Abstract
Febuxostat is a novel, potent, non-purine selective xanthine oxidase inhibitor, which in clinical trials demonstrated superior ability to lower and maintain serum urate levels below 6 mg/dL compared with conventionally used doses of allopurinol. Febuxostat was well tolerated in long term treatment in patients with hyperuricemia including those experiencing hypersensitity/intolerance to allopurinol. Dose adjustment appears unnecessary in patients with mild to moderate renal or liver insufficiency or advanced age. The most common adverse reactions reported were abnormal liver function tests, headache, and gastrointestinal symptoms, which were usually mild and transient. However, whether hepatotoxicity becomes a limitation in the use of febuxostat needs to be determined in further studies. An increased frequency of gout flares occurs for a prolonged period after treatment initiation, as with any aggressive lowering of serum urate, and prolonged prophylaxis with colchicine or NSAIDs is usually required. Febuxostat has been granted marketing authorization by the European Commission in early 2008 for the treatment of chronic hyperuricemia and gout. Febuxostat is the first major treatment alternative for gout in more than 40 years and is a promising alternative to allopurinol, although continued long-term surveillance on safety and efficacy is required.
Introduction
Gout occurs as an acute arthritis that appears when there is a sudden onset of inflammation in response to the accumulation of crystals of monosodium urate in or around a joint (CitationSchlesinger 2004), which is closely associated with hyperuricemia. With persistent urate crystal deposition, gout may progress from acute episodic attacks to a disabling chronic deforming arthropathy, with destructive deposits of urate crystals (tophi) in bones, joints, subcutaneous tissue, and other organs (CitationBecker 1988; CitationBecker et al 2005b; CitationTomlinson 2005). Renal damage may occur due to interstitial urate crystal deposition and urinary tract stones composed entirely or partly of monosodium urate and uric acid crystals (CitationBecker 1988; CitationSchlesinger 2004).
The development of gout can be divided into four stages: asymptomatic hyperuricemia, the gouty attack, the intercritical period, and chronic gouty arthritis. It has been recognized that hyperuricemia may exist for many years before the first clinical attack of gout (CitationFalasca 2006). Hyperuricemia has been thought to be the fundamental pathogenic biochemical aberration upon which various etiological factors predispose to the expression of the clinical disorder of gout (CitationTeng et al 2006). Moreover, hyperuricemia is also associated with chronic renal damage (CitationIseki et al 2004), some metabolic disturbances and risk factors for atherosclerotic cardiovascular disease including hypertension, overweight, insulin resistance, and hyperlipidemia (CitationSlot 1994), which may occur together as the metabolic syndrome. This may explain the recognized association between gout and cardiovascular disease (CitationAbbott et al 1988; CitationKrishnan et al 2006; CitationChoi and Curhan 2007), although there are no studies to show whether lowering plasma urate will reduce the cardiovascular risk in humans (CitationKim et al 2003; CitationWortmann 2005).
Multiple epidemiological studies from a diverse range of countries suggest that gout has increased in prevalence and incidence in the past few decades and the profile of clinical gout is becoming more complex (CitationRoddy et al 2007). The established risk factors for gout include genetic factors, excess alcohol consumption, purine-rich diet, metabolic syndrome, use of diuretics, and chronic renal failure (CitationRoddy et al 2007). Trends in lifestyle and dietary factors may partly explain the changes in the epidemiology of gout (CitationHak and Choi 2008). Furthermore, suboptimal management also contributes to the rising prevalence of clinically symptomatic chronic gout (CitationRoddy et al 2007).
Conventional dietary recommendations for gout have emphasized the restriction of purine intake. There are two main approaches: the traditional low purine, low protein, alcohol-restricted diet, and more recently, a weight-reducing, purine-unlimited, calorie- and carbohydrate-restricted diet, with increased proportional intake of both protein and unsaturated fats. However, a rigid purine restricted diet is of dubious therapeutic value and there are also no long-term studies of the efficacy of either approach (CitationFam 2002).
The treatment for an acute attack of gout is usually either colchicine or nonsteroidal anti-inflammatory drugs (NSAIDs). For chronic gout, prophylactic therapy to reduce the serum urate level is thought to be appropriate in patients who have more than two gout flares a year or in individuals with gouty complications, and this could reduce the likelihood of recurrent gout by 80% (CitationEmmerson 1996; CitationChoi et al 2005; CitationBruce 2006). The physiochemical properties of monosodium urate cause crystals to precipitate in body fluids if the concentration is greater than 6.8 mg/dL (400 μmol/L). Hence the target of urate lowering therapy is to reduce the serum urate concentration to below 6.0 mg/dL (353 μmol/L), which should prevent the recurrence of gouty attacks or at least reduce their frequency substantially and allow the remission in size of gouty tophi (CitationWortmann 2005).
It was previously considered that it may be useful to divide subjects with hyperuricemia into those who are urate overproducers from those who are underexcreters, based on the 24-hour uric acid excretion (CitationWortmann 2002). Underexcreters may be candidates for uricosuric drugs such as probenecid or benzbromarone, but probenecid has limited efficacy and/or safety in patients with renal impairment or prior urolithiasis, and the more potent uricosuric agent benzbromarone was associated with several cases of fulminant hepatic failure and is not generally available (CitationEmmerson 1996; CitationLi-Yu et al 2001; CitationBecker et al 2005b).
