Abstract
Advances in our understanding of the pathophysiology of migraine have resulted in important breakthroughs in treatment. For example, understanding of the role of serotonin in the cerebrovascular circulation has led to the development of triptans for the acute relief of migraine headaches, and the identification of cortical spreading depression as an early central event associated wih migraine has brought renewed interest in antiepileptic drugs for migraine prophylaxis. However, migraine still remains inadequately treated. Indeed, it is apparent that migraine is not a single disease but rather a syndrome that can manifest itself in a variety of pathological conditions. The consequences of this may be that treatment needs to be matched to particular patients. Clinical research needs to be devoted to identifying which sort of patients benefit best from which treatments, particularly in the field of prophylaxis. We propose four patterns of precipitating factors (adrenergic, serotoninergic, menstrual, and muscular) which may be used to structure migraine prophylaxis. Finally, little is known about long-term outcome in treated migraine. It is possible that appropriate early prophylaxis may modify the long-term course of the disease and avoid late complications.
Migraine headaches are the most frequent type of incapacitating headache and one of the most common reasons for consultation in neurology. However, these headaches have historically been poorly understood in terms of natural history, pathophysiology and prognosis. This unsatisfactory state of affairs had important consequences for the diagnosis and treatment of migraine, which have been frequently inadequate. Indeed, the first rational classification of headache semiology, providing an unambiguous definition of migraine was established relatively recently by the International Headache Society in 1988 (CitationHCCIHS 1988) (revised in 2004 [CitationHCCIHS 2004]). This provides a framework for the standardized diagnosis of migraine.
For this reason, there is little available data on long-term disease course from natural history cohorts which have used this diagnosis classification. In particular, although headache relief treatments, often with over-the-counter analgesic drugs, are widely used, the long-term management of migraine, involving prophylactic treatments and the implementation of strategies to prevent headache evolution to more severe disease and to promote remission, has been explored relatively little. This situation has changed somewhat over recent years with the introduction of standardized guidelines for the diagnosis and treatment of migraine headaches. Moreover, the introduction of triptans as a specific treatment for relief of migraine headaches has stimulated much research into the biology of migraine, leading to a better understanding of its pathophysiology. This articles reviews recent developments in our understanding of migraine and their consequences for improved management of migraine as a chronic disease.
Natural history of migraine
Migraine headache generally presents for the first time during adolescence, with a lower mean age of onset in males than in females (CitationUlrich et al 1999). However, migraine does occur in younger children (CitationAbu-Arefeh and Russell 1994; CitationAnnequin et al 2000) and may be under-diagnosed due to difficulties in assessing accurately symptomatology in this population.
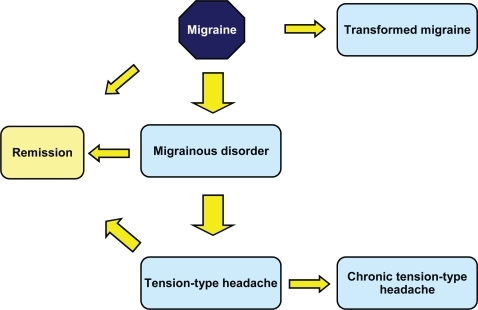
There have been few longitudinal studies investigating long-term outcome of migraine headache. However, given that the prevalence of migraine decreases with age, it can be expected that, in many cases, these headaches resolve spontaneously. Two recent studies have evaluated long-term outcome in larger populations whose original migraine diagnosis was established using the IHS criteria. The first study followed up 549 of 740 subjects originally diagnosed with episodic headaches in 1989 (CitationLyngberg et al 2005). Twelve years after the original diagnosis, 42% of subjects were in remission, 38% had less frequent headaches and 20% had more frequent headaches. Poor outcome was associated with high migraine frequency at baseline and age at onset younger than 20 years. For tension-type headache, remission was observed in 45% of subjects and transformation into a chronic tension-type headache was reported in 16%. The second study evaluated 1250 of 2051 subjects originally identified in 2003, of whom 398 fulfilled diagnostic criteria for migraine (CitationNachit-Ouinekh et al 2005). Ten years later, 37% stilled fulfilled these criteria, whereas 36% now fulfilled diagnostic criteria for migrainous disorder and 18% for other episodic headache types, the remainder being in complete remission. Remission or evolution to a less severe headache was more frequent in men and in older subjects.
Chronic headache presentations
These longitudinal studies identify a minority of subjects whose condition deteriorates with time, notably with an increased frequency of headaches. This corresponds to the notion of transformed migraine (CitationMathew et al 1982) or chronic daily headache. Many of these headaches may be iatrogenic and high levels of analgesic drug use have been described in up to three-quarters of cases (CitationMathew 1997) and are associated with a risk of transformation (CitationZwart et al 2003; CitationDowson et al 2005). Although analgesic drugs have been implicated most frequently in the development of chronic daily headaches, recent studies suggest that use of specific acute migraine treatments (triptans) may also lead to transformation of migraine (CitationLimmroth et al 2002). High caffeine use has also been described as a risk factor for transformation (CitationScher et al 2004). Psychiatric comorbidity, notable depression, may also be associated with an increased risk of transformation (CitationMathew 1997; CitationWang et al 2000).
In addition, tension-type headaches may also evolve into a chronic form. Chronic tension-type headaches, as defined in the IHS classification, occur with a frequency of over 180 days per year and are distinguished from transformed migraine (or chronic daily headache with migrainous features) by their characteristic symptom presentation (steady rather than pulsatile pain, bilateral rather than unilateral, etc). Some characteristic features of migraine, such as nausea, are less prevalent in subjects with chronic daily headache than in those with classical migraine headache (CitationSolomon et al 1992; CitationMathew 1993; CitationLanteri-Minet et al 2003). The overall prevalence of chronic daily headache in the general population is around 4% (CitationWang et al 2000; CitationScher et al 1998; CitationCastillo et al 1999; CitationLanteri-Minet et al 2003), with most studies reporting chronic tension-type headache to be more frequent than transformed migraine. Individuals presenting with these headache types represent a large segment of the population consulting specialist headache centres. Chronic daily headaches often remit spontaneously, and a one-year remission rate of 14% has been estimated from a general population sample (CitationScher et al 2003). Factors associated with a poor prognosis of chronic daily headache include older age, long duration of chronic headaches and medication overuse (CitationWang et al 2000; CitationLu et al 2001).
Consequences for treatment
The long-term outcome of migraine can thus be variable () and it is an important objective of therapy to ensure a favorable outcome (remission). This raises a number of important questions. Classical migraine can be managed effectively with acute or prophylactic treatments (see below) although analgesic drugs should be avoided as headache relief medication in order to reduce the risk of transformation to a chronic headache type. Certain of these drugs may be acquired over the counter and used as self-medication, and it is important to identify and discourage such habits in patients consulting for migraine headache. In patients whose headaches have already transformed into a chronic presentation, medication withdrawal can in many cases be sufficient to promote remission (CitationLinton-Dahlof et al 2000; CitationKavuk et al 2004).
Migrainous disorder can be considered as a ‘mild’ form of classical migraine and clinical experience suggests that these headaches respond to the same relief medications as the latter disorder, although this has not been evaluated specifically in randomized clinical trials. A methodical evaluation of symptoms is recommended in order to distinguish these headaches from tension-type headaches and to treat them appropriately.
It is not clear whether tension-type headache that is secondary to migraine headache (‘burnt-out’ migraine) differs from primary tension-type headaches in their physiopathology and in their response to treatments. For example, two randomized clinical trials have shown sumatriptan to be inefficacious in primary tension-type headaches (CitationBrennum et al 1996; CitationLipton et al 2000a) but their utility in secondary tension-type headaches evolving from migraine is unknown.
Another important unanswered question relates to whether early and intensive drug treatment of migraine headaches changes the probability of a favorable outcome. In another paroxystic neurological disorder, epilepsy, this is an established therapeutic principle, and merits investigation in long-term follow-up studies of patients presenting with and treated for migraine headaches.
Migraine as a syndrome
It is increasingly apparent that migraine is best understood as a syndrome expressed in several different subtypes of underlying disease. These various subtypes differ in their symptom presentation and are also likely to respond better to certain treatments than to others. A major challenge is to identify the most appropriate treatments for each subtype of migraine.
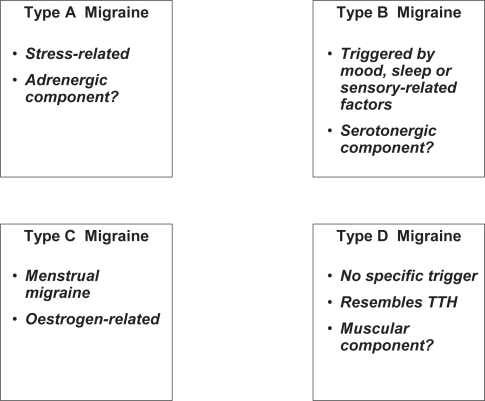
Familial hemiplegic migraine is probably only the first form of familial migraine whose genetic basis has been elucidated. This form of migraine headache can be considered a channelopathy in which symptoms arise due to a change in the gating kinetics of P/Q type calcium channels brought about by the mutations (CitationKraus et al 1998, Citation2000). The consequence of this may be an impairment of local GAB-Aergic inhibitory circuits (CitationCao and Tsien 2005). It may also be possible to define subtypes of migraine headache based on differences in precipitating factors. First of all, stress-induced headache, mediated by an adrenergic mechanism may be a specific migraine subtype. Stress is the most frequently cited precipitating factor and related to a particular pattern of physiological activation, namely activation of the hypothalamo-pituitary-adenocortical axis and increased sympathetic tone. This may lead to increased vasoconstrictor tone in the cerebral vasculature and thus sensitize individuals to migraine headache.
Mood and sleep disturbances are also common precipitating factors for migraine and these are associated with the central serotonergic system. Another group of precipitating factors relates to sensory stimuli such as particular smells (tobacco or perfume) or foodstuffs (cheese, alcohol, chocolate) and these may also implicate serotonergic mechanisms.
Menstrual migraine provides an example of a form of migraine with a specific precipitating factor, pathogenesis and treatment. Around half of women with migraine report menstruation to be a precipitating factor (CitationHenry et al 2002; CitationZivadinov et al 2003) and the frequency of migraine attacks varies over the reproductive cycle (CitationGranella et al 1993). A recent large prospective survey in a population of 153 women with migraine (CitationMacGregor 2004) identified a relative risk of migraine headache of 1.7 in the two days preceding menstruation and of 2.3 in the first three days of menstruation compared to all other times of the cycle. Menstrual migraine attacks have been reported to be of longer duration and to be more incapacitating than non-menstrual migraine (CitationCouturier et al 2003; CitationGranella et al 2004).
