Abstract
Changing from branded drugs to generic alternatives, or between different generic formulations, is common practice aiming at reducing health care costs. It has been suggested that antiepileptic drugs (AEDs) should be exempt from substitution because of the potential negative consequences of adverse events and breakthrough seizures. Controlled data are lacking on the risk of substitution. However, retrospective data from large medical claims databases suggest that switching might be associated with increased use of AED and non-AED medications, and health care resources (including hospitalization). In addition, some anecdotal evidence from patients and health care providers’ surveys suggest a potentially negative impact of substitution. Well-controlled data are needed to assess the real risk associated with substitution, allowing health care professionals involved in the care of patients with epilepsy to make informed decisions. This paper reviews currently available literature, based on which the authors suggest that the decision to substitute should be made on an individual basis by the physician and an informed patient. Unendorsed or undisclosed substitution at the pharmacy level should be discouraged.
Introduction
Changing from branded drugs to generic alternatives, or switching between different generic formulations, is common practice among physicians, pharmacists, and hospital formularies. Changes to generic drugs are based on the assumption that generic medicines provide an opportunity to obtain similar treatment at lower costs for patients and payers while liberating budgets for financing new, innovative medicines and diagnostic or therapeutic procedures. Indeed, pricing studies show that generic medications ensure lasting price reductions.Citation1
With antiepileptic drugs (AEDs), in the absence of controlled data, several concerns were raised when considering substitution, of which the most important is loss of seizure control as a result of changing serum drug levels.Citation2–Citation5 As a result of these potentially negative outcomes, several societies and specialist bodies (eg, UK National Institute for Health and Clinical Excellence [NICE]Citation6 and the American Academy of NeurologyCitation7,Citation8) discourage mandatory substitution of AEDs in specific patients and certain situations. Furthermore, regulatory bodies in several European countries have issued guidance or policies relating to nonsubstitution of certain AEDs, thus acknowledging epilepsy as a critical disease. For example, the Medical Products Agency (MPA; Läkemedelsverket) in Sweden specifies that lamotrigine, carbamazepine, phenytoin, valproic acid, and gabapentin cannot be changed for a generic version,Citation9,Citation10 while the Finnish National Agency for Medicines (Laakelaitos) does not include AEDs on its list of medicines substitutable by generic formulations.Citation11
Guidelines have been issued in the absence of well-controlled data being the gold standard for evaluating this question. In the absence of evidence-based decision-making, medical claims databases appear to have the highest level of unbiased data. These databases are limited by a number of factors which are outlined below, though results support findings derived from surveys of patients and physicians, pharmacokinetic studies, and case reports suggesting an overall cautious approach in substitution.
There is broad agreement amongst several professional societies (eg, the Deutsche Gesellschaft Für Epileptologie and the Italian Chapter of the League Against Epilepsy) that stable patients who are seizure-free, or whose seizures are well controlled on a given AED, should not be switched from branded to generic medication, or between different generic formulations, unless the physician considers this to be medically necessary and full disclosure is made by the treating physician to the patient.Citation12 Furthermore, substitution at the pharmacy level should not be performed without the physician’s approval and the informed consent of the patient. This article reviews presently available data relating to the potential medical impact of substitution of a generic AED for a branded product or for an alternative generic formulation of the same AED and areas of further research to further delineate the risks and benefits of substitution.
Impact of loss of seizure control: current data
It has long been suggested that specific disease categories should be established in which exemption should be granted from any reimbursement policies involving mandatory substitution of branded agents with a cheaper generic alternative.Citation13 One such category is for so-called “critical diseases” (ie, those with potentially serious outcomes if therapy should fail, or where polypharmacy may present difficulties), and the risk of loss of seizure control means that there is a strong case for epilepsy to be included in this category.
The occurrence of a breakthrough seizure in a patient with well-controlled disease requires seizure control to be re-established. Observational data are available from patients who have experienced seizures as a result of discontinuing AEDs. Though clearly different in its methodology, results suggest that regaining seizure control after a breakthrough seizure can be a lengthy process.Citation14
A single breakthrough seizure for a seizure-free patient has a substantial impact on safety, self-esteem, social interactions, and employment ().Citation15,Citation16 Another implication of a single or more breakthrough seizures is the loss of the patient’s driving license. For example, regulations in the UK state that patients may not drive after a seizure, and are unable to reapply for their license until 12 months after their last seizure.Citation17
Table 1 Potential impact of a single breakthrough seizure in a patient with well-controlled epilepsyCitation2,Citation5,Citation14,Citation53–Citation57
Clinical relevance of generic substitution
In most European counties and the United States, substitution of second-generation drugs is common and widespread. In order to understand the potential risk that a minority of patients face, it is important to understand both the clinical implications as well as the pharmacokinetic and pharmacodynamic effect of AEDs. Nowadays there is some evidence that high plasma levels might be associated with AEs and low plasma levels might trigger seizures.Citation18–Citation22 Before substituting one product for another, it is important that physicians consider the potential clinical consequences of over- and under-treatment.Citation23 While over-treatment is associated with adverse events, under-treatment may precipitate seizures that, as noted above, can destabilize the patient’s condition.