The urate-lowering therapy allopurinol, a xanthine oxidase (XO) inhibitor, has been the mainstay of prophylactic treatment for gout and conditions associated with hyperuricemia for many years (CitationWortmann 2002). It is the treatment of choice for urate overproducers, tophaceous gout, nephrolithiasis, or urate nephropathy, and in patients with renal insufficiency (CitationTeng et al 2006). In theory allopurinol should be effective in almost every patient with hyperuricemia if a sufficient dose is taken, but achieving normal serum urate levels may be difficult in patients with impaired renal function or in transplant recipients (CitationSchlesinger 2004). In practice approximately 20% of patients report side effects with allopurinol and 5% discontinue medication (CitationWortmann 2005). There is a rare but serious hypersensitivity reaction to allopurinol, which involves fever, rash, eosinophilia, hepatitis, and progressive renal insufficiency (CitationSchlesinger 2004; CitationWortmann 2005). This appears to be dose- or plasma concentration-dependent and can cause up to 20% mortality (CitationTeng et al 2006). Patients with renal insufficiency or receiving diuretic therapy are at increased risk of adverse effects (CitationWortmann 2005). It has been found that severe cutaneous adverse reactions, which include the drug hypersensitivity syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis are strongly associated with the HLA-B*5801 allele in Han Chinese patients, which could be developed as a screening test to identify the risk before commencing therapy (CitationHung et al 2005). As a result of frequent failure to reach target serum urate levels, or the intolerance to allopurinol in some patients, the development of an alternative potent serum urate lowering treatment has been a desirable goal. Febuxostat is a novel potent selective inhibitor of XO that appears to be well tolerated in all groups of patients, including those who are sensitive to allopurinol.
Pharmacology
Febuxostat, {TEI-6720, TMX-67, 2-[3-cyano-4-(2-methyl-propoxy) phenyl]-4-methyl-thiazole-5-carboxylic acid}, is a non-purine compound with the empiric chemical formula C16H16N2O3S and molecular weight of 316.38 (CitationBruce 2006). It is a selective inhibitor of XO that has been developed for the treatment of hyperuricemia and gout, as it was found to have a potent inhibitory activity for XO/xanthine dehydrogenase (XDH) during evaluation of a range of newly synthesized molecules (CitationKomoriya et al 1993). In humans, the xanthine oxidoreductase (XOR) enzyme catalyzes the last two steps in uric acid synthesis, the oxidation of hypoxanthine to xanthine and of xanthine to uric acid (hypoxanthine → xanthine → uric acid). Humans and the great apes lack the enzyme uricase which converts uric acid into allantoin (CitationOsada et al 1993). Some non-enzymatic conversion of uric acid to allantoin does take place and the ratio of serum urate to allantoin has been considered as a potential marker of oxidative stress (CitationBenzie et al 1999).
XOR is synthesized as XDH, which in mammals can easily be converted to XO by oxidation of sulfhydryl residues or by proteolysis (CitationOkamoto et al 2003). Febuxostat was shown to inhibit both the oxidized and reduced forms of XO, unlike allopurinol and oxypurinol, each of which binds only to one form of the enzyme (CitationOkamoto et al 2003; CitationTakano et al 2005). The molecular mechanism of inhibition of XO activity by febuxostat (Adenuric®, Ipsen, Paris, France) is by high affinity binding to the enzyme in a molecular channel leading to the molybdenum-pterin active site, whereas allopurinol exerts relatively weak competitive inhibition on activity of only the oxidized form of XO (CitationOkamoto et al 2003; CitationTakano et al 2005). In contrast to allopurinol and oxypurinol, febuxostat does not structurally resemble purines or pyrimidines and has no significant effect on the activities of other enzymes involved in purine and pyrimindine metabolism, which might relate to some of the adverse effects caused by allopurinol and its metabolites (CitationTakano et al 2005).
More recently, Okamoto and Nishino have reported that XOR inhibitors can be categorized into different types according to the crystal structure of the various inhibitors binding to XOR (CitationOkamoto and Nishino 2008). Febuxostat has numerous hydrogen bonds, salt bridges, and hydrophobic interactions with amino acids in the active site and almost completely fills the narrow channel leading to the molybdenum center of the enzyme, which is considered as a structure-based inhibitor, whereas allopurinol and oxypurinol are thought to be mechanism-based inhibitors (CitationOkamoto and Nishino 2008). The XO inhibitory activity and hypouricemic effect of febuxostat compared to allopurinol have been studied in vitro and in a variety of animal models (CitationKomoriya et al 1993; CitationOsada et al 1993; CitationHoriuchi et al 1999). All these studies demonstrated that febuxostat had a greater potency than allopurinol in reducing serum urate levels and/or allantoin levels. Furthermore, the pleiotropic effects of febuxostat dependent on its hypouricemic effect have also been investigated and these studies provided a foundation for later evaluations in humans (CitationSanchez-Lozada et al 2008a, Citationb, Citationc; CitationZhao et al 2008).
As increased fructose consumption is associated with hyperuricemia, the metabolic syndrome, and renal damage, Sanchez-Lozada et al evaluated whether febuxostat could alleviate some of the features of the metabolic syndrome as well as the renal hemodynamic alterations and afferent arteriolopathy induced by a high-fructose diet in rats. They found that a high-fructose diet was associated with hyperuricemia, hypertension, as well as increased plasma triglycerides and insulin. Febuxostat treatment in rats on a high-fructose diet resulted in significant lowering of blood pressure, serum urate, triglycerides, and insulin (p < 0.05 for all comparisons) compared with those without treatment, and it also significantly reduced glomerular pressure, renal vasoconstriction, and afferent arteriolar area relative to those without treatment. However, febuxostat treatment in rats on a normal diet had no significant effects (CitationSanchez-Lozada et al 2008a).
Considering the close association between prolonged hyperuricemia and renal disease, Sanchez-Lozada et al evaluated the effect of febuxostat on progressive renal disease in a 5/6 nephrectomy (5/6 Nx) model in Wistar rats with and without hyperuricemia induced by the uricase inhibitor oxonic acid (OA) (CitationSanchez-Lozada et al 2008b). The 5/6 Nx rats given OA and placebo developed hyperuricemia, renal vasoconstriction and glomerular hypertension in association with further aggravation of afferent arteriolopathy compared with those without OA-induced hyperuricemia. Febuxostat prevented OA-induced hyperuricemia and in 5/6 Nx rats with or without OA-induced hyperuricemia it ameliorated proteinuria, preserved renal function and prevented glomerular hypertension. Functional improvement was accompanied by preservation of afferent arteriolar morphology and reduced tubulointerstitial fibrosis. These results suggested that febuxostat prevented renal injury in 5/6 Nx rats with and without coexisting hyperuricemia and due to its effect on preserving preglomerular vessel morphology, normal glomerular pressure was maintained even in the presence of systemic hypertension (CitationSanchez-Lozada et al 2008b).