In women with ‘pure’ menstrual migraine (ie, those who only have headache attacks at the onset of menstruation and not at other times of the cycle), the onset of headache has been associated with rapidly falling plasma concentrations of estrogen (CitationSomerville 1972). Indeed, women with menstrual migraine show larger swings in estrogen levels across the menstrual cycle than did women without menstrual migraine (CitationEpstein et al 1975). Estrogen challenge experiments have shown that migraine can be precipitated following artificial elevations of plasma estrogen levels both in women with active menstrual migraine (CitationSomerville 1975) and in post-menopausal women with antecedents of menstrual migraine (CitationLichten et al 1996; CitationFacchinetti et al 2002). This hypothesis is also supported by the observation that women taking oral contraceptives often experience headache during the estrogen-free week and by reports of emergent migraine in women interrupting hormone replacement therapy (CitationMacGregor 2004). Exactly how fluctuations in estrogen levels trigger headache is, on the other hand, far from clear. Estrogens have a number of effects on the nervous system involving both genomic actions and direct effects on neuronal excitability (CitationMcEwen 2001). These latter may sensitize the nervous system to cortical spreading depression or modify gating of nociceptive stimuli in the trigeminal nucleus. Studies in experimental animals have provided evidence for a change in the sensitivity of the GABAA receptor over the course of the estrous cycle (CitationCarey et al 1992; CitationDiaz-Veliz et al 2000). It should also be pointed out that estrogens have direct effects on the cerebral vasculature (CitationLittleton-Kearney et al 2000) which may also contribute to the occurrence of migraine headaches. Menstrual migraine can be treated not only with headache medication active in all types of migraine, but also with specific hormonal treatments aimed at attenuating estrogen withdrawal (CitationLoder et al 2005). These are described in more detail below.
Finally, certain otherwise typical pulsatile migraine headaches may share features typical of tension-type headaches such as a sensation of muscular pressure or tension, and represent a mixed headache type. These headaches generally localise to the posterior occipital cortex and have a high probability of transformation into a chronic headache type. Whether such headaches are a transition stage in the deteriorating course of certain classical migraine headaches or represent a subtype of migraine with a distinct aetiopathology is unclear, but they are frequently associated with muscular diseases like fibromyalgia.
A proposed classification of migraine headaches based on triggering factors is presented in . The case of menstrual migraine, where a specific prophylaxis can be proposed may illustrate a general principle, and it will be interesting to see whether other prophylactic treatments may be particularly effective in migraineurs with particular patterns of precipitating factors. For example, beta-blockers such as propranolol, or alpha-blockers such as indoramine may be particularly effective in Type A migraine (as defined in ) whereas antiserotonergic drugs such as methysergide, dihydroergotamine or pizotofen may be more effective in Type B migraine. Hormone therapy is clearly useful in Type C (see below), and muscle relaxant and antidepressant drugs like amitriptyline seem effective in Type D, associated with other prophylactic migraine treatments where necessary. Adequately designed randomized clinical trials will be required to test these hypotheses. Of course, in medical practice patients presenting features typical of more than one headache type are frequently encountered. In these cases, the optimal therapeutic strategy may require association of two or three of these drugs.
Acute treatment of migraine headaches
Four classes of drug are used as acute treatments for the relief of headache. Nonsteroidal antiinflammatory drugs (NSAIDs) and analgesics represent non-specific headache relief medications and the triptans and ergot alkaloids migraine-specific treatments. NSAIDs are generally preferred to analgesics as non-specific treatments, since the latter drugs have a high perceived risk of leading to the development of chronic medication-overuse headaches (CitationZwart et al 2003). However, all acute treatments may to a greater or lesser extent lead to medication overuse headache and prophylactic treatments should be used in patients with frequent headaches to reduce headache frequency and thus recourse to acute medication. Triptans have come to supersede ergot alkaloids as specific headache relief treatments in many countries as they are perceived to have superior tolerability and efficacy.
Various treatment strategies for headache relief have been proposed that combine these drug classes in which patients initially take a non-specific treatment and then add a triptan, first-line/second-line strategies in which patients are initially prescribed an NSAID and then switched to a triptan in case of non-response, and stratified care, in which NSAIDs or triptans are differentially prescribed according to headache severity.
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used for acute headache relief both as prescription and over-the-counter medication (CitationLucas et al 2005). Numerous clinical trials of varying quality have been performed with these agents since the 1960’s.
Placebo-controlled trials have shown aspirin to be effective in the relief of migraine headache, either used alone (CitationBoureau et al 1994; CitationLange et al 2000; CitationMacGregor et al 2002; CitationLipton et al 2005b), in combination with metoclopramide (CitationChabriat et al 1994; CitationTfelt-Hansen et al 1995; CitationHenry et al 1995) or in combination with paracetamol and caffeine (CitationLipton et al 1998). Data from placebo-controlled trials suggests efficacy for ibuprofen (CitationKloster et al 1992; CitationNebe et al 1995; CitationSandrini et al 1998; CitationKellstein et al 2000; CitationCodispoti et al 2001), including two studies in children (CitationHamalainen et al 1997; CitationLewis et al 2002). Diclofenac has proved superior to placebo in several trials (CitationMassiou et al 1991; CitationDahlof and Bjorkman 1993; CitationThe Diclofenac-K/Sumatriptan Migraine Study Group 1999; CitationPeroutka et al 2004). An important advantage of diclofenac appears to be its rapidity of action. An intramuscular injectable form is also available and been shown to provide rapid headache relief (CitationKarachalios et al 1992; CitationBigal et al 2002). Ketoprofen has been compared to placebo in a larger study using a standard oral formulation (CitationDib et al 2002). This study demonstrated superior efficacy for ketoprofen.
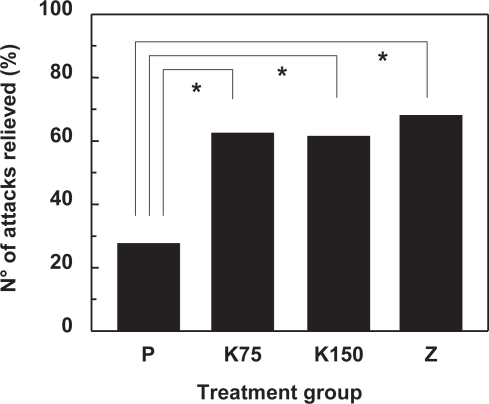
Comparative trials with triptans have been performed for oral aspirin compared to oral sumatriptan (CitationDiener et al 2004a), oral aspirin and metoclopramide compared to oral sumatriptan (CitationThe Oral Sumatriptan and Aspirin plus Metoclopramide Comparative Study Group 1992; CitationTfelt-Hansen et al 1995), intravenous aspirin compared to subcutaneous sumatriptan (CitationDiener 1999), oral aspirin and metoclopramide compared to oral zolmitriptan (CitationGeraud et al 2002), oral ibuprofen compared to oral sumatriptan (CitationDiener et al 2004a), oral diclofenac versus oral sumatriptan (CitationThe Diclofenac-K/Sumatriptan Migraine Study Group 1999), oral naproxen versus oral naratriptan (CitationStronks et al 2003), oral tolfenamic acid versus oral sumatriptan (CitationMyllyla et al 1998) and oral ketoprofen versus oral zolmitriptan (CitationDib et al 2002). With the exception of the asprin/zolmitriptan and noproxen/naratriptan studies, all these trials showed comparable efficacy for the NSAID and the triptan in most, if not all, endpoints. presents data on headache relief at two hours from the ketoprofen/zolmitriptan study. Given the comparable efficacy of NSAIDs to triptans and the considerably lower cost of the latter, many treatment guidelines suggest the use of an NSAID as first-line treatment for migraine headache.
Figure 3 Relief of migraine attack in 235 patients included in a comparative study of ketoprofen and zolmitriptan using a cross-over design (CitationDib et al 2002). Data are given as the percentage of the total number of attacks that were reduced in severity to mild or absent at two hours. Treatment groups are placebo (P), ketoprofen 75 mg (K75), ketoprofen 150 mg (K) and zolmitriptan 2.5 mg (Z). The asterisk indicates a significant difference (p < 0.0001; GEE model) between the bracketed groups.
Triptans
The introduction of the triptans as specific treatments for migraine headache represented an important breakthrough as these drugs were both more efficacious and better tolerated than previously available drugs (ergot alkaloids). The first demonstration of the potential benefit of triptans came from an open-label proof-of-concept study published in 1988 (CitationDoenicke et al 1988), subsequently confirmed in two pivotal randomized controlled trials (CitationEnsink 1991). Sumatriptan was first made available as an injectable subcutaneous formulation in 1992 and subsequently as oral tablets and as a nasal spray. Since the introduction of sumatriptan, six other members of this class have been made available. These are zolmitriptan, naratriptan, rizatriptan, eletriptan, frovatriptan and almotriptan. All triptans are 5-HT1B/D serotonin receptor agonists, acting in the cerebral vasculature to promote vasoconstriction and prevent the release of local vasoactive peptides responsible for inflammation and generation of the pain stimulus (CitationFerrari and Saxena 1992). Clinically, triptans have been shown to provide rapid, effective and sustained pain relief and a reduction in the intensity or duration of associated symptoms.
A meta-analysis has been performed concerning 53 clinical trials with these triptans used orally for the acute treatment of migraine (CitationFerrari et al 2001). This concluded that between 40% and 70% of patients treated with a triptan experienced a significant reduction in pain severity after two hours, whereas between 10% and 25% are pain-free and remain pain-free for up to 24 hours. In terms of tolerability, naratriptan and almotriptan were associated with the lowest numbers of treatment-emergent adverse events. Dose-response relationships were observed for all triptans that had been evaluated at multiple doses. Very similar conclusions were drawn from a second meta-analysis evaluating 54 clinical trials which determined efficacy in terms of numbers-needeed-to-treat (CitationOldman et al 2002). Another meta-analysis of 27 trials also used the numbers-needed-to-treat approach to assess cost-effectiveness, and found almotriptan to be the most cost-effective within the US healthcare system (CitationAdelman and Belsey 2003). This has also been the conclusion of a systematic review of pharmacoeconomic studies with the triptans (CitationLofland and Nash 2005). However, relative cost-effectiveness can vary considerably between different healhcare systems (CitationBelsey 2004).
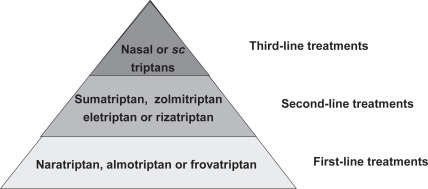
There are a number of factors entering into the choice of a given triptan for acute treatment, From the patient’s perspective, complete freedom from pain, rapid onset of action, no recurrence and absence of side effects are the most important criteria (CitationLanteri-Minet 2005). The TRIPSTAR study (CitationGoadsby et al 2004; CitationLipton et al 2005a) has attempted to categorise efficacy and tolerability attributes of triptans from extensive structured interviews with physicians and migraineurs. In a second step, individual drugs could be matched to this model. On the basis of such analyses, a stepwise heierarchy for the treatment of acute migraine with triptans can be proposed (). The best-tolerated drugs should be used (almotriptan, frovatriptan or naratriptan) as a first-line therapy. If these do not provide an adequate response, one of the other oral triptans should be tried. In case of continued treatment resistance, a nasal spray or injectable preparation should be proposed; This form is also proposed as first line in children.
Prophylaxis of migraine
Migraine prophylaxis should be considered when acute treatment is insufficient, either due to the severity of the headache disorder or to the ineffectiveness of treatment. Effective prophylaxis of migraine decreases recourse to acute treatment, thus reducing the risk of transformation to chronic headache and also decreases overall healthcare resource utilisation (CitationSilberstein et al 2003). Guidelines proposed by the American College of Physicians (CitationSnow et al 2002) recommend that prophylaxis be initiated in patients with two or more migraine headaches per month that produce disability lasting three or more days per month, contraindications or non-response to acute treatment, the use of relief medication more than twice a week or who present with certain specified atypical migraine disorders. Broadly similar recommendations have been published in a number of other national guidelines from, for example, Germany (CitationDiener et al 1998), the United Kingdom (CitationDowson et al 2002) and France (CitationGeraud et al 2004).
