similar.
Race and gender
The pharmacokinetics of echinocandins are similar among Caucasians, Blacks, Asians, and Hispanics. Dosage adjustments are not required based on race (CitationCancidas PI 2005; CitationMycamine PI 2005; CitationEraxis PI 2006).
Pharmacodynamics of echinocandins
The echinocandins exhibit concentration-dependent killing. In a murine model of systemic candidiasis, the pharmacodynamic parameter that predicted efficacy with caspofungin was the ratio of the area under the curve to the minimal inhibitory concentration (AUC/MIC) (CitationLouie et al 2005). Caspofungin efficacy against C. albicans persists even after serum concentrations fell below the MIC, suggesting that caspofungin concentrations in tissues remain high even after serum concentrations have declined (or that caspofungin displays a significant post-antifungal effect) (CitationLouie et al 2005). In fact, a study utilizing time-kill experiments found that when Candida is exposed to caspofungin for only one hour (followed by drug washout), virtually equivalent killing is achieved as when compared with caspofungin exposure for 24 hours. In addition, Candida growth is inhibited for at least 24 hours following drug washout (CitationClancy et al 2006). In another animal model with an experimental glucan synthase inhibitor, HMR 3270, antifungal efficacy was predicted by the ratio of the Cmax to the MIC (Cmax:MIC). A Cmax:MIC of 3 resulted in fungistatic activity but fungicidal activity was not observed until the Cmax:MIC ratio approached 10 (CitationAndes et al 2003). In the murine model of invasive pulmonary aspergillosis described above (CitationWiederhold et al 2004), Cmax:MEC was the parameter most closely associated with the reduction of pulmonary fungal burden. Finally, the activity of anidulafungin against C. albicans and glabrata in a neutropenic murine disseminated candidiasis model was found to be greatest when administered as large, infrequent doses (CitationAndes and Marchillo 2006).
Drug interactions with echinocandins
The echinocandins are not appreciable substrates, inhibitors, or inducers of cytochrome P450, nor do they interact with P-glycoprotein, as do some of the triazole antifungals. Although micafungin is a substrate for and a weak inhibitor of CYP3A in vitro, hydroxylation by CYP3A is not a major pathway for micafungin metabolism in vivo. (CitationMycamine PI 2005; CitationSakaeda et al 2005). As such, as a class, echinocandins are expected to demonstrate a low capability for producing drug–drug interactions. This has generally proven true. No effect on cyclosporine metabolism was noticed in vitro, and an in vivo study evaluating a potential interaction between cyclosporine and anidulafungin revealed only a clinically insignificant 22% increase in the AUC of anidulafungin following 4 days of concomitant cyclosporine therapy (CitationDowell et al 2005). Concurrent administration of rifampin or a variety of other (204 substrates, 140 inhibitors, and 40 inducers of CYP450) agents with anidulafungin does not affect the clearance of anidulafungin (CitationDowell et al 2004).
Several studies have evaluated the interaction of micafungin with various immunosuppressants. Although micafungin does not significantly affect the clearance (or AUC) of tacrolimus (CitationHebert, Blough, et al 2005), it increases the AUC of sirolimus by 21% and decreases the clearance of cyclosporine by 16% (CitationHebert, Townsend, et al 2005; CitationMycamine PI 2005). This alteration in cyclosporine clearance was deemed clinically insignificant by the investigators. However, since 5 of 28 (18%) subjects in the study experienced a clinically significant (>25%) change in clearance, the authors suggested monitoring cyclosporine levels during combination therapy with micafungin. The package insert also states that micafungin increases the AUC of nifedipine by 18%, entailing the need for close monitoring of an increased effect of nifedipine (CitationMycamine PI 2005).
Predictably, the most data exists with caspofungin. One group of authors hypothesized that since the metabolic transformation of caspofungin is slow, perhaps the process is limited by hepatocyte drug uptake transporters. Indeed, several in vitro experiments demonstrated that the hepatic transport protein OATP-1B1 may be responsible for hepatic uptake of caspofungin (CitationSandhu et al 2005). Since cyclosporine is a known substrate of this transporter, this offers a possible (albeit unproven) explanation for its interaction with caspofungin. Indeed, cyclosporine has been shown to increase the AUC of caspofungin by ~35% (CitationCancidas PI 2005). Similarly, rifampin (an inhibitor of OATP-1), has been shown to both inhibit and induce caspofungin metabolism. During the first day of rifampin co-administration, a transient 61% increase in the AUC of caspofungin is observed; however, when assessed after 14 days of rifampin administration, a 14%–31% reduction in caspofungin troughs is observed. Therefore, the authors recommend an increased dose of caspofungin during concomitant administration with rifampin (CitationStone et al 2004). A dosage increase is recommended in patients receiving other enzyme inducers, such as efavirenz, nevirapine, phenytoin, dexamethasone, and carbamazepine. Finally, tacrolimus AUC, peak, and 12-hour concentrations are decreased by approximately 20% during concomitant administration with caspofungin, potentially necessitating more frequent monitoring. The mechanism for this interaction has not been elucidated (CitationCancidas PI 2005).
When reconstituting anidulafungin, 20% dehydrated alcohol must be utilized (60 mL or 30 mL for 200 mg or 100 mg doses, respectively). This may be a concern for several patient populations, such as those susceptible to disulfuram reactions (for example, patients receiving concurrent metronidazole use), or for patients who are recovering alcoholics (CitationEraxis PI 2006).
Safety and adverse effects of echinocandins
All three echinocandins are generally well-tolerated. The most common adverse effects are listed in . While there appear to be some differences between the agents, it is important to keep in mind that caspofungin has been on the market much longer than both micafungin and anidulafungin. As such, it is difficult to derive conclusions or make valid comparisons between available echinocandins based on such small numbers of adverse effects. Finally, limited experience suggests that caspofungin and micafungin are safe to use in pediatric patients (CitationOdio et al 2004; Citationvan Burik et al 2004; CitationGroll et al 2005; CitationWalsh et al 2005). The safety and effectiveness of anidulafungin in pediatric patients has not been established (CitationEraxis PI 2006).
Table 1 Adverse effects of echinocandins (CitationSable et al 2002; CitationCarver 2004; CitationRaasch 2004; CitationKrause, Reinhardt, et al 2004; CitationCancidas PI 2005; CitationGroll et al 2005; CitationMycamine PI 2005; CitationEraxis PI 2006)
At the time of FDA approval, there were concerns regarding the safety of caspofungin when combined with cyclosporine. In initial studies utilizing a combination of cyclosporine and caspofungin in 12 healthy volunteers, 5 experienced elevations in serum aminotransferase levels ≤3 times the upper limit of normal (ULN). In addition, 3 of 4 healthy subjects who received caspofungin 70 mg daily for 10 days plus two 3 mg/kg doses of cyclosporine on day 10 experienced transient increases in alanine transferase (ALT) on day 11 that were <5 times the ULN. In the same study, 2 of 8 subjects who received caspofungin 35 mg daily for 3 days and two 3 mg/kg doses of cyclosporine on day 1 experienced increases in ALT<2 times ULN on day 2. As such, the package insert recommended that liver function tests be monitored closely when this combination is used (CitationCancidas PI 2005). However, recent data suggests that this interaction may be overstated. Three retrospective analyses of the use of caspofungin and cyclosporine in patients do not support a risk of clinically relevant hepatotoxicity (CitationMarr, Hachem et al 2004; CitationSanz-Rodriguez et al 2004; CitationGlasmacher et al 2006). A recent study further illustrates the lack of clinical significance of caspofungin-immunosuppressant interactions. Sixty-six patients from Phase II and III clinical trials received caspofungin in combination with cyclosporine (6 patients, for a mean of 14 days), tacrolimus (58 patients, 21 days), sirolimus (3 patients, 12 days), and mycophenolate (22 patients, 28 days). No patient on cyclosporine developed increased transaminases or discontinued therapy with caspofungin due to an adverse event. Two patients receiving sirolimus experienced transaminase elevations >2.5 times the ULN, and the incidence of transaminase elevations in the caspofungintacrolimus group was similar to the comparator agent combination (amphotericin B plus tacrolimus) (CitationKartsonis, Lipka, et al 2005).
One case report describes severe thrombocytopenia associated with caspofungin use in a patient with multiple aortic valve vegetations. The patient was receiving several agents known to cause thrombocytopenia, including piperacillin-tazobactam, heparin, 5-flucytosine, and amphotericin B deoxycholate; however, the time course (and subsequent rechallenges with several agents) were most consistent with caspofungin. However, the authors do not discuss the possible effects that the patient’s significant disease processes and comorbidities may have had on platelet counts. Nevertheless, the caspofungin package insert cites the incidence of decreased platelet counts to be 3.1% and 1.5% in patients receiving doses of 50 mg and 70 mg daily, respectively (CitationLynch and Wong-Beringer 2004).
It appears from clinical trials that caspofungin may have a higher propensity for causing histamine-induced reactions compared with other echinocandins (). These reactions may manifest as rash, facial swelling, pruritus, facial swelling, sensation of warmth, and/or bronchospasm (see http://www.cancidas.com/caspofungin_acetate/cancidas/hcp/product_highlights/tolerability/index.jsp). However, the package insert for micafungin states that patients may experience more frequent histamine-mediated reactions when the drug is infused more rapidly than 1 hour (Micafungin PI 2005). Possible histamine-mediated symptoms are infrequent when anidulafungin infusion rates do not exceed 1.1 mg/minute. However, more experience and comparative trials are needed to adequately assess the relative incidence for each agent.
It appears that certain structural alterations in the core molecule (ie, cyclic hexapeptides) of the echinocandins influence their ability to influence histamine release. Specifically, compounds with high proximal positive charge density demonstrated a high histamine-releasing potency when measured in mouse models with a histamine-radioligand-immunoassay (CitationWang et al 2003). In vitro studies in which human mononuclear, mast, and peripheral blood cells were incubated with caspofungin demonstrated a significant increase in histamine when incubated with peripheral and mast cells. Likewise, the activity of histamine N-methyltransferase, an enzyme which degrades histamine, was reduced by 33% in peripheral blood. Anidulafungin and micafungin were not studied in this in vitro system (CitationCleary et al 2003).
