Abstract
Anemia is a common, but underestimated and undertreated, complication of patients with cancer receiving chemo- or radiotherapy, and negatively affects their quality of life (QoL). Erythropoietic proteins (EPS) offer an effective treatment of cancer anemia and ameliorate QoL, although their use requires the correct targeting of hemoglobin increase to avoid thromboembolic complications. Currently the effort is focused on offering patients this effective treatment with reduced frequency of administration. Higher weekly single doses of recombinant human Epo (rHuEpo) either alpha or beta, instead of three times per week, have been proposed for the treatment. The pharmacokinetic and pharmacodynamic characteristics of the hyperglycosylated protein darbepoetin alpha permit even longer inter vals between administrations. Every other week or every three weeks schedules have shown results (erythropoietic response, reduction of transfusion requirements, and improvement of QoL) comparable with those of weekly rHuEpo.
Introduction
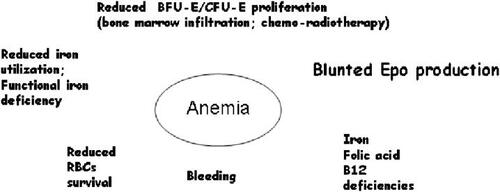
Anemia is a frequent complication in patients with cancer and affects quality of life (QoL) (CitationCella 1997; CitationHarper and Littlewood 2005) in at least 50%. Correct investigation of the reasons underlying anemia is mandatory for an adequate treatment (). A recent large survey (CitationLudwig et al 2004) has shown that prevalence and incidence were 39.3% of 14 912 enrolled and 57.3% of 13 628 analyzed patients respectively. Only 38.9% received a treatment of either transfusions or replacement therapy including recombinant human Epo (rHuEpo). Therefore an adequate treatment is not adopted in the majority patients with anemia of cancer.
A major step in the evaluation of anemia of chronic disorders, including cancer, was the recognition that erythropoietin production is often blunted and serum level inadequate to the degree of hemoglobin decrease because of toxicity, especially when platinum is used in therapy, or the disease itself (). Therefore, the availability of rHuEpo alpha and beta, and successively darbepoetin alpha, has offered an effective alternative to red cell transfusion in this setting. CitationBohlius and colleagues (2005) carried out a metanalysis of 27 randomized controlled trials, involving 3953 patients, that compared the use of rHuEpo and darbepoetin (plus transfusion if needed) with observation until red blood cell transfusion was required. This study was recently updated (CitationBohlius et al 2006) to include 57 trials with 9353 patients. The analysis of data indicates that administration of EPS reduces the relative risk for blood transfusions and the number of units transfused in cancer patients. For patients with baseline hemoglobin below 12 g/dL (mild anemia) there is strong evidence of improved hematological response and suggestive evidence of amelioration of QoL. On the other hand, the relative risk for thromboembolic complications increases by the treatment, while it remains uncertain whether and how rHuEpo and darbepoetin affect tumor response and overall survival. As for the disease outcome, the conclusion reflects the somewhat divergent results of a large randomized double blind versus placebo study suggesting a possible better outcome in patients treated with rHuEpo (CitationLittlewood et al 2001), and those of two different studies that have shown a risk of worst outcome of cancer disease in patients treated with rHuEpo (CitationHenke et al 2003; CitationLeyland-Jones 2003). The expression of Epo receptor (EpoR) has been demonstrated on several nonerythroid tumor cells (CitationArcasoy et al 2005), but this does not invariably translate into the receptor activation. Moreover it has been shown that antibodies currently used in immunoblotting and immunostaining techniques lack specificity (CitationElliott et al 2006), therefore the results should be evaluated with caution.