The medication classes available for the prophylactic treatment of migraine are presented in . The specific prophylactic agents act by a variety of pharmacological mechanisms, and a large number of molecules have been evaluated in clinical trials of varying quality. A review of all these trials prepared by the Quality Standards Subcommittee of the American Academy of Neurology, however, identified only four agents for which there was strong evidence of high efficacy in the prophylaxis of migraine (CitationSilberstein 2000). These four drugs were propranolol, timolol, amitriptyline and valproate.
Table 1 Prophylactic treatments for migraine
Beta-blockers
β-Blockers represent the oldest, widest used and best-documented prophylactic treatment for migraine. The utility of propranolol was first identified quite serendipitously in 1972 (CitationWeber and Reinmuth 1972) and since then has been the object of numerous clinical trials. A meta-analysis performed in 1991 of studies including 2403 patients included in placebo-controlled clinical trials reported a reduction of 44% in the frequency of headaches in patients receiving propranolol compared to 16% in the placebo group (CitationHolroyd et al 1991). A more recent evidence-based review performed for the Cochrane Collaboration (CitationLinde and Rossnagel 2004) extending the analysis to over five thousand patients included in 58 trials confirmed the superiority of proranolol to placebo in the prophylaxis of migraine. No clear dose-response relationship is observed with propranolol, necessitating up-titration of the dose until an adequate therapeutic response is observed. In most patients, this is achieved at a dose of 120–240 mg per day (CitationAndersson and Vinge 1990). Propranolol is considered to be the reference prophylactic treatment for migraine. Other β-blockers which have demonstrated superior efficacy for migraine prophylaxis compared to placebo include metoprolol (CitationKangasniemi et al 1987; CitationSteiner et al 1988), timolol (CitationTfelt-Hansen and Olesen 1984; CitationStellar et al 1984), altenolol (CitationForssman et al 1983; CitationJohannsson et al 1987) and nadolol (CitationFreitag and Diamond 1984). Comparable efficacy to propranolol has been shown for all four of these drugs (CitationKangasniemi and Hedman 1984; CitationOlsson et al 1984; CitationTfelt-Hansen and Olesen 1984; CitationStensrud and Sjaastad 1980; CitationRyan 1984; CitationSudilovsky et al 1987). On the other hand, β-blockers with partial agonist activity such as alprenolol (CitationEkbom 1975) or oxprenolol (CitationEkbom and Zetterman 1977), have not been shown to be beneficial.
The most common side-effects observed with β-blockers include nausea, dizziness, fatigue, depression and insomnia, although these are generally not severe and do not lead to treatment discontinuation. In the meta-analysis of trials with propranolol (CitationHolroyd et al 1991), the rate of study discontinuation for whatever reason was one in six.
Clonidine and other drugs acting at adrenoceptors
There have been sixteen controlled clinical trials of clonidine for the prophylaxis of migraine. Some of these have found small but significant treatment effects, but most have not (CitationSilberstein 2000). Two comparative studies suggest that clonidine is less efficacious than β-blockers (CitationKass and Nestvold 1980; CitationLouis et al 1985).
Antiserotoninergic drugs
A number of small, relatively old clinical trials tested dihydroergotamine in the prophylaxis of migraine (CitationBonuso et al 1983; CitationBousser et al 1988; CitationLangohr et al 1988; CitationMartucci et al 1983; CitationNeuman et al 1986; CitationPradalier et al 1988). However, a recent randomized, parallel group study versus placebo in 363 patients (CitationPradalier et al 2004a) which used a rigorous design following current clinical trial guidelines (CitationTfelt-Hansen et al 2000) did not reveal a statistically significant difference between the two treatments on the primary outcome measure (headache frequency), although differences in favor of dihydroergotamine were observed for several of the secondary efficacy measures evaluated in the study. In spite of the paucity of evidence, dihydroergotamine is one of the treatment frequently used for the prevention of migraine, for example in France (CitationLanteri-Minet et al 2000).
Concerning other ergot derivatives, three cross-over studies (CitationFrediani et al 1991; CitationBussone et al 1999; CitationMicieli et al 2001) have compared dihydroergocryptine to other prophylactic treatments (propranolol, flunarizine and dihydroergotamine). These studies all concluded that the drug may reduce headache frequency, but the absence of a comparison with a placebo group makes these studies difficult to interpret.
Methysergide is a semi-synthetic derivative of ergot alkaloids that is a relatively selective antagonist at 5-HT2 serotonin receptors. Methysergide was developed for the treatment of migraine in 1959 and the four clinical trials that demonstrated superior efficacy to placebo were performed in the 1960’s (CitationLance et al 1963; CitationShekelle and Ostfeld 1964; CitationPedersen and Moller 1966; CitationRyan 1968). More recently, comparative studies have been performed with propranolol (CitationSteardo et al 1982), flunarizine (CitationSteardo et al 1986) and 5-hydroxytryptophan (CitationTitus et al 1986). However, methysergide has severally potentially serious side-effects, notably retroperitoneal or retropleural fibrosis (CitationElkind et al 1968). This is generally associated with long-term, uninterrupted administration and occurs in around one out of every five thousand treated patients. For this reason, methysergide is only recommended for severe migraine that has not responded to other prophylactic treatments (CitationSilberstein 1998). When methysergide is used, the drug should be discontinued for three to four weeks after every six months of treatment.
Pizotifen (BC105, pizotyline) is extensively used in Europe as a prophylactic treatment for migraine. This serotonin receptor antagonist has been evaluated in several randomized, double-blind studies and shown to be superior to placebo for the prevention of migraine and to reduce utilisation of acute headache relief medication (CitationRyan 1968; CitationOsterman 1977; CitationBellavance and Meloche 1990). Head-to-head comparisons has shown its efficacy to be similar to that of the β-blocker metoprolol (CitationVilming et al 1985). Pizotifen has also been demonstrated to be useful for the treatment of paediatric migraine in a small placebo-controlled study (CitationGillies et al 1986). Like other 5-HT2 receptor antagonists, use of pizotifen is associated with significant weight gain, and treatment discontinuation rates for side-effects are high.
Other 5-HT receptor antagonists that have been evaluated in the prevention of migraine include cyproheptadine, lisuride, oxeterone, iprazochrome, 5-hydroxytryptophan (oxitriptan) and tropisetron. With the exception of tropisetron, these agents are antagonists at 5-HT2 receptors, although most of them are relatively aspecific and also act at other mono-amine receptors or ion channels. Cyproheptadine has been found to decrease the frequency of migraine attacks in a small early study (CitationLance et al 1970) and has subsequently become widely-used for prevention of migraine headaches in children (CitationBille et al 1977). Quite recently, a randomized, double-blind trial compared treatment with placebo, cyproheptadine, propranolol and the combination of the two active drugs (CitationRao et al 2000). Not only did this study confirm the benefit offered by cyproheptadine compared to placebo, but also indicated that the association of the two drugs resulted in a larger reduction in headache frequency than did the use of either drug alone. Lisuride was evaluated in two double-blind, placebo-controlled clinical trials (CitationSomerville and Herrmann 1978; CitationHerrmann et al 1978). Interest in oxeterone stems from four small, double-blind studies performed in Switzerland in the late ‘70s (Citationde Coster 1976; CitationWasserfallen 1976; CitationDufresne 1978; CitationFlorence 1978). Two randomized, placebo-controlled, double-blind studies of iprazochrome found the drug to be superior to placebo for the prevention of migraine (CitationKozubski and Prusinski 1999; CitationOsterman 1977); in the first of these, pizotifen was also studied and found to be more effective than iprazochrome. Placebo-controlled studies with 5-hydroxytryptophan have failed to provide convincing evidence of efficacy (CitationDe Benedittis and Massei 1985; CitationSantucci et al 1986) and comparative studies with methysergide (CitationTitus et al 1986) and propranolol (CitationMaissen and Ludin 1991) have found 5-hydroxytryptophan to be less effective than these two drugs. Two large, randomized, double-blind, placebo-controlled trials with the 5-HT3 receptor antagonist tropisetron have yielded inconclusive results (CitationFerrari et al 1991).
Calcium channel blockers
Calcium channel blocking drugs have evoked much interest in the prophylactic treatment of migraine due to their vasodilator effects in the cerebral vasculature (CitationMontastruc and Senard 1992). However, the only such drug that has demonstrated unequivocal efficacy is flunarizine, and it is debatable whether this property is related to calcium channel blockade at all. It has been suggested that a neuronal rather than a vascular action underlies its beneficial effects in migraine (CitationOlesen 1990). This drug has been evaluated in nine placebo-controlled clinical trials, although several of these are small or of poor quality. A meta-analysis of the most robust of these trials (CitationReveiz-Herault et al 2003) concluded that treatment flunarizine resulted in a greater reduction in headache frequency than placebo. Comparative trials have generally demonstrated a similar effect size as that obtained with propranolol (CitationLucking et al 1988; CitationLudin 1989; CitationGawel et al 1992; CitationDiener 1997) or metoprolol (CitationGrotemeyer et al 1988; CitationSorensen et al 1991). One small study which investigated combination therapy with propranolol and flunarizine failed to demonstrate superior efficacy for the combination compared with monotherpay with either drug alone (CitationBordini et al 1997).
The side effects described for treatment with flunarizine are somnolence, weight gain, and, in less frequently, depression. Treatment discontinuation rates due to side-effects have been high in many of the clinical trials. Due to the relatively poor side-effect profile of flunarizine compared to β-blockers, the use of this drug should be reserved for second-line treatment (CitationMontastruc and Senard 1992). Dotarizine is an analogue of flunarizine which has showed preliminary evidence of efficacy for the prophylaxis of migraine (CitationGaliano et al 1993).
Concerning other classes of calcium channel blocker, studies with dihydropyridines have been inconclusive (CitationMontastruc and Senard 1992). Nifedipine was found to offer no benefit over placebo in two randomized, double-blind clinical trials (CitationMcCarthy and Peroutka 1989; CitationShukla et al 1995), whereas nicardipine (CitationLeandri et al 1990) was reported to be effective. For nimodipine, two positive (CitationGelmers 1983; CitationHavanka-Kanniainen et al 1985), and three negative (CitationAnsell et al 1988; CitationMINES 1989a, Citation1989b) placebo-controlled studies have been reported. Study sizes were rather small in all cases. Preliminary evidence for the efficacy of verapamil has come from three small placebo-controlled trials, none of which included more than twenty-five patients (CitationSolomon 1989). Diltiazem has not been evaluated in randomized, double-blind controlled studies. The most recently evaluated calcium channel blocker is cyclandelate, for which three placebo-controlled trials have been performed, two of which included over 200 patients (CitationDiener et al 1996; CitationDiener et al 2001) and did not demonstrate superior efficacy to placebo in preventing headaches. The third, smaller (n = 25) study did find cyclandelate to be more efficacious than placebo (CitationSiniatchkin et al 1998).
