Abstract
There are variety of anticancer treatments including chemotherapeutic drugs, which are known to induce cell growth arrest and apoptosis through DNA damage and cytoskeleton toxicity. Meanwhile, histone deacetylase (HDAC) inhibitors could apply their antitumor activity through chromatin remodeling and gene expression modulation that affect the cell cycle and survival pathways. This paper proposes an anticancer three-drug compound and discusses several challenging issues in relation to designing multidrug compounds that could possibly lead to molecular-targeted therapies.
Introduction
There is accurate machinery that allows cells to recognize themselves and undertake specific tasks. This machinery represents the blueprint of various patterns of gene activation/inactivation throughout the cell cycle. It is mediated by numerous processes such as DNA methylation, adenosine triphosphate (ATP)-dependent chromatin remodeling, and post-translational modifications of histones, which include the acetylation and deacetylation of amino groups of lysine residues present in histone N-terminal tails. The set of chromatin modifying enzymes include one of the histone modification enzymes, such as histone acetyltransferase (HAT), histone deacetylase (HDAC), histone methyltransferase (HMT), histone demethylase (HDMT), and histone kinase. HATs act as transcriptional coactivators, whereas HDACs act as transcriptional corepressor enzymes. Lack of expression or repression leads to an irregular outcome for the cell, including altered genetic programs and increased rate of cell transformation.
A number of silenced tumor suppressor genes are shown to be lost due to epigenetic inactivation rather than sequence damages, although, epigenetic changes cooperate with genetic changes to initiate the development of a cancer since they are mitotically heritable.Citation1,Citation2 Further, epigenetic irregularities are pharmacologically reversible as oppose to genomic damage.Citation3 This fact provides an incentive to devote more efforts in designing combination drugs that eventually achieve epigenetic and molecular-targeted therapies.
Combination therapy
It is known that the whole epidermal growth factor receptor (EGFR) family of proteins contributes to tumor cell growth in numerous types of cancer. When activated by particular molecules that bind to their ligands, such receptors transmit a proliferation signal to the cell by initiating a cascade of internal molecular interactions. Cancer cells also secrete a variety of growth factors to attract the endothelial cells that build new blood vessels (ie, angiogenesis), which subsequently make tumors more difficult to treat.
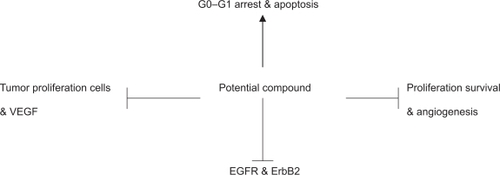
The tangle of signaling pathways leading from the receptors to the cellular processes that actually cause a cell to divide or to resist suicide despite DNA damage is highly complex. As depicted in , a potential drug compound may suppress the growth of cancer cells by inducing either G1 or G2 arrest, DNA synthesis, apoptosis, and should have antitumor activity against various types of cancer. Synergistic inhibition of cell proliferation and induction of apoptosis is an important factor towards effective combination therapy.
Figure 1 Representation of concomitant molecular changes after combination treatment with a potential compound: Combination therapies with agents that target endothelial cells to block angiogenesis, EGFR/ERBB2, and histone deacetylase inhibitors to prevent tumor adaptation in cancer treatment warrants experimental studies.
A combination between HDAC inhibitors like NVPLAQ824 and vascular endothelial growth factor (VEGF) receptor 2 kinase inhibitors like PTK787/ZK222584 has been demonstrated to have an antiangiogenic and antitumor effect.Citation4 This drug combination resulted in a greater antitumor and antiangiogenic effect in vitro and in vivo compared with single agents (ie, monotherapies).
