Abstract
Regular blood transfusions as supportive care for patients with chronic anemia inevitably lead to iron overload as humans cannot actively remove excess iron. The cumulative effects of iron overload cause significant morbidity and mortality if not effectively treated with chelation therapy. Based on a comprehensive clinical development program, the once-daily, oral iron chelator deferasirox (Exjade®) is approved for the treatment of transfusional iron overload in adult and pediatric patients with various transfusion-dependent anemias, including β-thalassemia and the myelodysplastic syndromes. Deferasirox dose should be titrated for each individual patient based on transfusional iron intake, current iron burden and whether the goal is to decrease or maintain body iron levels. Doses of >30 mg/kg/day have been shown to be effective with a safety profile consistent with that observed at doses <30 mg/kg/day. Recent data have highlighted the ability of deferasirox to decrease cardiac iron levels and to prevent the accumulation of iron in the heart. The long-term efficacy and safety of deferasirox for up to 5 years of treatment have now been established. The availability of this effective and generally well tolerated oral therapy represents a significant advance in the management of transfusional iron overload.
Iron overload and chelation therapy
The human body has many mechanisms to absorb, transfer and store essential dietary iron, but none to excrete excess amounts. It is therefore inevitable that patients who undergo regular transfusion therapy to treat chronic anemia, such as those with β-thalassemia and the myelodysplastic syndromes (MDS), will develop iron overload, since every unit of blood contains approximately 200 mg of iron.Citation1 Iron overload from transfusions may be exacerbated in some patients due to increased absorption of iron from the diet in response to ineffective erythropoiesis.Citation2 Excess iron is deposited in parenchymal tissue, including the liver, heart and endocrine system, which leads to ongoing damage and, ultimately, to organ failure.
Iron chelation therapy is the only viable option for the treatment of transfusional iron overload and can prevent further cellular iron uptake and reduce levels of morbidity and mortality in regularly transfused patients.Citation3–Citation5 Deferoxamine (DFO; Desferal®) is the current standard of care for iron chelation therapy; however, as DFO is a large molecule with a short half-life (20 to 30 minutes), treatment requires a demanding regimen of slow continuous infusion over 8 to 12 hours, 5 to 7 days/week, which often results in poor compliance.Citation6 The 3-times daily chelator deferiprone (Ferriprox®) was the first oral chelator to reach market and it is currently available in a number of countries outside the USA and Canada for the second-line treatment of iron overload in adult patients with thalassemia major for whom DFO therapy is contraindicated or inadequate.Citation7 The use of deferiprone has partly been restricted due to the occurrence of serious adverse events such as arthropathy (common), neutropenia and agranulocytosis (rare).Citation8,Citation9
Deferasirox (Exjade®), which became available in 2005, is an oral iron chelator that requires once-daily administration. It is currently approved in more than 90 countries for the treatment of chronic iron overload due to blood transfusions in pediatric and adult patients.Citation10 Deferasirox has been evaluated in patients with a wide range of underlying anemias and a wealth of clinical data is now available. This review will provide an update on the use of deferasirox, primarily focusing on the recent long-term efficacy and safety data available from the extension studies, new analyses in patients with MDS, aplastic anemia (AA) and Diamond-Blackfan anemia (DBA), as well as updates on the effects of deferasirox on cardiac iron levels.