Robust data on the incidence of generic substitution (ie, branded–generic or generic–generic) in practice and its impact on patients are limited. With the patent expiry of a number of AEDs, this issue has greatly increased in importance. While no prospective randomized controlled trials are yet available, and information on disease characteristics, baseline demographics, and reason for switch are limited, data from retrospective medical claims databases analyses can provide useful data and deserve to be analyzed. These data supplement case reports and series from pharmacist or physician practices, as well as pharmacokinetic studies.
Large-scale surveys and medical claims database analyses
In the United States, a case-control study with data from 1,664 patients in the Ingenix LabRx Database—containing longitudinal eligibility, pharmacy claims, and medical claims data from a US population of managed Medicare, Medicaid, and employed commercially insured patients with dependents – was performed.Citation24 Cases (individuals with epilepsy who received care during 2006 in an ambulance, emergency room or inpatient hospital with a primary epilepsy diagnosis) were 81% more likely to have been switched to a generic formulation compared with control patients (those who had a primary epilepsy diagnosis in a physician’s office during the same period; odds ratio, 1.81; 95% confidence interval [CI]: 1.25–2.63). Cases were also significantly more likely to be Medicaid recipients than controls (4.6% vs 1.8%; p = 0.002); when Medicaid recipients were removed from the analysis, the significant difference between cases and controls remained.
Data derived from a public payer pharmacy claims database in Ontario, Canada, regarding generic substitution and associated medical and financial consequences, were recently published.Citation25 In total, 1,354 patients received generic lamotrigine (403 receiving monotherapy and 951 receiving lamotrigine as part of a polytherapy regimen). Of these, 12.9% switched back to branded lamotrigine (11.7% in the monotherapy group and 13.4% in the polytherapy group). Switch-back rates were higher still for clobazam and valproate (), and were substantially higher than for statin (1.5%) or selective serotonin reuptake inhibitor (1.9%–2.9%) therapy. In patients who did not switch back from generic lamotrigine, the average daily dose was initially 255.3 mg, increasing to 271.1 mg while receiving the generic product (6.2% increase; p < 0.0001). Switching to a generic agent, regardless of whether the patient subsequently switched back, was also associated with significant increases in the use of AED and non-AED concomitant medications (+11.0%; p < 0.0001 and +15.6%; p < 0.0001, respectively).
Table 2 Switchback rates in database studies of brand-to-generic switching
In Ontario, mandatory substitution of generic drugs can be circumvented if a physician submits an adverse reaction form to the pharmacist, documenting that switching back to the branded product is medically necessary. A review of 71 pharmacies yielded 14 adverse reaction forms.Citation26 In 11 cases (79%), loss of seizure control while receiving generic lamotrigine was the primary reason for switching back to the branded formulation. In one case, anxiety, mood swings and dizziness were cited as additional reasons. In eight of 10 cases where an outcome was recorded, seizure control was recovered after branded lamotrigine was reinstated. As part of the same study, a chart review of data from six physicians (nine patients receiving generic lamotrigine; duration, 3–224 days) was carried out.Citation26 For eight of the nine patients, loss of seizure control was recorded as the reason for switching back to branded lamotrigine; seven of these patients regained control when switched back to branded lamotrigine.