Comparison of echinocandins by indication
Clinical trials
Febrile neutropenia
Caspofungin
To date, only caspofungin has been studied for empiric therapy in patients with febrile neutropenia; caspofungin received FDA approval for this indication based on the results of a noninferiority study in 1095 patients. These high-risk patients (~60% in both groups had a primary diagnosis of acute myelogenous leukemia) were randomized to receive infusions of either caspofungin (70 mg loading dose, followed by maintenance doses of 50 mg every 24 hours for a median of about 10 days) or liposomal amphotericin B (3.0 mg/kg of body weight). Of those receiving caspofungin and amphotericin B, respectively, 190/556 (33.9%) and 180/539 (33.7%) patients had a favorable response, defined as a composite score of 5 criteria: successful treatment of baseline fungal infection; absence of breakthrough fungal infection during therapy or within 7 days after the end of therapy; survival for 7 days after discontinuation of therapy; resolution of fever; and no premature discontinuation of drug due to lack of efficacy or toxicity. Overall, outcomes with caspofungin therapy were equivalent to therapy with liposomal amphotericin B. However, secondary analysis suggested that therapy with caspofungin therapy was significantly more successful than liposomal amphotericin B in the treatment of baseline infections: 14/27 (51.9%) versus 7/27 (25.9%), respectively, and resulted in a higher proportion of patients surviving at least 7 days. Liposomal amphotericin therapy was associated with a significantly higher rate of adverse drug reactions, including nephrotoxicity and infusion-related events (CitationWalsh et al 2004).
Prophylaxis in hematopoietic stem cell transplantation and high-risk patient populations
The incidence of invasive fungal infections in patients receiving hematopoietic stem cell transplantation (HSCT) is between 14% and 25%; these infections are associated with a high rate of mortality (CitationLin et al 2001; CitationWisplinghoff et al 2004). Numerous studies have examined the utility of antifungal agents in preventing invasive infection (CitationHamza et al 2004). Although fluconazole is FDA-approved for this indication, it lacks clinical activity against C. krusei and Aspergillus spp. (CitationDiflucan PI 2004). Potential advantages of echinocandins versus fluconazole for this indication include their expanded spectrum of activity against Candida species and Aspergillus, and their decreased potential for drug interactions. Potential disadvantages include their higher cost, lack of oral formulations, and lack of activity against emerging pathogens such as Scedosporium, Fusarium, and zygomyces.
Caspofungin
Recently, caspofungin has been evaluated as prophylaxis in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Intravenous caspofungin (50 mg daily) was compared with intravenous itraconazole (200 mg twice daily X 2 days, then 200 mg once daily). Success of therapy was defined as completion of prophylaxis (which was continued until any of the following: absolute neutrophil count >500 for 2 consecutive days; complete response; death; change in leukemia therapy; unacceptable toxicity; proven or probable invasive fungal infection; or 35 days of prophylaxis) without development of invasive fungal infection during the period of prophylaxis. The median length of prophylaxis was 21 days in both groups. Prophylaxis was effective in 44/86 (51%) of patients in the itraconazole group and 55/106 (52%) in the caspofungin group. Twelve patients developed invasive fungal infections: 5 in the itraconazole group (one patient with Aspergillus pneumonia and four patients with candidemia- one due to C. krusei, one due to C. albicans, and two due to C. glabrata) and 7 in the caspofungin group (2 patients with disseminated Trichosporon infection, two with Aspergillus pneumonia, one with Fusarium cellulitis, one with candidemia due to C. parapsilosis, and one with both candidemia due to C. albicans and C. glabrata and concurrent Aspergillus pneumonia). Mortality was similar in both groups. This study illustrates the disappointing efficacy of fungal prophylaxis in high-risk patients and how prophylaxis may enable organisms that are not susceptible to the prophylactic antifungal agent to cause infection (CitationMattiuzzi et al 2006).
Micafungin
Citationvan Burik and colleagues (2004) evaluated 882 adult and pediatric patients who had undergone allogeneic (for any indication) or autologous (for hematological malignancy) HSCT. Patients were randomized to receive infusions of either micafungin 50 mg daily or intravenous fluconazole 400 mg daily until the earliest of the following: ≤5 days after engraftment; day 42 after HSCT; the development of proven, probable, or suspected invasive fungal infection; the development of unacceptable drug toxicity; death; or withdrawal or discontinuation from study participation. Overall treatment success (defined as the absence of proven, probable, or suspected fungal infection throughout the period of prophylaxis, and through the end of a 4-week post-treatment period) was 80% in the micafungin arm, and 73.5% in the fluconazole arm. Breakthrough infections during prophylaxis included 1 case each of C. albicans, C. parapsilosis, and C. lusitaniae during micafungin therapy; following fluconazole therapy 1 case each of C. krusei and C. parapsilosis were observed. There was 1 case of probable aspergillosis and one case of fusariosis in the micafungin treatment arm, and 7 cases (4 proven and 3 probable) of aspergillosis among patients treated with fluconazole (p=0.071). The only episode of zygomycosis occurred in a patient treated with micafungin. Significantly fewer patients in the micafungin arm 64/425 (15%) versus the fluconazole arm 98/457 (21%) required empiric antifungal therapy. Mortality was decreased, although not significantly, in the micafungin arm (5.7% vs 4.2%, respectively). Based on this limited data, micafungin may provide an option for prophylaxis in patients undergoing HSCT (Citationvan Burik et al 2004).
The two available studies examining the efficacy of echinocandins in preventing infections in high-risk patients demonstrated remarkably different results. The micafungin study included significantly more patients, and achieved relatively high rates of successful outcomes (possibly due to the extremely high-risk patient population in the caspofungin group) compared with the caspofungin study. As such, it is difficult to directly compare the two agents. However, it does appear that both caspofungin and micafungin would be acceptable options in the prophylaxis of fungal infections in high-risk patients.
An important limitation of the study is that the duration of prophylaxis was too short to evaluate efficacy in the late post-engraftment period in allogeneic transplant recipients, when this population is at often at increased risk for invasive aspergillosis due to the use of corticosteroids, or the presence of graft-versus-host disease (GVHD) or cytomegalovirus (CMV) infection.
Invasive aspergillosis
Much of the data concerning the use of echinocandins in invasive aspergillosis is derived from trials using combinations of antifungal agents (discussed in more detail below). However, both caspofungin and micafungin have been studied as single-agent therapy in patients with invasive aspergillosis.
Caspofungin
CitationMaertens and colleagues (2004) evaluated the use of intravenous caspofungin (administered as a 70 mg loading dose on the first day, then 50 mg daily) in patients refractory to (86%) or intolerant of (14%) previous antifungal therapy. Response was determined by a panel of 3 experts in fungal infections. A “complete response” was defined as resolution of all signs, symptoms, and radiographic evidence of aspergillosis, while a “partial response” was defined as “clinically meaningful improvement” in the above characteristics. Overall, 37/83 (45%) patients had a favorable response (complete + partial). Of the patients intolerant to previous therapy, 9/12 (75%) had a favorable response, while 28/71 (39.4%) of those refractory to previous therapy responded (1/3 were refractory to >1 drug).
In a second, compassionate-use study, caspofungin was assessed in an additional 48 patients (of whom 3 were not evaluated at the end of therapy) with aspergillosis refractory to or intolerant of other therapy. An overall favorable response (using the same criteria as the previous study) was observed in 20/45 (44%) patients, with 9/45 (20%) exhibiting a complete response to therapy. Of 10 patients who received caspofungin in conjunction with another antifungal agent, only 1 patient survived, highlighting the severity of infection in these patients (CitationKartsonis, Saah, et al 2005).
There are limited data assessing caspofungin as first-line therapy in patients with proven (7/32 [22%]) or possible (25/32 [78%]) aspergillosis. An overall response rate of 18/32 (56%) was observed. Of the 18 responders, 12 (66%) experienced complete and 6 (33%) partial responses. Twelve of the 32 patients (38%) did not respond and 7 died of mycotic infection. All patients who were neutropenic received G-CSF therapy. Interestingly, 2/6 patients who did not respond to caspofungin responded to voriconazole therapy, and 6/6 of those with a partial or stable response to caspofungin responded to voriconazole (CitationCandoni et al 2005).
Additional case reports have corroborated the utility of caspofungin in a diverse group of infections and patient populations, including invasive fungal infections in immunocompromised hosts (CitationTaccone et al 2003; CitationCarby et al 2004; CitationIfran et al 2004). Several unique cases have shown promising results: subcutaneously disseminated aspergillosis in an allogeneic stem cell transplant patient, Aspergillus brain abscess in a diabetic patient, cerebral aspergillosis treated with amphotericin B deoxycholate plus caspofungin, and 3 patients with endophthalmitis treated with voriconazole + caspofungin (CitationChameuleau 2003; CitationColombo and Rosas 2003; CitationBreit et al 2005; CitationEhrmann et al 2005). There have also been reports of treatment failures with caspofungin. In one, a renal transplant patient treated with voriconazole and caspofungin for pulmonary aspergillosis developed infection due to Rhizopus oryzae, the patient passed away after less than one week of treatment with liposomal amphotericin B. In another case, an HSCT patient who developed pulmonary aspergillosis failed long-term treatment with varied combinations of liposomal amphotericin B, itraconazole, and caspofungin, but resolved following 7 months of voriconazole therapy (CitationBlin et al 2004; CitationEibl et al 2004).