Pharmacodynamics and pharmacokinetics of deferasirox
Chemistry and pharmacodynamic properties
Deferasirox represents a new class of tridentate iron chelators, the N-substituted bis-hydroxyphenyl-triazoles.Citation11 As a tridentate iron chelator, two molecules are required to form a stable complex with each iron (Fe3+) atom; the active molecule in deferasirox (ICL670) is highly lipophilic and 99% protein bound. Deferasirox has a high affinity and selectivity for Fe3+, which is approximately 14 and 21 orders of magnitude greater than its affinity for copper or zinc, respectively, which minimizes the potential for depletion of these trace elements.Citation12 Animal models in several species, such as rats, gerbils and marmosets, demonstrated that deferasirox could efficiently and selectively mobilize iron from various tissues such as hepatocytes and cardiomyocytes, and could also promote iron excretion.Citation11,Citation13,Citation14 Deferasirox is primarily metabolized by glucuronidation, with subsequent biliary excretion, and the deferasirox–iron complex is excreted in the feces.Citation15
Pharmacokinetic properties
Deferasirox is rapidly absorbed with a median tmax of 1 to 2 hours and a mean elimination half-life of 7 to 16 hours.Citation16 The plasma concentration of deferasirox is proportional to the administered doseCitation17,Citation18 and plasma levels are maintained within the therapeutic range over 24 hours following once-daily administration.Citation16 Cmax, area-under-curve and half-life are similar in children (aged < 12 years) and adolescents (aged ≥ 12 years). Exposure to deferasirox is approximately 20% to 30% lower in children and adolescents than in adults,Citation17 and is significantly lower in pediatric patients aged <6 years compared with older pediatric patients. These factors may add an additional margin for tolerability and mean that pediatric patients require higher deferasirox doses than adults to achieve comparable efficacy. As the bioavailability of deferasirox is affected by food when consumed concomitantly, it is recommended that it is administered at least 30 minutes before eating.Citation19
Efficacy of deferasirox
Introduction to deferasirox clinical trials
The clinical efficacy of deferasirox has been thoroughly evaluated in patients with a wide range of transfusion-dependent anemias, including β-thalassemia, MDS, sickle cell disease (SCD), AA, DBA and other rare anemias. Five pivotal 1-year core studies were conducted in more than 1000 patients,Citation16,Citation20–Citation23 while more than 900 patients were enrolled into subsequent extension studies and will receive treatment with deferasirox for up to a further 4 years. A wealth of data are also now available from a global program of additional studies, the largest of which include ESCALATOR (237 patients with β-thalassemia)Citation24,Citation25 and EPIC (1744 patients with various underlying anemias)Citation26 ().
Table 1 Trial design and key efficacy results from the ESCALATOR and EPIC studies
Factors impacting on deferasirox efficacy
The pivotal 1-year clinical studies demonstrated that the efficacy of deferasirox is dependent on both dose and ongoing transfusional iron intake.Citation16,Citation20–Citation23,Citation27 Although doses of 5 and 10 mg/kg/day are able to effectively remove iron they are generally insufficient to balance the iron uptake from regular blood transfusions. Doses of 20 to 30 mg/kg/day are able to maintain or reduce body iron burden depending on iron intake.
For most patients the recommended starting dose for deferasirox is 20 mg/kg/day,Citation18 however this dose can be modified based on iron intake, current iron burden and a patient’s individual therapeutic goal (ie, whether the aim is to decrease or maintain body iron levels);Citation10 local prescribing information should be consulted. If necessary, deferasirox dose adjustments should be made in steps of 5 to 10 mg/kg/day every 3 to 6 months based on serum ferritin trends. Data from the ESCALATOR study suggested that a starting dose of 20 mg/kg/day is insufficient to decrease iron burden in heavily iron-overloaded patients, since dose increases above 20 mg/kg/day were required in 185/237 patients (78%) in the initial 1-year treatment period.Citation24 Further dose increases were performed in 137/233 patients (59%) in the extension study, with increases to >30 mg/kg/day necessary in 112 patients; significant improvements in liver iron concentration (LIC) and serum ferritin were observed following these dose increases ().Citation25 The importance of timely and appropriate deferasirox dose adjustments to enable patients to achieve target serum ferritin levels has also been highlighted in the extension phases of the pivotal studies. Patients who initially received deferasirox 5/10 mg/kg/day had increases in serum ferritin during the core 1-year treatment period, however a gradual decline in iron burden to below baseline levels was observed once doses were subsequently increased.Citation28,Citation29 shows that doses of >20 mg/kg/day were necessary before patients were able to achieve a significant reduction in serum ferritin.Citation28
Figure 1 Mean liver iron concentration (LIC) and median serum ferritin at 1 year and end of study. Reproduced with permission. Taher A, El-Beshlawy A, Elalfy M, et al. Haematologica. 2009;94(Suppl 2):abstr 209.Citation25 Obtained from Haematologica/the Hematology Journal website http://www.haematologica.org with kind permission of the Ferrata Storti foudation, Pavia, Italy.
