Abstract
Procarbazine HCl is a ‘nonclassical’ oral alkylating anticancer agent that was first synthesized in the late 1950s. It has been used in the treatment of many cancers, but its main use is in the treatment of Hodgkin’s lymphoma and brain tumors and, to a lesser extent, Non-Hodgkin’s lymphoma and primary central nervous system lymphoma. Procarbazine is a prodrug that undergoes metabolic transformation into active intermediates that are thought to inhibit DNA, RNA, and protein synthesis. Early use of procarbazine in combination with mechlorethamine, vincristine, and prednisone (MOPP) was effective in the treatment of advanced Hodgkin’s lymphoma, but late toxic effects such as secondary cancer and infertility led to its replacement by other regimens. However, its recent reintroduction in the dose-intensified BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) regimen has yielded very promising findings. Procarbazine alone, or more commonly combined in the PCV (procarbazine, lomustine [CCNU], and vincristine) regimen, is also effective in treating gliomas comprising astrocytomas, glioblastomas, and oligodendrogliomas. The most common side effects of procarbazine are gastrointestinal disturbances, myelosuppression, and central nervous system effects. In conclusion, the use of procarbazine in combination with other drugs means that it remains a major anticancer drug in the management of Hodgkin’s lymphoma and gliomas.
Introduction
Procarbazine HCl is a ‘nonclassical’ oral alkylating agent that belongs to the same family as dacarbazine and hexamethylamine (CitationZeller et al 1963). It contains an N-methyl group that is essential for its activity, but not the chloroethyl group that is present in nitrogen mustard-type alkylating agents (CitationNewell et al 1987). It was first synthesized in the late 1950s during a search for a new monoamine oxidase inhibitor, but was soon developed as an anticancer agent (CitationKenis et al 1966; CitationMartin and Schubert 1966; CitationLivingston and Carter 1970; CitationFriedman 2001) and has since been used in the treatment of many cancers including Hodgkin’s lymphomas, non-Hodgkin’s lymphoma, brain tumors, multiple myeloma, primary central nervous system lymphoma, malignant melanoma, and lung cancer.
Procarbazine was first granted approval in 1965 and was subsequently marketed in around 84 countries. It has been widely deployed in the treatment of a number of cancers and a considerable body of clinical experience with this drug has accumulated. The introduction of safer combination therapies led to the gradual supplantation of procarbazine. During recent years, there has been a re-emergence of interest in procarbazine combinations with other chemotherapeutic agents, specifically for the treatment of Hodgkin’s lymphoma and gliomas and, to a lesser extent, non-Hodgkin’s lymphoma and primary central nervous system lymphoma. This is partly prompted by the unique mechanism of action of procarbazine; this agent has multiple sites of action and is not cross-resistant with other alkylating agents, cytostatics or radiotherapy (Matche et al 1963; CitationBrunner and Young 1965).
Reintroduction of procarbazine in the dose-intensified BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) regimen has yielded impressive therapeutic results in the treatment of Hodgkin’s lymphoma and, in combination with lomustine (CCNU) and vincristine in the PCV (procarbazine, CCNU, and vincristine) regimen, it shows considerable promise in the treatment of gliomas.
Preclinical studies
Pharmacology
Procarbazine is a prodrug that undergoes a complex metabolic transformation into active intermediates. Although these intermediates have been characterized, their precise mechanism of action remains unclear. They are thought to inhibit DNA, RNA, and protein synthesis (CitationBerneis et al 1963; CitationRutinshauser and Bollag 1963). Studies suggest that procarbazine may inhibit transmethylation of the methyl groups of methionine into t-RNA; the subsequent lack of functional t-RNA could cause cessation of protein synthesis, and consequently DNA and RNA synthesis. DNA may also be damaged directly by hydrogen peroxide, formed during auto-oxidation of procarbazine, which may attack the protein sulphydryl groups in residual protein that is tightly bound to DNA. These effects are specific for the S and G2 phases of the cell cycle. Drug resistance mechanisms to procarbazine also indicate that the methylating pathway is important in its mechanism of action and may help to develop new therapeutic strategies. Potential resistance mechanisms involve O6-alkylguanine DNA alkyltransferase (AGT)-mediated repair of O6-methylguanine activity and hMSH2 mismatch-repair deficiency (CitationPegg 1990; CitationFriedman et al 1997).
In vivo, significant antitumor activity has been demonstrated against Ehrlich carcinoma and Crocker sarcoma S180 in mice, and Walker carcinoma 256, Guerin uterine carcinoma T8, and intracerebral leukemia L1210 in rats (CitationBollag and Grunberg 1963).
Toxicology
Mutagenic effects of procarbazine have been demonstrated in a variety of bacterial and mammalian test systems, and it has been shown to inhibit fetal development in rats (CitationMalek et al 2003). Unscheduled DNA synthesis in the testes of rabbits has also been reported (CitationZbinden 1980). As might be expected with an agent that inhibits DNA, RNA, and/or protein synthesis, reduced fertility has been seen in male mice given procarbazine (CitationChryssanthou et al 1983).
Carcinogenic effects of procarbazine have been reported in mice, rats, and monkeys.
Pharmacokinetics and drug disposition
Procarbazine is quickly and completely absorbed from the gastrointestinal tract following oral administration, with peak plasma levels achieved after 0.5–1 hour (CitationSupko et al 2002; CitationPreiss et al 2006). It readily crosses the blood-brain barrier and peak cerebrospinal fluid levels are reached after 0.5–1.5 hours.