American Society of Hematology/American Society of Clinical Oncology (ASCO) (CitationRizzo et al 2002), CitationNational Comprehensive Cancer Network (NCCN) (2006), and European Organisation for Research and Treatment of Cancer (EORTC) (CitationBokemeyer et al 2004) proposed guidelines for the use of erythropoietic proteins (EPS) in anemia of cancer. These indicate that scope of the treatment is not only the erythropoietic response (hemoglobin increase), but also the improvement of QoL, negatively affected by the anemia, and prevention of transfusion. The target hemoglobin level should be 12–13 g/dL in order to reduce the risk of hypertension and thromboembolic events associated with this therapy (CitationBokemeyer et al 2004). However the efficacy of EPS relies on the presence of adequate iron availability for the necessity of erythropoiesis, therefore preliminary evaluation and monitoring of transferrin saturation are mandatory. If transferring saturation is ≤20% intravenous should be administered (CitationAuerbach et al 2004)
The spectrum of EPS includes a large variety of products, such as erythropoietin delta (CitationDeicher and Horl 2004) and omega (CitationBren et al 2002), with registration limited to anemia of kidney failure, while pegylated Epo beta (CitationOsterborg et al 2004) and a pegylated synthetic peptide, Hematide (CitationStead et al 2006), are still under clinical evaluation. The only approved and currently marketed EPS for treatment of anemia in cancer patients are rHuEpo alpha and beta, and darbepoetin alpha. Data on rHuEpo are briefly mentioned in this review, which will focus especially on the activity of darbepoetin in the treatment of patients with cancer anemia.
rHuEpo
Treatment with EPS in the setting of anemia of chronic disorders, including cancer, requires higher doses (3- to 4-fold) than in the treatment of anemia of kidney failure, due to the inhibition of endogenous Epo production, of bone marrow activity, or both. Therefore a typical schedule of 150 U/Kg (10 000U) of rHuEpo three times per week with rHuEpo alpha or beta is adopted in cancer patients instead of 40–50 ml/Kg used in chronic kidney failure. Response rate varies largely in relation to the disease. Commonly hemoglobin levels improve in 25% to 30% of patients with myelodysplastic syndrome (MDS) (CitationAlessandrino et al. 2002), but in subgroups of patients with favourable characteristics (International Prognostic Scoring System [IPSS] low/intermediate 1 risk, reduced or no transfusion support, endogenous Epo levels <200 mU/ml) this rate can increasee to 50% with daily (CitationICSG 1998; CitationAlessandrino et al 2002) or every other day (CitationTer pos et al 2003) administrations of 10 000 U. CitationHellstrom-Lindberg and colleagues (1998) demonstrated that the percentage of responders may increase if rHuEpo is used in combination with granulocyte-colony stimulating factor (G-CSF), a result recently confirmed by Balleari and colleagues (2005), and this protocol proved to be effective also in patients with higher endogenous Epo levels (up to 500m U/mL). In the MDS setting, it is particularly important to define correctly the criteria of response to evaluate the efficacy of treatment. A revised version has recently been published by the International Working Group (CitationCheson et al 2006). In patients with nonmyeloid malignancies, including non-Hodgkin lymphoma and multiple myeloma (MM), response is obtained in 50% to 70 % of patients (CitationRizzo et al 2002), with the highest response rate in MM. CitationGabrilove and colleagues (2001) reported that a single weekly dose (QW) of rHuEpo alpha (40 000–60 000U) was as effective as the three times per week protocol, a result confirmed by others (CitationShasha et al 2003; CitationChang et al 2005; CitationWitzig et al 2005). Similarly, QW dosing (30 000U) (CitationCazzola et al 2003) of rHuEpo beta are as effective as 10 000 U x 3 per week.
CitationHenry and colleagues (2006) reported that a further extension of the interval between administration of rHuEpo alpha using 80 000 U Q2W was as effective as 40 000 QW with comparable safety.
Whether the measurement of endogenous Epo levels is a useful tool to predict the response in patients with solid cancer or lymphoproliferative disorders is questionable (CitationLittlewood et al 2003; CitationLudwig et al 2004). These are inappropriately low in approximately 25% of all patients with MM, and increase to 50% in patients with stage III disease and 60% in those with renal impairment (CitationBeguin et al 1992). Moreover, in some patients bone marrow infiltration participate to the poor response to rHuEpo (CitationBeguin 1995).
In conclusion rHuEpo is convenient and effective treatment capable of reducing transfusion support, increasing hemoglobin level, and improving QoL.
Darbepoetin alpha
Darbepoetin alpha is a novel hyperglycosilated erythropoietic protein that, in comparison with rHuEpo alpha, contains 5 N-linked carbohydrate chains versus 3 and 22 sialic acid residues versus 14. These characteristics determine a 3 times longer half-life (25.3 h vs 8.45 h), which increases in vivo biological activity in a murine model (CitationEgrie and Browne 2001), and permits less frequent administrations. The roles of carbohydrate and sialic acid residues in deter mining the pharmacokinetics and phar macodynamics of darbepoetin alpha have been elucidated by CitationElliot and colleagues (2004). Although less effective than rHuEpo in vitro in inducing colony-forming unit-erythroid proliferation (due to sialic acid residues), the longer half life confers an advantage in vivo, and the reduced affinity for EpoR fur ther participates to maintain erythropoietic concentrations of darbepoetin over the time.