Antiepileptic drugs
Although interest in the potential of antiepileptic drugs for the treatment of migraine headache goes back over thirty years (CitationRompel and Bauermeister 1970), there has been a renewal of interest in such drugs over the last decade. Most of the large randomized clinical trials in migraine prophylaxis which have used current standards of clinical trial methodology (IHSCCT 1991) have concerned this class of drug, which represents the principal innovation in the field for over twenty-five years. Antiepileptic drugs reduce neuronal excitability and thus reduce the cortical spreading depression that is believed to be an early event in the development of migraine headache (CitationWelch 2003). A number of studies using transcranial magnetic stimulation or magnetoencephalography (CitationBowyer et al 2005; CitationMulleners et al 2002) have shown that antiepileptic drugs do effectively reduce cortical hyper-excitability in patients with migraine.
A number of antiepileptic drugs was tested over the past twenty years (CitationKrymchantowski et al 2002; CitationSilberstein and Goadsby 2002; CitationChronicle and Mulleners 2004; CitationYoung et al 2004). These include valproate, topiramate, gabapentin, lamotrigine, carbamazepine, clonazepam, tiagabine, leviracetam and zonisamide. Recent, large-scale controlled trials are however limited to the first four drugs, and only valproate and topiramate have been approved for the prophylaxis of acute migraine in some countries. Studies performed with tiagabine (CitationDrake et al 1999; CitationFreitag et al 1999), levetiracetam (CitationDrake et al 2001; CitationKrusz 2001a) and zonisamide (CitationDrake et al 2001; CitationKrusz 2001b) did not include a control group and thus allow no conclusions to be drawn as to their real clinical benefit.
The first clinical trial dates from 1970, when CitationRompel and Bauermeister (1970) evaluated the efficacy of carbamazepine in a randomized, placebo-controlled, cross over study, and observed an improvement in 84% of patients during treatment with carbamazepine compared to 27% during the placebo phase, and a corresponding reduction in the number of migraine attacks from 186 under placebo to 30 under carbamazepine.
The first study evaluating the efficacy of valproate, and using a placebo-controlled double-blind randomized cross-over design in twenty-nine patients (CitationHering and Kuritzky 1992). Patients received a low dose of valproate (400 mg) for eight weeks. Reduction of the frequency, severity and duration of migraine attacks was observed in 86.2% of the patients studied. A number of subsequent randomized, controlled studies have valproate in the prophylaxis of migraine headache (CitationJensen et al 1994; CitationMathew et al 1995; CitationKlapper 1997; CitationFreitag et al 2002), and concluded that this drug is an efficacious, well-tolerated and easy-to-use medication. Notably, the findings were replicated in a much larger study including 107 patients and using a parallel-group design (CitationMathew et al 1995). A reduction of at least 50% in headache frequency compared to baseline was observed in 48% of patients receiving valproate compared to 14% of those receiving placebo. The severity of residual headaches was also reduced in the valproate treatment group. However, there was a higher rate of adverse events and of treatment discontinuation in patients receiving valproate. A dose-ranging study comparing fixed doses of 500 mg, 1000 mg, or 1500 mg with placebo (CitationKlapper 1997) showed similar efficacy between all three doses, and recommended that the 500 mg/day dose should be used in most patients. A subsequent study, in which responses were related to plasma levels of valproate, also concluded that for most patients, doses over 600 mg/day would provide no additional benefit over lower doses.
It should be noted that intravenous valproate has also been evaluated in several randomized, controlled studies (CitationEdwards et al 2001; CitationTanen et al 2003; CitationLeniger et al 2005) for the acute treatment of migraine, following the publication of an extensive uncontrolled case series suggesting the interest of such a treatment (CitationMathew et al 2000). Although efficacious, it appeared less active than intravenous lysine-acetylsalicylic acid or prochlorperazine. The tolerability and commodity of use of intravenous valproate in this indication are obviously inferior compared to triptans or NSAIDS and this treatment is unlikely to be of widespread use.
Although treatment with valproate is generally well-tolerated, this drug is associated with an elevated teratogenic risk (CitationTomson and Battino 2005). In patients with epilepsy, this risk has been estimated in a number of dedicated retrospective and prospective surveys. For example, in a recent analysis of data from the Swedish Medical Birth Registry and the Swedish Registry of Congenital Malformations, the rate of major malformations in neonates exposed to valproate in utero was 9.7% compared to 1.0% in the Swedish general population (CitationWide et al 2004). There is some evidence that this risk is dose-related, being particularly high with valproate doses over 800–1000 mg/day. In patients with migraine, of whom women of child-bearing age represent the majority, this risk clearly needs to be taken into account in assessing the risks and benefits of treatment with valproate. Unlike what is observed for epilepsy, where this may be the only drug that offers complete seizure freedom, in migraine, valproate is just one of a number of agents that offers a similar level of headache relief.
The most data on the utility of antiepileptic drugs in the prophylaxis of migraine has been obtained for topiramate. The interest of this drug was first suggested in a series of open-label studies (CitationKrusz and Scott 1999; CitationMathew et al 2002; CitationSchuaib et al 1999; CitationVon Seggern et al 2002; CitationYoung et al 2002) and subsequently confirmed in two randomized controlled pilot studies (CitationStorey et al 2001; CitationEdwards et al 2003).
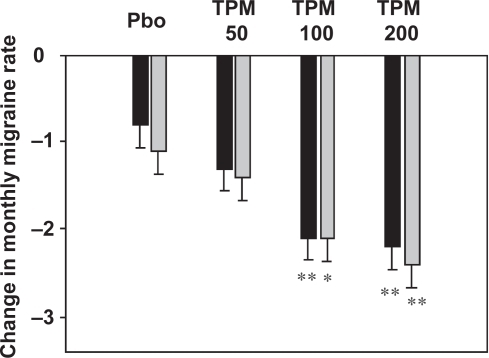
Two large pivotal randomized Phase III clinical trials were performed and the results used to obtain marketing authorisation for topiramate. These evaluated three doses of topiramate (50 mg/day, 100 mg/day and 200 mg/day) in over 900 adult subjects using an identical design (CitationBrandes et al 2004; CitationSilberstein et al 2004). These studies demonstrated a reduction in the monthly frequency of migraine headaches of 30% to 50% following initiation of topiramate treatment (). The effect of topiramate was dose-related and usually was observable within the first month of treatment. Since then, several other randomized, controlled studies have generated data largely consistent with the results of the pivotal studies (CitationSilvestrini et al 2003; CitationDiener et al 2004b; CitationMei et al 2004).
Figure 5 Efficacy of topiramate in the prophylaxis of migraine. Three different doses of topiramate (TPM; 50 mg/day, 100 mg/day, and 200 mg/day) are compared with placebo (Pbo). Data represent the change in monthly migraine frequency compared to a pre-treatment baseline period. The asterisks indicate a statistically significant difference from placebo (*p < 0.01; **p < 001). Data are taken from CitationBrandes and colleagues (2004) (grey columns) and CitationSilberstein and colleagues (2004) (black columns).
However, a number of potentially serious side-effects are associated with topiramate (CitationJones 1998), and it is important to consider safety issues carefully before and during treatment of patients with migraine with this antiepileptic drug. Many of these side-effects are related to inhibition of carbonic anhydrase, including metabolic acidosis, formation of kidney stones, acute myopia associated with secondary angle closure glaucoma, paraesthesia and oligohidrosis. In addition, impairments of cognitive function (slowing of thought processes) and fatigue are commonly reported. Topiramate use is also associated with significant weight loss, which may also be detrimental to self-image and quality of life. The incidence of these side-effects appears to be dose-related and can be mitigated by slowly titrating the dose upwards.
Two randomized, placebo-controlled studies have assessed the efficacy and safety of gabapentin in the prophylaxis of migraine. One study evaluated doses of 900 mg/day to 2400 mg/day and included 143 subjects (CitationMathew et al 2001), whilst the other evaluated a fixed dose of 1200 mg/day in 63 subjects (CitationDi Trapani et al 2000). The larger study demonstrated a higher proportion of patients achieving a reduction in the monthly migraine rate of at least 50% in the gabapentin-treated patients compared to those receiving placebo, and a significant reduction in the frequency and severity of migraine headaches was reported in the smaller study. Nonetheless, somnolence and dizziness were observed in a significant minority of patients. A subsequent randomized, open-label study found essentially similar findings (CitationJimenez-Hernandez et al 2002).
Lamotrigine has been studies in three open-label studies (CitationD’Andrea et al 1999; CitationLampl et al 1999; CitationPascual et al 2004) and one randomized, placebo controlled study (CitationSteiner et al 1997). Although the open-label studies suggested that lamotrigine could be of benefit, particularly in relieving aura, the placebo-controlled study, which included 110 patients, found no evidence that the efficacy of lamotrigine was superior to placebo.
Clonazepam has been evaluated in a small, randomized, placebo-controlled double-blind, cross-over study (CitationStensrud and Sjaastad 1979). This alternated four-week treatment-free run-in periods with four-week treatment periods where the patients received placebo, clonazepam 1 mg or clonazepam 2 mg. A significant reduction in headache frequency compared to the run-in period was observed for the two clonazepam-treatment periods but not for the placebo period.
Compared with β-blockers, randomized clinical trials of valproate (CitationKaniecki 1997) and topiramate (CitationDiener et al 2004b) have shown these two drugs to have similar efficacy to propranolol, although the β-blocker was better tolerated. Due to this difference in tolerability, antiepileptic drugs should be considered principally as second-line treatments in patients who have failed to respond adequately to β-blockers. There is little information on the utility of combining antiepileptic drugs and β-blockers. An open-label study which evaluated the combination of propranolol or nadolol with valproate in 52 subjects who had failed to respond to the individual drugs in monotherapy (CitationPascual et al 2003). This study demonstrated that over half the treated subjects responded to the combination treatment. A second open-label study investigated add-on treatment with topiramate in patients who responded inadequately to current prophylactic treatment, principally with propranolol or flunarizine (CitationMartinez et al 2003). Again, headache frequency decreased after initiation of combination treatment. These interesting observations deserve confirmation in randomized, double-blind studies.
Antidepressants
The utility of certain antidepressants for the prophylaxis of migraine dates from the only 1970s (CitationGomersall and Stuart 1973). The most widely studied antidepressant is amitriptyline and this agent appears to be the most efficacious (CitationColombo et al 2004). It is possible that amitriptyline is active in the prophylaxis of migraine by virtue of an action at voltage-sensitive ion channels controlling neuronal excitability. However, this drug causes sedation which may not be desirable in patients taking this drug every day for migraine prophylaxis. In this respect, other antidepressants with a more acceptable side-effect profile may be preferred. For example, venlafaxine was shown to be reduce headache frequency in a retrospective case series (CitationAdelman et al 2000) and has since shown similar efficacy to amitriptyline and superior efficacy to placebo in two recent randomized, double-blind studies (CitationBulut et al 2004; CitationOzyalcin et al 2005).
Other older antidepressants for which there is some evidence for efficacy in the prophylaxis of migraine include clomipramine (CitationNoone 1980; CitationLangohr et al 1985), opipramol (CitationJacobs 1972) and mianserin (CitationMonro et al 1985). However, these relatively old studies generally included small numbers of patients, were not always double-blinded, and did not use the design and outcome criteria currently recommended for randomized clinical trials in migraine (CitationTfelt-Hansen et al 2000).