The half-life of procarbazine is about 10 minutes and it is rapidly and extensively metabolized by the liver. Oxidation of procarbazine to azo-procarbazine occurs by microsomal cytotchrome P450 oxidoreductase, as well as by mitochondrial monoamine oxidase enzymatic conversion. Plasma levels of azo-procarbazine are notably higher than those of procarbazine itself. Azo-procarbazine is microsomally oxidized to azoxyderivatives. Of these, methylazoxy-procarbazine appears to be the major cytotoxic compound, showing several-fold greater cytotoxicity than procarbazine, azo-procarbazine, and benzyazoxy-procarbazine (CitationErikson et al 1989). Plasma levels of methylazoxy-procarbazine peak after about 1.5 hours and it has a half-life of around 1 hour.
About 70% of the dose is renally excreted in the first 24 hours; the major urinary metabolite is the biologically inactive N-isopropylterphthalamic acid. The majority of the remainder is excreted via the respiratory system, with only minimal fecal excretion. Impaired renal or hepatic function can delay elimination of procarbazine and its active metabolites.
Clinical efficacy
Lymphomas
Hodgkin’s lymphoma
Hodgkin’s lymphoma affects approximately 8000 new patients each year in the USA and around 1500 in the UK, occurring most commonly in young adults and in people who are more than 55 years old (CitationGlaser and Jarrett 1996; CitationACS 2006). Although the management of the disease has improved considerably in recent years, around 20% to 30% of patients still experience disease progression or relapse.
Early studies reported overall response rates of 53% to 69% with single agent procarbazine in patients with advanced Hodgkin’s lymphoma (CitationBrunner and Young 1965; CitationLivingston and Carter 1970). However, these responses were generally very short-lived and there were few complete responses. In the early 1970s, procarbazine was used successfully in combination with three other drugs in the regimen known as MOPP (mechlorethamine [nitrogen mustard], Oncovin® [vincristine], procarbazine, and prednisone) in the treatment of advanced Hodgkin’s lymphoma (CitationDeVita et al 1970). A complete remission rate of 84% was attained, with relapse-free and overall survival rates of 66% and 48%, respectively, after more than 20 years. Unfortunately, although the acute toxic effects of MOPP are manageable, it is associated with significant long-term toxicity. For example, more than 90% of men, and 80%–100% of women over 25 years old, become irreversibly infertile (CitationPazdur et al 2003). There is also a risk of secondary acute leukemia, which is frequently resistant to treatment and has an extremely poor prognosis (CitationNg et al 2002). A number of MOPP-like regimens were developed in an attempt to reduce this toxicity. It would appear that procarbazine plays an important role in MOPP, as lower complete remission rates of shorter duration were reported with MOPP-like regimens that did not contain procarbazine (CitationNicholson et al 1970; CitationLuce et al 1971).
ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) subsequently became the regimen of choice for advanced disease after showing superior efficacy and less long-term toxicity than MOPP and MOPP hybrid regimens (CitationCanellos et al 1992; CitationDuggan et al 2003). The combination of MOPP plus epidoxirubicin, bleomycin, vinblastine, lomustine, doxorubicin, and vindesine (MOPPEBVCAD) has recently been suggested to be as effective as ABVD and more effective than the Stanford V schedule (doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, and prednisone) (CitationGobbi et al 2005, Citation2006). However, despite lower toxicity than normally expected with MOPP analogues, the incidence of second cancers remains somewhat higher than with ABVD. It is, nevertheless, a good option for patients who are not suitable for high-dose chemotherapy.
C-MOPP/ABV, a hybrid chemotherapy in which cyclophosphamide is substituted for mechlorethamine (which has been implicated as the cause of secondary malignancies) has shown beneficial effects (CitationMontoto et al 2000). In 73 patients with advanced disease, 57 (78%) achieved a complete response and 7 further patients who achieved partial response reached complete response after radiotherapy (CitationMonoto et al 2000). The overall survival rate after 4 years was 92%. In another study in which dacarbazine was added to the regimen (C-MOPP-ABVD), a response rate of 93% and a complete response rate of 84% was achieved amongst the 67 patients (CitationTakenaka et al 2000).
A recent report of the long-term follow-up of 533 patients with advanced Hodgkin’s lymphoma indicated that ABVPP (doxorubicin, bleomycin, vinblastine, procarbazine, and prednisone) was associated with a better survival probability than MOPP-ABV (CitationFerme et al 2006). The 10-year overall survival estimates were 90% for ABVPP, 78% for MOPP-ABV, 82% for MOPP-ABV and radiotherapy, and 77% for ABVPP and radiotherapy (p = 0.03).