The Canadian province of Quebec allows the continued use of branded drugs – even when a generic alternative is available – for up to 15 years after listing in the Régie de l’Assurance-Maladie du Québec (RAMQ) formulary. Analysis of the RAMQ database (January 2006 to October 2007) in an open-cohort design, classifying the observation into periods of brand, single-generic and multiple-generic use, provided information on 948 patients observed for an average of 665 days.Citation27 Within one year of observation, newer AEDs (topiramate, lamotrigine, and gabapentin) and older AEDs (divalproex, clobazam, clonazepam, valproate and carbamazepine) showed generic switch rates of 30.5% and 18.2%, respectively, which were lower than with non-AEDs (35.9%). There was also a higher switchback rate for AEDs (14.7% and 19.2%) versus non-AEDs (7.8%). Of patients receiving generic AEDs, approximately 25%–50% took two or more different generic versions of a drug. Compared with patients receiving non-AED agents, patients taking AEDs were significantly less likely to switch to a generic alternative (hazard ratio [HR], 0.77; 95% CI: 0.70–0.84; p < 0.0001) but more likely to switch back (HR, 1.88; 95% CI: 1.52–2.33; p < 0.0001). Compared with continuous use of a branded AED, the risk of hospitalization was 1.28 times higher after single brand–generic switch (p < 0.0059) and even more pronounced after a generic–generic switch, with an almost 3.8-fold higher risk (p < 0.0001). The risk of head injury or fracture for a single brand–generic switch was 1.53 times higher, and for multiple generic switches was 5.4 times higher (both p < 0.0001). Additionally, compared with brand use only, single and multiple switches were associated with significantly higher pharmacy use (other AEDs and non-AEDs) and increased lengths of hospital stay.
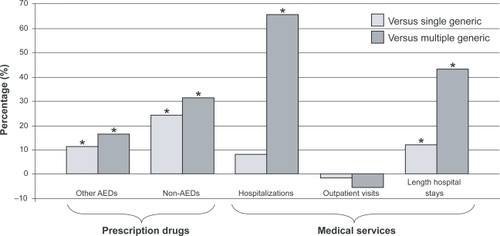
As part of the analysis of the Quebec database, data on patients switching from branded to generic topiramate were also evaluated. Similar to the findings with lamotrigine in the Ontario database, substantial increases in use of prescription drugs and hospitalizations were reported, particularly in those who received more than one generic formulation ().Citation27 Overall, 23% of patients who received generic topiramate received at least two different generic versions. After adjustment, multiple generic use was associated with 21% higher total health care costs (adjusted cost ratio [CR], 1.21; adjusted p = 0.0420), whereas the difference between single generic and brand periods was not statistically significant (adjusted p = 0.9715). The lower costs of generic topiramate compared with the brand were counterbalanced, however, by higher costs for other drugs, (single generic versus brand: adjusted CR, 0.95; adjusted p = 0.3053; multiple generic versus brand: adjusted CR, 1.14; adjusted p = 0.0926) and marginally higher costs for medical services (not statistically significant).
Figure 1 Use of health care sevices in patients receiving generic topiramate, adjusted vs branded topiramate use.Citation27
Abbreviation: AED, antiepileptic drug.
Pharmacokinetic studies
Several pharmacokinetic studies, in healthy volunteers and patients with epilepsy, have been conducted to compare parameters such as maximum serum concentration (Cmax) and exposure (area under the curve; AUC) for branded and generic AEDs, and many have identified wide variations in the pharmacokinetic profiles of some generic formulations (reviewed by Crawford and colleagues).Citation3 Notably, some generic formulations of carbamazepine and phenytoin have been identified that fail to meet bioequivalence standards.Citation15,Citation28–Citation31 More recently, a pilot study was carried out in eight outpatients who reported problems attributed to a switch from branded to generic lamotrigine, and one patient who requested pharmacokinetic information ahead of a proposed switch (all patients had received branded lamotrigine for at least two weeks before enrollment).Citation22 Daily blood profiles were obtained at 3- or 4-hour intervals on day 3 after admission, while receiving branded lamotrigine. A second profile was then carried out at least seven days after switching to generic lamotrigine. In five of the nine patients, lamotrigine pharmacokinetic parameters increased or decreased by more than 10%. Complaints reported by these patients included increased seizure frequency or relapse, as well as ataxia, falls and vertigo.
Case reports and case study series
Case reports and case study series are the lowest level of evidence and are open to bias to report negative consequences only. However, they have often reported an increase in severe adverse events after changing to a generic AED.Citation18–Citation20
In an open-label crossover study, 14 patients who had been receiving a branded carbamazepine formulation for at least 35 days were changed to a generic alternative.Citation18 Clinically relevant adverse events, including dizziness, nausea, ataxia, diplopia, and nystagmus, were reported in nine of the 14 patients, occurring on the day of the change or one day after. Severe adverse events occurred in seven patients. In all but one patient, the occurrence of adverse events was correlated with an increased Cmax and AUC of carbamazepine and its active metabolite, carbamazepine-10, 11-epoxide, after changing to the generic formulation.Citation18 AEDs that have been implicated in increases in seizure frequency after generic substitution include phenytoin,Citation21 carbamazepine,Citation5,Citation18,Citation19,Citation32 valproate,Citation33 and primidone,Citation34 with some authors attributing this to reduced bioavailability with the generic formulation.