Using different animal models, the same group of researchers also examined the effect of febuxostat on hyperuricemia-induced hypertension and renal damage (CitationSanchez-Lozada et al 2008c). They found that in OA-induced hyperuricemic rats, febuxostat lowered uric acid and ameliorated systemic and glomerular hypertension as well as mesangial matrix expansion and the development of preglomerular arteriolar disease as indicated by a reduction of the arteriolar area and media-to-lumen ratio. In normal rats, febuxostat tended to lower uric acid but had no effect on blood pressure, renal hemodynamics, and afferent arteriole morphology. The authors suggested that febuxostat merits further evaluation for the treatment of hypertension and renal alterations induced by hyperuricemia (CitationSanchez-Lozada et al 2008c).
More recently, CitationZhao et al (2008) reported the effects of chronic XO inhibition by febuxostat or allopurinol on the progression of chronic heart failure (CHF) induced by left coronary artery ligation in rabbits, which have low intrinsic myocardial XO activity similar to humans. One day after coronary ligation, rabbits were assigned to early treatment with vehicle or febuxostat for 49 days or to delayed-treatment with vehicle for 21 days followed by either febuxostat or allopurinol for 28 days. Treatment with febuxostat initiated shortly after myocardial infarction delayed or prevented the onset of CHF, but XO inhibition with either drug initiated after establishment of the disease had no cardiac protection effects (CitationZhao et al 2008).
Pharmacokinetics
After oral administration, about 85% of febuxostat is absorbed rapidly with a time (tmax) to reach peak plasma concentrations (Cmax) of approximately 1 hour in healthy human subjects (CitationBruce 2006). In a phase I study in which oral febuxostat doses of 40, 70, and 120 mg were given to healthy male and female adults (n = 154), Cmax values of 1.53, 3.08, and 4.47 μg/mL occurred at tmax of about 1 hour, with area under the plasma concentration-time curve (AUC) values of 4.00, 6.93, and 11.31 μg h/mL, respectively. The elimination half-life (t1/2) values tended to increase (from 3.8 to 9.1 hours) with increasing doses and were longer after multiple doses (6.3 to 11.9 hours) compared with single doses (CitationBecker et al 2004a). Escalating dose pharmacokinetic studies of single and multiple doses of febuxostat in healthy volunteers have shown that Cmax and AUC exhibit an approximately linear relation with single doses of 10 to 240 mg and 10 to 120 mg, respectively, and nearly dose proportional increases were noted for both Cmax and AUC after multiple oral doses with a mean apparent total clearance of 10 to 12 L/h and an apparent volume of distribution at steady state of 33 to 64 L (CitationBecker et al 2004a; CitationKhosravan et al 2006b). The AUC increased more than proportionally between the groups receiving 120 and 240 mg daily suggesting some enterohepatic recirculation (CitationBecker et al 2004a; CitationKhosravan et al 2006b) ().
Table 1 Pharmacokinetics of febuxostat
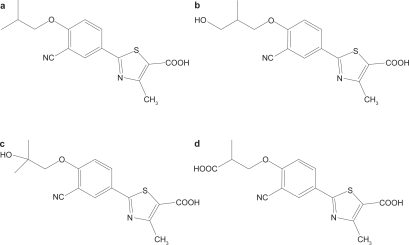
Febuxostat is highly bound to albumin in blood (~99%) and appears to have a low to medium apparent volume of distribution at steady state of approximately 0.7 L/kg (CitationMayer et al 2005). The metabolism of febuxostat occurs predominantly in the liver by glucuronidation to produce the acyl-glucuronide metabolite (22%–44% of the dose), and to a much lesser extent (2%–8%), to produce oxidative metabolites, 67M-1, 67M-2, and 67M-4 () by cytochrome P450 enzymes (CitationKhosravan et al 2006b). Approximately 25% to 45% of the drug was excreted in urine mainly as the conjugate with only about 1% to 6% being eliminated as the unchanged drug. An additional 2% to 8% of the dose was excreted as oxidative metabolites, either unchanged or as conjugates. Age and gender did not have statistically or clinically significant effects on the pharmacokinetics, pharmacodynamics or safety of febuxostat in healthy individuals after daily oral dosing with febuxostat at 80 mg for 7 days, which suggested that no dose adjustment should be necessary when administering febuxostat based on age or gender (CitationKhosravan et al 2008b). Patients with gout and/or hyperuricemia treated with oral febuxostat at 10 mg daily for 2 weeks, then 20 mg daily for a further 4 weeks had similar AUC and Cmax values for febuxostat after the last dose as those reported for the same dose in healthy male adults (CitationKomoriya et al 2003).
Figure 1 Chemical structures of (a) febuxostat and metabolites (b) 67M-1, (c) 67M-2, and (d) 67M-4. Adapted with permission from Khosravan R, Grabowski BA, Wu JT, et al. 2006b. Pharmacokinetics, pharmacodynamics and safety of febuxostat, a non-purine selective inhibitor of xanthine oxidase, in a dose escalation study in healthy subjects. Clin Pharmacokinet, 45:821–41. Copyright © 2006 Wolters Kluwer.
Renal impairment
Although renal clearance may only contribute to a small degree to the total systemic clearance of febuxostat, the effects of renal impairment on the pharmacokinetics and pharmacodynamics of febuxostat have been evaluated considering this is of clinical importance as many patients with gout may have impaired renal function. A clinical study was conducted to examine the pharmacokinetics and pharmacodynamics of febuxostat in individuals (n = 15) with mild (creatinine clearance [Clcr] 50–80 mL/min) or moderate (Clcr 30–50 mL/min) impairment in renal function, compared with healthy (Clcr ≥ 80 mL/min) participants (CitationHoshide et al 2004). The difference in plasma AUC for unchanged febuxostat after single oral doses of 20 mg febuxostat was less than 2-fold across the groups, indicating that renal impairment had little clinical impact on the pharmacokinetic profile. Changes in plasma urate levels from pre-dose levels were not significant in this study (CitationHoshide et al 2004).