The utility of selective-serotonin reuptake inhibitors in the prophylaxis of migraine is much less clear. Early studies performed in the 1980’s in Scandinavia failed to demonstrate clear efficacy for femoxitine (CitationAndersson and Petersen 1981; CitationZeeberg et al 1981; CitationKangasniemi et al 1983; CitationOrholm et al 1986) and more recent studies with sertraline (CitationLandy et al 1999) and citalopram have also been disappointing (CitationRampello et al 2004). On the other hand, there is some weak evidence for the efficacy of fluvoxamine (CitationBank 1994) and fluoxetine (CitationAdly et al 1992; CitationSaper et al 1995; CitationSteiner et al 1998; Citationd’Amato et al 1999).
Of particular interest is the use of amitriptyline, and potentially other antidepressants, in the treatment of chronic daily headache. A meta-analysis of thirty-eight clinical trials (CitationTomkins et al 2001) concluded that all classes of antidepressant drug evaluated (tricyclics, selective serotonin reuptake inhibitors and serotonin receptor antagonists) were all effective in reducing the frequency of both migraine-type and tension-type chronic daily headaches with an effective size that was considered as large. This allowed a reduction in the use of analgesics as headache relief medication. Since analgesic use is considered to be an important factor in the development and maintenance of chronic headaches, and a major determinant of poor prognosis, prophylaxis with antidepressants may improve clinical outcome over the long-term as well as reducing headache frequency in the short-term.
Hormone therapy for menstrual migraine
Oral contraception with progestins can be effective in reducing the frequency of menstrual migraine headaches (CitationBradley et al 1968; CitationSomerville and Carey 1970), presumably by suppression of the menstrual cycle. However, oral contraceptive regimens using continuous progestin exposure seem interesting but are no longer widely used, and current standard oral contraceptives have not been found to be particularly efficacious (CitationSilberstein and Merriam 1999). Use of long-acting estrogen preparations such as gels (Citationde Lignieres et al 1986; CitationDennerstein et al 1988; CitationMacGregor and Hackshaw 2002; CitationMacGregor et al 2006) or patches (Citationde Lignieres and Bousser 1992; CitationPfaffenrath 1993; CitationPradalier et al 2004b) have also been reported to be effective for migraine prophylaxis and may be more suitable formulations in this indication. These estrogens initiated at the end of the luteal phase and maintained until the end of the menstrual period compensate for the rapid fall of endogenous estrogen concentrations that is thought to precipitate migraine headaches. Two small clinical trials of phytoestrogens (CitationBurke et al 2002; CitationFerrante et al 2004) suggested that these agents may also be effective in the treatment of menstrual migraine.
Conclusions
Advances in our understanding of the pathophysiology of migraine have resulted in important breakthroughs in treatment. Notably, understanding of the role of serotonin in the cerebrovascular circulation led to the development of triptans for the acute relief of migraine headaches, and the identification of cortical spreading depression as an early central event associated wih migraine has brought renewed interest in antiepileptic drugs for migraine prophylaxis.
Nevertheless, several important questions remain. Firstly, while it is increasingly recognized that migraine is not a single disease but rather a syndrome that can manifest itself in a variety of pathological conditions, the consequences of this for treatment are not understood. Clinical research needs to be devoted to identifying which sort of patients benefit best from which treatments, particularly in the field of prophylaxis. One possible approach that merits exploration is to try and match prophylactic treatments to particular patterns of precipitating factors, as has already proved successful for the treatment of menstrual migraine In this respect, the proposal of four types of migraine (adrenergic, serotoninergic, menstrual, and muscular) may contribute to the optimisation of migraine prophylaxis.
The interest of stratifying treatment strategies by headache severity is also at an early stage. Studies such as DISC (CitationLipton et al 2000b; CitationSculpher et al 2002) suggest that this could be a useful approach, but treatment stratification remains under-exploited in routine care of patients with migraine. Further prospective studies would help characterising patient severity groups that would benefit from particular strategies.
Treatment resistance is frequent with both acute and prophylactic treatments of migraine. Even with triptans, probably the most effective class of antimigraine drug, up to one-third of patients fail to respond adequately. The reasons underlying treatment resistance need to be better understood, and strategies identified to avoid serial treatment failures when the patient first comes to the clinic.
Moreover, there is a lack of information on the benefit of combination treatment using different drug classes. This approach may be of particular use in patients with more severe hedaches and in those showing mixed headache types. In this respect, and bearing in mind potential artefacts due to the open-label design of the study, the results of the combination study with propranolol and valproate imply that these two agents can be used in association to provide greater efficacy in particular patients. Further studies such as this using a randomized, double-blind design are required to provide the data necessary to orientate treatment decisions about combinations of migraine prophylaxis agents.
Finally, little is known about long-term outcome in treated migraine. It is possible that appropriate early prophylaxis may modify the long-term course of the disease and avoid late complications, as has been shown, for example, in another paroxystic neurological condition, epilepsy. This is particularly relevant for antiepileptic drugs that are thought to be of benefit in migraine by an action on cortical excitability. If this is the case, then early aggressive migraine prophylaxis may be merited. Although medication overuse can be a predisposing factor to developing a chronic headache syndrome, risk thresholds remain poorly quantified, particularly for specific acute migraine treatments such as the triptans.
Although migraine is much better defined than it was twenty years ago, and more efficacious and better tolerated treatments are on offer, there is still a long way to go before we can provide patients with individualized and rational long-term therapeutic management strategies that take into account the heterogeneity of the migraine condition and its variable clinical course.
Disclosure
The author reports no conflicts of interest in this work.
References
- Abu-ArefehIRussellG1994Prevalence of headache and migraine in schoolchildrenBr Med J30976597950559
- AdelmanJUBelseyJ2003Meta-analysis of oral triptan therapy for migraine: number needed to treat and relative cost to achieve relief within 2 hoursJ Manag Care Pharm9455214613361
- AdelmanLCAdelmanJUVon SeggernR2000Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: A retrospective study in a clinical settingHeadache405728010940096
- AdlyCStraumanisJChessonA1992Fluoxetine prophylaxis of migraineHeadache3210141551787
- AnderssonKEVingeE1990Beta-adrenoceptor blockers and calcium antagonists in the prophylaxis and treatment of migraineDrugs39355731970289
- AnderssonPGPetersenEN1981Propranolol and femoxetine, a HT-uptake inhibitor, in migraine prophylaxis. A double-blind crossover studyActa Neurol Scand6428087032183
- AnnequinDTourniaireBMassiouH2000Migraine and headache in childhood and adolescencePediatr Clin North Am476173110835994
- AnsellEFazzoneTFestensteinR1988Nimodipine in migraine prophylaxisCephalalgia8269723064919
- BankJ1994A comparative study of amitriptyline and fluvoxamine in migraine prophylaxisHeadache3447687960733
- BellavanceAJMelocheJP1990A comparative study of naproxen sodium, pizotyline and placebo in migraine prophylaxisHeadache3071052074163
- BelseyJD2004Cost effectiveness of oral triptan therapy: a trans-national comparison based on a meta-analysis of randomised controlled trialsCurr Med Res Opin206596915140331
- BigalMEBordiniCASpecialiJG2002[Intramuscular diclofenac in the acute treatment of migraine: a double-blind placebo controlled study]Arq Neuropsiquiatr60410512131942
- BilleBLudvigssonJSannerG1977Prophylaxis of migraine in childrenHeadache17613324951
- BonusoSDi StasioEBaroneP1983Timed-release dihydroergotamine in the prophylaxis of mixed headache. A study versus amitriptylineCephalalgia3Suppl 117586352047
- BordiniCAArrudaMACiciarelliMC1997Propranolol vs flunarizine vs flunarizine plus propranolol in migraine without aura prophylaxis. A double-blind trialArq Neuropsiquiatr55536419629401
- BoureauFJoubertJMLasserreV1994Double-blind comparison of an acetaminophen 400 mg-codeine 25 mg combination versus aspirin 1000 mg and placebo in acute migraine attackCephalalgia14156618062355
- BousserMGChickJFuseauE1988Combined low-dose acetylsalicylic acid and dihydroergotamine in migraine prophylaxis. A double-blind, placebo-controlled crossover studyCephalalgia8187923197098
- BowyerSMMasonKMMoranJE2005Cortical hyperexcitability in migraine patients before and after sodium valproate treatmentJ Clin Neurophysiol2265715689715
- BradleyWGHudgsonPFosterJB1968Double-blind controlled trial of a micronized preparation of flumedroxone (Demigran) in prophylaxis of migraineBr Med J353134877801
- BrandesJLSaperJRDiamondM2004Topiramate for migraine prevention: a randomized controlled trialJAMA2919657314982912
- BrennumJBrinckTSchriverL1996Sumatriptan has no clinically relevant effect in the treatment of episodic tension-type headacheEur J Neurol32328
- BulutSBerilgenMSBaranA2004Venlafaxine versus amitriptyline in the prophylactic treatment of migraine: randomized, double-blind, crossover studyClin Neurol Neurosurg10744815567552
- BurkeBEOlsonRDCusackBJ2002Randomized, controlled trial of phytoestrogen in the prophylactic treatment of menstrual migraineBiomed Pharmacother56283812224599
- BussoneGCerboRMartucciN1999Alpha-dihydroergocryptine in the prophylaxis of migraine: a multicenter double-blind study versus flunarizineHeadache394263111279920
- CaoYQTsienRW2005Effects of familial hemiplegic migraine type 1 mutations on neuronal P/Q-type Ca2+ channel activity and inhibitory synaptic transmissionProc Natl Acad Sci U S A1022590515699344
- CareyMPBillingAEFryJP1992Fluctuations in responses to diazepam during the oestrous cycle in the mousePharmacol Biochem Behav41719251594639
- CastilloJMunozPGuiteraV1999Epidemiology of chronic daily headache in the general populationHeadache39190615613213