As part of a large survey of AED use in the United States, physicians were asked to submit case report forms regarding patients who experienced loss of seizure control after switching to a generic AED.Citation35 Fifty patients, well controlled on a branded AED (phenytoin, valproate, carbamazepine, gabapentin, or zonisamide), subsequently experienced a breakthrough seizure or increased seizure frequency after switching to the generic alternative without other provoking factors. Of 26 patients in whom serum AED levels were known before and after generic substitution, 21 were found to have lower levels at the time of the breakthrough seizure while receiving generic medication. Within one week of seizure, 72% of patients had been switched back to the branded product, with 91% switched back within one month. At the time of case review, 44 of the 46 patients switched back to the branded product had regained seizure control. Loss of seizure control had a negative impact on quality of life, including loss of driving privileges (n = 30), and missed school/work days (n = 9).
Patient and physician surveys of generic substitution
To gain insight into the incidence and impact of generic prescribing in “real-world” practice, numerous surveys of patients and health care professionals have been carried out.
Patient surveys
A study of general practices in the UK identified 2,285 patients with epilepsy who were receiving carbamazepine, phenytoin, or sodium valproate.Citation36 A questionnaire was sent to these patients, and those who recalled taking a generic agent (defined as a drug supplied by a different pharmaceutical manufacturer) during the previous two years were interviewed by their practice if they reported a problem with the control of their epilepsy after substitution. Of the patients contacted, 1,333 (59%) responded, and 251 (19%) of respondents had experienced a change in medication supplier. In total, some form of problem (ie, reduced seizure control or increased side effects) was reported by 29% of patients who changed medication. This included 11% who reported “validated” problems (ie, an increase in seizure frequency or side effects with no other medical or psychological cause identifiable by the GP), of which an increase in side effects or “feeling worse” was the most common problem (21/27 patients), followed by an increase in seizure frequency (8/27 patients) and first seizure in over 12 months (one patient). In addition, 10% of patients reported “unproven” problems (other likely medical or psychological explanations were identified by the GP), while follow-up was incomplete in 9%. The majority of problems (88%) were reported in patients who were changed from a branded to a generic product, or between generic products.
In a Canadian survey of 83 patients with epilepsy, 14 (17%) reported that they had been changed from a branded AED to a generic alternative.Citation37 Of these, two patients (14%) reported that they had had problems after the change. In a US survey of 82 patients (or their parents), 96% agreed that switching between forms of the same AED may cause an increase in seizures or adverse effects, with only 38% feeling that medication switching is safe.Citation38 In addition, 43% of patients reported having problems with formulation switching, and 48% reported knowing other patients with problems.
A larger, international survey of patient opinions regarding generic AEDs was carried out in Canada, the UK, Germany, France, and Spain in 2004.Citation39 Of 974 patients included in the analysis, just over half were aware of the term “generic” (52%). In subsequent questions, ”generic” was defined for all patients as “a less expensive and clinically similar alternative to a name-branded prescription medication.” Overall, 58% felt uncomfortable about receiving a generic AED. Breakthrough seizures were attributed to generic medication by 23% of patients.
In a recent US study, 550 patients with epilepsy were questioned about their perceptions of generic AEDs.Citation40 Overall, one-third of patients (34%) linked breakthrough seizures with generic substitution, while two-thirds (65%) had concerns about the efficacy of generic agents.
Physician surveys
In the Canadian survey described above, 46 neurologists were also surveyed about their attitudes towards generic substitution of AEDs.Citation37 Only around half of respondents felt that generic substitution of various AEDs was safe, although values ranged from 30% for lamotrigine to 67% for primidone. A larger-scale study was carried out in the United States, in which 6,420 neurologists received a postal survey questionnaire; of these, 301 (4.7%) responded.Citation41 Breakthrough seizures after changing from a branded AED to a generic alternative were reported by 68% of the neurologists who responded, while 56% reported an increase in adverse events after substitution. When considering a change between different generic preparations of the same AED, 33% of neurologists reported breakthrough seizures and 27% reported an increase in adverse events attributable to a change from one generic AED to another. Among the reported consequences of generic substitution were an increase in the need for consultations, a greater number of sick days, and a higher incidence of injury to patients. Perhaps most worryingly, 10% of physicians stated that their relationship with the patient had been undermined by the change to a generic AED. Neurologists were also questioned in the study about their responses to generic substitution, the most common of which was to specify “dispense as written” or a similar instruction on future prescriptions.