Another parallel-group, open-label, multiple-dose study compared the safety, pharmacokinetics, and pharmacodynamics of febuxostat (80 mg/d), administered orally for 7 days, in individuals with normal renal function (Clcr > 80 mL/min/1.73 m; n = 11), or mild (Clcr 50–80 mL/min/1.73 m; n = 6), moderate (Clcr 30–49 mL/min/1.73 m; n = 7), or severe (Clcr 10–29 mL/min/1.73 m; n = 7) renal impairment. Mean unbound febuxostat tmax and Cmax values in subjects with various degrees of renal impairment were similar to those in subjects with normal renal function, whereas the mean AUC(24 h) unbound and t1/2z (t1/2 obtained by a non-compartment analysis from the terminal slope of the concentration-time curve) values tended to increase with increasing renal impairment. For the Cmax and AUC(24 h) values for the quantifiable active metabolites 67M-1, 67M-2, and 67M-4, with the exception of Cmax for 67M-1, regression analyses indicated a significant linear relationship with Clcr, suggesting that plasma exposure to the metabolites as well as febuxostat was generally increased in proportion to the degree of renal impairment. Although plasma exposure to febuxostat and its metabolites was generally higher in subjects with increasing degrees of renal impairment, the percentage reductions in serum urate were comparable, with a mean value of 55% to 64% by day 7 irrespective of renal function. The 80-mg once-daily dose of febuxostat was safe and well tolerated and the investigators concluded that no dose adjustment is required based on differences in renal function (CitationMayer et al 2005).
Hepatic impairment
As hepatic metabolism plays an important role in febuxostat elimination the effects of hepatic impairment on the pharmacokinetics, pharmacodynamics, and safety of febuxostat were investigated in a phase I study in individuals with normal (n = 11), mildly impaired (Child-Pugh class A; n = 8) or moderately impaired (Child-Pugh class B; n = 8) hepatic function. Subjects took oral febuxostat 80 mg daily for 7 days. There were smaller reductions in serum urate in the patients with hepatic impairment than in those with normal hepatic function but no differences in the pharmacokinetic parameters of febuxostat or its metabolites, suggesting the dosage may not need to be reduced in patients with mild or moderate hepatic dysfunction (CitationKhosravan et al 2006a). However, the safety and efficacy of febuxostat in patients with severe hepatic impairment has not been studied. Liver toxicity was not reported in any of the animal studies.
Drug interactions
Febuxostat is highly bound to albumin in blood and is mainly metabolized by UDP-glucuronosyltransferase (UGT) 1 and 2 families and to a lesser extent by cytochrome P450 (CYP) enzymes. In vitro studies showed the presence of ibuprofen or warfarin did not change the plasma protein binding of febuxostat and febuxostat did not influence the plasma protein binding of ibuprofen or warfarin, suggesting that febuxostat was unlikely to cause a drug-drug interaction by displacement of protein binding (CitationMukoyoshi et al 2008). Examination of the inhibitory effect of febuxostat on CYP enzymes suggested that it had minimal inhibitory effect on the activities of any CYP (CitationMukoyoshi et al 2008). Based on the in vitro study that showed febuxostat had a weak inhibitory effect on CYP2D6, but no effect on CYP1A2, CYP2C9, CYP2C19 or CYP3A4, CitationKhosravan et al (2005b) examined the effect of multiple doses of febuxostat 120 mg daily on the pharmacokinetics of desipramine, a substrate for CYP2D6. A small increase in total exposure to desipramine was found suggesting that CYP2D6-mediated metabolism was mildly inhibited, but this was considered clinically insignificant (CitationKhosravan et al 2005b). The potential interactions between febuxostat and other medications which might be concurrently used in patients with hyperuricemia and gout have been evaluated in some studies.
Four phase I, two-period, crossover studies were performed in healthy male and female subjects to evaluate the effect of food and antacid on the pharmacokinetics and/or pharmacodynamics of febuxostat. Food decreased rate and extent of absorption of febuxostat, but this decrease was not associated with a clinically significant change in the pharmacodynamic effect. With antacid there was a decrease in the absorption rate of febuxostat, but no effect on the total extent of febuxostat absorption. These findings suggested that febuxostat can be taken with food or antacid without a significant effect on the response (CitationKhosravan et al 2008a).
CitationGrabowski et al (2005) reported that a single dose of hydrochlorothiazide 50 mg did not alter the pharmacokinetics of febuxostat, but caused a slightly higher serum uric acid concentration that was not considered to be clinically significant. Khosravan et al evaluated the potential drug interactions between febuxostat and other medication for treatment or prevention of acute gout attacks, including colchicine, indomethacin and naproxen (CitationKhosravan et al 2005a, Citation2006c). Febuxostat had no effect on the plasma pharmacokinetics of colchicine, indomethacin, and naproxen and none of these medications had clinically significant effects on the pharmacokinetics of febuxostat. Therefore, based on the plasma pharmacokinetic data in healthy subjects, febuxostat can be administered with colchicine, indomethacin or naproxen with no dose adjustments.
Safety and efficacy in clinical trials
The in vitro and animal studies with febuxostat showed it was more potent than allopurinol in reducing serum urate levels without any major additional adverse effects (CitationTomlinson 2005). A series of clinical trials from phase I to phase III have been carried out to evaluate the safety and efficacy of febuxostat in humans ().
Table 2 Efficacy of febuxostat in phase III and long term clinical trials
A phase I, multiple-dose, placebo-controlled, dose-escalation study was conducted in healthy male and female adults in the United States with a majority of Caucasian subjects (n = 142) (CitationBecker et al 2004a; CitationKhosravan et al 2006b). Febuxostat was given orally for 2 weeks in daily doses in the range of 10 to 240 mg. Febuxostat resulted in significant decreases in serum and urinary urate concentrations and increases in serum and urinary xanthine concentrations. The percentage decreases in serum urate concentrations ranged from 27% to 76% (net change: 1.34–3.88 mg/dL), which were proportional to dosage for 10 to 120 mg doses but higher doses (>120 mg) did not appear to have a greater effect. Percentage decreases in serum uric acid were 27%, 35%, 37.5%, 40%, 47%, 50%, 60%, 69%, 70%, 72%, and 76% for the 10, 20, 30, 40, 50, 70, 90, 120, 160, 180, and 240 mg doses, respectively. Proportional increases in serum xanthine concentrations, decreases in urinary uric acid excretion, and increases in urinary xanthine and hypoxanthine excretion were also observed for doses of 10 mg to 120 mg. Adverse events were mild or moderate and self-limited, but not uncommon. The incidence of adverse events tended to be higher with higher doses of febuxostat. No deaths or serious adverse events were observed (CitationKhosravan et al 2006b).