A QW administration of darbepoetin is sufficient for sustained stimulation of erythropoiesis. The percentage of cancer patients with hematopoietic response (hemoglobin >2 g/dL or >12 g/dL in the absence of red blood cell [RBC] transfusion) is dose-related, and time to hemoglobin response reduced compared with three times per week rHuEpo administration (CitationSmith et al 2001; CitationGlaspy et al 2002). Maximum concentration (Cmax) is reached at week 2 after chemotherapy, when the pool of erythroid cells is likely to be reduced as a consequence of chemotherapy (CitationHeatherington et al 2001). This suggests that the clearance of darbepoetin is possibly due to binding to EpoR expressed on erythroid progenitor and precursor cells (CitationGlaspy et al 2005). Finally, there is a trend toward increasing serum concentration of darbepoetin over time, as at cycle 3 concentration was doubled compared with cycle 1. When tested in a randomized study versus placebo in patients with lung cancer (CitationVasteenkiste et al 2002), QW 150 µg darbepoetin reduced transfusion support (27% vs 52%) and increased hematopoietic response (66% vs 24%). Similar results were reported by CitationHedenus and colleagues. (2003) in patients with non-Hodgkin lymphomas, and these authors observed a response to darbepoetin in 69% of patients with basal serum Epo <100 mU/mL versus 44% in those with basal values >100mU/mL. QoL also improved, as shown by a Functional Assessment of Cancer Therapy-Fatigue (FACT-F) score increased in 56% of patients treated with darbepoetin and 44% in the placebo group, although the difference was not significant. Recently CitationLittlewood and colleagues (2006) confirmed the efficacy of darbepoetin in ameliorating fatigue and QoL in patients with lymphoproliferative disorders, in a multicenter randomized trial.
Currently in the QW schedule, darbepoetin is administered at the dose of 2.25 µg/Kg (150 mg fixed dose), to be increased to 4.50 µg/Kg (300 µg fixed dose) if response is not observed at week four.
Darbepoetin (150 µg increased to 300 µg if no response is observed) determines a substantial response also in patients with low-risk MDS (CitationMusto et al 2005; CitationPatton et al 2005) and may offer the advantage of less frequent administrations. In a recent series of 66 patients with low-intermediate 1 risk according to IPSS, CitationMannone and colleagues (2006) obtained an overall erythroid response of 71%, comparable with that observed in patients treated with the rHuEpo alpha plus G-CSF combination.
Darbepoetin every-other-week
The pharmacokinetic characteristics of the Darbepoetin suggest that Q2W or longer intervals between administrations are made possible. In an open-label study, CitationVadhan-Raj and colleagues (2003) evaluated the ability of darbepoetin alpha to reverse chemotherapy-induced anemia in cancer patients, induce changes in fatigue and functional capacity and their relation to hemoglobin increase in patients with hemoglobin ≤11 g/dL with nonmyeloid malignancy receiving multicycle chemotherapy. Darbepoetin alpha was administered at a starting dosage of 3 µg/kg Q2W for up to eight doses (16 weeks). The interim analysis included a total of 1174 patients: mean increase in hemoglobin was 1.7 g/dL (ITT analysis) and 2.1 g/dL for those patients receiving the full 16 weeks of therapy. The Kaplan-Meier estimate of the proportion of subjects with a hematopoietic response (increase in hemoglobin ≥2 g/dL and/or ≥12 g/dL) was 84%. To summarize the results of this study: a) darbepoetin determined an hemoglobin response (greater in patients with hemoglobin <10 g/dL) during treatment; b) the FACT-F subscale score increased by a mean of 6.8 points (26%) during the study; c) improvements in fatigue score paralleled the increases observed in hemoglobin. In conclusion the efficacy of the Q2W regimen of darbepoetin alpha is no different from that of weekly or three times per week rHuEpo. CitationGlaspy and colleagues (2002) tested the administration of darbepoetin in a randomized multi-arm study in which different doses of darbepoetin (3 µg/kg, 5 µg/kg, 7 µg/kg, 9 µg/kg) Q2W were given to patients on chemotherapy, and compared with the activity of a fixed weekly dose (40 000) of rHuEpo alpha. 60 000 U weekly could be used if hemoglobin increase was <1 g/dL. The study showed that darbepoetin Q2W is as effective as rHuEpo QW, and doses of darbepoetin higher than 5 µg/kg do not increase the response. The feasibility of Q2W approach was confirmed by CitationPatton and colleagues (2004) in a retrospective observational cohort study in which patients with chemotherapy-induced anemia received darbepoetin 100 µg/kg QW, darbepoetin 200 µg/kg Q2W or rHuEpo (40 000 U QW), and by other authors (CitationSchwartzberg et al 2004; CitationSenecal et al 2005; CitationWaltzman et al 2005).