The German Hodgkin’s Study Group developed a new combination regimen known as BEACOPP, based on a statistical model that considers tumor growth and chemotherapeutic effects (CitationLoeffler et al 1998). The dose of procarbazine is 100 mg/m2 over 7 days, which is half that delivered over 14 days in the MOPP regimen. Both regimens are given on a 21-day cycle. In a randomized study, 1195 patients with newly diagnosed Hodgkin’s lymphoma in an unfavorable stage (IIB, IIIA, IIIB, or IV) were randomized to receive eight cycles of COPP-ABVD (alternating cyclophosphamide, vincristine, procarbazine, and prednisone with doxorubicin, bleomycin, vinblastine, and dacarbazine), standard-dose BEACOPP, or increased-dose BEACOPP (), each followed by local radiotherapy when indicated (CitationDiehl et al 2003). The outcome of treatment and five-year survival rates are shown in . The increased-dose BEACOPP regimen was significantly (pd ≤ 0.002) more effective than COPP-ABVD in terms of freedom from treatment failure (87% vs 69%) and overall survival (91% vs 83%) at 5 years, as well as early progression (2% vs 10%). Standard-dose BEACOPP was also significantly (p = 0.04) more effective than COPP-ABVD for freedom from treatment failure at 5 years (76% vs 69%). When the two BEACOPP regimens were compared with each other, the increased-dose regimen resulted in a significantly lower rate of early progression (2% vs 8%) and a significantly (p < 0.001) greater freedom from treatment failure at 5 years (87% vs 76%). Acute hematological effects were more common with the increased-dose BEACOPP regimen. The actuarial rate of secondary acute leukemia five years after diagnosis of Hodgkin’s disease was also significantly (p = 0.03) higher with increased-dose BEACOPP (2.5%) compared with standard-dose (0.6%), and COPP-ABVD (0.4%).
Table 1 COPP-ABVD, standard-dose BEACOPP, and increased-dose BEACOPP dosage regimens (CitationDiehl et al 2003)
Table 2 Outcome of treatment and five-year survival rates with COPP-ABVD, standard-dose BEACOPP, and increased-dose BEACOPP (CitationDiehl et al 2003)
The acute hematological toxicity of standard-dose and dose-intensified BEACOPP was investigated in 858 patients with advanced Hodgkin’s lymphoma (CitationEngel et al 2000). Dose-limiting toxicity occurred in 25% of cycles of dose-intensified treatment, but severe thrombocytopenia and leukocytopenia were rare. Myelosuppression was the most important dose-limiting factor. The findings indicated that, despite some increase in hemotoxicity, moderate dose escalation was safe for most patients receiving granulocyte colony-stimulating factor (G-CSF) and standard supportive treatment.
BEACOPP has also been used successfully as salvage treatment in patients with early stage Hodgkin’s lymphoma who relapsed after extended field radiotherapy. A retrospective analysis of 107 patients treated mainly with COPP-ABVD-like regimens (n = 74) or BEACOPP (n = 24) showed that BEACOPP was significantly (p = 0.02) superior to the other regimens, with 100% of patients achieving complete remission compared with 85% of those given COPD-ABVD-like regimens, and 67% given other regimens including radiotherapy (CitationRüffer et al 2005). BEACOPP was also a significant (p = 0.03) prognostic factor for freedom from second failure.
In a study in children and adolescents with advanced disease, induction treatment with dose-intensified BEACOPP improved early tumor control, with a 45% response rate (>70% reduction in disease burden) after 2 cycles and a 72% response rate after 4 cycles (CitationKelly et al 2002).
Non-Hodgkin’s lymphoma
Single-agent procarbazine is less active in non-Hodgkin’s lymphoma than in Hodgkin’s lymphoma, resulting in overall response rates of only around 36%–40% (CitationLivingston and Carter 1970), although it has a role to play for patients who fail standard regimens or who cannot tolerate or refuse such treatment (CitationChaar et al 2006). Combination regimens containing procarbazine, such as MOPP and COPP (cyclophosphamide, Oncovin® [vincristine], procarbazine, and prednisone), have resulted in more beneficial effects, particularly in patients with advanced stage nodular mixed or lymphocytic poorly differentiated disease (CitationLowenbraun et al 1970; CitationDeVita et al 1975; CitationLongo et al 1984; CitationEzdinli et al 1985). The best response rates to regimens containing procarbazine are seen with intensive cyclic multi-drug combinations such as ProMACE-MOPP (procarbazine, methotrexate with leucovorin, doxorubicin, cyclophosphamide, and etoposide). In a randomized, controlled trial, 221 patients with diffuse intermediate- to high-grade non-Hodgkin’s lymphoma received ProMACE-MOPP for 6 cycles or the newer generation more aggressive MACOP-B (methotrexate with leucovorin, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin) for 12 weeks (CitationSertoli et al 1994). There was no significant difference between the ProMACE-MOPP and MACOP-B groups in terms of complete remission rate (49.1% and 52.3%), three-year overall survival rate (45.2% and 52.3%), and three-year progression-free survival rate (36.4% and 36.1%). Toxicity was also similar with both treatments. THP-COPBLM (pirarubicin, cyclophosphamide, vincristine, prednisone, bleomycin, and procarbazine) combined with G-CSF in 42 patients with intermediate- or high-grade non-Hodgkin’s lymphoma resulted in a complete remission rate of 88% and a partial remission rate of 12% (CitationNiitsu and Umeda 1998). The 3-year survival rate was 72% and the 3-year event-free survival rate was 58%. In 51 older patients (e ≥ 55 years old) with intermediate-grade non-Hodgkin’s lymphoma, COPBLAM-I (cyclophosphamide, vincristine, prednisone, bleomycin, doxorubicin, and procarbazine) resulted in a complete remission rate of 65% after a median follow-up of 2.4 years (CitationShpilberg et al 1994). The projected 3-year overall survival and progression-free survival rates were 58% and 77%, respectively.
Primary central nervous system lymphoma
Primary central nervous system lymphoma is an aggressive non-Hodgkin’s lymphoma arising in the brain, eyes, spinal cord, or leptomeninges. The incidence has increased markedly over the last two decades, both in patients with and without immunodeficiency (CitationCorn et al 1997).