- ChabriatHJoireJEDanchotJ1994Combined oral lysine acetylsalicylate and metoclopramide in the acute treatment of migraine: a multicentre double-blind placebo-controlled studyCephalalgia142973007954760
- ChronicleEMullenersW2004Anticonvulsant drugs for migraine prophylaxisCochrane Database Syst Rev3CD00322615266476
- CodispotiJRPriorMJFuM2001Efficacy of nonprescription doses of ibuprofen for treating migraine headache. a randomized controlled trialHeadache416657911554954
- ColomboBAnnovazziPOComiG2004Therapy of primary headaches: the role of antidepressantsNeurol Sci25Suppl 3S171515549531
- CouturierEGBomhofMANevenAK2003Menstrual migraine in a representative Dutch population sample: prevalence, disability and treatmentCephalalgia23302812716349
- d’AmatoCCPizzaVMarmoloT1999Fluoxetine for migraine prophylaxis: a double-blind trialHeadache39716911279947
- D’AndreaGGranellaFCadaldiniM1999Effectiveness of lamotrigine in the prophylaxis of migraine with aura: an open pilot studyCephalalgia1964610099862
- DahlofCBjorkmanR1993Diclofenac-K (50 and 100 mg) and placebo in the acute treatment of migraineCephalalgia13117238495453
- De BenedittisGMasseiR1985Serotonin precursors in chronic primary headache. A double-blind cross-over study with L-5-hydroxytryptophan vs placeboJ Neurosurg Sci29239483913752
- de CosterJ1976[Oxetorone in the treatment of migrainous cephalalgia]Schweiz Rundsch Med Prax65879821005299
- de LignieresBBousserMG1992MigraineLancet3406121351646
- de LignieresBVincensMMauvais-JarvisP1986Prevention of menstrual migraine by percutaneous oestradiolBr Med J (Clin Res Ed)2931540
- DennersteinLMorseCBurrowsG1988Menstrual migraine: a double-blind trial of percutaneous estradiolGynecol Endocrinol2113203055819
- Di TrapaniGMeiDMarraC2000Gabapentin in the prophylaxis of migraine: a double-blind randomized placebo-controlled studyClin Ter151145810958046
- Diaz-VelizGButronSBenavidesMS2000Gender, estrous cycle, ovariectomy, and ovarian hormones influence the effects of diazepam on avoidance conditioning in ratsPharmacol Biochem Behav668879210973530
- DibMMassiouHWeberM2002Efficacy of oral ketoprofen in acute migraine: a double-blind randomized clinical trialNeurology581660512058095
- DienerHC1997[Migraine – diagnosis, differential diagnosis and therapy]Ther Umsch5464709139407
- DienerHC1999Efficacy and safety of intravenous acetylsalicylic acid lysinate compared to subcutaneous sumatriptan and parenteral placebo in the acute treatment of migraine. A double-blind, double-dummy, randomized, multicenter, parallel group study. The ASASUMAMIG Study GroupCephalalgia195818 discussion 542.10448545
- DienerHCBruneKGerberWD1998[Treatment of migraine attacks and migraine prophylaxis: recommendations of the German Migraine and Headache Society]Med Monatsschr Pharm213099531789
- DienerHCBussoneGde LianoH2004aPlacebo-controlled comparison of effervescent acetylsalicylic acid, sumatriptan and ibuprofen in the treatment of migraine attacksCephalalgia249475415482357
- DienerHCFohMIaccarinoC1996Cyclandelate in the prophylaxis of migraine: a randomized, parallel, double-blind study in comparison with placebo and propranolol. The Study groupCephalalgia1644178902255
- DienerHCKruppPSchmittT2001Cyclandelate in the prophylaxis of migraine: a placebo-controlled studyCephalalgia21667011298666
- DienerHCTfelt-HansenPDahlofC2004bTopiramate in migraine prophylaxis – results from a placebo-controlled trial with propranolol as an active controlJ Neurol2519435015316798
- DoenickeABrandJPerrinVL1988Possible benefit of GR43175, a novel 5-HT1-like receptor agonist, for the acute treatment of severe migraineLancet11309112897560
- DowsonAJDodickDWLimmrothV2005Medication overuse headache in patients with primary headache disorders: epidemiology, management and pathogenesisCNS Drugs194839715962999
- DowsonAJLipscombeSSenderJ2002New guidelines for the management of migraine in primary careCurr Med Res Opin184143912487508
- DrakeMGreathouseNArmentbrightA2001Levetiracetam for preventive treatment of migraine [abstract]Cephalalgia21373
- DrakeMKayAKnappM1999An open-label trial of tiagabine for migraine prophylaxis [abstract]Headache39352
- DrakeMEGreathouseNIArmentbrightAD2001Preventive treatment of migraine with zonisamide [abstract]Cephalalgia21374
- DufresneJJ1978[Double-blind study of oxetorone in patients with migraine or migrainous headache]Schweiz Rundsch Med Prax67114852674095
- EdwardsKRNortonJBehnkeM2001Comparison of intravenous valproate versus intramuscular dihydroergotamine and metoclopramide for acute treatment of migraine headacheHeadache419768011903525
- EdwardsKRPotterDLWuSC2003Topiramate in the preventive treatment of episodic migraine: a combined analysis from pilot, double-blind, placebo-controlled trialsCNS Spectr84283212858132
- EkbomK1975Alprenolol for migraine prophylaxisHeadache15129321097368
- EkbomKZettermanM1977Oxprenolol in the treatment of migraineActa Neurol Scand561814331835
- ElkindAHFriedmanAPBachmanA1968Silent retroperitoneal fibrosis associated with methysergide therapyJAMA206104145695652
- EnsinkFB1991Subcutaneous sumatriptan in the acute treatment of migraine. Sumatriptan International Study GroupJ Neurol238Suppl 1S6691646291
- EpsteinMTHockadayJMHockadayTD1975Migraine and reporoductive hormones throughout the menstrual cycleLancet1543847017
- FacchinettiFNappiRETirelliA2002Hormone supplementation differently affects migraine in postmenopausal womenHeadache42924912390622
- FerranteFFuscoECalabresiP2004Phyto-oestrogens in the prophylaxis of menstrual migraineClin Neuropharmacol271374015190238
- FerrariMDRoonKILiptonRB2001Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trialsLancet35816687511728541
- FerrariMDSaxenaPR1992Clinical effects and mechanism of action of sumatriptan in migraineClin Neurol Neurosurg94 SupplS7371320526
- FerrariMDWilkinsonMHirtD1991Efficacy of ICS 205-930, a novel 5-hydroxytryptamine3 (5-HT3) receptor antagonist, in the prevention of migraine attacks. A complex answer to a simple question. ICS 205-930 Migraine Study GroupPain45283911876437
- FlorenceJ1978[Clinical study of oxetorone in catamenial migraine. Comparison of two posological diagrams]Schweiz Rundsch Med Prax6713237683962
- ForssmanBLindbladCJZbornikovaV1983Atenolol for migraine prophylaxisHeadache23188906350226
- FredianiFGrazziLZanottiA1991Dihydroergokryptine versus dihydroergotamine in migraine prophylaxis: a double-blind clinical trialCephalalgia11117211909603
- FreitagFDiamondSSolomonG1999The prophylaxis of migraine with the GABA-agonist tiagabine: a clinical report [abstract]Cephalalgia19354
- FreitagFGCollinsSDCarlsonHA2002A randomized trial of divalproex sodium extended-release tablets in migraine prophylaxisNeurology581652912058094
- FreitagFGDiamondS1984Nadolol and placebo comparison study in the prophylactic treatment of migraineJ Am Osteopath Assoc8434376150909
- GalianoLMatías-GuiuJHorgaJ1993Dotarizine: a double-blind trial in propylactic treatment of migraineCephalalgia13Suppl 13251
- GawelMJKreeftJNelsonRF1992Comparison of the efficacy and safety of flunarizine to propranolol in the prophylaxis of migraineCan J Neurol Sci1934051393843
- GelmersHJ1983Nimodipine, a new calcium antagonist, in the prophylactic treatment of migraineHeadache2310696347970
- GeraudGCompagnonARossiA2002Zolmitriptan versus a combination of acetylsalicylic acid and metoclopramide in the acute oral treatment of migraine: a double-blind, randomised, three-attack studyEur Neurol47889811844897
- GeraudGLanteri-MinetMLucasC2004French guidelines for the diagnosis and management of migraine in adults and childrenClin Ther2613051815476911
- GilliesDSillsMForsytheI1986Pizotifen (Sanomigran) in childhood migraine. A double-blind controlled trialEur Neurol253253510125
- GoadsbyPJDodickDWFerrariMD2004TRIPSTAR: prioritizing oral triptan treatment attributes in migraine managementActa Neurol Scand1101374315285768
- GomersallJDStuartA1973Amitriptyline in migraine prophylaxis. Changes in pattern of attacks during a controlled clinical trialJ Neurol Neurosurg Psychiatry36684904731336
- GranellaFSancesGAllaisG2004Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centresCephalalgia247071615315526
- GranellaFSancesGZanferrariC1993Migraine without aura and reproductive life events: a clinical epidemiological study in 1300 womenHeadache3338598376100
- GrotemeyerKHSchlakeHPHusstedtIW1988[Prevention of migraine with metoprolol and flunarizine. A double-blind crossover study]Nervenarzt59549523054595
- HamalainenMLHoppuKValkeilaE1997Ibuprofen or acetaminophen for the acute treatment of migraine in children: a double-blind, randomized, placebo-controlled, crossover studyNeurology4810379008503
- Havanka-KanniainenHHokkanenEMyllylaVV1985Efficacy of nimodipine in the prophylaxis of migraineCephalalgia539433886153
- [HCCIHS] Headache Classification Committee of the International Headache Society1988Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial painCephalalgia8Suppl 7196
- [HCCIHS] Headache Classification Committee of the International Headache Society2004Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Second Edition.Cephalalgia24Suppl 11160
- HenryPAurayJPGaudinAF2002Prevalence and clinical characteristics of migraine in FranceNeurology59232712136063
- HenryPHiesse-ProvostODillenschneiderA1995[Efficacy and tolerance of an effervescent aspirin-metoclopramide combination in the treatment of a migraine attack. Randomized double-blind study using a placebo]Presse Med2425487899379
- HeringRKuritzkyA1992Sodium valproate in the prophylactic treatment of migraine: a double-blind study versus placeboCephalalgia128141576648
- HerrmannWMKristofMSastreM1978Preventive treatment of migraine headache with a new isoergolenyl derivativeJ Int Med Res647682363486
- HolroydKAPenzienDBCordingleyGE1991Propranolol in the management of recurrent migraine: a meta-analytic reviewHeadache31333401830566
- International Headache Society Committee on Clinical Trials in Migraine1991Guidelines for controlled trials of drugs in migraine. First editionCephalalgia11112
- JacobsH1972A trial of opipramol in the treatment of migraineJ Neurol Neurosurg Psychiatry3550044559028
- JensenRBrinckTOlesenJ1994Sodium valproate has a prophylactic effect in migraine without aura: a triple-blind, placebo-controlled crossover studyNeurology44647518164818
- Jimenez-HernandezMDTorrecillas NarvaezMDFriera AcebalG2002[Effectiveness and safety of gabapentin in the preventive treatment of migraine]Rev Neurol35603612389143
- JohannssonVNilssonLRWideliusT1987Atenolol in migraine prophylaxis a double-blind cross-over multicentre studyHeadache2737243308768
- JonesMW1998Topiramate–safety and tolerabilityCan J Neurol Sci25S1359706735
- KangasniemiPAndersenARAnderssonPG1987Classic migraine: effective prophylaxis with metoprololCephalalgia723183322569
- KangasniemiPHedmanC1984Metoprolol and propranolol in the prophylactic treatment of classical and common migraine. A double-blind studyCephalalgia49166428749