CitationHerrington and colleagues (2005) derived data from 1444 patients treated with darbepoetin alpha and 1341 with epoetin alpha. The most common initial dosages were 200 µg Q2W for darbepoetin alpha (61%), and 40 000 U QW epoetin alpha (72%). The dosage was escalated for 22% of darbepoetin alpha recipients and 23% of epoetin alpha recipients at a median of six weeks after the initial dose. The mean change from baseline in hemoglobin concentration after 12 weeks of therapy was similar for both groups, as well as the percent of patients receiving red-blood-cell transfusions during treatment.
Other schedules
The possibility to further prolong the interval between administrations of darbepoetin in patients with cancer chemotherapy-induced anemia has also been explored. CitationKotasek and colleagues (2003), in a dose finding (from 4.5 µg/kg to 15 µg/kg by 2.5 µg/kg steps) placebo-controlled study, observed that at all doses given darbepoetin Q3W reduced transfusion requirements compared with placebo, and hemoglobin increase was dose-dependent, with an increase ≥1 g/dL at doses of 6.75 mg/kg or greater. Fact-F fatigue score also improved with increasing hemoglobin concentration (p=0.0023), and safety profile was comparable in darbepoetin- and placebo-treated patients.
CitationGlaspy and coworkers (2005) studied whether timing in respect to chemotherapy influences eff icacy of darbepoetin Q3W. A dose of 6.75 µg/kg Q3W was administered synchronously (starting on chemotherapy day 1, patients 38) or asynchronously (starting on chemotherapy day 15, patients 43) with concurrent chemotherapy Q3W (platinum- and nonplatinum-based regimens) in patients with nonmyeloid malignancies (40% breast cancer). Primary endpoints were mean change in hemoglobin after 6 weeks from baseline and proportion of patients with hemoglobin increase from baseline were 1 g/dL. Secondary endpoints at the entire treatment period (16 weeks) were the proportion of patients with hemoglobin increase of hemoglobin >2 g/dL or a level of >12 g/dL in the absence of RBC transfusion, time to erythropoietic response, proportion of patients who had a RBC transfusion from week 5 to end of treatment. The overall erythropoietic response was 74%, and there was no difference between groups in the proportion of patients who achieved an increase in hemoglobin equal to or >1 g/dL, and time to erythropoietic response. Only 19% of patients required transfusion support. These results seem to be comparable with those obtained with darbepoetin QW administration. Two data suggest that the binding to receptor plays a role in the clearance of EPS in patients with chemotherapy-induced anemia: a) pharmacokinetic analysis carried out in a subset of patients indicated that endogenous erythropoietin concentration increases and lasts for approximately 1 week following chemotherapy administration; b) synchronous administration of darbepoetin alpha was associated with a 1.3-fold increase in the darbepoetin alpha area-under-the-curve compared with asynchronous administration. The feasibility of the Q3W schedule has been confirmed by CitationBoccia and colleagues (2006)
A direct comparison between fixed 500 µg Q3W at fixed dose and 2.25 µg/kg QW was carried out by CitationCanon and colleagues (2006) in a non inferiority study active-controlled phase III study, in patients with nonmyeloid malignancies. The incidence of transfusion was 19% of 353 patients in the group treated with 500 mg Q3W and 28% of 352 patient in the QW group. The target hemoglobin level was reached in a similar proportion of patients and increase in FACT-F score was also superimposable, without differences in toxicity.
An Q4W administration is probably possible using an adequate dosing of darbepoetin (CitationSmith et al 2003). A hyperglycosylated analog of darbepoetin (AMG 114) with longer half life is currently in phase I clinical study.