Many HDAC inhibitors mediate their antitumor activity by chromatin remodeling and then followed by gene expression modulation. The antiproliferative effect of an HDAC inhibitor on tumor cells is supposed to correlate with the induction of a specific gene expression that mediate cell cycle arrest and/or apoptosis. In addition, HDAC inhibitors may also repress gene expression by inducing protein acetylation. For example, induction of p21 and inhibition of survivin expression may compromise the proliferation and differentiation of endothelial cells.Citation4
Mitogen-activated protein kinases (MAPK) and AKT (a serine/threonine kinase also known as protein kinase B) pathways play important roles in cell proliferation,Citation5 and therefore a compound that induces apoptosis is also supposed to inhibit AKT and/or MAPK activation. In addition, MAPK plays a role of a signaling mediator of EGFR in blockade of apoptosis. This presumes a significant contribution of MAPK in cell survival, since it is also activated by EGF stimulation independent of epidermal receptor growth factor 2 (ErbB2) and epidermal receptor growth factor 3 (ErbB3) signaling in human colon cancer (GEO) cells.Citation6 Unknown mechanisms or pathways that react to the compensatory activation of EGFR in response to the down-regulation of ErbB2 phosphorylation need to be explored. Hence, this study investigates the combination of an EGFR inhibitor with an HDAC inhibitor and subsequently analyze the corresponding level of inhibitory effects on ErbB2 phosphorylation. This combination therapy is discussed and analyzed later in this paper.
A drug’s antitumor and antiangiogenesis effect needs to be correlated with the down-regulation of angiogenesis-related genes such as VEGF and survivin.Citation7,Citation8 This type of drug also needs to modulate the expression of multiple genes that contribute in tumor development and angiogenesis, and it is required to induce other inhibition of VEGF signaling and angiogenesis as it is depicted in . Further, the design of a potential compound with an antitumor effect must affect tumor growth by acting on independent and parallel pathways. A combination therapy should induce cell cycle arrest by gene expression modulation in epithelial tumor and endothelial cells. As a result, the newly designed drug has the potential to be tailored for individual patients. For instance, this therapy can target patients with tumors that are dependent upon VEGF, angiogenesis-related genes, EGFR, and ErbB2. This type of drug represents a potential molecular targeted therapy that is also called key personalized medicine product.
Clinical trials of multidrug compounds
During a phase I study, a combination of trabectedin and pegylated liposomal doxorubicin (PLD) was tested in 36 patients with advanced malignancies.Citation9 An overall response rate of 16.7% was reported including one complete response (CR) and five partial responses (PR), and 39% had stable disease (SD). This study also confirmed that trabectedin combined with PLD provides a potential clinical advantage, and it is generally well tolerated at therapeutic doses in pre-treated patients with various tumor types.
A combination of immunosuppressive agents cyclophosphamide (CYC) and imatinib was evaluated in five patients with advanced scleroderma-related interstitial lung disease.Citation10 This combination was tolerated and without major effects in all patients. From the two patients who completed one year of treatment, only one patient with mild restrictive lung disease showed improvement in pulmonary function. A phase I/II study tested the combination of gefitinib and rofecoxib in 42 patients with nonsmall cell lung cancer.Citation11 This study reported 2.3% CRs, 4.7% PRs, and 28.5% SDs. In addition, the treatment was also reported to be generally tolerated.
Thirty-one open-angle glaucoma patients who were insufficiently controlled on latanoprost monotherapy, were given dorzolamide/timolol (DTFC), latanoprost/timolol fixed combination (LTFC), or a combination of DTFC and latanoprost.Citation12 This study showed that the latter therapy considerably decreased the intraocular pressure (IOP). In a similar study, a combination therapy of brimonidine and timodol was effective in decreasing the IOP as opposed to monotherapy with brimonidin or timodol.Citation13
In a phase I study, a combination of DNA-hypomethylating agent (5-AZA) and an HDAC inhibitor (valproic acid) was assigned to 55 patients with advanced cancer.Citation14 This report showed 25% SDs in which the disease stabilized from four to 12 months, and a considerable decrease in global DNA methylation and induction of histone acetylation were also observed. A phase II study of epigenetic therapy with hydralazine and magnesium valproate was reported.Citation15 Seventeen patients were evaluable for toxicity and 15 for responses. Primary sites included cervix (3), breast (3), lung (1), testis (1), and ovarian (7) carcinomas. The results showed 26.7% PRs and 53% SDs. The main toxicity was hematologic-related. Further, global DNA methylation, HDAC activity, and promoter demethylation were observed. A phase II trial was conducted to investigate clinical and molecular responses mediated by a histone deacetylase inhibitor (Depsipeptide FK228) in lung cancer patients. Nineteen patients were evaluable for toxicity and 18 for responses. This report showed neither significant cardiac toxicities nor objective responses,Citation16 since small does were assigned. However, a combination with other compounds warrants further evaluation in lung cancer patients.