More recently, 606 physicians who treat patients with epilepsy (74% neurologists, including epileptologists, and 26% GPs) were questioned about perceptions of generic medication.Citation40 Concern about an increase in breakthrough seizures in patients switched from a branded AED to a generic or who are consistently switched among generic formulations of the same AED was expressed by 88% of physicians. In addition, 55% were “very” or ”extremely” concerned about the level of epilepsy control as a result of generic substitution. When 312 French private neurologists and hospital specialists in epilepsy were questioned, most indicated that they felt uncomfortable with generic substitution, with only a few actually prescribing generic AEDs.Citation42 Few respondents, however, indicated on prescriptions that substitution should not be carried out. One-third of participants reported the occurrence of breakthrough seizures (n = 70) or new adverse events (n = 75) after generic substitution, and 70% stated that additional telephone consultations were required with patients who had switched.
Physician responses to generic AED substitution were also assessed in a cross-sectional, telephone-based survey of 435 GPs, neurologists and epileptologists in Canada, the UK, France, Germany and Spain.Citation39 In total, respondents stated that 65% of AED prescriptions were for brand-name drugs, particularly in France and Spain, where more than 90% of prescriptions were for branded agents. Generic substitution without the physician’s consent was opposed by 55% of respondents, and 27% believed that a patient had experienced a breakthrough seizure as the result of a change to a generic agent. Overall, 31% of physicians reported that they felt uncomfortable about prescribing generic AEDs.Citation39
Similar results were obtained in an Internet-based survey of members of the German, Austrian, and Swiss sections of the International League Against Epilepsy, and members of the Deutsche Gesellschaft Für Epileptologie.Citation43 Of 2,800 physicians contacted, more than 600 responded, with around 80% reporting experience with generic AEDs. Of these, approximately half reported problems with their usage, primarily in the form of additional use of health care resources (telephone contacts, visits, hospital admissions, calls for emergency doctors, or visits to emergency rooms). Around half of the physicians also reported that their experiences with generic AEDs had led them to change their prescribing behavior, as well as increasing patient counseling and blood level monitoring. Current criteria for approval of generic AEDs were considered to be inappropriate by 50% of respondents, while 90% considered it unacceptable that pharmacists are able to substitute a generic agent for a branded drug without consultation.Citation43
Pharmacist survey
A survey of 112 community pharmacists in the United States found that 87% agreed that switching between forms of the same AEDs may cause an increase in seizures or adverse effects, even though 96% felt that medication switching in general was safe.Citation38 Half of pharmacists questioned (51%) knew of patients who had experienced problems when changing formulations.
Awareness of generic substitution
In patients who experience sudden, unexpected loss of seizure control, physicians and pharmacists may look for explanations such as poor compliance with the medication regimen, inappropriate dose modification or drug–drug interactions. Brand–generic or generic–generic substitution should not, however, be overlooked.
Physicians
Physicians may greatly underestimate the number of generic prescriptions that are distributed by pharmacists. In a survey of physicians attending two major US epilepsy and neurology congresses, most estimated that the rate was 30% or 50%.Citation44 Independent audit revealed, however, that the overall substitution rate was 68%. In a recent US survey of 660 physicians who treat patients with epilepsy, 25% of physicians were unaware that a pharmacist may substitute a generic medication for a brand prescription without physician consent, even though 85% of physicians believed that such substitution of AEDs without physician consent is medically inappropriate and unacceptable.Citation40 On average, physicians reported that 55% of their AED prescriptions used for the treatment of epilepsy specified “dispense as written” (brand only), although this practice was far more common among neurologists (61% of prescriptions) than GPs (36%). In a Canadian survey, there was again some lack of information concerning pharmacy dispensing procedures, with 10 of 46 neurologists (22%) unaware that a generic could be substituted by the pharmacist even when a brand name was specified on the prescription.Citation37 Perhaps surprisingly, only 41% of pharmacists in the US survey knew that problems resulting from formulation switching should be reported as adverse drug events to the US Food and Drug Administration (FDA), and only 27% had used the FDA’s MedWatch for reporting of safety information and adverse events, even though 79% of pharmacists were aware of the program.Citation38
Patients
Results from surveys show that awareness of generic prescribing by individual patients is very low. In large US and international surveys of patient opinions regarding generic AEDs, only 34%–38% of patients were aware that pharmacies could substitute a generic medication without the physician’s consent.Citation39,Citation40 Similarly, a US survey of 82 patients found that fewer than half (47%) knew that problems with formulation switching should be reported as adverse drug events to the FDA, and only 6% knew of the FDA MedWatch program.Citation38 In a Canadian survey of 83 patients with epilepsy, 22% did not know whether they had ever been changed from a branded drug to a generic alternative.Citation37
Discussion
Until recently, data regarding the clinical effects of brand–generic or generic–generic substitution were limited to single-case or small patient-series reports, patient or physician surveys. For example, Crawford (2006) stated that evidence was “limited,” consisting of “mainly case reports with some pharmacokinetic studies.”Citation3 There was also a large body of unpublished, anecdotal evidence that substitution of AED formulations was associated with efficacy or safety issues. Indeed, all the authors of this paper have treated patients who have experienced problems with adverse events or breakthrough seizures, such as those detailed above, after switching between branded and generic or between two generic formulations. While this anecdotal evidence was highly suggestive of a link between generic substitution and adverse effects, there remained a need for methodologically robust studies to evaluate the relationship. More recently, patent expiry of frequently used AEDs has led to increased interest, including generation of more robust data to estimate clinical effects of brand–generic or generic–generic substitution. While these data are far from ideal—double-blind randomized controlled trials are lacking, and claims databases do not provide information on disease characteristics and lack important patient information (eg, seizure frequency, disease duration, etc)—medical claims database analyses still provide useful information about the care of patients with epilepsy and associated medical costs. These recent publications therefore represent some advance in the state of knowledge regarding generic substitution when compared with the previous, largely anecdotal, evidence.