Micafungin
Clinical data regarding the use of micafungin for the treatment of aspergillosis is expanding. Administration of intravenous micafungin 300 mg daily for 50 days was successful in the treatment of pulmonary infection with A. fumigatus in a patient with acute myeloid leukemia who had required repeated and prolonged courses of intravenous amphotericin B deoxycholate 1 mg/kg/day (CitationYokote et al 2004). In another case, a patient with acute lymphoblastic leukemia complicated by invasive pulmonary aspergillosis responded to 75–150 mg daily of intravenous micafungin. The higher dose resulted in increased serum concentrations of micafungin, and was associated with improvement in signs and symptoms of infection (CitationOta et al 2004). A Phase II study in Japan enrolled patients with presumed or documented infection due to Aspergillus or Candida spp. Patients received intravenous micafungin at doses of 25–150 mg daily for 13–56 days. Response was defined as improvement in radiologic findings without clinical deterioration for invasive pulmonary aspergillosis (IPA); as improvement in both parameters for patients with chronic necrotizing pulmonary aspergillosis (CNPA); and as either radiologic improvement without clinical decline, or clinical improvement without radiologic decline, in patients with pulmonary aspergilloma (PA). Overall, 24/41 (59%) patients responded: 6/10 with IPA, 6/9 with CNPA, and 12/22 with PA (CitationKohno et al 2004). In a recent, open-label, noncomparative, international study in 225 adult and pediatric patients (of whom 66 (29%) were neutropenic at baseline) the use of intravenous micafungin was evaluated in 29 (13%), as primary therapy, and in 192 (85%) patients refractory to, or in 4 (2%) intolerant to previous antifungal therapy. The dosage of micafungin was 75 mg/day initially (1.5 mg/kg/day in patients ≤40 kg); the dosage was increased in increments of 75 mg/day (1.5 mg/kg/day in patients ≤40 kg) if cultures remained positive, progression of disease was evident, or no improvement was observed. Response was determined by a panel of 3 experts in fungal infections. The mean daily dose in adults was 111 mg/day (median 97 mg/day). The mean duration of treatment was 54 days. Of 96 patients whose doses were not escalated, 30 (31%) had a favorable response. Of 192 refractory patients, 148 (77%) had received a lipid preparation of amphotericin B, 86 (45%) amphotericin B deoxycholate, 66 (34%) itraconazole, 7 (4%) caspofungin, 5 (3%) voriconazole, and 5 (3%) posaconazole. Combination therapy (micafungin added to previous failing therapy) was utilized in 191 patients. Overall, 80 (36%) of patients had a favorable (complete + partial) response; and additional 25 (11%) of patients experienced stabilization of their infection. Of 29 patients who received micafungin as primary therapy, 11 had a favorable response (5/17 of those receiving combination therapy and 6/12 of those receiving micafungin alone). In the 34 patients receiving micafungin alone (18 refractory, 12 as primary therapy, and 4 due to prior drug toxicity), 15 (44%) had a favorable response (CitationDenning et al 2006). This study, very similar to the study by Maertens and colleagues with caspofungin, suggests that micafungin has clinical efficacy similar to that of caspofungin in the treatment of invasive aspergillosis.
Micafungin has also been studied in pediatric patients (<16 years old) with proven or probable invasive aspergillosis. In a noncomparative study, 58 patients (4 newly diagnosed, 54 refractory to prior therapy of whom 43% had undergone an allogeneic bone marrow transplant, and 47% had undergone chemotherapy) with a mean age of 9 ± 4 years were treated with an initial dose of 1.5 mg/kg/day of micafungin; 30 patients received further dose escalation, and the mean treatment dose was 2.0 ± 1.2 mg/kg/day. Only 2 patients received micafungin alone, while the others received combination therapy with other antifungals (the majority, 47, receiving liposomal amphotericin B). Overall response (compete + partial) was obtained in 26/58 (45%) patients. Of these, 9 (16%) had a complete response, and 17 (29%) had a partial response. 5/21 (24%) of those infected with A. fumigatus and 11/20 (55%) with A. flavus responded. These response rates are similar to those of the trials described above, and as such, micafungin (especially in combination) is an option for the treatment of invasive aspergillosis in pediatric patients (CitationFlynn et al 2006).
Despite the available data encompassing very low numbers of patients, the above studies are still significant indicators of efficacy in a devastating disease. As evidence of this, caspofungin received FDA approval for the treatment invasive aspergillosis in patients who are refractory to or intolerant of other therapies based solely on the results of the Maertens study (83 patients!). The 2 studies evaluating the use of echinocandins as first-line therapy for invasive aspergillosis are interesting, and demonstrate similar responses for caspofungin and micafungin. Possibly the most urgent need for additional data is in clinical trials examining the use of the echinocandins in the first-line or combination therapy of invasive aspergillosis.
Esophageal candidiasis
Caspofungin and micafungin have been studied extensively, and anidulafungin, less extensively, in patients with esophageal candidiasis.
Caspofungin
Intravenous doses of caspofungin (35 mg, 50 mg, or 70 mg daily) were compared with intravenous fluconazole 200mg daily or amphotericin B 0.5 mg/kg/d for the treatment of clinically or microbiologically fluconazole-resistant Candida esophagitis. Of 31 patients, 14 (45%) were refractory to fluconazole and 17 (55%) had fluconazole MICs of ≥16 μg/mL. Overall, 7/11 (64%) of fluconazole-refractory patients treated with caspofungin responded, and 11/14 patients (79%) whose Candida isolates had decreased susceptibility to fluconazole (including 5/6 (83%) patients with fluconazole MICs ≥64 μg/mL) treated with caspofungin responded (CitationKartsonis et al 2002).
In a compassionate-use study with caspofungin in 21 patients with invasive mucosal candidiasis (17 esophageal, 4 oropharyngeal), 19 (91%) of whom had HIV disease and were refractory to other therapy, 18/21 (86%) of patients had a favorable response (CitationKartsonis et al 2004). A subsequent study analyzed 120 patients with endoscopically and microbiologically documented esophageal candidiasis from 4 phase II/III caspofungin trials; C. albicans was isolated in 109/110 of isolates, and was the sole isolate in 77%. Caspofungin (50 mg daily) was administered for a median of 12 days in 120 patients. Symptoms resolved in 117/123 (95%) patients, within a median of 4 days. Response rates were not significantly different for patients with CD4 counts greater than or less than 50 cells/mm3. However, 17% of patients experienced relapse within 2 weeks of discontinuation of therapy (CitationDinubile et al 2002).
Following these studies, in a randomized, double-blind study comparing intravenous therapy with fluconazole (200 mg daily) with caspofungin (50 mg daily) once daily for 7 to 21 days, no significant difference in favorable response rates (66/81 [81%] versus 80/94 [85%] patients, respectively), were observed. Symptoms resolved by the fifth day of treatment in the majority of patients. Relapse was observed within 4 weeks following discontinuation of therapy in 12/72 (17%) and 18/64 (28%) of patients receiving fluconazole and caspofungin, respectively (CitationVillanueva et al 2002).
Intravenous therapy for 2 weeks with caspofungin (50mg or 70 mg daily) was compared with amphotericin B deoxycholate 0.5 mg/kg/day in 122 patients with oropharyngeal or esophageal candidiasis, the majority of whom were HIV-infected. Patients with possible fluconazole resistance were excluded. The median time to symptom resolution (4 days) was similar in all groups. Endoscopic success was achieved in 74%, 89%, and 63% in patients treated with caspofungin 50 mg, 70 mg, and amphotericin B, respectively, at the 14-day post-treatment follow-up. Significantly more adverse events occurred in the amphotericin B arm, although only 1 was deemed serious. Interestingly, the authors found no appreciable increases in adverse events between the 2 doses of caspofungin which showcases the future possibility of a valuable option of increasing doses in non-responders without expectation of a higher rate of side effects (CitationVillanueva et al 2001). In a similar study, intravenous caspofungin (35 mg, 50 mg, or 70 mg daily) or conventional amphotericin B (0.5 mg/kg of body weight once daily) were administered for 7 to 14 days. A modestly higher proportion of patients in each of the caspofungin groups (74% to 91%) achieved favorable responses compared with amphotericin B recipients (63%); however, there was considerable overlap in the 95% confidence intervals surrounding these point estimates (CitationArathoon et al 2002).
Micafungin
Micafungin was studied in 120 South African patients with HIV-related esophageal candidiasis: groups of 20 patients were randomized to one of 6 dosing regimens (12.5 mg, 25 mg, 50 mg, 75 mg, or 100 mg per day). Clinical response was found to be dose dependent: 6/18 (33.3%) of patients in the 12.5 mg daily dosage group experienced clinical clearing, compared with 18/19 (94.7%) of patients in the 100 mg daily dosage group. In addition, all patients at dose schemes of 50 mg or greater exhibited endoscopic improvement. Of concern, only 84 patients were included in the per-protocol analysis, as 13 patients discontinued therapy due to adverse events, and 16 patients were excluded due to a negative histology or cytology, thus signifying a lack of firm diagnosis. Despite this, most adverse events were considered mild to moderate, consisting mostly of gastrointestinal disturbances, liver function test abnormalities, and rash (CitationPettengell 2004).
A double-blind, randomized, noninferiority study compared response rates of micafungin (intravenous doses of 50 mg, 100 mg, or 150 mg/daily) with intravenous fluconazole (200 mg) for 14–21 days in 245 adult HIV-positive patients. Once again, a dose-dependent response was shown for micafungin, with a 69% documented cure in the 50 mg arm and 90% cure in the 150 mg arm. Fluconazole exhibited a 87% success rate. Based on these findings, micafungin was determined to be noninferior to fluconazole when administered at doses of 100 mg and 150 mg. 50% of patients had improvement by 3 days of treatment, and 75% were improved by day 7 (Citationde Wet et al 2004).
Finally, a randomized, double-blind, noninferiority study compared a minimum of 14 days of therapy with intravenous micafungin 150 mg daily with intravenous fluconazole 200 mg daily. As expected, the majority of patients had a diagnosis of HIV (94%), and C. albicans was most often implicated as the cause of infection (98%). The mean number of days on study medication was similar for both groups (about 14 days), as were the rates of endoscopic cure (87.7% for micafungin, 88.0% for fluconazole), and clinical success (94.2% and 94.6%, respectively). Again, improvement was often discernible within 3–5 days, and the rate of relapse was higher, although not significantly so, in the micafungin arm (15.2% vs 11.3% through week 4) (Citationde Wet et al 2005).
In a recent multicenter, multinational, double-blind, randomized, noninferiority study in 452 patients with esophageal candidiasis, alternate day dosing of intravenous 300 mg of micafungin was as effective as daily intravenous doses of either 150 mg of micafungin or 50 mg of caspofungin (CitationBuell et al 2005).
Anidulafungin
In a randomized, double-blind, noninferiority study in 601 patients, anidulafungin (100 mg intravenously on day 1 followed by 50 mg daily thereafter) was comparable to oral fluconazole (200 mg on day 1, 100 mg daily thereafter) for the treatment of esophageal candidiasis. The rates of endoscopically-confirmed success were 98.8% and 97.2% for fluconazole and anidulafungin, respectively. Of concern, a 2-week follow-up revealed that only 64.4% of patients in the anidulafungin group compared with 89.5% of patients taking fluconazole had sustained success. This may have been compounded, however, by the finding that more patients took antiretrovirals during treatment in the fluconazole arm (CitationKrause, Simjee, et al 2004).