The initial phase I/II trial of bortezomib and melphalan in 35 patients, who had progressed on relapsed or refractory multiple myeloma (MM) was reported.Citation17 Responses were observed in 68% of the studied population including 6% CRs, 9% immunofixation-positive CRs, 32% PRs, and 21% minimal response (MR). The combination of bortezomib and melphalan was reported to have a controllable toxicity and represent a compelling therapy candidate for MM patients. In a later report, a phase I/II study was conducted to assess the combination of arsenic trioxide/bortezomib/ascorbic acid (ABC) in 22 patients who had progressed on MM.Citation18 This study showed that responses were only observed in 27% of the heavily pretreated study population including 9% PRs and 18% MRs. The ABC treatment was reported to be well tolerated by the majority of patients.
One thousand ninety-one patients with type 2 diabetes were treated with the combination therapy of sitagliptin and metformin.Citation19 This regimen was reported tolerable amongst all patients and provided an additive glycemic improvements. Similar results were obtained when 217 type 2 diabetes patients were treated with the combination therapy of sulfonylurea and metformin.Citation20
A novel farnesyl protein transferase inhibitor (BMS-214662) combined with paclitaxel and carboplatin was assigned in 30 patients with solid tumors.Citation21 The purpose of this study was to investigate the toxicities, pharmacokinetics, and pharmacodynamics of BMS-214662 when combined with the other two compounds. This report showed no particular pharmacokinetic interaction between BMS-214662 and paclitaxel, and the combination of BMS-214662 with paclitaxel and carboplatin was normally tolerated in solid tumors. This phase I trial also reported 3.3% PRs related to a taxane-resistant esophageal cancer, 6.6% PRs related to endometrial and ovarian cancer, and 26.6% SDs. A phase I study also investigated the combination of P-glycoprotein inhibitor (MS209) with docetaxel in patients with solid tumors.Citation22 This study showed the absence of strong pharmacokinetic interaction between the two compounds at moderate dose levels, and the combination therapy had a limited effect on docetaxel toxicity or pharmacokinetics.
In a phase Ib study, the combination of patupilone and carboplatin was tested in 26 patients with recurrent platinum-sensitive ovarian cancer.Citation23 Tumor response was assessable by response evaluation criteria in solid tumors in 17 patients including 6% CRs and 59% PRs. This report showed that the treatment was generally tolerable and proved to have an antitumor activity.
The link between human epidermal growth factor receptor 2 (Her-2) and VEGF was reported to have a major role in predicting clinical outcome in primary breast cancer.Citation24 Consequently, various combination therapies were assessed against these two proteins when treating breast cancers with Her-2 overexpression. A phase I study using a combination of bevacizumab and trastuzumab in nine patients was reported.Citation25 The reported results show 11% CRs, 44% PRs, and 22% SDs.
A number of trials of bevacizumab combined with other anticancer compounds were assigned in patients with metastatic breast cancer (MBC).Citation26 This report discussed the combination of bevacizumab and chemotherapy including docetaxel which is known for its synergistic suppression of capillary vessel formation. It also discussed a study where 21 patients with inflammatory and locally advanced breast cancer (LABC) were assigned a combination therapy of bevacizumab and doxorubicin. This particular report showed 67% PRs, 24% SDs, and 9% PDs. Additional phase II trial was conducted in 49 patients to assess the vascular effects on tumor regression when using a monotherapy with docetaxel as opposed to a combination therapy of bevacizumab and docetaxel during the treatment of LABC. This study reported 14.3% CRs, 65.3% PRs, and 10% disease progression. A phase III study of a monotherapy with capecitabine and a combination of capecitabine and bevacizumab was conducted in 462 patients with MBC.Citation27 This report showed that the combination therapy increased the objective response rate from 9.1% to 19.8% as opposed to the monotherapy.