Although the use of radiotherapy prior to methotrexate improves survival in these patients, it is also associated with neurotoxic effects, such as severe cognitive dysfunction and ataxia (CitationDeAngelis et al 1989). This is a particular problem because at least half of all patients are at least 60 years old at the time of diagnosis. To avoid this problem, a number of chemotherapeutic regimens have been investigated as sole therapy. Procarbazine crosses the blood-brain barrier and has therefore been included in some of these regimens.
In a study of 52 patients treated with 5 cycles of high-dose methotrexate, procarbazine, and vincristine, with or without whole-brain radiotherapy, the objective response rate was 90% and the median survival 60 months (CitationAbrey et al 2000). The median survival was similar amongst the older patients (>60 years old), irrespective of whether or not they received radiotherapy, but late neurotoxicity was significantly (p = 0.0004) more common in those who received radiotherapy. Another study, conducted by the European Organisation for Research and Treatment of Cancer (EORTC) Brain Tumour Group in 50 elderly patients (median age 72 years), investigated the use of high-dose methotrexate, lomustine, procarbazine, methylprednisolone, and intrathecal chemotherapy with methotrexate and cytarabine (CitationHoang-Xuan et al 2003). The objective response rate was 48% (42% complete and 6% partial responses); the median duration of the response in the patients with complete response was 27 months; the median survival was 14.3 months; and the one-year progression-free survival was 40%. The majority of patients had improved or preserved cognitive function and Karnofsky performance scores (KPS) until relapse. A decline in cognitive function and KPS attributed to late neurotoxicity occurred in only 8% and 12% of patients, respectively. In the long-term responders who were in remission 2 years after the start of therapy, only one patient showed a decline in cognitive function or performance status due to delayed neurotoxicity. This outcome was superior to that expected with radiotherapy alone in elderly patients and considerably reduced the risk of delayed neurotoxicity.
Blood-brain barrier disruption (BBBD) results in enhanced chemotherapy delivery. In 74 patients who underwent BBBD preceded by systemic cyclophosphamide and followed by intra-arterial methotrexate in combination with procarbazine and dexamethasone, a complete response was achieved in 65% (n = 48) and a partial response in 19% (n = 14) (CitationMcAllister et al 2000). Amongst the 36 patients who continued to show a complete response after one year, none had any evidence of cognitive loss.
Procarbazine has also been used as part of the PCV (procarbazine, CCNU, and vincristine) regimen. Although the prognosis at relapse is often poor for patients suffering from primary central nervous system lymphoma, treatment of 7 relapsed patients with PCV resulted in a complete response in 4 and a partial response in 2 (CitationHerrlinger et al 2000).
Brain tumors—glioma/astrocytoma
Gliomas comprise about half of all primary brain tumors and include astrocytomas, glioblastomas, and oligodendrogliomas. The dosage details for the PCV and intensified PCV regimens are shown in . A summary of the main clinical trials into the use of single-agent procarbazine and procarbazine-containing regimens in patients with glioma is provided in .
Table 3 PCV and intensified PCV regimens
Table 4 Use of single-agent procarbazine or procarbazine-containing regimens to treat glioma
Interest in the use of procarbazine emerged around 1990 when data from the Northern California Oncology Group was reanalyzed to compare the effects of carmustine (BCNU) with that of PCV following radiation/oral hydroxyurea in patients with glioblastoma multiforme or other anaplastic gliomas (CitationLevin et al 1990). PCV treatment resulted in longer survival and time to tumor progression than BCNU, with the difference achieving statistical significance in the group of patients with anaplastic gliomas.
In a randomized study of 171 patients with grade III/IV astrocytoma given PCV, with or without radiotherapy, the median time to progression was 21 weeks and the median survival time was 53 weeks (CitationSandberg-Wollheim et al 1991). Overall, 18% of patients survived for at least two years. Survival analysis showed that the best responses were achieved in patients less than 50 years old who received both PCV and radiotherapy (median time to progression and survival time of 81 and 124 weeks, respectively).
A durable response to PCV was reported by CitationKim and colleagues (1996) in 32 patients with grade III/IV oligoastrocytoma or anaplastic oligodendroglioma. The PCV therapy was administered every 6 weeks for a total of at least 124 cycles. The median duration of follow-up review from the start of chemotherapy was 19.3 months. Grade 3 or 4 hematological toxicity was experienced by nine (31%) of 29 patients. Ten patients had delayed treatment due to treatment-related toxicities (34.5%). Ninety-one percent of the 32 patients responded to the therapy. These included 10 patients with a complete response and 19 with a partial response. The median time to progression was 15.4 months for all patients and 23.2 months for those with Grade III tumors. The median time to progression for patients with Grade III oligoastrocytomas was 13.8 months; for those with Grade IV oligoastrocytoma it was 12.4 months, and for those with anaplastic oligodendrogliomas it was 63.4 months (p = 0.0348). These patients survived a median of 49.8 months, 16 months, and 76 or more months, respectively, from the start of chemotherapy (p = 0.0154), thus showing that PCV therapy provides durable responses in patients with Grade III or IV oligoastrocytomas.