Initial results from large Canadian database studies appear to support the effects of generic substitution that have been suspected from anecdotal reports for many years: that there may be an apparent association with increased seizure frequency, morbidity, and use of health care services, with a number of patients requiring a switch back to their previous formulation.Citation25,Citation27,Citation45 The risk of seizures, injuries and health care use appears to be further increased with generic–generic switching. Smaller pharmacokinetic studies have demonstrated that patients who switch formulations experience variations in serum drug levels that can lead both to over-treatment and adverse events, as well as under-treatment,Citation18–Citation20,Citation22 which might be a possible explanation for the findings discussed above.
Surveys of patients and physicians, while far less robust than the large database studies discussed above and open to bias, can provide a useful “real-world” view of substitution, and appear to provide corroboration for the findings of database analyses. One important finding of such surveys is the relatively low level of awareness among physicians and patients regarding the ability of pharmacists to make generic substitutions without the explicit consent of the prescriber and the patient.Citation37,Citation39,Citation40,Citation44 With this in mind, several European and US professional bodies have called for an end to generic substitution of AEDs without informed consent of the patient and physician,Citation7,Citation8,Citation12,Citation46–Citation49 while others, including the Scottish Intercollegiate Guidelines Network,Citation50 NICECitation6 and the Irish Epilepsy Association (Brainwave),Citation51 recommend that generic substitution of AEDs should not be carried out at all, thus acknowledging epilepsy as a critical disease. As noted above, it is important to realize that issues with substitution for the individual patient may not apply only when switching from a branded product to a generic agent, but also when switching from one generic formulation to another.Citation27 Furthermore, generic drugs may differ from the original branded product and from each other in appearance (eg, changes to color or shape), which can lead to confusion or even anxiety for patients.Citation36,Citation37,Citation52
Ideally, the effects of generic substitution would be evaluated in a prospective manner—such as in randomized, controlled trials using, for example, a cross-over design and of sufficient duration to overcome natural fluctuations in serum AED levels. An enriched population might be most likely to show differences if it captured the emergence of adverse events as well as seizures. The study would have to rely on pharmacokinetic data comparing generics at the upper and lower end of bioequivalence. However, whether seizure-free patients are willing to enter a study with the potential hazard of experiencing adverse events and breakthrough seizures remains to be seen.
A possible alternative would be a prospective, observational study in patients considered by their physician to be eligible for generic substitution, to include both pharmacokinetic measurements and clinical outcomes. Such a study would be easier to conduct as it would reflect current daily practice, although it would definitely be hampered by its open-label design and dependent on patients’ and physicians’ expectations about potential outcomes. Therefore, double-blinded trial designs with the objective of looking at the occurrence of adverse events and/or seizures would be the preferred option to investigate this question further.
As the patents of AEDs continue to expire, there will be increasing pressure on prescribers and pharmacists to reduce medication costs by switching to generic alternatives, or between different generic formulations as costs and supplies vary. It is important, therefore, that health care workers are aware of the potential consequences of substitution and consider each patient on an individual basis regarding their eligibility for a change of formulation.
Acknowledgements
Editorial assistance with this manuscript was provided by Dr Daniel Booth (BioScript Stirling Ltd.), who was funded by Janssen–Cilag EMEA, and Barbara Schauble, MD PhD and Maren Gaudig, MSc, both employees of Janssen–Cilag Gemany.