Conclusions
Anemia is a common, underestimated and undertreated complication of cancer, especially in patients treated with chemotherapy. Provided that other factors, especially iron, are in sufficient amount to support their activity, EPS offer a simple, effective and generally safe treatment of anemia. The targets of this treatment should be: a) reduction or, possibly, elimination of transfusion support (therefore the beginning of treatment should not be delayed if the patient’s hemoglobin levels is 10–11 g/dL; b) an adequate hemoglobin level (12–13 g/dL) that also would also improve QoL could be improved in patients with cancer, but avoiding higher values that could expose the patient to thromboembolic complication. The guidelines released by EORTC, NCCN, and ASCO are useful tools for these purposes.
From the large amount of data available in the literature it can be concluded that darbepoetin is as effective as rHuEpo (60% to 80% of patients respond to therapy) in stimulating erythropoiesis in patients with cancer. Pharmakokinetic characteristics make darbepoetin ideal for a reduced frequency of administration and more convenient for the patient by adjusting dosage and frequency to reach and maintain the target hemoglobin level. In consideration that chemotherapy is most commonly given every three week, a supplemental Biologics License Application has been submitted by the producer to the Food and Drug Administration for Q3W administration. The European Agency (EMEA) has granted darbepoetin approval for extended dosing once every three weeks interval in the treatment of cancer chemotherapy-induced anemia in 2004. In this setting there is no evidence that 500 µg are more effective than 300 µg in the Q3W schedule because a direct comparison of these doses is not available.
Less frequent dosing of rHuEpo, either alpha or beta, than 3 times per week proved to be effective, and adequate dosing could make the Q3W interval possible even with these erythropoietic agents.
The preliminary data of efficacy of the new pegylated Epo beta (Continuous Erythropoiesis Stimulating Activity – CERA) (CitationOsterborg et al 2004) in a Q3W administration, and the pharmacokinetics of hematide, confirm the trend toward a reduced frequency of administration of erythropoietic proteins. The development of drugs targeting the hypoxia-inducible factor through the inactivation of the degradation enzyme prolyl-4-hydroxylase and with different (oral) route of administration are underway and could offer new perspectives in the control of anemia of cancer patients.
Disclosures
Alberto Grossi is consultant for Shire Italia.
References
- AlessandrinoEPAmadoriSBarosiGEvidence- and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes. A statement from the Italian Society of HematologyHaematologica200287128630612495903
- ArcasoyMOAminKChouSCErythropoietin and erythropoietin receptor expression in head and neck cancer: relationship to tumor hypoxiaClin Cancer Res20051120715671524
- AuerbachMBallardHTroutJRIntravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trialJ Clin Oncol2004221301715051778
- BalleariERossiEClavioMErythropoietin plus granulocyte colony-stimulating factor is better than erythropoietin alone to treat anemia in low-risk myelodysplastic syndromes: results from a randomized single-centre studyAnn Hematol2006851748016408206
- BeguinYVernaMLooMErythropoiesis in multiple myeloma: defective red cell production due to inappropriate erythropoietin productionBr J Haematol199282648531482651
- BeguinYErythropoiesis and erythropoietin in multiple myelomaLeuk Lymphoma19951841221
- BocciaRMalikIARajaVDarbepoetin alpha administered every three weeks is effective for the treatment of chemotherapy-induced anemiaOncologist20061144091716614237
- BohliusJLangensiepenSSchwarzerGRecombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysisJ Natl Cancer Inst2005974899815812074
- BohliusJWilsonJSeidenfeldJErythropoietin or darbepoetin for patients with cancerCochrane Database Syst Rev20063CD00340716856007
- BokemeyerCAaproMSCourdiAEORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancerEur J Cancer20044022011615454245
- BrenAKandusAVarlJA comparison between epoetin omega and epoetin alpha in the correction of anemia in hemodialysis patients: a prospective, controlled crossover studyArtif Organs20022691711879235