Summary
Based on currently available literature, there are no clear distinctions between the echinocandins when used in the treatment of esophageal candidiasis. All 3 agents have proven noninferior to the current standard of care (fluconazole), and all 3 carry a concern of significant relapse/reinfection rates. In rabbits, poor penetration of anidulafungin into saliva (although penetration into esophageal tissue was quite high) has been demonstrated; whether this is true for other echinocandins or in humans is not known (CitationPetraitis et al 2001). Until a comparative class study is available, no clear distinction is evident based on clinical efficacy.
Invasive candidiasis
Caspofungin
Caspofungin has been studied extensively in patients with invasive candidiasis. Prior to its licensure, caspofungin was evaluated in 16 patients with invasive candidiasis who were refractory to or intolerant of other antifungal therapy: 8/16 (50%) of the patients had acute leukemia/lymphoma, and 5/16 (31%) were diabetic. Sites of infection were widespread, including four patients with chronic disseminated disease. A favorable response was observed in 13/15 patients (one patient died of staphylococcal sepsis prior to evaluation) (CitationKartsonis, Killar, et al 2005). Following this, a randomized, double-blind trial compared caspofungin (50 mg daily) with amphotericin B deoxycholate (0.6–0.7 mg/kg/day) in 239 patients with invasive candidiasis. A successful outcome was achieved in 73.9% and 61.7% of patients receiving caspofungin and amphotericin B, respectively (95.6% Confidence Interval [CI]: −0.7 to 26.0); response rates were consistent across most infection sources, including 7 patients with Candida endophthalmitis. Mortality was similar with either therapy (34.2% with caspofungin, 30.4% with amphotericin B) (CitationMora-Duarte et al 2002). These findings led to the subsequent FDA approval of caspofungin for this indication.
Several case reports have highlighted the use of intravenous caspofungin for uncommon or off-label indications. One case documents resolution of non-albicans Candida endocarditis without valve replacement, using a 70 mg loading dose followed by 50 mg daily for 6 weeks. The only other antifungal agent the patient had received was intravenous fluconazole for 4 days prior to initiation of caspofungin (CitationRajendram et al 2005). In another case, a patient with endocarditis due to C. guilliermondii was cured with surgery and 6 weeks of caspofungin monotherapy (50 mg daily) (CitationNevado et al 2005). These two cases present an encouraging picture for the use of caspofungin, even as monotherapy, for the treatment of fungal endocarditis. A combination of liposomal amphotericin B and caspofungin was successful in treating a case of Candida endocarditis without valve replacement (CitationJimenez-Exposito et al 2004). However, another patient with C. albicans endocarditis did not respond to caspofungin monotherapy and surgery, and developed likely fungal brain abscesses. The patient subsequently improved on liposomal amphotericin B + oral fluconazole therapy. This study highlighted concerns that the poor central nervous system (CNS) penetration of caspofungin might allow for dissemination of fungi to the CNS (CitationPrabhu and Orenstein 2004). However, in another case report, a patient with C. albicans meningitis refractory to amphotericin B (1 mg/kg/day), intravenous fluconazole and intrathecal amphotericin B (1 ml of 0.25 mg/ml 3 times per week) improved dramatically following caspofungin monotherapy (CitationLiu et al 2004). Finally, 2 case reports present conflicting data concerning the use of caspofungin in the treatment of Candida endophthalmitis. In one case, a patient failing caspofungin monotherapy had undetectable intravitreous concentrations of caspofungin; the patient was subsequently cured after therapy with amphotericin B lipid complex (5 mg/kg/day) (CitationGauthier et al 2005). However, another patient was cured after a 28-day course of caspofungin monotherapy (initially 50mg daily, later decreased to 35 mg daily due to moderate hepatic impairment). Intravitreous drug concentrations of caspofungin were not obtained (CitationSarria et al 2005). These case reports are interesting, but do not provide sufficient data upon which to form decisive conclusions regarding the use of caspofungin in endocarditis, meningitis, and endopthalmitis.
There are limited data regarding the use of caspofungin monotherapy in pediatric patients with invasive candidiasis. Encouraging results were observed when caspofungin was substituted for amphotericin B deoxycholate in 9 neonates with persistent infection. All blood cultures were sterilized within 3 to 7 days of initiating caspofungin, and in one case, an atrial vegetation was eradicated (CitationOdio et al 2004). Another case report also documents a successful use of caspofungin in a patient with amphotericin B- and fluconazole-resistant Candida glabrata endocarditis (CitationMrowczynski and Wojtalik 2004). Caspofungin has also been added without serious side effects to failing regimens in a variety of pediatric patients, including a low-birth-weight neonate, several bone marrow transplant recipients, and a 3-year old child with persistent candidemia, with generally positive results (CitationFranklin et al 2003; CitationHesseling et al 2003; CitationCastagnola et al 2004; CitationWertz and Pretzlaff 2004).
Micafungin
Data regarding the efficacy of micafungin for the treatment of invasive candidiasis are emerging. An open-label study in Japan documented a 100% response (6/6 patients) in the treatment of candidemia. However, one patient with disseminated candidiasis did not respond to treatment (CitationKohno et al 2004). In an open-label, noncomparative trial in 126 patients with candidemia, micafungin therapy produced a complete or partial response in 83.3% of patients; however, since dose escalation (with dosages up to 200 mg/day) was allowed, and the majority of patients received concurrent antifungal agents, the clinical applicability of this trial is limited (CitationOstrosky-Zeichner et al 2005). A double-blind, noninferiority study comparing intravenous micafungin (100 mg daily) with liposomal amphotericin B (3 mg/kg/day) for 2-4 weeks was undertaken in mainly non-neutropenic (12.9% vs 10.5% of patients, respectively), mostly candidemic (~85% in both arms) patients. Micafungin treatment was considered effective (clinical plus mycological response) in 89.6% of patients (181/202) compared with 89.5% (170/190) in the amphotericin B group. 62.4% and 58.9% of patients, respectively, were infected with non-albicans Candida species. Both groups were equal with regard to eradication of C. glabrata and C. parapsilosis, and catheters were removed in an equal frequency in both groups. The amphotericin B group had a significantly higher incidence of side effects, including infusion-related reactions and increases in serum creatinine. As such, the investigators concluded that micafungin was noninferior to amphotericin B, but displayed a significantly more favorable side effect profile (CitationRuhnke et al 2005). A randomized, 1:1:1, double blind, noninferiority trial comparing 2 doses of micafungin (100 mg/day and 150 mg/day) with caspofungin (70 mg loading dose, then 50 mg/day) was recently presented in abstract form. The primary efficacy endpoint was clinical and microbiological response at the end of intravenous therapy, with a pre-specified margin for noninferiority of −15%. 593 patients received at least one dose of study drug, and the three groups had similar baseline characteristics. Overall success was 73.9% for micafungin 100 mg/day, 70.3% for micafungin 150mg/day, and 71.4% for caspofungin. Based on these results, micafungin was determined noninferior to caspofungin. In addition, this study showed no advantage of micafungin at 150 mg/day compared with 100 mg/day. The safety profiles for the 3 treatments were similar. Further analysis of response stratified by organism are pending (CitationBetts et al 2006).
Micafungin (2 mg/kg/day) was recently compared with liposomal amphotericin B (3 mg/kg/day) for the treatment of invasive candidiasis in pediatric patients (≤15 years old) in a randomized, double-blind study. The study included 98 patients: the majority had candidemia (92% in the micafungin group, 94% in the amphotericin B group), with 30 (63%) and 35 (70%) patients, respectively, infected with non-albicans species. Overall treatment success (clinical and mycological response) in the modified intention-to-treat patients was similar for the two agents, 35/48 (72.9%) for micafungin and 38/50 (76.0%) for amphotericin B. No significant differences were found when responses were analyzed according to patient age, neutropenic status, safety profiles, or in 12-week survival. As such, micafungin may be considered in pediatric patients with candidiasis, especially those with candidemia (CitationArrieta et al 2006).
Anidulafungin
In a Phase II, dose-ranging study, 123 patients with invasive candidiasis were randomized to 50 mg, 75 mg, or 100 mg daily. Of the 68 evaluable patients (94% of which had candidemia only), success rates were 84%, 90%, and 89% in each group, respectively (CitationKrause, Reinhardt, et al 2004). Eradication rates were also dose-dependent (74%, 85%, and 89%, respectively). MICs for both C. albicans and non-albicans isolates were similar, except for C. parapsilosis isolates, which comprised 9.5% of isolates. The MIC range (4–8 μg/mL) for this organism was significantly greater than those of other isolates. However, as described above with caspofungin, high MICs did not predict treatment failure: 6 of 7 infections due to isolates of C. parapsilosis with MICs of 4–8 μg/mL were eradicated (CitationPfaller, Diekema, et al 2005). Based on these results, a randomized, double-blind Phase III trial compared intravenous anidulafungin (200 mg loading dose X 1, then 100 mg daily) with intravenous fluconazole (800 mg loading dose X 1, then 400 mg daily) in 256 patients was conducted. Patients in either arm could switch to oral fluconazole after 10 days. Approximately 3% of patients were neutropenic, and 89% had only candidemia. About 40% of isolates were of non-albicans spp. in both arms. Treatment success required both a clinical and microbiological response. A statistically significantly greater response was found in the anidulafungin arm in the microbiological intent-to-treat arm at the end of intravenous therapy (75.6% vs 60.2%). At the same time point, 6.3% of patients in the anidulafungin arm had persistence of infection, compared with 14.4% in the fluconazole arm. A secondary analysis at a two-week follow-up again revealed statistical superiority in the anidulafungin arm (64.6% vs 49.2%), with similar results again at six weeks (55.9% vs 44.1%). A subset analysis revealed that anidulafungin retained its statistical superiority only in patients with APACHE II scores >20 (CitationReboli et al 2005).
Summary
At this time, the only distinction that can be drawn between the echinocandin agents and their utility in invasive candidiasis is that caspofungin and anidulafungin, to date, are the only agents to have FDA approval. However, based on limited information, micafungin also appears to be effective. As such, it is difficult to discern the preferred echinocandin for the treatment of invasive candidiasis. Studies which directly compare the agents would help delineate any differences.