- KangasniemiPJNyrkeTLangAH1983Femoxetine – a new 5-HT uptake inhibitor – and propranolol in the prophylactic treatment of migraineActa Neurol Scand6826276606930
- KanieckiRG1997A comparison of divalproex with propranolol and placebo for the prophylaxis of migraine without auraArch Neurol54114159311358
- KarachaliosGNFotiadouAChrisikosN1992Treatment of acute migraine attack with diclofenac sodium: a double-blind studyHeadache32981001551795
- KassBNestvoldK1980Propranolol (Inderal) and clonidine (Catapressan) in the prophylactic treatment of migraine. A comparative trialActa Neurol Scand6135166998250
- KavukIKatsaravaZSeleklerM2004Clinical features and therapy of medication overuse headacheEur J Med Res9565915689304
- KellsteinDELiptonRBGeethaR2000Evaluation of a novel solubilized formulation of ibuprofen in the treatment of migraine headache: a randomized, double-blind, placebo-controlled, dose-ranging studyCephalalgia202334310999673
- KlapperJ1997Divalproex sodium in migraine prophylaxis: a dose-controlled studyCephalalgia1710389137847
- KlosterRNestvoldKVilmingST1992A double-blind study of ibuprofen versus placebo in the treatment of acute migraine attacksCephalalgia1216971 discussion 128.1623513
- KozubskiWPrusinskiA1999[Controlled study of iprazochrome effectiveness (Divascan) in prophylactic treatment of migraine]Neurol Neurochir Pol333697610463251
- KrausRLSinneggerMJGlossmannH1998Familial hemiplegic migraine mutations change alpha1A Ca2+ channel kineticsJ Biol Chem2735586909488686
- KrausRLSinneggerMJKoschakA2000Three new familial hemiplegic migraine mutants affect P/Q-type Ca(2+) channel kineticsJ Biol Chem27592394310734061
- KruszJ2001aLevetiracetam as prophylaxis for resistant headaches [abstract]Cephalalgia21373
- KruszJ2001bZonisamide in the treatment of headache disorders [abstract]Cephalalgia21374
- KruszJScottV1999Topiramate in the treatment of chronic migraine and other headaches [abstract]Headache39363
- KrymchantowskiAVBigalMEMoreiraPF2002New and emerging prophylactic agents for migraineCNS Drugs166113412153333
- LamplCBuzathAKlingerD1999Lamotrigine in the prophylactic treatment of migraine aura – a pilot studyCephalalgia19586310099861
- LanceJWAnthonyMSomervilleB1970Comparative trial of serotonin antagonists in the management of migraineBr Med J2327304393372
- LanceJWFineRDCurranDA1963An evaluation of methysergide in the prevention of migraine and other vascular headachesMed J Aust50814813928400
- LandySMcGinnisJCurlinD1999Selective serotonin reuptake inhibitors for migraine prophylaxisHeadache39283215613191
- LangeRSchwarzJAHohnM2000Acetylsalicylic acid effervescent 1000 mg (Aspirin) in acute migraine attacks; a multicentre, randomized, double-blind, single-dose, placebo-controlled parallel group studyCephalalgia20663711128825
- LangohrHDGerberWDKoletzkiE1985Clomipramine and metoprolol in migraine prophylaxis – a double-blind crossover studyHeadache25107133886599
- LangohrHDReineckeMGerberWD1988[Dihydroergotamine and flunarizine in the prevention of migraine. A comparative double-blind study]Fortschr Med10665669703278960
- Lanteri-MinetM2005What do patients want from their acute migraine therapy?Eur Neurol53Suppl 13915920331
- Lanteri-MinetMAlchaarHBessonG2000[Pharmaco-epidemiological study on the prophylactic treatment of migraine. National inquiry on attitude to prescription practices by primary care physicians and neurologists in Franceo]Rev Neurol (Paris)15611061211139726
- Lanteri-MinetMAurayJPEl HasnaouiA2003Prevalence and description of chronic daily headache in the general population in FrancePain102143912620605
- LeandriMRigardoSSchizziR1990Migraine treatment with nicardipineCephalalgia1011162245455
- LenigerTPagelerLStudeP2005Comparison of intravenous valproate with intravenous lysine-acetylsalicylic acid in acute migraine attacksHeadache4542615663612
- LewisDWKellsteinDDahlG2002Children’s ibuprofen suspension for the acute treatment of pediatric migraineHeadache42780612390641
- LichtenEMLichtenJBWhittyA1996The confirmation of a biochemical marker for women’s hormonal migraine: the depoestradiol challenge testHeadache36367718707555
- LimmrothVKatsaravaZFritscheG2002Features of medication overuse headache following overuse of different acute headache drugsNeurology591011412370454
- LindeKRossnagelK2004Propranolol for migraine prophylaxisCochrane Database Syst Rev3CD00322515106196
- Linton-DahlofPLindeMDahlofC2000Withdrawal therapy improves chronic daily headache associated with long-term misuse of headache medication:a retrospective studyCephalalgia206586211128824
- LiptonRBCutrerFMGoadsbyPJ2005aHow treatment priorities influence triptan preferences in clinical practice: perspectives of migraine sufferers, neurologists, and primary care physiciansCurr Med Res Opin214132415811210
- LiptonRBGoldsteinJBaggishJS2005bAspirin is efficacious for the treatment of acute migraineHeadache452839215836564
- LiptonRBStewartWFCadyR2000a2000 Wolfe Award. Sumatriptan for the range of headaches in migraine sufferers: results of the Spectrum StudyHeadache407839111135021
- LiptonRBStewartWFRyanREJr1998Efficacy and safety of acetaminophen, aspirin, and caffeine in alleviating migraine headache pain: three double-blind, randomized, placebo-controlled trialsArch Neurol5521079482363
- LiptonRBStewartWFStoneAM2000bStratified care vs step care strategies for migraine: the Disability in Strategies of Care (DISC) Study: A randomized trialJAMA284259960511086366
- Littleton-KearneyMTAgnewDMTraystmanRJ2000Effects of estrogen on cerebral blood flow and pial microvasculature in rabbitsAm J Physiol Heart Circ Physiol279H12081410993786
- LoderEWBuseDCGolubJR2005Headache and combination estrogen-progestin oral contraceptives: integrating evidence, guidelines, and clinical practiceHeadache452243115836597
- LoflandJHNashDB2005Oral serotonin receptor agonists: a review of their cost effectiveness in migrainePharmacoeconomics232597415836007
- LouisPSchoenenJHedmanC1985Metoprolol v. clonidine in the prophylactic treatment of migraineCephalalgia5159653899370
- LuSRFuhJLChenWT2001Chronic daily headache in Taipei, Taiwan: prevalence, follow-up and outcome predictorsCephalalgia21980611843870
- LucasCChaffautCArtazMA2005FRAMIG 2000: medical and therapeutic management of migraine in FranceCephalalgia252677915773824
- LuckingCHOestreichWSchmidtR1988Flunarizine vs propranolol in the prophylaxis of migraine: two double-blind comparative studies in more than 400 patientsCephalalgia8 Suppl82163180198
- LudinHP1989Flunarizine and propranolol in the treatment of migraineHeadache29219242654068
- LyngbergACRasmussenBKJorgensenT2005Prognosis of migraine and tension-type headache: a population-based follow-up studyNeurology65580516116119
- MacGregorEA2004Oestrogen and attacks of migraine with and without auraLancet Neurol33546115157850
- MacGregorEADowsonADaviesPT2002Mouth-dispersible aspirin in the treatment of migraine: a placebo-controlled studyHeadache422495512010380
- MacGregorEAFrithAEllisJ2006Prevention of menstrual attacks of migraine: a double-blind placebo-controlled crossover studyNeurology6721596317190936
- MacGregorEAHackshawA2002Prevention of migraine in the pill-free interval of combined oral contraceptives: a double-blind, placebo-controlled pilot study using natural oestrogen supplementsJ Fam Plann Reprod Health Care28273116259812
- MaissenCPLudinHP1991[Comparison of the effect of 5-hydroxytryptophan and propranolol in the interval treatment of migraine]Schweiz Med Wochenschr1211585901947955
- MartinezHRLondonoOCantu-MartinezL2003Topiramate as an adjunctive treatment in migraine prophylaxisHeadache431080414629243
- MartucciNMannaVMattesiP1983Ergot derivatives in the prophylaxis of migraine: a multicentric study with a timed-release dihydroergotamine formulationCephalalgia3Suppl 115156352046
- MassiouHSerrurierDLasserreO1991Effectiveness of oral diclof-enac in the acute treatment of common migraine attacks: a double-blind study versus placeboCephalalgia1159631860132
- MathewNT1993Transformed migraineCephalalgia, 13 Suppl127883
- MathewNT1997Transformed migraine, analgesic rebound, and other chronic daily headachesNeurol Clin15167869058404
- MathewNTKailasamJMeadorsL2002Prophylaxis of migraine, transformed migraine, and cluster headache with topiramateHeadache4279680312390644
- MathewNTKailasamJMeadorsL2000Intravenous valproate sodium (depacon) aborts migraine rapidly: a preliminary reportHeadache40720311091289
- MathewNTRapoportASaperJ2001Efficacy of gabapentin in migraine prophylaxisHeadache411192811251695
- MathewNTSaperJRSilbersteinSD1995Migraine prophylaxis with divalproexArch Neurol5228167872882
- MathewNTStubitsENigamMP1982Transformation of episodic migraine into daily headache: analysis of factorsHeadache226687085263
- McCarthyBGPeroutkaSJ1989Comparative neuropharmacology of dihydroergotamine and sumatriptan (GR 43175)Headache2942022547733
- McEwenBS2001Invited review: Estrogens effects on the brain: multiple sites and molecular mechanismsJ Appl Physiol91278580111717247
- MeiDCapuanoAVollonoC2004Topiramate in migraine prophylaxis: a randomised double-blind versus placebo studyNeurol Sci252455015624081
- MicieliGCavalliniAMarcheselliS2001Alpha-dihydroergocryptine and predictive factors in migraine prophylaxisInt J Clin Pharmacol Ther391445111332869
- [MINES] Migraine-Nimodipine European Study Group1989aEuropean multicenter trial of nimodipine in the prophylaxis of classic migraine (migraine with aura)Headache2963942
- [MINES] Migraine-Nimodipine European Study Group1989bEuropean multicenter trial of nimodipine in the prophylaxis of common migraine (migraine without aura)Headache296338
- MonroPSwadeCCoppenA1985Mianserin in the prophylaxis of migraine: a double-blind studyActa Psychiatr Scand Suppl320981033901676
- MontastrucJLSenardJM1992[Calcium channel blockers and prevention of migraine]Pathol Biol (Paris)4038181353873
- MullenersWMChronicleEPVredeveldJW2002Visual cortex excitability in migraine before and after valproate prophylaxis: a pilot study using TMSEur J Neurol9354011784374
- MyllylaVVHavankaHHerralaL1998Tolfenamic acid rapid release versus sumatriptan in the acute treatment of migraine: comparable effect in a double-blind, randomized, controlled, parallel-group studyHeadache3820179563211
- Nachit-OuinekhFDartiguesJFChrysostomeV2005Evolution of migraine after a 10-year follow-upHeadache451280716324159
- NebeJHeierMDienerHC1995Low-dose ibuprofen in self-medication of mild to moderate headache: a comparison with acetylsalicylic acid and placeboCephalalgia1553158706118
- NeumanMDemarezJPHarmeyJL1986Prevention of migraine attacks through the use of dihydroergotamineInt J Clin Pharmacol Res61133514491
- NooneJF1980Clomipramine in the prevention of migraineJ Int Med Res8Suppl 349527009254
- OldmanADSmithLAMcQuayHJ2002Pharmacological treatments for acute migraine: quantitative systematic reviewPain972475712044621
- OlesenJ1990Calcium antagonists in migraine and vertigo. Possible mechanisms of action and review of clinical trialsEur Neurol30Suppl 2314 discussion 39–41.2180716