A number of other studies found no marked beneficial effects of PCV therapy on survival (CitationPrados et al 1999; CitationKappelle et al 2001; CitationMRC 2001) and so there has been an increasing tendency to use procarbazine as a salvage option. However, little data are available in the literature regarding this strategy (CitationRodriguez et al 1989; CitationNewton et al 1990; CitationBrandes et al 1999; CitationNewlands et al 2003). Response rates of 15% to 25% were reported with single-agent procarbazine in patients with recurrent glioblastoma or anaplastic astrocytoma (CitationRodriguez et al 1989; CitationNewton et al 1990). An overall response rate of 30% was achieved with combined procarbazine and tamoxifen in patients with high-grade gliomas that recurred after surgery and radiotherapy (CitationBrandes et al 1999). Compared with patients suffering from glioblastoma, those with anaplastic astrocytoma had a significantly longer median time to progression (33 vs 13 weeks) and median survival time (57 vs 27 weeks).
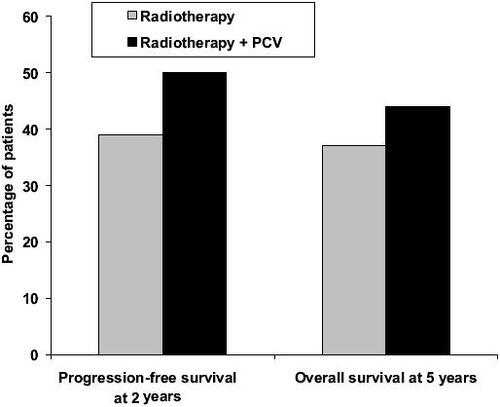
Recent results of adjuvant PCV in newly diagnosed patients with anaplastic oligodendroglioma are extremely encouraging (CitationBuckner et al 2003; CitationStege et al 2005; Citationvan den Bent et al 2005, Citation2006; Yamamoto et al 2005). PCV resulted in tumor regression in a meaningful proportion of newly diagnosed low-grade oligodendroglioma, with or without loss of 1p/19q. In a phase III EORTC study (Citationvan den Bent et al 2005, Citation2006), 6 cycles of adjuvant PCV following radiotherapy in 368 patients with highly anaplastic oligodendroglioma prolonged the progress-ion-free survival from 13 to 23 months compared with radiotherapy alone (p = 0.0018) (). Overall survival was also longer with adjuvant PCV, but the difference did not reach statistical significance (). The percentage of patients with progression-free survival after 2 years and overall survival after 5 years also showed advantages for adjuvant PCV treatment (). Recent evidence suggests that PCV may also be beneficial in patients with symptomatic low-grade oligodendroglioma (CitationCatenoix et al 2006).
Figure 1 Median progression-free survival and overall survival in patients with newly diagnosed anaplastic oligodendroglioma treated with radiotherapy alone or radiotherapy plus PCV (procarbazine, CCNU, and vincristine) (Citationvan den Bent et al 2006).
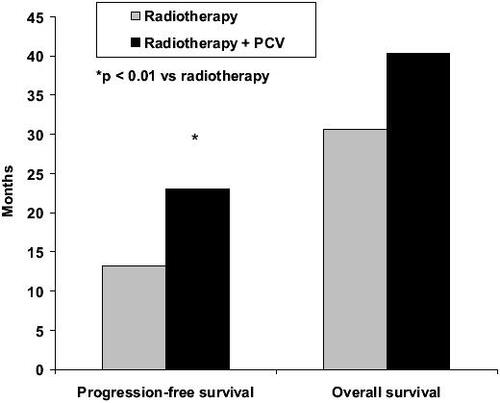
Figure 2 Percentage of patients with progression-free survival after 2 years and overall survival after 5 years in patients with newly diagnosed anaplastic oligodendroglioma treated with radiotherapy alone or radiotherapy plus PCV (procarbazine, CCNU, and vincristine) (Citationvan den Bent et al 2006).
Beneficial effects were reported with 6-thioguanine, procarbazine, dibromodulcitol, lomustine, and vincristine (TPDCV) in 42 children and young people with malignant astrocytomas (CitationLevin et al 2000). Of the 17 patients with glioblastoma multiforme, 13 died, the median time to progression was 49 weeks and median survival was 85 weeks. Amongst the remaining 25 patients with nonglioblastoma malignant astrocytoma, 14 died and the median time to progression was 224 weeks. The median survival was not reached in this group after a median follow-up of 494 weeks. The BBSFOP protocol, which comprises seven cycles of three drug pairs (carboplatin-procarbazine, cisplatin-etoposide and vincristine-cyclopho-sphamide), was used as first line treatment to avoid radiotherapy in 21 children (<5 years old) with high-grade glioma (CitationDufour et al 2006). The 5-year progression-free survival was 35% and the 5-year overall survival was 59%, with a median follow-up of 5.2 years. Of the 12 children still alive, 10 have not required radiotherapy.
Procarbazine, nimustine, and vincristine given in combination with hyperfractionated radiotherapy resulted in an objective response in 3 out of 8 patients with high-grade glioma (Ogawa et al 2006). The median time to progression was 10.7 months and the median survival time of all patients was 15.0 months. The treatment was safe and well tolerated.
Recent evidence indicates that enzyme-inducing anti-seizure medications have no effect on the pharmacokinetics of procarbazine in patients with glioma, but that there may be an increased risk of hepatic dysfunction with this drug combination (CitationGrossman et al 2006).
Safety and tolerability
Gastrointestinal disturbances (eg, nausea and vomiting), myelosuppression (eg, leukopenia, anemia, and thrombopenia) and central nervous system effects (eg, headache, depression, nervousness, and insomnia) are the most common side-effects seen with procarbazine, followed by neuromuscular problems such as tremor, weakness, and parasthesia. Procarbazine has also been associated with secondary nonlymphoid malignancies, including acute leukemia and solid tumors such as lung cancer, when used in combination therapy. Azoospermia and adverse effects on female gonadal function may also be a problem. Rare side-effects include hemolysis in patients with glucose 6-phosphate dehydrogenase deficiency, hypersensitivity reactions, eosinophilia, and pulmonary infiltrates.