Combination therapy with echinocandins and other antifungal agents
The unique mechanism of action of the echinocandins has rekindled an interest in utilizing combination antifungal chemotherapy. Most examples of additive or synergistic effects make theoretical sense, such as flucytosine + amphotericin B in cryptococcal meningitis and rifampin or an aminoglycoside in combination with a β-lactam antibiotic for selected bacteria. In the same way, an echinocandin in combination with a polyene or azole antifungal makes theoretical sense, given the different mechanisms of action. However, a logical theory does not exclude the possibility for antagonism when two agents are combined. The various combinations have been tested against several fungi, including Trichosporon asahii in a murine model (CitationSerena et al 2005), Scedosporium spp in vitro (CitationYustes and Guarro 2005), Cryptococcus spp. in vitro (CitationJohnson et al 2004), and Mucor in a successful patient case (CitationSpellberg et al 2005). Several extensive reviews have been published recently on combination therapy for fungal infection (CitationLewis and Kontoyiannis 2001; CitationJohnson et al 2004; CitationBaddley and Pappas 2005). The only randomized, blinded study evaluating the efficacy of combination therapy involves fluconazole versus fluconazole + amphotericin B deoxycholate for candidemia. This study found a trend towards improved outcomes in the combination therapy group (CitationRex et al 2003). However, at the present, most interest concerning combination therapy lies in the treatment of aspergillosis, given the continued high mortality of these infections. The results of in vitro and animal studies for the most relevant fungi are summarized in .
Table 3 Echinocandin-containing combination antifungal therapy (in vitro and animal data) (CitationJohnson et al 2004)
Caspofungin
Several small series of patients have reported successful treatment of refractory infections with combinations of caspofungin and itraconazole, voriconazole, or lipid preparations of amphotericin B. One study retrospectively assessed the response of 48 patients with invasive aspergillosis who received combination therapy with liposomal amphotericin B and caspofungin in whom 17 (35%) had received combination therapy as initial therapy, while an additional 31 (65%) patients had progressive disease on liposomal amphotericin B alone. The overall response rate was 42% (22% in patients with documented infection, and 60% in those with possible infection). In those patients whose disease had progressed on liposomal amphotericin B monotherapy, the response rate was 18% and 57%, respectively. 5/13 (38%) patients with neutropenia persisting through the study period responded to combination therapy (CitationKontoyiannis et al 2003). In a similar study, which included 85 patients with progression of disease despite 72 hours of appropriate therapy, 33 (39%) patients demonstrated a complete or partial response. However, 13 patients received triple therapy (micafungin, amphotericin B, and an azole) (CitationRatanatharathorn et al 2002). Investigators from the University of Washington examined the outcomes of 47 patients who failed amphotericin B formulations and received voriconazole alone or in combination with caspofungin. Compared with single-agent therapy, combination therapy significantly improved 3-month survival and reduced mortality (CitationMarr, Boeckh, et al 2004). Another study analyzed the efficacy of caspofungin combined with polyene formulations (16 patients), itraconazole (7), or voriconazole (30) in patients refractory to (87%) or intolerant of (13%) prior therapy, of whom 7 (13%) patients had disseminated infection, and 43 (81%) had pulmonary infections. The average duration of therapy was 31 days (range 1–196), with only 10 patients (19%) receiving therapy for <7 days. Efficacy, determined by an independent expert assessment, was achieved in 29/55 patients (55%) at the end of combination therapy, and 25/51 (49%) after 84 days of therapy. At the end of combination therapy, efficacy was achieved in 18/30 (60%) in the caspofungin–voriconazole group, compared with 3/7 (43%) in the caspofungin–itraconazole arm and 8/16 (50%) in the caspofungin–amphotericin B arm. Efficacy was similar in patients refractory to prior therapy (54%) and in those intolerant (57%), as well as in patients who were either neutropenic or non-neutropenic at study outset (57% vs 52%, respectively) (CitationMaertens et al 2005).
CitationSingh and colleagues (2006) compared a historical control group of 47 solid organ transplant recipients treated as primary therapy for aspergillosis with liposomal amphotericin B monotherapy from 1999–2002, to 40 patients treated from 2003–2005 with voriconazole plus caspofungin (CitationSingh et al 2006). 90-day mortality was significantly lower in the combination therapy versus the monotherapy group (51% vs 67.5%, respectively). In addition, in transplant recipients with renal failure, and in those with A. fumigatus infection, combination therapy was independently associated with an improved 90-day survival. As such, voriconazole + caspofungin may be preferred in solid organ transplant patients with invasive aspergillosis (CitationSingh et al 2006). However, the results do not prove the superiority of combination therapy, especially since a previous randomized, controlled trial proved the superiority of voriconazole to amphotericin B deoxycholate for aspergillosis (CitationHerbrecht et al 2002). However, the control group may have experienced other differences in medical management from the case group. As such, a prospective trial which compares combination therapy versus voriconazole monotherapy would be more telling. In addition, a more recent study of 146 patients, 32% of whom were solid organ transplant recipients, found that primary combination therapy did not affect mortality or rates of favorable response (CitationKubin et al 2006).
Micafungin
A recent study of 98 bone marrow transplant recipients with invasive aspergillosis shows similar findings with micafungin-containing combination therapy. Initial dosing of micafungin was 75 mg/day, with escalation to a mean dose of 105 ± 60 mg/day. Response (complete and partial) was seen in 25/98 (26%) patients, the majority (83) of whom were refractory to previous therapy (CitationKontoyiannis et al 2006).
Micafungin combination therapy has also been evaluated in a case series involving 6 patients with pulmonary aspergillosis (2 with probable infection). Patients received intravenous amphotericin B deoxycholate (0.8–1.5 mg/kg/day) and micafungin (150–300 mg daily) for 14–90 days. 4/6 patients showed response in both radiological and mycological studies. 5/6 were determined to have treatment success (determined by the occurrence of one or more of the following without deterioration in any criteria: complete or partial response in clinical symptoms, radiological findings, and mycological tests) (CitationMiyazaki et al 2005).
Summary
To our knowledge, only one randomized clinical trial has evaluated combination therapy for the treatment of fungal infections (CitationRex et al 2003). In vitro and animal data are of especially limited use in invasive fungal diseases, due to the numerous patient-specific factors that greatly affect patient outcomes. In addition, the available clinical studies evaluating combination therapy are fraught with limitations and discrepancies. New studies have not rectified these issues, and add to a confusing mix of diverse disease states, drug combinations, and study designs. This has made the topic very controversial, and at this time, practice seems to be based more on preference and theory than on evidence. This is evidenced by the varied opinions of experts in the field (CitationChamolis and Kontoyannis 2006; CitationMunoz et al 2006; CitationLeather and Wingard 2006). Thus, firm recommendations regarding the use of combination therapy can not be given at this time. However, based on the available evidence, it appears that the use of caspofungin in combination with either voriconazole or amphotericin B should be considered by clinicians in patients with Aspergillus infections refractory to single-agent therapy, or those with disseminated disease. This benefit, although not certain at this point, is logical, when one considers the mechanisms of action of the different antifungal agents, as well as caspofungin’s proven efficacy in refractory aspergillosis. At this time, micafungin has shown similar trends in one trial and a case series, while anidulafungin has only been tested in vitro models. The evidence concerning combination therapy in infections due to other fungi is too limited to extrapolate clinically.
Dosage/administration
The dosages for the treatment of FDA-approved indications in adults are listed in . For all echinocandins, slow infusions (1 hour for caspofungin and micafungin, a rate ≤1 mg/minute for anidulafungin) are recommended. Pharmacokinetic data support the use of once daily intravenous dosages for all echinocandins. Micafungin is the sole echinocandin for which a loading dose is not recommended. In addition, optimal dosages of micafungin have not been established in either esophageal or systemic infections. Thus far, dosages for the treatment of esophageal candidiasis in HIV-infected patients have ranged from 50 mg to 150 mg daily, while dosages of up to 300 mg daily have been utilized for the treatment of invasive aspergillosis.
Table 4 Adult dosing of echinocandins (CitationKrause, Reinhardt, et al 2004; CitationCancidas PI 2005; CitationMycamine PI 2005; CitationReboli et al 2005; CitationRuhnke et al 2005; CitationEraxis PI 2006)
For all echinocandins, dosage adjustments are not required based on race, for the elderly, or for patients with severe renal dysfunction. Anidulafungin does not require any dose adjustment in patients with severe hepatic dysfunction, and micafungin does not require dosage adjustment in moderate dysfunction (has not been studied in severe dysfunction). The caspofungin maintenance dose, however, should be reduced to 35 mg daily in patients with moderate to severe hepatic dysfunction.
Availability
As of April 2006, caspofungin, micafungin, and anidulafungin are all FDA-approved in the US (). Caspofungin and micafungin are also available in Japan and Europe.
Table 5 Formulations of echinocandins (CitationCancidas PI 2005; CitationMycamine PI 2005; CitationEraxis PI 2006)
Summary
The echinocandins are important and exciting agents because of their novel mechanism of action, low incidence of serious adverse effects, and low potential for drug–drug interactions. As a class, they demonstrate potent activity against Candida spp., and are an option for the treatment of infections due to these organisms. In addition, caspofungin is a viable option for the treatment of refractory aspergillosis. Although micafungin is not FDA-approved for this indication, recent data suggests that it may also be effective. Likewise, caspofungin- or micafungin-containing combination therapy should be a considereation for the treatment of severe infections due to Aspergillus spp.. Although the echinocandins share many common properties, data regarding their differences are emerging at a rapid pace. However, there continues to be a dire need for additional data concerning the echinocandins. Specifically, head-to-head comparison trials would help delineate differences between in vitro (theory) and in vivo clinical practice. More data are needed concerning echinocandin use as initial therapy for invasive aspergillosis. In addition, considering the substantial cost and somewhat theoretical benefit of combination therapy, controlled trials analyzing this use of echinocandins are desperately needed. Finally, continued data regarding the use of echinocandins for the treatment of unusual infections (meningitis, endopthalmitis, endocarditis, etc) would also help clarify the echinocandins’ place in the antifungal armamentarium.