References
- Pharmaceutical Forum. Second progress report, 26 June 2007. [cited 2008 Nov 15]. Available from: http://ec.europa.eu/enterprise/phabiocom/docs/pf_20070626_progr_report.pdf
- NashefLFishDRGarnerSSanderJWShorvonSDSudden death in epilepsy: a study of incidence in a young cohort with epilepsy and learning difficultyEpilepsia199536118711947489695
- CrawfordPFeelyMGubermanAKramerGAre there potential problems with generic substitution of antiepileptic drugs? A review of issuesSeizure20061516517616504545
- TrimbleMRGeneric prescribingHum Psychopharmacol1987212
- WeltyTEPickeringPRHaleBCAraziRLoss of seizure control associated with generic substitution of carbamazepineAnn Pharmacother1992267757771611158
- StokesTShawEJJuarez-GarciaACamosso-StefinovicJBakerRClinical guidelines and evidence review for the epilepsies: Diagnosis and management in adults and children in primary and secondary careLondonRoyal College of General Practitioners2004
- American Academy of NeurologyAssessment: generic substitution for antiepileptic medication. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of NeurologyNeurology199040164116432234417
- LiowKBarkleyGLPollardJRHardenCLBazilCWPosition statement on the coverage of anticonvulsant drugs for the treatment of epilepsyNeurology2007681249125017438213
- MPA (Läkemedelsverket)Substitutable medical productsUppsalaLäkemedelsverket2006
- MPA (Läkemedelsverket)Neurontin and gabapentin nycomed - why aren’t they interchangeable?UppsalaLäkemedelsverket2004
- National Agency for Medicines (Laakelaitos)The National Agency for Medicines’ updated (November 2005) principles for the preparation of interchangeable drugs that are authorized for saleHelsinkiLaakelaitos2005
- PeruccaEAlbaniFCapovillaGBernardinaBDMichelucciRZaccaraGRecommendations of the Italian League against Epilepsy working group on generic products of antiepileptic drugsEpilepsia200647Suppl 5162017239100
- LamyPPGeneric equivalents: issues and concernsJ Clin Pharmacol1986263093163700686
- SchmidtDLoscherWUncontrolled epilepsy following discontinuation of antiepileptic drugs in seizure-free patients: a review of current clinical experienceActa Neurol Scand200511129130015819708
- FeelyMCrawfordPKramerGGubermanARisk management in epilepsy: generic substitution and continuity of supplyEur J Hosp Pharm Sci2005118387
- EUCAREEuropean White Paper on EpilepsyEpilepsia200344Suppl 6188
- National Society for EpilepsyEpilepsy Getting around: driving and travelChalfont St PeterNational Society for Epilepsy2006
- MayerTMayTWAltenmüllerDMSandmannMWolfPClinical problems with generic antiepileptic drugsClin Invest Drug1999181726
- GilmanJTAlvarezLADuchownyMCarbamazepine toxicity resulting from generic substitutionNeurology199343269626978255480
- Di BonaventuraCFattouchJFabbriniGManfrediMPrencipeMGiallonardoTASwitching from branded to generic antiepileptic drugs as a confounding factor and unpredictable diagnostic pitfall in epilepsy managementEpileptic Disord2007946546618055310
- BurkhardtRTLeppikIEBlesiKScottSGapanySRCloydJCLower phenytoin serum levels in persons switched from brand to generic phenytoinNeurology2004631494149615505173
- NielsenKADahlMTommerupEWolfPComparative daily profiles with different preparations of lamotrigine: a pilot investigationEpilepsy Behav20081312713018406211
- SchmidtDStrategies to prevent overtreatment with antiepileptic drugs in patients with epilepsyEpilepsy Res200252616912445961
- ZachryWMDoanQDClewellJDSmithBJCase-control analysis of ambulance, emergency room, or inpatient hospital events for epilepsy and antiepileptic drug formulation changesEpilepsia200950349350018616554
- AndermannFDuhMSGosselinAParadisPECompulsory generic switching of antiepileptic drugs: high switchback rates to branded compounds compared with other drug classesEpilepsia20074846446917346246
- MakusKGMcCormickJIdentification of adverse reactions that can occur on substitution of generic for branded lamotrigine in patients with epilepsyClin Ther20072933434117472825
- LeLorierJDuhMSParadisPEThe risks of multiple-generic substitution of antiepileptic drugs: the case of topiramatePoster #PND46 presented at ISPOR 13th Annual International Meeting2008 May 3–7Toronto, ON, Canada