Since procarbazine is a weak monoamine oxidase inhibitor, there is a possibility of interactions with food containing high levels of tyramine (eg, beer, cheese, wine, bananas) and with certain drugs such as sympathomimetics and decongestants. Possible effects include hypertensive crisis, intracranial bleeding, and headache. Procarbazine can also interact with other medications. For example, central nervous system depressants (eg, anesthetics, barbiturates, narcotic analgesics), drugs with anticholinergic effects (eg, tricyclic antidepressants), and antihypertensive agents should all be used with caution and in reduced dosage in patients taking procarbazine due to possible potentiation effects. Alcohol should also be avoided as disulfiram-like effects such as sweating and facial flushing may occur.
Discussion and place in therapy
Procarbazine is a long established anticancer medicine. Although there is renewed interest in this agent, it is worthwhile to remember the many patient-years of experience in many different oncological indications which exist; few ‘new’ cancer therapies have the benefit of such experience.
Although there was a long period in which interest in procarbazine waned due to safety concerns and the development of other cancer treatments, interest is currently re-emerging, mainly in combination with other agents for the treatment of Hodgkin’s lymphoma and gliomas, for which procarbazine activity is high. Some of the early combination therapies which used procarbazine (such as MOPP) were effective, but their use was limited by late toxicity. The new generation of procarbazine-containing combination therapies have been optimized to maximize efficacy and minimize the late-toxic effects seen with the first combinations.
Procarbazine looks likely to continue in the anticancer armamentarium for some time to come and, as a component of the BEACOPP combination, become part of the proposed ‘gold standard’ treatment for Hodgkin’s lymphoma (CitationMassoud et al 2004). Recent results of adjuvant PCV in newly diagnosed patients with anaplastic oligodendroglioma are also extremely encouraging. Despite its long history, procarbazine is still an important drug in the treatment of Hodgkin’s lymphoma and gliomas.
References
- AbreyLEYahalomJDeAngelisLMTreatment for primary CNS lymphoma: the next stepJ Clin Oncol20001831445010963643
- [ACS] American Cancer SocietyCancer facts and figures 2006 [online]2006 Accessed on 28 December 2006. URL: http://www.cancer.org/downloads/STT/CAFF2006PWSecured.pdf
- BerneisKKoflerMBollagWThe degradation of DNA by a new tumor inhibiting compoundExperientia196319132313971061
- BollagWGrunbergEThe degradation of deoxyribonucleic acid by new tumour inhibiting compounds: the intermediate formation of hydrogen peroxideExperientia196319130113968615
- BrandesAAErmaniMTurazziSProcarbazine and high-dose tamoxifen as a second-line regimen in recurrent high-grade gliomas: a phase II studyJ Clin Oncol1999176455010080610
- BrandesAATosoniAVastolaFEfficacy and feasibility of standard procarbazine, lomustine, and vincristine chemotherapy in anaplastic oligodendroglioma and oligoastrocytoma recurrent after radiotherapy. A Phase II studyCancer200410120798515372474
- BrunnerKWYoungCWA methylhydrazine derivative in Hodgkin’s disease and other malignant neoplasms. Therapeutic and toxic effects studied in 51 patientsAnn Intern Med196563698614305970
- BucknerJCGesmeDJrO’FallonJRPhase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalitiesJ Clin Oncol200321251512525516
- CanellosGPAndersonJRPropertKJChemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, MOPP alternating with ABVDN Engl J Med19923271478841383821
- CatenoixHHonnoratJCartalat-CarelS[Long-term outcome in patients with symptomatic low-grade oligodendrogliomatous tumors treated by cytotoxic agents.]Rev Neurol200616210697517086143
- ChaarBTSalemPPetruskaPJProcarbazine for non-Hodgkin’s lymphomaLeukemia Lymphoma2006476374016690522
- ChryssanthouCPWallachRCAtchisonMMeiotic chromosomal changes and sterility produced by nitrogen mustard and procarbazine in miceFertil Steril198339971026848396
- CornBWMarcusSMTophamAWill primary central nervous system lymphoma be the most frequent brain tumor diagnosed in the year 2000?Cancer1997792409139191531
- CoyleTBaptistaJWinfieldJMechlorethamine, vincristine and procarbazine chemotherapy for recurrent high-grade glioma in adults: a phase II studyJ Clin Oncol199082014182230893
- DeAngelisLMDelattreJYPosnerJBRadiation-induced dementia in patients cured of brain metastasesNeurology198939789962725874
- DeVitaVTJrCanellosGPChabnerBAdvanced diffuse histiocytic lymphoma, a potentially curable diseaseLancet197512485046388
- DevitaVTJrSerpickAACarbonePPCombination chemotherapy in the treatment of advanced Hodgkin’s diseaseAnn Intern Med197073881955525541
- DiehlVFranklinJPfreundschuhMStandard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s diseaseN Engl J Med200334823869512802024
- DufourCGrillJLellouch-TubianaAHigh-grade glioma in children under 5 years of age: A chemotherapy only approach with the BBSFOP protocolEur J Cancer20064229394516962317