References
- AbruzzoGKAndersonJWDouglasCMThe paradoxical in vitro response of Candida albicans to caspofungin [abstract M-2155]2005Abstracts of the 45th Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 16–19, 2005Washington, DC: American Society for Microbiology455
- AbruzzoGKFlatteryAMGillCJEvaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosisAntimicrob Agents Chemother199741233389371329
- AndesDMarchilloKIn vivo pharmacodynamic characterization of a new echinocandin, anidulafungin, against Candida albicans and Candida glabrata in the neutropenic murine disseminated candidiasis model [abstract A-1109]2006Abstracts of the 46th Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 2730, 2006San Francisco, CA: American Society for Microbiology24
- AndesDMarchilloKLowtherJIn vivo pharmacodynamics of HMR 3270, a glucan synthase inhibitor, in a murine candidiasis modelAntimicrob Agents Chemother20034711879212654645
- [Anonymous]Anidulafungin: ECB, LY 303366, v-echinocandin, VEC, VER 002, VER-02Drugs R & D2003416773
- ArathoonEGGotuzzoENoriegaLMRandomized, double-blind, multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiasesAntimicrob Agents Chemother200246451711796357
- ArrietaACTelles FilhoFBerezinEA randomized, double-blind trial comparing micafungin (mcfg) and liposomal amphotericin b (l-amb) in pediatric patients with invasive candidiasis [abstract M1308b]2006Abstracts of the 46th Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 27–30, 2006San Francisco, CA: American Society for Microbiology
- BachmannSPPattersonTFLopez-RibotJLIn vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistanceJ Clin Microbiol20024022283012037093
- BachmannSPVandeWalleKRamageGIn vitro activity of caspofungin against Candida albicans biofilmsAntimicrob Agents Chemother2002463591612384370
- BachmannSPRamageGVendeWalleKAntifungal combinations against Candida albicans biofilms in vitroAntimicrob Agents Chemother2003473657914576141
- BaddleyJWPappasPGAntifungal combination therapy: clinical potentialDrugs20056514618016033288
- BalashovSVParkSPerlinDSAssessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1Antimicrob Agents Chemother20065020586316723566
- BarchiesiFSpreghiniETomassettiSEffects of caspofungin against Candida guilliermondii and Candida parapsilosisAntimicrob Agents Chemother20065027192716870764
- BartizalKGillCJAbruzzoGKIn vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872)Antimicrob Agents Chemother1997412326329371328
- BettsRFRotsteinCTalwarDComparison of micafungin and caspofungin for candidemia or invasive candidiasis [abstract M1308a]2006Abstracts of the 46th Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 27–30, 2006San Francisco, CA: American Society for Microbiology
- BlinNMorineauNGaillardFDisseminated mucormycosis associated with invasive pulmonary aspergillosis in a patient treated for post-transplant high-grade non-Hodgkin’s lymphomaLeuk Lymphoma2004452161315370266
- BoucherHWGrollAHChiouCCNewer systemic antifungal agents: pharmacokinetics, safety and efficacyDrugs2004641997202015341494
- BowmanJCHicksPSKurtzMBThe antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitroAntimicrob Agents Chemother20024630011212183260
- BreitSMHariprasadSMMielerWFManagement of endogenous fungal endophthalmitis with voriconazole and caspofunginAm J Ophthalmol20051391354015652837
- BuellDKovandaLDrakeTAlternative day dosing of micafungin in the treatment of esophageal candidiasis [abstract M-719–2005]2005Abstracts of the 45th Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 16–19, 2005Washington, DC: American Society for Microbiology
- Cancidas PICancidas package insert [online]2005Rahway, NJMerck Accessed on 3 January 2006. URL: http://www.cancidas.com/cancidas/shared/documents/english/pi.pdf
- CandoniAMestroniRDamianiDCaspofungin as first line therapy of pulmonary invasive fungal infections in 32 immunocompromised patients with hematologic malignanciesEur J Haematol2005752273316104879
- CarbyMRHodsonMEBannerNRRefractory pulmonary aspergillosis treated with caspofungin after heart-lung transplantationTranspl Int200417545815365605
- CarverPLMicafunginAnn Pharmacother20043817072115340133
- CastagnolaEMachettiMCappelliBCaspofungin associated with liposomal amphotericin B or voriconazole for treatment of refractory fungal pneumonia in children with acute leukaemia or undergoing allogeneic bone marrow transplantClin Microbiol Infect200410255715008948
- ChameuleauMEDDeenikWZweegmanSSuccessful treatment of subcutaneously disseminated aspergillosis with caspofungin acetate in an allogeneic peripheral blood stem cell transplantation patientHaematologica200388ECR1012681983
- ChamolisGKontoyiannisDPThe rationale of combination antifungal therapy in severely immunocompromised patients: empiricism versus evidence-based medicineCurr Opin Infect Dis200619380516804387
- ChandrasekarPHCutrightJLManavathuEKAspergillus: Rising frequency of clinical isolation and continued susceptibility to antifungal agents, 1994–1999Diagn Microbiol Infect Dis200141211411777662
- ChoiJHBrummerEStevensDACombined action of micafungin, a new echinocandin, and human phagocytes for antifungal activity against Aspergillus fumigatusMicrobes Infect20046383915050966
- ClancyCJHuangHChengSCharacterizing the effects of caspofungin on Candida albicans, Candida parapsilosis, and Candida glabrata isolates by simultaneous time-kill and postantifungal-effect experimentsAntimicrob Agents Chemother20065025697216801448
- ClarkTAHajjehRARecent trends in the epidemiology of invasive mycosesCurr Opin Infect Dis2002155697412821832
- ClearyJDSchwartzMRogersPDEffects of amphotericin B and caspofungin on histamine expressionPharmacotherapy2003239667312921242
- ClemonsKVEspirituMParmarRAssessment of the paradoxical effect of caspofungin in therapy of candidiasisAntimicrob Agents Chemother20065041293716569843
- CocuaudCRodierMHDaniaultGAnti-metabolic activity of caspofungin against Candida albicans and Candida parapsilosis biofilmsJ Antimicrob Chemother2005565071216040622
- ColomboALRosasRCSuccessful treatment of an Aspergillus brain abscess with caspofungin: case report of a diabetic patient intolerant of amphotericin BEur J Clin Microbiol Infect Dis200322575612938012
- DannaouiELortholaryODromerFIn vitro evaluation of double and triple combinations of antifungal drugs against Aspergillus fumigatus and Aspergillus terreusAntimicrob Agents Chemother200448970814982791
- de WetNLlanos-CuentasASuleimanJA randomized, double-blind, parallel-group, dose-response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV-positive patientsClin Infect Dis200439842915472817
- de WetNTBesterAJViljoenJJA randomized, double blind, comparative trial of micafungin (FK463) vs. fluconazole for the treatment of oesophageal candidiasisAliment Pharmacol Ther20052189990715801925
- DenningDWEchinocandin antifungal drugsLancet200336211425114550704
- DenningDWMarrKALauWMMicafungin (FK463) alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosisJ Infect2006 (in press)
- DeresinskiSCStevensDACaspofunginClin Infect Dis20033614455712766841
- Diflucan PIDiflucan package insert [online]2004New York, NYPfizer Accessed on 3 January 2006. URL: http://www.pfizer.com/pfizer/download/uspi_diflucan.pdf
- DinubileMJLupinacciRJBermanRSResponse and relapse rates of candidal esophagitis in HIV-infected patients treated with caspofunginAIDS Res Hum Retroviruses200218903812230933
- DouglasCMD’IppolitoJASheiGJIdentification of the FKS1 gene of Candida albicans as the essential target of 1,3-beta-D-glucan synthase inhibitorsAntimicrob Agents Chemother199741247199371352
- DowellJAKnebelWLuddenTPopulation pharmacokinetic analysis of anidulafungin, an echinocandin antifungalJ Clin Pharmacol200444590815145966
- DowellJAStogniewMKrauseDAssessment of the safety and pharmacokinetics of anidulafungin when administered with cyclosporineJ Clin Pharmacol2005452273315647416
- EhrmannSBastidesFGissotVCerebral aspergillosis in the critically ill: two cases of successful medical treatmentIntensive Care Med2005317384215782314
- EiblMAunerHWZinke-CerwenkaWHigh-risk AML complicated by pulmonary aspergillosis: successful treatment with nonmyeloablative stem cell transplantation and long-term administration of voriconazoleAnn Hematol200483133614530879
- Eraxis PIEraxis package insert [online]2006New York, NYPfizer Accessed on 20 September 2006. URL: http://www.fda.gov/cder/foi/label/2006/021632s000,021948s000lbl.pdf
- FeldmesserMKressYMednickAThe effect of the echinocandin analogue caspofungin on cell wall glucan synthesis by Cryptococcus neoformansJ Infect Dis20001821791511069257
- FlynnPMSeibelNArrietaATreatment of invasive aspergillosis (ia) in pediatric patients (pts) with micafungin (mica) alone or in combination with other systemic antifungal agents [abstract M-891]2006Abstracts of the 46th Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 27–30, 2006San Francisco, CA: American Society for Microbiology407
- FranklinJAMcCormickJFlynnPMRetrospective study of the safety of caspofungin in immunocompromised pediatric patientsPediatr Infect Dis J200322747912938676
- GardinerRESouteropoulosPParkSCharacterization of Aspergillus fumigatus mutants with reduced susceptibility to caspofunginMed Mycol200543Suppl 1S29930516110824
- GauthierGMNorkTMPrinceRSubtherapeutic ocular penetration of caspofungin and associated treatment failure in Candida albicans endophthalmitisClin Infect Dis200541e27816007519
- GhannoumMAChenABuhariMMulti-echinocandin resistant Candida parapsilosis: an emerging pathogen [abstract M-722a]2005Abstracts of the 45th Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 16–19, 2005Washington, DC: American Society for Microbiology
- Gil-LamaignereCSalvenmoserSHessRMicafungin enhances neutrophil fungicidal functions against Candida pseudohyphaeAntimicrob Agents Chemother2004482730215215137
- GlasmacherACornelyOAOrloppKCaspofungin treatment in severely ill, immunocompromised patients: a case-documentation study of 118 patientsJ Antimicrob Chemother2006571273416308418
- GoicoecheaMFiererJJohnsSTreatment of candidal cholangitis with caspofungin therapy in a patient with a liver transplant: documentation of biliary excretion of caspofunginClin Infect Dis2004381040115034841
- GrollAHStergiopoulouTRoilidesEMicafungin: pharmacology, experimental therapeutics and clinical applicationsExpert Opin Investig Drugs200514489509