A pre-plan for an anticancer three-drug compound design
A specific dose of dual ErbB1/ErbB2 inhibitor lapatinib (GW572016) was needed to induce poly(adenosine diphosphate-ribose) polymerase (PARP) cleavage and DNA fragmentation to inhibit activation of EGFR and ErbB2 as well as the downstream MAPK and AKT pathways.Citation28 Various experiments validated that a concentration higher than the inhibitory concentration (IC)50 determines the induction of apoptosis. Conversely, small inhibitory effects on EGFR or ErbB2 phosphorylation in tumor cells were observed when concentrations are below the IC50 value.Citation29
Furthermore, it was reported that for some chronic, physiological diseases, synergism or antagonism at low concentration level also contribute towards treatment.Citation30 However, for infectious diseases or cancer therapies, synergism at high effect levels including equilibrium dialysis (ED)90, ED95, or ED99, are considered to be more therapeutically appropriate as opposed to low effect levels such as ED30 or ED50.
The number of events, which occur during the process of killing cancer cells through the combination of two cytotoxic agents, is unknown. Evaluating synergism or antagonism and the explanation of how or why it happens represents different issues.Citation30 Using the latest technological tools, it is possible to design the primary, secondary, and tertiary structure of an enzyme or a receptor as depicted in ,Citation8 however, it is still hard to design an inhibitor for the drug development process. In addition, the prediction of synergism or antagonism for enzymes or receptors is even more complex.
Figure 2 Complex receptor-ligand: Delta-Ex3 (a Survivin isoform) and XY2 (an HRas ligand). This complex has been properly visualized using CheVi (R) (a Linux-based Chemical Visualizer), where XY2 ligand is shown docked along Delta-Ex3’s surface and deeply embedded in a particular active site.
A pre-plan for conducting a three-drug combination therapy helps to seamlessly analyze two-drug compounds before reaching the three-drug compound. To demonstrate this scenario, I propose an experiment to design an anticancer three-drug combination composed of the following compounds: (1) an HDAC inhibitor “HDAC/INH”, (2) a VEGF receptor kinase inhibitor “VEGFR/INH”, and (3) an EGFR/ERbB2 inhibitor “EGFR/ERbB2/INH”. illustrates the design of the proposed experiment, in which each inhibitor refers to a drug. This proposal includes a set of three two-drug compounds, one three-drug compound, and a set of three three-drug compounds given as two-drug compounds consecutively.
Figure 3 Design of the proposed three-drug combination experiment.
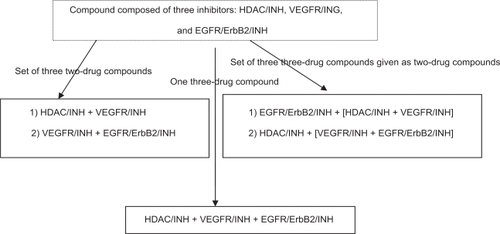
In this experiment, it is proposed to use three drugs as two drugs as they are represented within square brackets in . The benefit of this process is to gather valuable information when combining two drugs, which can be vital in spawning a three-drug compound. It embodies a process to generate novel compounds that incorporates the cytotoxicity factors of both compounds, while it helps to identify the most valuable candidate that satisfies the constraints of preclinical models. This process also helps to bridge the gap between preclinical models and clinical outcome through accurate prediction of toxicity levels.