- DugganDBPetroniGRJohnsonJLRandomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: report of an Intergroup trialJ Clin Oncol2003216071412586796
- EngelCLoefflerMSchmitzHAcute hematologic toxicity and practicability of dose-intensified BEACOPP chemotherapy for advanced stage Hodgkin’s disease. German Hodgkin’s Lymphoma Study Group (GHSG)Ann Oncol20001111051411061603
- EriksonJMTweedieDJDucoreJMCytotoxicity and DNA damage caused by the azoxy metabolites of procarbazine in L1210 tumor cellsCancer Res198949127332908840
- EzdinliEZAndersonJRMelvinFModerate versus aggressive chemotherapy of nodular lymphocytic poorly differentiated lymphomaJ Clin Oncol19853769753839262
- FermeCMounierNCasasnovasOLong-term results and competing risk analysis of the H89 trial in patients with advanced-stage Hodgkin lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte (GELA)Blood200610746364216478882
- FriedmanHSChabnerBALongoDLAlkylating agents in cancer chemotherapy and biotherapy20014th editionPhiladelphiaLippincott Williams & Wilkins415
- FriedmanHSJohnsonSPDongQMethylator resistance mediated by mismatch repair deficiency in a glioblastoma multiforme xenograftCancer Res199757293369230204
- GlaserSLJarrettRFThe epidemiology of Hodgkin’s diseaseBaillieres Clin Haematol1996940168922237
- GobbiPGBrogliaCLevisAMOPPEBVCAD chemotherapy with limited and conditioned radiotherapy in advanced Hodgkin’s lymphoma: 10-year results, late toxicity, and second tumorsClin Cancer Res2006125293516428496
- GobbiPGLevisAChisesiTABVD versus modified Stanford V versus MOPPEBVCAD with optional and limited radiotherapy in intermediate- and advanced-stage Hodgkin’s lymphoma: final results of a multicenter randomized trial by the Intergruppo Italiano LinfomiJ Clin Oncol200523919820716172458
- GrossmanSACarsonKABatchelorTTThe effect of enzyme-inducing antiseizure drugs on the pharmacokinetics and tolerability of procarbazine hydrochlorideClin Cancer Res20061251748116951236
- HerrlingerUBruggerWBambergMPCV salvage chemotherapy for recurrent primary CNS lymphomaNeurology2000541707810762527
- HildebrandJde WitteOSahmoudTResponse of recurrent glioblastoma and anaplastic astrocytoma to dibromodulcitol, BCNU and procarbazine - a phase II studyJ Neurooncol199837155609524094
- Hoang-XuanKTaillandierLChinotOChemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor GroupJ Clin Oncol20032127263112860951
- JeremicBJovanovicDDjuricLJAdvantage of post-radiotherapy chemotherapy with CCNU, procarbazine and vincristine (mPCV) over chemotherapy with VM-26 and CCNU for malignant gliomasJ Chemother1992412361321239
- KappelleACPostmaTJTaphoornMJPCV chemotherapy for recurrent glioblastoma multiformeNeurol20015611820
- KellyKMHutchinsonRJSpostoRFeasibility of upfront dose-intensive chemotherapy in children with advanced-stage Hodgkin’s lymphoma: preliminary results from the Children’s Cancer Group Study CCG-59704Ann Oncol200213Suppl 11071112078889
- KenisYDe SmedtJTagnonHJThe action of Natulan in 94 cases of solid tumorsEur J Cancer196625175962294
- KimLHochbergFHThorntonAFProcarbazine, lomustine, and vincristine (PCV) chemotherapy for grade III and grade IV oligoastrocytomasJ Neurosur1996856027
- KyritsisAPYungWKJaeckleKACombination of 6-thioguanine, procarbazine, lomustine and hydroxyurea for patients with recurrent malignant gliomasNeurosurgery19963992168905746
- LevinVALambornKWaraWPhase II study of 6-thioguanine, procarbazine, dibromodulcitol, lomustine and vincristine chemotherapy with radiotherapy for treating malignant glioma in childrenNeuro-oncol2000222811302250
- LevinVASilverPHanniganJSuperiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas. NCOG 6G61 final reportInt J Radiat Oncol Biol Phys19901832142154418
- LevinVAUhmJHJaeckleKAPhase III randomized study of postradiotherapy chemotherapy with alpha-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N’-cyclohexyl-N-nitrosurea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiformeClin Cancer Res2000638788411051233
- LevinVAWaraWMDavisRLPhase III comparison of BCNU and the combination of procarbazine, CCNU and vincristine administered after radiotherapy with hydroxyurea for malignant gliomasJ Neurosurg198563218232991486
- LivingstonRBCarterSKSingle agents in cancer chemotherapy1970New YorkIFI/Plenum Data Corp.31836
- LoefflerMHasencleverDDiehlVModel based development of the BEACOPP regimen for advanced Hodgkin’s disease. German Hodgkin’s Lymphoma Study GroupAnn Oncol19989738
- LongoDLYoungRCHubbardSMProlonged initial remission in patients with nodular mixed lymphomaAnn Intern Med198410065166370065
- LowenbraunSDeVitaVTSerpickAACombination chemotherapy with nitrogen mustard, vincristine, procarbazine, and prednisone in lymphosarcoma and reticulum cell sarcomaCancer1970251018254910254
- LuceJKGambleJFWilsonHECombined cyclophosphamide vincristine, and prednisone therapy of malignant lymphomaCancer197128306174935774
- McAllisterLDDoolittleNDGuastadisegniPECognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphomaNeurosurgery200046516110626935