- GrollAHAttarbaschiASchusterFRTreatment with caspofungin in immunocompromised paediatric patients: a multicentre surveyJ Antimicrob Chemother20065735273516431856
- GudlaugssonOGillespieSLeeKAttributable mortality of nosocomial candidemia, revisitedClin Infect Dis2003371172714557960
- HakkiMStaabJFMarrKAEmergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapyAntimicrob Agents Chemother2006502522416801435
- HamzaNSGhannoumMALazarusHMChoices aplenty: antifungal prophylaxis in hematopoietic stem cell transplant recipientsBone Marrow Transplant2004343778915247928
- HebertMFBloughDKTownsendRWConcomitant tacrolimus and micafungin pharmacokinetics in healthy volunteersJ Clin Pharmacol20054510182416100295
- HebertMFSmithHEMarburyTCPharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunctionJ Clin Pharmacol20054511455216172179
- HebertMFTownsendRWAustinSConcomitant cyclosporine and micafungin pharmacokinetics in healthy volunteersJ Clin Pharmacol2005459546016027407
- HerbrechtRDenningDWPattersonTFVoriconazole versus amphotericin B for primary therapy of invasive aspergillosisN Engl J Med20023474081512167683
- HernandezSLopez-RibotJLNajvarLKCaspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitisAntimicrob Agents Chemother2004481382315047549
- HesselingMWeindlingMNealTFirst reported use of caspofungin in an extremely low-birth-weight neonateJ Matern Fetal Neonatal Med20031421214694977
- HeynKTredupASalvenmoserSEffect of voriconazole combined with micafungin against Candida, Aspergillus, and Scedosporium spp. and Fusarium solaniAntimicrob Agents Chemother2005495157916304192
- IfranAKaptanKBeyanCEfficacy of caspofungin in prophylaxis and treatment of an adult leukemic patient with invasive pulmonary aspergillosis in allogeneic stem cell transplantationMycoses200448146815743435
- ItoMNozuRKuramochiTProphylactic effect of FK463, a novel antifungal lipopeptide, against Pneumocystis carinii infection in miceAntimicrob Agents Chemother20004422596210952565
- Jimenez-ExpositoMJTorresGBaraldesANative valve endocarditis due to Candida glabrata treated without valvular replacement: a potential role for caspofungin in the induction and maintenance treatmentClin Infect Dis200439e70315472836
- JohnsonMDMacDougallCOstrosky-ZeichnerLCombination antifungal therapyAntimicrob Agents Chemother20044869371514982754
- KahnJNHsuMRacineFCaspofungin susceptibility in Aspergillus and non-Aspergillus molds: inhibition of glucan synthase and reduction of ˜-D-1,3 glucan levels in cultureAntimicrob Agents Chemother20065022141616723587
- KarlowskyJAHobanDJZhanelGGIn vitro interactions of anidulafungin with azole antifungals, amphotericin B and 5-fluorocytosine against Candida speciesInt J Antimicrob Agents200627174716414247
- KartsonisNDiNubileMJBartizalKEfficacy of caspofungin in the treatment of esophageal candidiasis resistant to fluconazoleJ Acquir Immune Defic Syndr200231183712394797
- KartsonisNDiNubileMBradshawSCaspofungin (cas) for treatment (rx) of Candida urinary tract infections(uti): review of the cas clinical database [abstract 135]2003Abstracts of the 41st Interscience Concerence on Antimicrobial Agents and ChemotherapySeptember 14–17, 2003Chicago: American Society for Microbiology49
- KartsonisNASaahALipkaCJSecond-line therapy with caspofungin for mucosal or invasive candidiasis: results from the caspofungin compassionate-use studyJ Antimicrob Chemother2004538788115044431
- KartsonisNKillarJMixsonLCaspofungin susceptibility testing of isolates from patients with esophageal candidiasis or invasive candidiasis: relationship of MIC to treatment outcomeAntimicrob Agents Chemother20054936162316127030
- KartsonisNLipkaJBradshawSSafety of caspofungin (cas) during concomitant immunosuppressant (is) therapy [abstract M-956]2005Abstracts of the 45th Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 16–19, 2005Washington, DC: American Society for Microbiology420
- KartsonisNASaahAJLipkaCJSalvage therapy with caspofungin for invasive aspergillosis: results from the caspofungin compassionate use studyJ Infect20055019620515780413
- KatiyarSKVermitskyJPEdlindTDGenetic analysis of caspofungin susceptibility in Candida glabrata [abstract M-1589]2005Abstracts of the 45th Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 16–19, 2005Washington, DC:American Society for Microbiology439
- KetkoAKSobelJDAkinsRAGene overexpression analysis of echinocandin resistance in Candida albicans [abstract M-1758]2006Abstracts of the 46th Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 27–30, 2006San Francisco, CA: American Society for Microbiology434
- KleinLKLiLDesign and preparation of cyclopeptamine antifungal agentsCurr Pharm Des19995577110066884
- KohnoSMasaokaTYamaguchiHA multicenter, open-label clinical study of micafungin (FK463) in the treatment of deep-seated mycosis in JapanScand J Infect Dis2004363727915287383
- KontoyiannisDPHachemRLewisREEfficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignanciesCancer200398292912872348
- KontoyiannisDPRatanatharathornVVan BurikJMicafungin (mcf) alone or in combination with other licensed antifungal therapy (olat) in bone marrow transplant (bmt) recipients with invasive aspergillosis (ia) [abstract M-878]2006Abstracts of the 46th Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 2730, 2006San Francisco, CA: American Society for Microbiology404
- KrauseDSReinhardtJVazquezJAPhase 2, randomized, dose-ranging study evaluating the safety and efficacy of anidulafungin in invasive candidiasis and candidemiaAntimicrob Agents Chemother2004482021415155194
- KrauseDSSimjeeAEvan RensburgCA randomized, double-blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasisClin Infect Dis200439770515472806
- KrishnanSManavathuEKChandrasekarPHA comparative study of fungicidal activities of voriconazole and amphotericin B against hyphae of Aspergillus fumigatusJ Antimicrob Chemother2005559142015824093
- KubinCJLukoseTLoganAImpact of primary combination therapy on outcomes in invasive aspergillosis (ia) [abstract M-899]2006Abstracts of the 46th Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 27–30, 2006San Francisco, CA:American Society for Microbiology409
- KuhnDMGeorgeTChandraJAntifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandinsAntimicrob Agents Chemother20024617738012019089
- KurtzMBHeathIBMarrinanJMorphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-b-D-glucan synthaseAntimicrob Agents Chemother199438148097979276
- KurtzMBAbruzzoGFlatteryACharacterization of echinocandin-resistant mutants of Candida albicans: genetic, biochemical, and virulence studiesInfect Immunity1996643244518757860
- LeatherHLWingardJRIs combination antifungal therapy for invasive aspergillosis a necessity in hematopoietic stem-cell transplant recipients?Curr Opin Infect Dis200619371916804386
- LewisREKontoyiannisDPRationale for combination antifungal therapyPharmacotherapy2001218 Pt 2149S-64S
- LewisREKontoyiannisDPMicafungin in combination with voriconazole in Aspergillus species: a pharmacodynamic approach for detection of combined antifungal activity in vitroJ Antimicrob Chemother2005568879216188916
- LinSJSchranzJTeutschSMAspergillus case-fatality rate: Systematic review of the literatureClin Infect Dis2001323586611170942
- LiuKHWuCJChouCHRefractory candidal meningitis in an immunocompromised patient cured by caspofunginJ Clin Microbiol2004425950315583351
- LouieADezielMLiuWPharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activityAntimicrob Agents Chemother20054950586816304173
- LynchJWong-BeringerACaspofungin: a potential cause of reversible severe thrombocytopeniaPharmacotherapy20042414081115628837
- MaertensJRaadIPetrikkosGEfficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapyClin Infect Dis20043915637115578352
- MaertensJGlasmacherAHerbrechtRMulticenter, noncomparative study of caspofungin (cas) combined with other antifungals in adults with invasive aspergillosis (ia) refractory (r) or intolerant (i) to prior therapy (rx): final data [abstract M-954]2005Abstracts of the 45th Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 16–19, 2005Washington, DC: American Society for Microbiology420
- MaligieMASelitrennikoffCPCryptococcus neoformans resistance to echinocandins: (1,3)beta-glucan synthase activity is sensitive to echinocandinsAntimicrob Agents Chemother2005492851615980360
- MarrKABoeckhMCarterRACombination antifungal therapy for invasive aspergillosisClin Infect Dis20043979780215472810
- MarrKAHachemRPapanicolaouGRetrospective study of the hepatic safety profile of patients concomitantly treated with caspofungin and cyclosporin ATranspl Infect Dis200431101615569226
- MattiuzziGNAlvaradoGGilesFJOpen-label, randomized comparison of itraconazole versus caspofungin for prophylaxis in patients with hematologic malignanciesAntimicrob Agents Chemother200650143716377679
- McNeilMMNashSLHajjehRATrends in mortality due to invasive mycotic diseases in the United States, 1980–1997Clin Infect Dis200133641711486286
- MikamoHSatoYTamayaTIn vitro antifungal activity of FK463, a new water-soluble echinocandin-like lipopeptideJ Antimicrob Chemother200046485710980180
- MillerCDLomaestroBWParkSProgressive esophagitis caused by Candida albicans with reduced susceptibility to caspofunginPharmacotherapy2006268778016716141
- MiyazakiYMoriMTashiroTClinical efficacy for the combination of micafungin with amphotericin B against pulmonary aspergillosis [abstract M-1052]2005Abstracts of the 45th Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 16–19, 2005Washington, DC: American Society for Microbiology
- Mora-DuarteJBettsRRotsteinCComparison of caspofungin and amphotericin B for invasive candidiasisN Engl J Med20023472020912490683
- MoudgalVLittleTBoikovDMultiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditisAntimicrob Agents Chemother200549767915673762
- MrowczynskiWWojtalikMCaspofungin for Candida endocarditisPediatr Infect Dis J20042337615071306
- MunozPSinghNBouzaETreatment of solid organ transplant patients with invasive fungal infections: should a combination of antifungal drugs be used?Curr Opin Infect Dis2006193657016804385
- MurdochDPloskerGLAnidulafunginDrugs20046422495815456342
- Mycamine PIMycamine package insert [online]2005Osaka, JapanFujisawa Accessed on 3 January 2006. URL: http://www.astellas.us/docs/mycamine.pdf