- CanonJLVansteenkisteJGyörgy BodokyGRandomized, double-blind, active-controlled trial of every-3-week darbepoetin alpha for the treatment of chemotherapy-induced anemiaJ Natl Cancer Inst2006982738416478746
- CazzolaMBeguinYKloczkoJOnce-weekly epoetin beta is highly effective in treating anaemic patients with lymphoproliferative malignancy and defective endogenous erythropoietin productionBr J Haematol20031223869312877665
- CellaDThe Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigueSemin Hematol199734Suppl.213199253779
- ChangJCoutureFYoungSWeekly epoetin alpha maintains hemoglobin, improves quality of life, and reduces transfusion in breast cancer patients receiving chemotherapyJ Clin Oncol200523259760515452188
- ChesonBDGreenbergPLBennettJMClinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasiaBlood20061084192516609072
- DeicherRHorlWHDifferentiating factors between erythropoiesis-stimulating agents: a guide to selection for anaemia of chronic kidney diseaseDrugs20046449950914977387
- EgrieJCBrowneJKDevelopment and characterization of novel erythropoiesis stimulating protein (NESP)Br J Cancer200184Suppl.131011308268
- ElliottSBusseLBassMEAnti-Epo receptor antibodies do not predict Epo receptor expressionBlood20061071892516249375
- ElliottSEgrieJBrowneJControl of rHuEPO biological activity: the role of carbohydrateExp Hematol20043211465515588939
- GabriloveJLCleelandCSLivingstoneRBClinical evaluation of once-weekly dosing of epoetin alpha in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosingJ Clin Oncol20011928758211387360
- GlaspyJHenryDPatelREffects of chemotherapy on endogenous erythropoietin levels and the pharmacokinetics and erythropoietic response of darbepoetin alpha: a randomised clinical trial of synchronous versus asynchronous dosing of darbepoetin alphaEur J Cancer2005411140915911237
- GlaspyJAJadejaJSJusticeGDarbepoetin alpha given every 1 or 2 weeks alleviates anaemia associated with cancer chemotherapyBr J Cancer2002872687612177793
- HarperPLittlewoodTAnemia of cancer: impact on patient fatigue and long-term outcomeOncology200569suppl 22716244504
- HeatheringtonACSchullerJMercerAJPharmakokinetics of novel erythropoiesis stimulating protein (NESP) in cance patients: preliminary reportBr J Cancer200184suppl.1111611308269
- HedenusMAdrianssonMSan MiguelJEfficacy and safety of darbepoetin alpha in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled studyBr J Haematol200312239440312877666
- Hellstrom-LindbergEAhlgrenTBeguinYTreatment of anemia in myelodysplastic syndromes with granulocyte colony-stimulating factor plus erythropoietin: results from a randomized phase II study and long-term follow-up of 71 patientsBlood19989268759639501
- HenkeMLaszigRRubeCErythropoietin to treat head and neck cancer patients with anemia undergoing radiotherapy: randomised, double-blind, placebo controlled trialLancet200336212556014575968
- HenryDHGordanLCharuVRandomized, open-label comparison of epoetin alpha extended dosing (80 000 U Q2W) vs weekly dosing (40 000 U QW) in patients with chemotherapy-induced anemiaCurr Med Res Opin20062214031316834839
- HerringtonJDDavidsonSLTomitaDKUtilization of darbepoetin alpha and epoetin alpha for chemotherapy-induced anemiaAm J Health Syst Pharm200562546215658073
- [ICSG] Italian Cooperative Study Group for rHuEpo in Myelodysplastic SyndromesA randomized double-blind placebo-controlled study with subcutaneous recombinant human erythropoietin in patients with low-risk myelodysplastic syndromesBr J Haematol1998103107049886322
- KotasekDStegerGFaughtWDarbepoetin alpha administered every 3 weeks alleviated anaemia in patients with solid tumors receiving chemotherapy; results of a double-blind, placebo-conrolled, randomised studyEur J Cancer20033920263412957457
- Leyland-JonesBBEST Investigators and Study GroupBreast cancer trial with erythropoietin terminated unexpectedlyLancet Oncol200344596012901958
- LittlewoodTJBajettaENortierJWEpoetin alpha study group. Effects of epoetin alpha on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trialJ Clin Oncol20011928657411387359
- LittlewoodTJKallichJDSan MiguelJEfficacy of darbepoetin alpha in alleviating fatigue and the effect of fatigue on quality of life in anemic patients with lymphoproliferative malignanciesJ Pain Symptom Manage200633172516632079