- MalekFAMoritzKUFanghanelJEffects of prenatal procarbazine administration on intrauterine development in ratsAnn Anat20031851171912725435
- MartinHSchubertJCCytostatic treatment of polycythemia vera with a methylbenzylhydrazine derivativeDtsch Med Wochenschr1966915575900941
- MassoudMArmandJPRibragVProcarbazine in haematology: an old drug with a new life?Eur J Cancer2004401924715315798
- MontotoSCamosMLopez-GuillermoAHybrid chemotherapy consisting of cyclophosphamide, vincristine, procarbazine, prednisone, doxorubicin, bleomycin and vinblastine (C-MOPP/ABV) as first-line treatment for patients with advanced Hodgkin diseaseCancer2000882142810813727
- [MRC] Medical Research Council Brain Tumor Working PartyRandomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trialJ Clin Oncol2001195091811208845
- NewellDGescherAHarlandSN-methyl antitumor agents: a distinct class of anticancer drugs?Cancer Chemother Pharmacol198719911023552281
- NewlandsESFosterTZaknoenSPhase I study of temozolamide (TMZ) combined with procarbazine (PCB) in patients with gliomasBr J Cancer2003892485112865911
- NewtonHBJunckLBrombergJProcarbazine chemotherapy in the treatment of recurrent malignant astrocytomas after radiation and nitrosourea failureNeurol19904017436
- NgAKBernardoMVWellerESecond malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long-term risks and risk factorsBlood200210019899612200357
- NicholsonWMBeardMECrowtherDCombination chemotherapy in generalized Hodgkin’s diseaseBr Med J197037105427500
- NiitsuNUmedaMBiweekly THP-COPBLM (pirarubicin, cyclophosphamide, vincristine, prednisone, bleomycin and procarbazine) regimen combined with granulocyte colony-stimulating factor (G-CSF) for intermediate- and high-grade non-Hodgkins’s lymphomaLeukemia1998121457609737696
- PazdurRCoiaLRHoskinsWJCancer management: a multidisciplinary approach2003New YorkThe Oncology Group
- PeggAEMammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agentsCancer Res1990506119292205376
- PradosMDScottCCurranWJJrProcarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma: A retrospective review of radiation therapy oncology group protocols comparing survival with carmustine or PCV adjuvant chemotherapyJ Clin Oncol19991733899510550132
- PreissRBaumannFRegenthalRPlasma kinetics of procarbazine and azo-procarbazine in humansAnti-Cancer Drugs200617758016317293
- RodriguezLAPradosMSilverPReevaluation of procarbazine for the treatment of recurrent malignant central nervous system tumorsCancer198964242032555038
- RüfferJUBallovaVGlossmannJBEACOPP and COPP/ABVD as salvage treatment after primary extended field radiation therapy of early stage Hodgkins disease— results of the German Hodgkin Study GroupLeuk Lymphoma2005461561716236610
- RutinshauserABollagWCytological investigations of a new class of cytotoxic agents methylhydrazine derivativesExperientia196319131213975694
- Sandberg-WollheimMMalmstromPStrombladLA randomized study of chemotherapy with procarbazine, vincristine, and lomustine with and without radiation therapy for astrocytoma grades 3 and/or 4Cancer1991682292049748
- SertoliMRSantiniGChisesiTMACOP-B versus ProMACE-MOPP in the treatment of advanced diffuse non-Hodgkin’s lymphoma: results of a prospective randomized trial by the non-Hodgkin’s Lymphoma Cooperative Study GroupJ Clin Oncol1994121366747517442
- ShpilbergOShiffJChetritAThe cyclophosphamide, vincristine, prednisone, bleomycin, doxorubicin, and procarbazine (COPBLAM-I) regimen for intermediate-grade non-Hodgkin’s lymphoma. Long term follow-up in 51 patientsCancer1994743029337525042
- StegeEMKrosJMde BruinHGSuccessful treatment of low-grade oligodendroglial tumors with a chemotherapy regimen of procarbazine, lomustine, and vincristineCancer2005103802915637687
- SupkoJGHeXWhitneyCPharmacokinetics of procarbazine and metabolites involved in its bioactivation in patients with recurrent anaplastic astrocytoma [abstract]Proc Am Soc Clin Oncol2002212086
- TakenakaTMikuniCMiuraAAlternating combination chemotherapy C-MOPP (cyclophosphamide, vincristine, procarbazine, prednisone) and ABVd (adriamycin, bleomycin, vinblastine, dacarbazine) in clinical stage II-IV Hodgkin’s disease: a multicenter phase II study (JCOG 8905). The Lymphoma Study Group of the Japan Clinical Oncology GroupJpn J Clin Oncol2000301465210798542
- van den BentMJDelattreJ-YBrandesAAFirst analysis of EORTC trial 26951, a randomized phase III study of adjuvant PCV chemotherapy in patients with highly anaplastic oligodendrogliomaJ Clin Oncol2005231503
- van den BentMJCarpentierAFBrandesAAAdjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trialJ Clin Oncol20062427152216782911
- YungWKAlbrightREOlsonJA phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapseBr J Cancer2000835889310944597
- ZbindenGUnscheduled DNA synthesis in the testis, a secondary test for the evaluation of chemical mutagensArch Toxicol198046139497235990
- ZellerPGutmannHHegedusBMethylhydrazine derivatives, a new class of cytotoxic agentsExperientia19631912914003433