Based on the results from the above phase I study, the doses for phase II studies were selected and tested in patients with gout. A phase II double-blind, placebo-controlled, multicenter study included 153 patients, aged 23 to 80 years, with gout and baseline serum urate ≥8.0 mg/dL (CitationBecker et al 2005b). Subjects were randomized to febuxostat (40 mg, 80 mg, 120 mg) or placebo once daily for 28 days with colchicine (0.6 mg twice daily) prophylaxis for 14 days prior to and 14 days after randomization. The subjects achieving the primary outcome of serum urate <6.0 mg/dL on day 28, were 0%, 56%, 76%, and 94% for placebo, 40 mg, 80 mg, and 120 mg dose febuxostat groups, respectively (p < 0.001 for each treatment group vs. placebo). Compared with baseline, decreases in serum urate at day 28 were 2%, 37%, 44%, and 59% for placebo, 40-, 80-, and 120-mg dose groups, respectively (p < 0.001 for each treatment group vs placebo). Gout flares occurred with similar frequency in the placebo (37%) and 40 mg febuxostat (35%) groups and with increased frequency in the higher dosage febuxostat groups (43% with 80 mg; 55% with 120 mg). During colchicine prophylaxis, gout flares occurred less frequently (8%–13%). Incidences of treatment-related adverse events were similar in the febuxostat and placebo groups (CitationBecker et al 2005b).
A long-term, open-label extension of the above 4-week double-blind phase II study included 116 patients and 69 patients continued for at least 2 years (CitationSchumacher et al 2004; CitationWortmann et al 2004). Subjects were initially given febuxostat 80 mg/day with titration to either 40 or 120 mg based on serum urate and adverse events, achieving a stable dose after 28 weeks. Colchicine (0.6 mg twice daily) prophylaxis was implemented during the initial phase of treatment. Most patients (74%–81%) maintained serum urate levels of <6.0 mg/dL throughout the study period. Any treatment-related adverse events were mild-to-moderate with the most common being diarrhea, headache, and abnormal liver function test results. No serious adverse event was considered to be related to febuxostat. Analysis of a small number of patients (n = 9) in this extension study with gouty tophi revealed that tophus volume increased in patients with serum urate >6.0 mg/dL and decreased in patients with serum urate <6.0 mg/dL (CitationBecker et al 2004c). Febuxostat was also efficacious, safe and well tolerated in a subgroup of allopurinol-intolerant patients included in the trial extension (n = 8) (CitationBecker et al 2004b). More recently, the investigators further reported the data from 61 subjects with long-term treatment with febuxostat for at least 4 years (FOCUS) (CitationSchumacher et al 2006). Across all doses, the proportion of subjects with serum urate <6.0 mg/dL were 78%, 76%, 84%, and 90% at year 1, 2, 3, and 4, respectively. The overall incidence of gout flares requiring treatment declined markedly by 2 years and continued over 4 years of febuxostat treatment. After the first year of stable dose, subjects, on average, had <1 gout flare per year. 26 subjects entered the study with a palpable tophus; 20 (77%) of these subjects had no tophus detectable at the examinations during the study. The most common treatment-related adverse events for all febuxostat groups were diarrhea, gastrointestinal motility disorders, headache, abnormal liver function tests, and hyperlipidemia. This longest clinical study of febuxostat therapy to date showed that long-term treatment with febuxostat reducing and maintaining serum urate <6.0 mg/dL was accompanied by beneficial clinical outcomes, including reduction in gout flares and tophus volume (CitationSchumacher et al 2006). They also found that 4 years treatment with febuxostat was safe, effective, and well tolerated in 6 allopurinol-intolerant patients, and 3 subjects with tophi identified at the time of enrollment had disappearance of their tophi by the fourth year of febuxostat treatment (CitationBecker et al 2006).
The Febuxostat versus Allopurinol Controlled Trial (FACT), a phase III, randomized, double-blind, 52-week, multicenter trial, compared the safety and efficacy of febuxostat with allopurinol in adult subjects with gout (CitationBecker et al 2005a). A total of 762 patients with chronic gout and serum urate levels ≥8.0 mg/dL were randomized to receive once-daily doses of either 80 or 120 mg febuxostat or 300 mg allopurinol for 52 weeks and 760 received at least one dose of the study drug during the study period. Naproxen (250 mg twice daily) or colchicine (0.6 mg once daily) was provided for gout flare prophylaxis during the 2-week washout period and first 8 weeks of the study. The primary endpoint of serum urate <6.0 mg/dL at all the last three measurements at monthly intervals up to 52 weeks was reached in 53%, 62%, and 21% of the febuxostat 80 mg and 120 mg and allopurinol 300 mg groups, respectively (p < 0.001 for the comparison of each febuxostat group with the allopurinol group). Although the incidence of gout flares gradually decreased with continued treatment, the overall incidence during weeks 9 through 52 was similar in all groups: 64% of patients receiving 80 mg of febuxostat, 70% of those receiving 120 mg of febuxostat, and 64% of those receiving allopurinol. The median reduction in tophus area was 83% and 66% in patients receiving 80 mg or 120 mg of febuxostat and 50% in those receiving allopurinol (p > 0.05). More patients in the high-dose febuxostat group than in the allopurinol group (p = 0.003) or the low-dose febuxostat group discontinued the study. The investigators concluded that febuxostat 80 mg or 120 mg daily was more effective than allopurinol 300 mg daily in reducing serum urate (CitationBecker et al 2005a).