- LittlewoodTJZagariMPallisterCBaseline and early treatment factors are not clinically useful for predictingindividual response to erythropoietin in anemic cancer patientsOncologist200389910712604736
- LudwigHVan BelleSBarrett-LeePThe European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patientsEur J Cancer200440229330615454256
- MannoneLGardinCQuarreMCHigh-dose darbepoetin alpha in the treatment of anaemia of lower risk myelodysplastic syndrome results of a phase II studyBr J Haematol20061335131916681638
- MustoPLanzaFBalleariEDarbepoetin alpha for the treatment of anaemia in low-intermediate risk myelodysplastic syndromesBr J Haematol2005128204915638854
- [NCCN] National Comprehensive Cancer NetworkClinical practice guidelines in oncology, v.2.2006. Cancer and treatment-related anemia [online]2006 Accessed on 25 Sept 2006. URL: http:/www.enccn.com/professionals/physician_gls/PDF/anemia.pdf
- OsterborgABrandbergYMolostovaVRandomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin Beta, in hematologic malignanciesJ Clin Oncol20022024869412011126
- OsterborgAHellmannAJuan Luis SteegmannJLCERA (Continuous Erythropoietin Receptor Activator): Dose-response trial of subcutaneous (SC) administration once every 3 weeks (Q3W) to patients with aggressive non-Hodgkin’s lymphoma and anemia receiving chemotherapy [abstract]Blood200410446th meeting of the American Society of HematologyDecember 4–7San Diego, CA4225
- PattonJReevesTWallaceJEffectiveness of Darbepoetin alpha versus Epoetin alpha in patients with chemotherapy-induced anemia treated in clinical practiceOncologist20049451815266098
- PattonJFSullivanTMunYA retrospective cohort study to assess the impact of therapeutic substitution of darbepoetin alpha for epoetin alpha in anemic patients with myelodysplastic syndromeJ Support Oncol200534192616350429
- RizzoJDLichtinAEWoolfSHUse of erythropoietin in patients with cancer: evidence-based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of HematologyJ Clin Oncol200220408310712351606
- SchwartzbergLSYeeLKSenecalFMA randomized comparison of every-2-week darbepoetin alpha and weekly epoetin alpha for the treatment of chemotherapy-induced anemia in patients with breast, lung, or gynecologic cancerOncologist2004969670715561813
- SenecalFMYeeLGabrailNTreatment of chemotherapy-induced anemia in breast cancer: results of a randomized controlled trial of darbepoetin alpha 200 microg every 2 weeks versus epoetin alpha 40,000 U weeklyClin Breast Cancer200564465416381629
- ShashaDGeorgeMJLouisBOnce-weekly dosing of epoetin-alpha increases hemoglobin and improves quality of life in anemic cancer patients receiving radiation therapy either concomitantly or sequentially with chemotherapyCancer2003981072912942577
- SmithREJrJaiyesimiIAMezaLANovel erythropoiesis stimulating protein (NESP) for the treatment of anaemia of chronic disease associated with cancerBr J Cancer200184Suppl.1243011308271
- SmithREJrTchekmedyianNSChanDA dose- and schedule-finding study of darbepoetin alpha for the treatment of chronic anaemia of cancerBr J Cancer2003881851812799626
- SteadRBLambertJWesselsDEvaluation of the safety and pharmacodynamics of Hematide, a novel erythropoietic agent, in a phase 1, double-blind, placebo-controlled, dose-escalation study in healthy volunteersBlood20061081830416720830
- TerposEMougiouAKouraklisAThe Greek MDS Study GroupProlonged administration of erythropoietin increases erythroid response rate in myelodysplastic syndromes: a phase II trial in 281 patientsBr J Haematol20031181748012100145
- Vadhan-RajSMirtschingBCharuVAssessment of hematologic effects and fatigue in cancer patients with chemotherapy-induced anemia given darbepoetin alpha every two weeksJ Support Oncol20031131815352656
- VansteenkisteJPirkerRMassutiBDouble-blind, placebo-controlled, randomized phase iii trial of darbepoetin alpha in lung cancer patients receiving chemotherapyJ Natl Cancer Inst20029412112012189224
- WaltzmanRCrootCJusticeGRRandomized comparison of epoetin alpha (40,000 U weekly) and darbepoetin alpha (200 microg every 2 weeks) in anemic patients with cancer receiving chemotherapyOncologist2005106425016177289
- WitzigTESilbersteinPTLoprinziCLPhase III, randomized, double-blind study of epoetin alpha compared with placebo in anemic patients receiving chemotherapyJ Clin Oncol20052326061715452187