Challenges in translating experimental studies into clinical trials
The drug development process is characterized by being high-risk, long-term, and costly. From a pool of 5,000 compound candidates entering the first development phase, only 5 will reach clinical testing.Citation31 Furthermore, a new drug enters phase 1 testing, only after it endures at least 10 years of preclinical experimentation. It has been reported that in the USA, the population of adult cancer patients who are eligible to participate in clinical trials is between 2% and 3%. Since the resources are very limited, drug sponsors search for cases from other parts of the world.Citation32
Novel drug developments need to use an improved method to define the optimal biological dose rather than the maximum tolerated dose. These methods and studies need to consider additional factors such as pharmacokinetics and pharmacodynamics of the compound instead of merely tumor size. The design of preclinical models based on these considerations and others such as appropriate endpoints and surrogate markers of response will lead to prospective clinical trials. This will allow preclinical models to predict clinical outcome with an adequate accuracy.
There are numerous challenges in translating experimental findings into clinical trials including a growing need to identify improved biomarkers that accurately predict the risk of the compound-related toxicity. This is associated with the quality of the preclinical models in predicting the corresponding toxicity. Hence, the use of nonclinical systems including animals, tissues slices, cell cultures, and in silico models, will not always help to accurately predict toxicity levels in humans. Human tumor xenografts grown subcutaneously have been used in preclinical testing when performed in immune deficient mice.Citation33 These models were successful in cytotoxic agents.Citation34 However, the validity of these kinds of experiments did not reach the expected predictive indicator of clinical activity except when pharmacokinetically clinically equivalent drug doses are tested.Citation35 Biomarkers that measure the molecular changes that lead to toxicity are considered to provide a useful early detection of toxicity. This type of biomarkers will help investigators to conduct a predictable and successful transition from preclinical studies to clinical trials.
Conclusion
This paper describes an anticancer drug design that combines three types of drugs as well as the challenges that lay ahead in setting up this type of experiment. The proposed three-drug compound warrants further experiments and clinical tests to demonstrate its candidacy among anticancer drugs. An analysis of toxicity is also required to be conducted on the newly designed compound. This analysis includes whether any two drugs, within the thee-drug compound, have overlapping or nonoverlapping toxicity, which includes gastrointestinal toxicity, cardiotoxicity, renal toxicity, and neurotoxicity. However, the generation of newly designed compounds with nonoverlapping toxicities may not be valuable since this will increase treatment costs and decrease patient compliance.
Disclosure
The author reports no conflict of interest in this work.
References
- EstellerMCancer epigenomicsNat Rev Genet2007828629817339880
- JonesPABaylinSBThe epigenomics of cancerCell200712868369217320506
- YooCBJonesPAEpigenetic therapy of cancerNat Rev Drug Disc200653750
- QianDZWangXKachhapSKThe histone deacetylase inhibitor NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584Cancer Res2004646626663415374977
- VenkateswarluSDawsonDMSt ClairPAutocrine heregulin generates growth factor independence and blocks apoptosis in colon cancer cellsOncogene200221788611791178
- JacksonJGSt ClairPSliwkowskiMXBlockade of epidermal growth factor- or heregulin- dependent ErbB2 activation with the anti-ErbB2 monoclonal antibody 2C4 has divergent downstream signaling and growth effectsCancer Res2004642601260915059917
- GaspariniGLongoRFanelliMCombination of antiangiogenic therapy with other anticancer therapies: results, challenges, and open questionsJ Clin Oncol20051295131115718328
- EzzianeZMolecular docking and analysis of survivin delta-ex3 isoform proteinThe Open Medicinal Chemistry Journal200821620