Another large phase III study, the APEX study, involving 1067 subjects (78% Caucasians) with gout and serum urate levels ≥8.0 mg/dL compared the safety and efficacy of febuxostat to allopurinol and placebo (CitationSchumacher et al 2005). This study included 40 subjects with moderately impaired renal function (serum creatinine 1.6 to ≤2.0 mg/dL) and a high dose of febuxostat for safety evaluation. Subjects were randomized to receive placebo, febuxostat 80 mg, 120 mg, or 240 mg (safety dose) or allopurinol once daily in a 1:2:2:1:2 ratio for 28 weeks. No dose adjustments based on renal function were made in the febuxostat treatment groups, but subjects randomized to allopurinol received 300 mg/day if serum creatinine was ≤1.5 mg/dL and 100 mg/day for serum creatinine 1.6 to ≤2.0 mg/dL. The primary endpoint of the proportion of subjects with serum urate <6.0 mg/dL at each of the last 3 visits was achieved in 48%, 65%, 69%, 22%, and 0% in febuxostat 80 mg, 120 mg, 240 mg, allopurinol 300/100 mg, and placebo groups, respectively (p < 0.05 for the comparison of each febuxostat group with the allopurinol group and placebo group) and 44%, 45%, 60%, 0%, and 0%, respectively, for subjects with moderate renal impairment for each group. After 28 weeks, 76%, 87%, 94%, 41%, and 1% of patients in febuxostat 80 mg, 120 mg, 240 mg, allopurinol 300/100 mg, and placebo groups had serum urate <6.0 mg/dL (p < 0.05 for the comparison of each febuxostat group with the allopurinol group and placebo group). Adverse events including liver function abnormalities, headache, nausea and vomiting, abdominal pain, and dizziness, were similar in incidences across treatment groups. There were no increases in adverse events in subjects with moderate renal insufficiency including those receiving febuxostat 240 mg daily. These results suggested that febuxostat (80 mg, 120 mg, or 240 mg) demonstrated superior efficacy compared with placebo and allouprinol (300 or 100 mg) in reducing and maintaining serum urate <6.0 mg/dL. Febuxostat was generally safe and well tolerated at all doses (CitationSchumacher et al 2005).
Clinical benefits of long-term urate-lowering therapy with febuxostat have been evaluated in the EXCEL trial, which is an extension study of 2 Phase III trials mentioned above (FACT and APEX) (CitationWortmann et al 2006). This long-term open-label study was designed to determine if subjects treated with febuxostat or allopurinol maintaining serum urate <6.0 mg/dL was associated with reduction in gout flare frequency and tophus resolution. A total of 1086 subjects completing the above two phase III trials were enrolled. At the time of interim analysis, 75% (815/1086) of subjects completed ≥16 months in EXCEL; the durations of exposure to initial treatment were 492, 428, and 271 days, respectively, for febuxostat 80 mg, 120 mg, and allopurinol 300/100 mg. The incidence of gout flares decreased gradually from 1.4, 1.72, 1.49 fares per year in the first year to 0.19, 0.0, and 0.11 flares per year in the third year for febuxostat 80 mg, 120 mg, and allopurinol 300/100 mg groups, respectively. The most common febuxostat treatment-related adverse events were abnormal liver function tests, headache, hypertension, diarrhea, and arthralgia/stiffness, whereas the most common treatment-related adverse events for allopurinol were abnormal liver function tests and rash (CitationWortmann et al 2006).
The effects of lower doses of febuxostat were evaluated in Japanese subjects compared to placebo or allopurinol in some studies (CitationKamatani et al 2003, Citation2004). A phase II, multicenter, double-blind study was conducted to assess the efficacy and safety of doses of febuxostat in 128 patients with gout or hyperuricemia (serum urate levels ≥8.0 mg/dL). Participants were randomized to receive once-daily doses of placebo or febuxostat (10 mg, 20mg, or 40 mg; n = 32, per group). Patients assigned to the febuxostat groups were administered 10 mg febuxostat for the first 2 weeks, followed by 10 mg, 20 mg, or 40 mg for a subsequent 6-week treatment period, while individuals assigned to the placebo group received placebo throughout the 8-week trial. Serum urate levels of <6.0 mg/dL were achieved in 0%, 22%, 31.5%, and 41.9% of patients with placebo, 10 mg, 20 mg, and 40 mg febuxostat groups, respectively. There was no significant difference in response rate between gout and hyperuricemia patients or between urate overproducers or underexcreters. Febuxostat was safe and well tolerated at all doses (CitationKamatani et al 2003).
A further phase III study compared the safety and efficacy of febuxostat with allopurinol in 256 Japanese subjects with gout or hyperuricemia with serum urate levels ≥8.0 mg/dL (CitationKamatani et al 2004). In this randomized, multicenter, double-blind study, patients received either febuxostat (10 mg once daily) or allopurinol (100 mg once daily) for a 12-week introductory phase before doses were increased for a further 44 days of treatment with febuxostat (40 mg once daily) or allopurinol (100 mg twice daily). The decrease in serum urate levels after 8 weeks of treatment was significantly (p < 0.001) greater in the febuxostat group (40.5%), compared with the allopurinol group (33.9%). Furthermore, 82% of patients in the febuxostat arm achieved serum urate levels of ≤6.0, compared with 69% in the allopurinol arm (p < 0.05). Adverse reactions were reported in 11% of patients in the allopurinol group and 8.6% in the febuxostat group. The majority of adverse events were transient and mild in severity (CitationKamatani et al 2004).
Summary
Febuxostat has been shown to be safe and effective in lowering serum urate according to the available clinical data. Doses of 80 mg of febuxostat are more effective in lowering serum urate than doses of 300 mg of allopurinol. Febuxostat has shown to be well tolerated in long term treatment in patients experiencing hypersensitity/intolerance to allopurinol.