- OlssonJEBehringHCForssmanB1984Metoprolol and propranolol in migraine prophylaxis: a double-blind multicentre studyActa Neurol Scand7016086391066
- OrholmMHonorePFZeebergI1986A randomized general practice group-comparative study of femoxetine and placebo in the prophylaxis of migraineActa Neurol Scand7423593538753
- OstermanPO1977A comparison between placebo, pizotifen and 1-isopropyl-3-hydroxy-5-semicarbazono-6-oxo-2.3.5.6-tetrahydroindol (Divascan) in migraine prophylaxisActa Neurol Scand561728327746
- OzyalcinSNTaluGKKiziltanE2005The efficacy and safety of venlafaxine in the prophylaxis of migraineHeadache451445215705120
- PascualJCamineroABMateosV2004Preventing disturbing migraine aura with lamotrigine: an open studyHeadache441024815546267
- PascualJLeiraRLainezJM2003Combined therapy for migraine prevention? Clinical experience with a beta-blocker plus sodium valproate in 52 resistant migraine patientsCephalalgia23961214984228
- PedersenEMollerCE1966Methysergide in migraine prophylaxisClin Pharmacol Ther752065328472
- PeroutkaSJLyonJASwarbrickJ2004Efficacy of diclofenac sodium softgel 100 mg with or without caffeine 100 mg in migraine without aura: a randomized, double-blind, crossover studyHeadache441364114756851
- PfaffenrathV1993Efficacy and safety of percutaneous estradiol vs placebo in menstrual migraineCephalalgia13Suppl 13244
- PradalierADryJLoisyB1988[Comparative study of indoramin versus dihydroergotamine in the preventive treatment of migraine]Therapie4329373055407
- PradalierALanteri-MinetMGeraudG2004aThe PROMISE study: PROphylaxis of MIgraine with SEglor (dihydroergotamine mesilate) in French primary careCNS Drugs1811496315581385
- PradalierAVincentDBeaulieuP2004bCorrelation between oestradiol plasma level and therapeutic effect on menstrual migraineRoseFNew Advances in Headache Research4th EditionLondon, UKSmith-Gordon
- RampelloLAlvanoAChiechioS2004Evaluation of the prophylactic efficacy of amitriptyline and citalopram, alone or in combination, in patients with comorbidity of depression, migraine, and tension-type headacheNeuropsychobiology50322815539864
- RaoBSDasDGTaraknathVR2000A double blind controlled study of propranolol and cyproheptadine in migraine prophylaxisNeurol India48223611025624
- Reveiz-HeraultLCardonaAFOspinaEG2003[Effectiveness of flunarizine in the prophylaxis of migraine: a meta-analytical review of the literature]Rev Neurol369071212766861
- RompelHBauermeisterPW1970Aetiology of migraine and prevention with carbamazepine (Tegretol): results of a double-blind, cross-over studyS Afr Med J4475804905910
- RyanRE1968Double-blind crossover comparison of bc-105, methysergide and placebo in the prophylaxis of migraine headacheHeadache8118264892617
- RyanRESr1984Comparative study of nadolol and propranolol in prophylactic treatment of migraineAm Heart J108115696148878
- SandriniGFranchiniSLanfranchiS1998Effectiveness of ibuprofen-arginine in the treatment of acute migraine attacksInt J Clin Pharmacol Res18145509825271
- SantucciMCortelliPRossiPG1986L-5-hydroxytryptophan versus placebo in childhood migraine prophylaxis: a double-blind crossover studyCephalalgia615573533271
- SaperJRSilbersteinSDLakeAE3rd1995Fluoxetine and migraine: comparison of double-blind trialsHeadache352337775182
- ScherAIStewartWFLibermanJ1998Prevalence of frequent headache in a population sampleHeadache3849750615613165
- ScherAIStewartWFLiptonRB2004Caffeine as a risk factor for chronic daily headache: a population-based studyNeurology632022715596744
- ScherAIStewartWFRicciJA2003Factors associated with the onset and remission of chronic daily headache in a population-based studyPain10681914581114
- SchuaibAAhmedFMuratogluM1999Topiramate in migraine prophylaxis: a pilot study [abstract]Cephalalgia1937980
- SculpherMMillsonDMeddisD2002Cost-effectiveness analysis of stratified versus stepped care strategies for acute treatment of migraine: The Disability in Strategies for Care (DISC) StudyPharmacoeconomics209110011888361
- ShekelleRBOstfeldAM1964Methysergide in the Migraine SyndromeClin Pharmacol Ther5201414155162
- ShuklaRGargRKNagD1995Nifedipine in migraine and tension headache: a randomised double blind crossover studyJ Assoc Physicians India4377028773038
- SilbersteinSMerriamG1999Sex hormones and headache 1999 (menstrual migraine)Neurology53S31310487507
- SilbersteinSD1998MethysergideCephalalgia18421359793694
- SilbersteinSD2000Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of NeurologyNeurology557546210993991
- SilbersteinSDGoadsbyPJ2002Migraine: preventive treatmentCephalalgia2249151212230591
- SilbersteinSDNetoWSchmittJ2004Topiramate in migraine prevention: results of a large controlled trialArch Neurol61490515096395
- SilbersteinSDWinnerPKChmielJJ2003Migraine preventive medication reduces resource utilizationHeadache43171812603635
- SilvestriniMBartoliniMCocciaM2003Topiramate in the treatment of chronic migraineCephalalgia23820414510929
- SiniatchkinMGerberWDVeinA1998Clinical efficacy and central mechanisms of cyclandelate in migraine: a double-blind placebo-controlled studyFunct Neurol1347569584874
- SnowVWeissKWallEM2002Pharmacologic management of acute attacks of migraine and prevention of migraine headacheAnn Intern Med137840912435222
- SolomonGD1989Verapamil in migraine prophylaxis – a five-year reviewHeadache2942572668225
- SolomonSLiptonRBNewmanLC1992Clinical features of chronic daily headacheHeadache3232591526762
- SomervilleBW1972The role of estradiol withdrawal in the etiology of menstrual migraineNeurology22355655062827
- SomervilleBW1975Estrogen-withdrawal migraine. I. Duration of exposure required and attempted prophylaxis by premenstrual estrogen administrationNeurology25239441167630
- SomervilleBWCareyHM1970The use of continuous progestogen contraception in the treatment of migraineMed J Aust1104354193905
- SomervilleBWHerrmannWM1978Migraine prophylaxis with Lisuride hydrogen maleate–a double blind study of Lisuride versus placeboHeadache18759348647
- SorensenPSLarsenBHRasmussenMJ1991Flunarizine versus metoprolol in migraine prophylaxis: a double-blind, randomized parallel group study of efficacy and tolerabilityHeadache3165071769820
- SteardoLBonusoSDi StasioE1982Selective and non-selective beta-blockers: are both effective in prophylaxis of migraine? A clinical trial versus methysergideActa Neurol (Napoli)41962047136885
- SteardoLMaranoEBaroneP1986Prophylaxis of migraine attacks with a calcium-channel blocker: flunarizine versus methysergideJ Clin Pharmacol2652483531248
- SteinerTJAhmedFFindleyLJ1998S-fluoxetine in the prophylaxis of migraine: a phase II double-blind randomized placebo-controlled studyCephalalgia1828369673809
- SteinerTJFindleyLJYuenAW1997Lamotrigine versus placebo in the prophylaxis of migraine with and without auraCephalalgia17109129137848
- SteinerTJJosephRHedmanC1988Metoprolol in the prophylaxis of migraine: parallel-groups comparison with placebo and dose-ranging follow-upHeadache2815233277926
- StellarSAhrensSPMeibohmAR1984Migraine prevention with timolol. A double-blind crossover studyJAMA2522576806387197
- StensrudPSjaastadO1979Clonazepam (rivotril) in migraine prophylaxisHeadache193334511533
- StensrudPSjaastadO1980Comparative trial of Tenormin (atenolol) and Inderal (propranolol) in migraineHeadache2020476993420
- StoreyJRCalderCSHartDE2001Topiramate in migraine prevention: a double-blind, placebo-controlled studyHeadache419687511903524
- StronksDLTulenJHBussmannHB2003Effects of naratriptan versus naproxen on daily functioning in the acute treatment of migraine: a randomized, double-blind, double-dummy, crossover studyHeadache438455212940805
- SudilovskyAElkindAHRyanRESr1987Comparative efficacy of nadolol and propranolol in the management of migraineHeadache2742163312113
- TanenDAMillerSFrenchT2003Intravenous sodium valproate versus prochlorperazine for the emergency department treatment of acute migraine headaches: a prospective, randomized, double-blind trialAnn Emerg Med418475312764341
- Tfelt-HansenPBlockGDahlofC2000Guidelines for controlled trials of drugs in migraine: second editionCephalalgia207658611167908
- Tfelt-HansenPHenryPMulderLJ1995The effectiveness of combined oral lysine acetylsalicylate and metoclopramide compared with oral sumatriptan for migraineLancet34692367564725
- Tfelt-HansenPOlesenJ1984Effervescent metoclopramide and aspirin (Migravess) versus effervescent aspirin or placebo for migraine attacks: a double-blind studyCephalalgia4107116375873
- The Diclofenac-K/Sumatriptan Migraine Study Group1999Acute treatment of migraine attacks: efficacy and safety of a nonsteroidal anti-inflammatory drug, diclofenac-potassium, in comparison to oral sumatriptan and placeboCephalalgia192324010376168
- [TOSAM] The Oral Sumatriptan and Aspirin plus Metoclopramide Comparative Study Group1992A study to compare oral sumatriptan with oral aspirin plus oral metoclopramide in the acute treatment of migraineEur Neurol32177841317294
- TitusFDavalosAAlomJ19865-Hydroxytryptophan versus methysergide in the prophylaxis of migraine. Randomized clinical trialEur Neurol2532793536521
- TomkinsGEJacksonJLO’MalleyPG2001Treatment of chronic headache with antidepressants: a meta-analysisAm J Med111546311448661
- TomsonTBattinoD2005Teratogenicity of antiepileptic drugs: state of the artCurr Opin Neurol181354015791143
- UlrichVGervilMFengerK1999The prevalence and characteristics of migraine in twins from the general populationHeadache391738015613211
- VilmingSStandnesBHedmanC1985Metoprolol and pizotifen in the prophylactic treatment of classical and common migraine. A double-blind investigationCephalalgia517233986895
- Von SeggernRLMannixLKAdelmanJU2002Efficacy of topiramate in migraine prophylaxis: a retrospective chart analysisHeadache42804912390645
- WangSJFuhJLLuSR2000Chronic daily headache in Chinese elderly: prevalence, risk factors, and biannual follow-upNeurology54314910668689
- WasserfallenJM1976[Double-blind study with oxetorone in cephalalgia (author’s transl)]Schweiz Rundsch Med Prax6510214794862
- WeberRBReinmuthOM1972The treatment of migraine with propranololNeurology2236694552716
- WelchKM2003Contemporary concepts of migraine pathogenesisNeurology61S2814581652
- WideKWinbladhBKallenB2004Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population-based register studyActa Paediatr93174615046269
- YoungWBHopkinsMMShechterAL2002Topiramate: a case series study in migraine prophylaxisCephalalgia226596312383061
- YoungWBSiowHCSilbersteinSD2004Anticonvulsants in migraineCurr Pain Headache Rep82445015115645
- ZeebergIOrholmMNielsenJD1981Femoxetine in the prophylaxis of migraine – a randomised comparison with placeboActa Neurol Scand6445296753446
- ZivadinovRWillheimKSepic-GrahovacD2003Migraine and tension-type headache in Croatia: a population-based survey of precipitating factorsCephalalgia233364312780762
- ZwartJADybGHagenK2003Analgesic use: a predictor of chronic pain and medication overuse headache: the Head-HUNT StudyNeurology61160412874392