- von MehrenMSchilderRJChengJDA phase I study of the safety and pharmacokinetics of trabectedin in combination with pegylated liposomal doxorubicin in patients with advanced malignanciesAnn Oncol200819101802180918497430
- SabnaniIZuckerMJRosensteinEDA novel therapeutic approach to the treatment of scleroderma-associated pulmonary complications: safety and efficacy of combination therapy with imatinib and cyclophosphamideRheumatology2009481495218815156
- O’ByrneKJDansonSDunlopDCombination therapy with gefitinib and rofecoxib in patients with platinum-pretreated relapsed non-small-cell lung cancerJ Clin Oncol200725223266327317664473
- KonstasAGPMikropoulosDDimopoulosATSecond-line therapy with dorzolamide/timolol or latanoprost/timolol fixed combination versus adding dorzolamide/timolol fixed combination to latanoprost monotherapyBr J Ophthalmol2008921498150218703549
- SherwoodMBCravenERChonCTwice-daily 0.2% brimo-nidine−0.5% timolol fixed-combination therapy vs monotherapy with timolol or brimonidine in patients with glaucoma or ocular hypertension: A 12-month randomized trial for the Combigan Study Groups I and IIArch Ophthalmol20061241230123816966616
- BraitehFSorianoAOGarcia-ManeroGPhase I study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancersClin Can Res20081462966301
- CandelariaMGallardo-RincónDArceCA phase II study of epigenetic therapy with hydralazine and magnesium valproate to overcome chemotherapy resistance in refractory solid tumorsAnn Oncol20071891529153817761710
- SchrumpDSFischetteMRNguyenDMClinical and molecular responses in lung cancer patients receiving romidepsinClin Can Res200814188198
- BerensonJRYangHHSadlerKPhase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myelomaJ Clin Oncol200624693794416418495
- BerensonJRMatousJSwiftRAPhase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myelomaClin Can Res20071317621768
- GoldsteinBJFeinglosMNLuncefordJKEffect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetesDiabetes Care2007301979198717485570
- RosenstockJSugimotoDStrangePInsulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naïve patientsDiabetes Care20062955455916505505
- DyGKBruzekLMCroghanGAA phase I trial of the novel farnesyl protein transferase inhibitor, BMS-214662, in combination with paclitaxel and carboplatin in patients with advanced cancerClin Can Res20051118771883
- DiérasVBonneterreJLaurenceVPhase I combining a p-glycoprotein inhibitor, MS209, in combination with docetaxel in patients with advanced malignanciesClin Can Res20051162566260
- ForsterMKayeSOzaAA Phase Ib and pharmacokinetic trial of patupilone combined with carboplatin in patients with advanced cancerClin Can Res20071341784184
- KonecnyGEPegramMVenkatesanNMActivity of the dual kinase inhibitor lapatinib (GW572016) against HER-2 overexpressing and trastuzumab-treated breast cancer cellsCancer Res20066631630163916452222
- PegramMYeonCKuNPhase I combined biological therapy of breast cancer using two humanized monoclonal antibodies directed against HER-2 protooncogene and vascular endothelial growth factor (VEGF) Proc. SABCS 2004 [abstract 3039]Breast Cancer Res Treat2005902193214
- MilanoANastiGLaffaioliRVFirst line targeted therapies in breast cancer: focus on bevacizumabBiologics: Targets and Therapy200711310
- MillerKDChapLIHolmesFARandomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancerJ Clin Oncol2005a23792a799a15681523
- ZhouYSongLHu YiPBlockade of EGFR and ErbB2 by the novel dual EGFR and ErbB2 tyrosine kinase inhibitor GW572016 sensitizes human colon carcinoma GEO cells to apoptosisCancer Res200666140441116397255
- RusnakDWLackeyKAffleckKThe effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivoMol Cancer Ther200112859412467226
- ChouTCTheoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studiesPharmacolo Rev200658621681
- Tufts Center for the Study of Drug DevelopmentRecent news from the Tufts Center for the Study of Drug Development2001 [Cited Jan 10, 2009]. Available from: http://csdd.tufts.edu/NewsEvents.
- ScheinPSSchefflerBBarriers to efficient development of cancer therapeuticsClin Can Res2006121132433248
- NewellPVillanuevaAFriedmanSLExperimental models of hepatocellular carcinomaJ Hepatol20084885887918314222
- HuynhHSooKCChowPKXenografts of human hepatocellular carcinoma: a useful model for testing drugsClin Can Res20061243064314
- KerbelRSHuman tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improvedCancer Biol Ther20032S134S13914508091