Dose adjustment does not seem to be necessary in patients with mild to moderate renal/liver insufficiency or advanced age according to data from these particular groups of subjects. The most common adverse reactions reported were abnormal liver function tests, headache, and gastrointestinal symptoms, which were usually mild and transient. Hepatotoxicity was not a feature in the animal studies, but whether it becomes a limitation in the clinical use of febuxostat needs to be determined in further studies. As with other urate-lowering therapies, the rapid decrease in serum urate associated with initiation of treatment with febuxostat caused a number of patients to experience acute gout flares. This appeared to be more frequent with the more potent serum urate-lowering effects of higher doses of febuxostat, but this increased incidence of gout attacks tended to decline with ongoing treatment and could be attenuated with concomitant prophylaxis during the initiation of febuxostat therapy. In February 2008, 80 mg and 120 mg of febuxostat film-coated tablets (Adenuric®) were granted marketing authorization by the European Commission for the treatment of chronic hyper-uricemia in conditions in which urate deposition has already occurred (including a history or presence of tophus and/or gouty arthritis), which is the first major treatment alternative for gout in more than 40 years (CitationCHMP 2008; CitationIpsen 2008). A concern on potential serious cardiovascular adverse events was noted in the European Commission statement, but this was not apparent in the published clinical trial reports.
In summary, febuxostat is a promising urate-lowering therapy as an alternative to allopurinol for the treatment of hyperuricemia and gout, although further observation on post-marketing safety and efficacy of long term treatment with febuxostat in patients with gout or hyperuricemia and with other complications is required.
Disclosures
The authors have no conflicts of interest to disclose.
References
- AbbottRDBrandFNKannelWB1988Gout and coronary heart disease: the Framingham StudyJ Clin Epidemiol41237423339376
- BeckerMA1988Clinical aspects of monosodium urate monohydrate crystal deposition disease (gout)Rheum Dis Clin North Am14377943051156
- BeckerMAKisickiJKhosravanR2004aFebuxostat (TMX-67), a novel, non-purine, selective inhibitor of xanthine oxidase, is safe and decreases serum urate in healthy volunteersNucleosides Nucleotides Nucleic Acids231111615571211
- BeckerMASchumacherHRJrWortmannRL2005aFebuxostat compared with allopurinol in patients with hyperuricemia and goutN Engl J Med35324506116339094
- BeckerMASchumacherHRJrWortmannRL2006Allopurinol intolerant patients treated with febuxostat for 4 yearsACR/ARHP Annual Scientific Meeting
- BeckerMASchumacherHRWortmannRLJr2005bFebuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with goutArthritis Rheum529162315751090
- BeckerMASchumacherRWortmannR2004bFebuxostat, a novel non-purine selective inhibitor of xanthine oxidase, therapy in allopurinol intolerant patientsArthritis Rheum50803/103
- BeckerMASchumacherRWortmannR2004cMagnetic resonance imaging of gouty tophi during treatment with febuxostat, a non-purine selective inhibitor of xanthine oxidaseArthritis Rheum50802/103
- BenzieIFChungWTomlinsonB1999Simultaneous measurement of allantoin and urate in plasma: analytical evaluation and potential clinical application in oxidant:antioxidant balance studiesClin Chem45901410352002
- BruceSP2006Febuxostat: a selective xanthine oxidase inhibitor for the treatment of hyperuricemia and goutAnn Pharmacother4021879417132810
- CHMP2008Committee for medicinal products for human use summary of positive opinion for Adenuric [online] 21 February 2008. URL: http://www.emea.europa.eu/pdfs/human/opinion/Adenuric_8075108en.pdf
- ChoiHKCurhanG2007Independent impact of gout on mortality and risk for coronary heart diseaseCirculation11689490017698728
- ChoiHKMountDBReginatoAM2005Pathogenesis of goutAnn Intern Med14349951616204163
- EmmersonBT1996The management of goutN Engl J Med334445518552148
- FalascaGF2006Metabolic diseases: goutClin Dermatol2449850817113968
- FamAG2002Gout, diet, and the insulin resistance syndromeJ Rheumatol291350512136887
- GrabowskiBAKhosravanRWuJT2005Effect of hydrochlorothiazide on pharmacokinetics and pharmacodynamics of febuxostatArthritis Rheum52S1034
- HakAEChoiHK2008Lifestyle and goutCurr Opin Rheumatol201798618349748
- HoriuchiHOtaMKobayashiM1999A comparative study on the hypouricemic activity and potency in renal xanthine calculus formation of two xanthine oxidase/xanthine dehydrogenase inhibitors: TEI-6720 and allopurinol in ratsRes Commun Mol Pathol Pharmacol1043071910741381
- HoshideSTakahashiYIshikawaT2004PK/PD and safety of a single dose of TMX-67 (febuxostat) in subjects with mild and moderate renal impairmentNucleosides Nucleotides Nucleic Acids231117815571212
- HungSIChungWHLiouLB2005HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinolProc Natl Acad Sci U S A1024134915743917
- Ipsen2008Adenuric® (febuxostat) receives marketing authorisation in the European Union Ipsen Press Release 5 May 2008 [online]. URL: http://www.ipsen.com/articles/investorrelations/regulatedinformation/20080505___autorisation_adenuric_eu_10.pdf
- IsekiKIkemiyaYInoueT2004Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohortAm J Kidney Dis446425015384015
- KamataniNFujimoriSHadaT2003Phase II dose-response clinical trial using febuxostat (TMX-67), a novel-type xanthine oxidase/xanthine dehydrogenase inhibitor, for gout and hyperuricemiaArthritis Rheum48S530
- KamataniNFujimoriSHadaT2004Febuxostat, a novel non-Purine selective inhibitor of xanthine oxidase, in an allopurinol-controlled phase III clinical trial in Japanese subjects with gout or hyperuricemiaArthritis Rheum50804/103