- MeyerMCStraughnABMhatreRMShahVPWilliamsRLLeskoLJThe relative bioavailability and in vivo-in vitro correlations for four marketed carbamazepine tabletsPharm Res199815178717919834004
- MeyerMCStraughnABJarviEJWoodGCPelsorFRShahVPThe bioinequivalence of carbamazepine tablets with a history of clinical failuresPharm Res19929161216161488405
- SilpakitOAmornpichetkoonMKaojarernSComparative study of bioavailability and clinical efficacy of carbamazepine in epileptic patientsAnn Pharmacother1997315485529161646
- SoryalIRichensABioavailability and dissolution of proprietary and generic formulations of phenytoinJ Neurol Neurosurg Psychiatry1992556886911527539
- HartleyRAleksandrowiczJNgPCMcLainBBowmerCJForsytheWIBreakthrough seizures with generic carbamazepine: a consequence of poorer bioavailability?Br J Clin Pract1990442702732206824
- MacDonaldJTBreakthrough seizure following substitution of Depakene capsules (Abbott) with a generic productNeurology19873718853120036
- WyllieEPippengerCERothnerADIncreased seizure frequency with generic primidoneJAMA1987258121612173626006
- BergMJGrossRATomaszewskiKJZingaroWMHaskinsLSGeneric substitution in the treatment of epilepsy: case evidence of breakthrough seizuresNeurology20087152553018695164
- CrawfordPHallWWChappellBCollingsJStewartAGeneric prescribing for epilepsy. Is it safe?Seizure19965158777549
- GubermanACormanCGeneric substitution for brand name antiepileptic drugs: a surveyCan J Neurol Sci200027374310676586
- McAuleyJWChenAYElliottJOShnekerBFAn assessment of patient and pharmacist knowledge of and attitudes toward reporting adverse drug events due to formulation switching in patients with epilepsyEpilepsy Behav200914111311718768168
- HaskinsLSTomaszewskiKJCrawfordPPatient and physician reactions to generic antiepileptic substitution in the treatment of epilepsyEpilepsy Behav200579810515961350
- BergMJGrossRAHaskinsLSZingaroWMTomaszewskiKJGeneric substitution in the treatment of epilepsy: Patient and physician perceptionsEpilepsy Behav200813469369918589000
- WilnerANTherapeutic equivalency of generic antiepileptic drugs: results of a surveyEpilepsy Behav2004599599815582850
- BirabenADeTBSemahFRouaudT[Use of generic anti-epilepsy drugs in France: survey of neurologists and review of the literature]Rev Neurol (Paris)200716345546117452947
- KrämerGSteinhoffBJFeuchtMPfäfflinMMayTWExperiences with generic drugs in epilepsy patients. Results of an internet-based questionnaire study in Germany, Austria, and SwitzerlandAkt Neurol200633431438
- WilnerANPhysicians underestimate the frequency of generic carbamazepine substitution: results of a survey and review of the problemEpilepsy Behav2002352252512609245
- LeLorierJDuhMSParadisPEClinical consequences of generic substitution of lamotrigine for patients with epilepsyNeurology2008702179218618505997
- Epilepsy ActionPosition statement – consistency of supplyLeedsBritish Epilepsy Association2005
- AFSSAPSPress release. Substitution des médicaments antiépileptiquesParisAgence française de sécurité sanitaire des produits de santé2008
- KrämerGDennigDSchmidtDGenerics in antiepileptic drug therapy: what has to be consideredAkt Neurol200532275278
- LFCERecommendations on the use of generics for the treatment of epilepsyBronFrench Chapter of the International League Against Epilepsy2008
- Scottish Intercollegiate Guidelines NetworkDiagnosis and management of epilepsy in adults A national guidelineEdinburghRoyal College of Physicians2003
- BrainwaveSubmission to the Oireachtas Joint Committee on Health and Children in respect of the proposed substitution by generic AEDs (anti-epileptic drugs) of branded AEDsEpilepsy News20083745
- HeaneyDCSanderJWAntiepileptic drugs: generic versus branded treatmentsLancet Neurol2007646546817434101
- KitsonAShorvonSClinical Standards Advisory Group Services for patients with epilepsyLondonDepartment of Health2000
- ShorvonSDThe epidemiology and treatment of chronic and refractory epilepsyEpilepsia199637Suppl 2S1S38641240
- HannaNJBlackMSanderJWThe National Sentinel Clinical Audit of Epilepsy-Related Death Epilepsy – death in the shadowsNorwichHMSO2002
- AnonEpilepsy: safety, excess mortality and sudden deathEpilepsia200344Suppl 6192012919335
- TomsonTWalczakTSillanpaaMSanderJWSudden unexpected death in epilepsy: a review of incidence and risk factorsEpilepsia200546Suppl 11546116393182