- NevadoJDe AlarconAHernandezACaspofungin: a new therapeutic option for fungal endocarditisClin Microbiol Infect20051124815715728
- OdioCMArayaRPintoLECaspofungin therapy of neonates with invasive candidiasisPediatr Infect Dis J2004231093715626944
- OdioCMPintoLEAlfaroBPharmacokinetics of caspofungin in six premature neonates with invasive candidiasis at a neonatal intensive care unit [poster LB-16]2005The 45th Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 16–19, 2005Washington, DC
- OlsonJAAdler-MooreJPTreatment of Candida glabrata infection in immunosuppressed mice by using a combination of liposomal amphotericin B with caspofungin or micafunginAntimicrob Agents Chemother200549489590216304150
- OsherovNMayGSAlbertNDOverexpression of Sbe2p, a golgi protein, results in resistance to caspofungin in Saccharomyces cerevisiaeAntimicrob Agents Chemother2002462462912121919
- Ostrosky-ZeichnerLKontoyiannisDRaffalliJInternational, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemiaEur J Clin Microbiol Infect Dis2005246546116261306
- OtaSTanakaJKahataKSuccessful micafungin (fk463) treatment of invasive pulmonary aspergillosis in a patient with acute lymphoblastic leukemia in a phase II studyInt J Hematol200479390315218972
- PacettiSAGeloneSPCaspofungin acetate for treatment of invasive fungal infectionsAnn Pharmacother20033790812503942
- PaderuPParkSPerlinDSCaspofungin uptake is mediated by a high-affinity transporter in Candida albicansAntimicrob Agents Chemother2004483845915388444
- PappasPGRexJHLeeJA prospective observational study of candidemia: Epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patientsClin Infect Dis2003376344312942393
- ParkSPaderuPPerlinDSHuman serum modulates the antifungal efficacy of echinocandin drugs [abstract M-307]2006Abstracts of the 46th Interscience Conference on Antimicrobial Agents and ChemotherapySeptember 27–30, 2006San Francisco, CA: American Society for Microbiology400
- PetraitieneRPetraitisVGrollAAntifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemiaAntimicrob Agents Chemother200246122311751105
- PetraitisVPetraitieneRGrollAHDosage-dependent antifungal efficacy of V-echinocandin (LY303366) against experimental fluconazole-resistant oropharyngeal and esophageal candidiasisAntimicrob Agents Chemother200145471911158743
- PettengellKMynhardtJKluytsTFK463 South African Study Group. Successful treatment of oesophageal candidiasis by micafungin: a novel systemic antifungal agentAliment Pharmacol Ther2004204758115298643
- PfallerMADiekemaDJEffectiveness of anidulafungin in eradicating Candida species in invasive candidiasisAntimicrob Agents Chemother2005494795716251335
- PfallerMABoykenLHollisRJIn vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazoleJ Clin Microbiol2005435425716272464
- PfallerMABoykenLHollisRJIn vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillanceJ Clin Microbiol200644760316517851
- PhilipAOdabasiZRodriguezJIn vitro synergy testing of anidulafungin with itraconazole, voriconazole, and amphotericin B against Aspergillus spp. and Fusarium sppAntimicrob Agents Chemother2005493572416048988
- PrabhuRMOrensteinRFailure of caspofungin to treat brain abscesses secondary to Candida albicans prosthetic valve endocarditisClin Infect Dis2004391253415486856
- RaaschRHAnidulafungin: review of a new echinocandin antifungal agentExpert Rev Anti Infect Ther2004249950815482216
- RajendramRAlpNJMitchellARCandida prosthetic valve endocarditis cured by caspofungin therapy without valve replacementClin Infect Dis200540e72415825018
- RamageGVandeWalleKBachmannSPIn vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studiesAntimicrob Agents Chemother2002463634612384379
- RatanatharathornVFlynnPvan BurikJAMicafungin in combination with systemic antifungal agents in the treatment of refractory aspergillosis in bone marrow transplant patients (Abstract 2472)2002Program and abstracts of the American Society of Hematology 44th annual meeting (Philadelphia)September 6–10, 2002Washington, DC: American Society of Hematology
- ReboliARotsteinCPappasPAnidulafungin vs. fluconazole for treatment of candidemia and invasive candidiasis (c/ic) [abstract M-718]2005Abstracts of the 45th Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 16–19, 2005Washington, DC: American Society for Microbiology418
- RexJHPappasPGKarchmerAWA randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjectsClin Infect Dis2003361221812746765
- RuhnkeMKuseEChetchotisakdPComparison of micafungin and liposomal amphotericin B for invasive candidiasis [abstract M-722c]2005Abstracts of the 45th Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 16–19, 2005Washington, DC: American Society for Microbiology
- SableCANguyenBYTChodakewitzJASafety and tolerability of caspofungin acetate in the treatment of fungal infectionsTranspl Infect Dis20024253012123423
- SandhuPLeeWXuXHepatic uptake of the novel antifungal agent caspofunginDrug Metab Dispos2005336768215716364
- SakaedaTIwakiKKakumotoMEffect of micafungin on cytochrome P450 3A4 and multidrug resistance protein 1 activities, and its comparison with azole antifungal drugsJ Pharm Pharmacol2005577596415969931
- Sanz-Rodriguez, Lopez-DuarteMJuradoMSafety of the concomitant use of caspofungin and cyclosporin A in patients with invasive fungal infectionsBone Marrow Transplantation200434132015122312
- SarriaJCBradleyJCHabashRCandida glabrata endophthalmitis treated successfully with caspofunginClin Infect Dis200540e46815714407
- Schuetzer-MuehlbauerMWillingerBKrapfGThe Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofunginMol Microbiol2003482253512657057
- SeibelNLSchwartzCArrietaASafety, tolerability, and pharmacokinetics of Micafungin (FK463) in febrile neutropenic pediatric patientsAntimicrob Agents Chemother20054933172416048942
- SerenaCPastorJGilgadoFEfficacy of micafungin in combination with other drugs in a murine model of disseminated trichosporonosisAntimicrob Agents Chemother20054949750215673724
- SinghNLimayeAForrestGCombination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational studyTransplantation2006813320616477215
- SoustreJRodierMHImbert-BouyerSCaspofungin modulates in vitro adherence of Candida albicans to plastic coated with extracellular matrix proteinsJ Antimicrob Chemother200453522514749343
- SpellbergBEdwardsJIbrahimANovel perspectives on mucormycosis: pathophysiology, presentation, and managementClin Microbiol Rev2005185566916020690
- StevensDAEspirituMParmarRParadoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrationsAntimicrob Agents Chemother20044834071115328104
- StevensDAIchinomiyaMKoshiYEscape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for ˜-1,6-glucan synthesis inhibition by caspofunginAntimicrob Agents Chemother2006503160116940118
- StevensDAWhiteTCPerlinDSStudies of the paradoxical effect of caspofungin at high drug concentrationsDiagn Microbiol Infect Dis200551173815766602
- StoneJHollandSLiSEffect of hepatic insufficiency on the pharmacokinetics of caspofungin [abstract A-14]2001Abstracts of the 41st Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 16–19, 2001Chicago, IL, USA
- StoneJAMigoyaEMHickeyLPotential for interactions between caspofungin and nelfinavir or rifampinAntimicrob Agents Chemother20044843061415504857
- TacconeFSMarechalRMeulemanNCaspofungin salvage therapy in a neutropenic patient with probable invasive aspergillosis: a case reportSupport Care Cancer200311742413680323
- TrickWEFridkinSKEdwardsJRSecular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999Clin Infect Dis2002356273012173140
- van BurikJARatanatharathornVStepanDEMicafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantationClin Infect Dis20043914071615546073
- VillanuevaAArathoonEGGotuzzoEA randomized double-blind study of caspofungin versus amphotericin for the treatment of candidal esophagitisClin Infect Dis20013315293511588698
- VillanuevaAGotuzzoEArathoonEGA randomized double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasisAm J Med2002113294912361815
- WalshTJAdamsonPCSeibelNLPharmacokinetics, safety, and tolerability of caspofungin in children and adolescentsAntimicrob Agents Chemother20054945364516251293
- WalshTJTepplerHDonowitzGRCaspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropeniaN Engl J Med2004351139140215459300
- WangWLiQHasvoldLSteinerBDiscovery, SAR, synthesis, pharmacokinetic and biochemical characterization of A-192411: a novel fungicidal lipopeptide-(I)Bioorganic Medicinal Chemistry Letters2003134899312565957
- WertzKKPretzlaffRKCaspofungin in a pediatric patient with persistent candidemiaPediatr Crit Care Med20045181314987350
- WiederholdNPLewisREThe echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacyExpert Opin Investig Drugs200312121
- WiederholdNPKontoyiannisDPChiJPharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activityJ Infect Dis200419014647115378439
- WiederholdNPGraybillJRNajvarLKIn vitro pharmacodynamics of anidulafungin (afg) and caspofungin (cfg) against clinical isolates of Candida glabrata including strains with elevated MICs to cfg [abstract M-2160]2005Abstracts of the 45th Interscience Conference on Antimicrobial Agents and ChemotherapyDecember 1619, 2005Washington, DC: American Society for Microbiology456
- WiederholdNPKontoyiannisDPAttenuation of the activity of caspofungin at high concentrations against Candida albicans: possible roles of cell wall integrity and calcineurin pathwaysAntimicrob Agents Chemother2005a495146816304189
- WisplinghoffHBischoffTTallentSMNosocomial bloodstream infections in US hospitals: Analysis of 24, 179 cases from a prospective nationwide surveillance studyClin Infect Dis2004393091715306996
- YekutielAShalitIShadkchanYIn vitro activity of caspofungin combined with sulfamethoxazole against clinical isolates of Aspergillus sppAntimicrob Agents Chemother20044832798315328085
- YokoteTAkiokaTOkaSSuccessful treatment with micafungin of invasive pulmonary aspergillosis in acute myeloid leukemia, with renal failure due to amphotericin B therapyAnn Hematol20048364614661114
- YustesCGuarroJIn vitro synergistic interaction between amphotericin B and micafungin against Scedosporium sppAntimicrob Agents Chemother200549349850016048969
- ZaasAKAlexanderBDEchinocandins: role in antifungal therapy, 2005Exp Opin Pharmacother20056165768