- KhosravanRGrabowskiBAMayerMD2006aThe effect of mild and moderate hepatic impairment on pharmacokinetics, pharmacodynamics, and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidaseJ Clin Pharmacol468810216397288
- KhosravanRGrabowskiBAWuJT2006bPharmacokinetics, pharmacodynamics and safety of febuxostat, a non-purine selective inhibitor of xanthine oxidase, in a dose escalation study in healthy subjectsClin Pharmacokinet458214116884320
- KhosravanRGrabowskiBWuJT2008aEffect of food or antacid on pharmacokinetics and pharmacodynamics of febuxostat in healthy subjectsBr J Clin Pharmacol653556317953718
- KhosravanRKukulkaMJWuJT2008bThe effect of age and gender on pharmacokinetics, pharmacodynamics, and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidaseJ Clin Pharmacol4810142418635756
- KhosravanRMayerMDWuJT2005aEffect of concomitant administration of febuxostat and colchicine on pharmacokinetics of febuxostat and colchicine at steady stateArthritis Rheum52supplS1023
- KhosravanRWuJTJoseph-RidgeN2006cPharmacokinetic interactions of concomitant administration of febuxostat and NSAIDsJ Clin Pharmacol468556616855070
- KhosravanRPErdmanKBVernilletLPP2005bEffect of febuxostat on pharmacokinetics of desipramine, a CYP2D6 substrate, in healthy subjectsClin Pharmacol Ther77P43PI137
- KimKYRalph SchumacherHHunscheE2003A literature review of the epidemiology and treatment of acute goutClin Ther25159361712860487
- KomoriyaKHoshideSTakedaK2003Pharmacokinetics and pharmacodynamics of febuxostat (TMX-67), a non-purine selective inhibitor of xanthine oxidase/xanthine dehydrogenase (NPSIXO) in patients with gout and/or hyperuricemiaNucleosides Nucleotides Nucleic Acids2311192215571213
- KomoriyaKHoshideSTakedaK2004Pharmacokinetics and pharmacodynamics of febuxostat (TMX-67), a non-purine selective inhibitor of xanthine oxidase/xanthine dehydrogenase (NPSIXO) in patients with gout and/or hyperuricemiaNucleosides Nucleotides Nucleic Acids2311192215571213
- KomoriyaKOsadaYHasegawaM1993Hypouricemic effect of allopurinol and the novel xanthine oxidase inhibitor TEI-6720 in chimpanzeesEur J Pharmacol250455608112406
- KrishnanEBakerJFFurstDE2006Gout and the risk of acute myocardial infarctionArthritis Rheum5426889616871533
- Li-YuJClayburneGSieckM2001Treatment of chronic gout. Can we determine when urate stores are depleted enough to prevent attacks of gout?J Rheumatol285778011296962
- MayerMDKhosravanRVernilletL2005Pharmacokinetics and pharmacodynamics of febuxostat, a new non-purine selective inhibitor of xanthine oxidase in subjects with renal impairmentAm J Ther12223415662289
- MukoyoshiMNishimuraSHoshideS2008In vitro drug-drug interaction studies with febuxostat, a novel non-purine selective inhibitor of xanthine oxidase: plasma protein binding, identification of metabolic enzymes and cytochrome P450 inhibitionXenobiotica3849651018421623
- OkamotoKEgerBTNishinoT2003An extremely potent inhibitor of xanthine oxidoreductase. Crystal structure of the enzyme-inhibitor complex and mechanism of inhibitionJ Biol Chem27818485512421831
- OkamotoKNishinoT2008Crystal structures of mammalian xanthine oxidoreductase bound with various inhibitors: allopurinol, febuxostat, and FYX-051J Nippon Med Sch752318360072
- OsadaYTsuchimotoMFukushimaH1993Hypouricemic effect of the novel xanthine oxidase inhibitor, TEI-6720, in rodentsEur J Pharmacol24118388243554
- RoddyEZhangWDohertyM2007The changing epidemiology of goutNat Clin Pract Rheumatol3443917664951
- Sanchez-LozadaLGTapiaEBautista-GarciaP2008aEffects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndromeAm J Physiol Renal Physiol294F710818216151
- Sanchez-LozadaLGTapiaESotoV2008bEffect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemiaNephron Physiol1086978
- Sanchez-LozadaLGTapiaESotoV2008cTreatment with the xanthine oxidase inhibitor febuxostat lowers uric acid and alleviates systemic and glomerular hypertension in experimental hyperuricaemiaNephrol Dial Transplant2311798518048425
- SchlesingerN2004Management of acute and chronic gouty arthritis: present state-of-the-artDrugs64239941615481999
- SchumacherHRJrBeckerMAWortmannRL2006The FOCUS trial 48-month interim analysis: long-term clinical outcomes of treatment with febuxostat in subjects with gout in an ongoing phase 2, open-label extension studyACR/ARHP Annual Scientific Meeting
- SchumacherHRBeckerMAJrWortmannRL2005Febuxostat vs allopurinol and placebo in subjects with hyperuricemia and gout: the 28-week APEX studyArthritis Rheum52S680
- SchumacherHRJrWortmannRBeckerMA2004A phase 2, long term open-label safety and efficacy study of febuxostat, a novel non-purine, selective inhibitor of xanthine oxidaseArthritis Rheum50800/180
- SlotO1994[Hyperuricemia]Ugeskr Laeger15623964018009701
- TakanoYHase-AokiKHoriuchiH2005Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenaseLife Sci7618354715698861
- TengGGNairRSaagKG2006Pathophysiology, clinical presentation and treatment of goutDrugs6615476316956303
- TomlinsonB2005Febuxostat (Teijin/Ipsen/TAP)Curr Opin Investig Drugs6116878
- WortmannRBeckerMASchumacherHRJr2004Gout flare prophylaxis during management of chronic gout with febuxostat, a non-purine selective inhibitor of xanthine oxidaseArthritis Rheum50801/103
- WortmannRL2002Gout and hyperuricemiaCurr Opin Rheumatol14281611981327
- WortmannRL2005Recent advances in the management of gout and hyperuricemiaCurr Opin Rheumatol173192415838244
- WortmannRLBeckerMASchumacherHRJr2006Effect of febuxostat or allopurinol on the clinical manifestations of gout: reduction in gout flares and tophus size over time in the EXCEL trialACR/ARHP Annual Scientific Meeting
- ZhaoLRocheBMWessaleJL2008Chronic xanthine oxidase inhibition following myocardial infarction in rabbits: effects of early versus delayed treatmentLife Sci8249550218215719