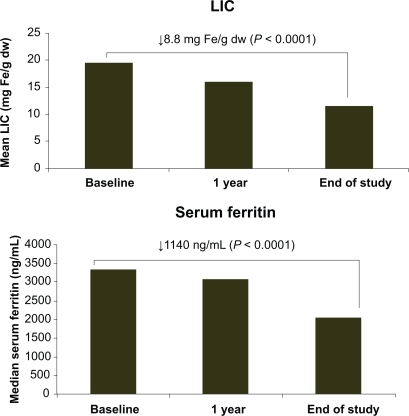
Figure 2 Mean dose and median serum ferritin during long-term deferasirox treatment in patients with β-thalassemia.Citation28
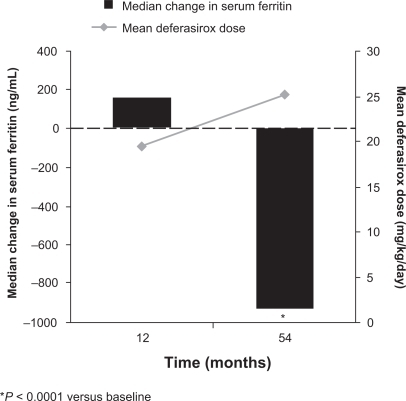
The dosing approach used in the EPIC study accounted for iron intake as patients received initial deferasirox doses of 10 to 30 mg/kg/day depending on transfusion history, followed by dose titration in steps of 5 to 10 mg/kg/day every 3 months according to serum ferritin trends and safety markers.Citation26,Citation27 Changes in serum ferritin over the 1-year treatment period were reflective of dosage adjustments and mean iron intake ().Citation26 Significant decreases in serum ferritin were observed in each overall disease cohort ().Citation30–Citation34 These data from the EPIC study show that it is important to regularly monitor transfusional iron intake and serum ferritin levels during deferasirox therapy in order to optimize dosing.
Figure 3 Median change from baseline in serum ferritin in patients enrolled in the EPIC study.Citation26
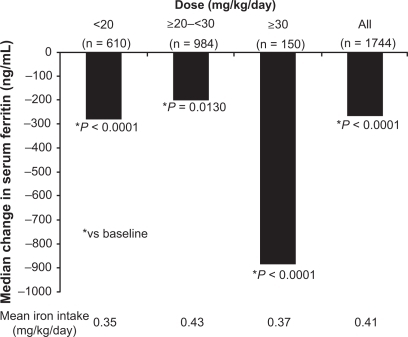
Table 2 Efficacy of deferasirox across various transfusion-dependent anemias: data from the EPIC trial
Some patients will require escalation to >30 mg/kg/day to achieve therapeutic goals. A retrospective analysis of 228 patients who received deferasirox doses of >30 mg/kg/day in the extension studies and ESCALATOR demonstrated a significant reduction in median serum ferritin of 370 ng/mL (P < 0.001) from pre-dose-escalation to the time-of-analysis.Citation35 These findings have important implications for patients who are heavily transfused and may require higher doses of deferasirox to reduce body iron burden.
Efficacy across different underlying anemias
β-thalassemia major
As complications of iron overload have been most widely studied in β-thalassemia major, this population was the primary focus of the deferasirox clinical trial program. The efficacy of deferasirox in this population was clearly demonstrated in the pivotal 1-year Phase III study (n = 296), where doses of 20 or 30 mg/kg/day provided dose-dependent changes in LIC and serum ferritin.Citation20 These data were confirmed in several trials including EPIC, where 937 patients with β-thalassemia major were enrolled and where a significant decrease in serum ferritin was observed after 1 year of deferasirox treatment ().Citation30 In the ESCALATOR study, the proportion of β-thalassemia major patients with a LIC of <7 mg Fe/g dry weight (dw) increased from 9.9% at baseline, to 26.2% at 1 year and 44.4% at the end of study (median of 2.7 years).Citation25 With appropriate dose adjustments, deferasirox continues to be effective over the long term in patients with β-thalassemia major, a finding that is supported by data from the extension studies over a median treatment period of 4.5 years ().Citation28
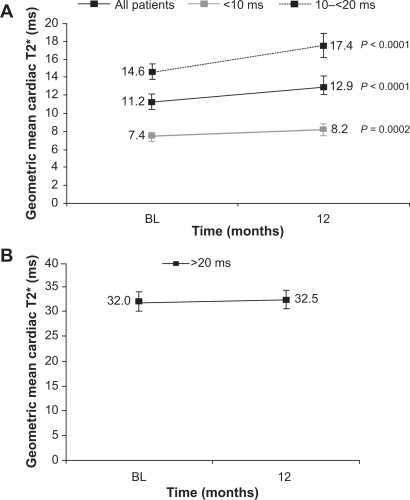
Cardiac failure related to myocardial iron overload is the leading cause of death in regularly transfused patients with β-thalassemia major.Citation4,Citation36 Evidence for the ability of deferasirox to remove cardiac iron in patients with β-thalassemia major has been demonstrated in a number of studies.Citation37–Citation41 The largest of these was a substudy of EPIC, where deferasirox significantly improved myocardial T2* in patients with mild, moderate and severe myocardial siderosis (11.2 ms at baseline to 12.9 ms at 12 months; P < 0.0001) ().Citation41 Left ventricular ejection fraction (LVEF) is a useful surrogate marker of cardiac function and is often used when evaluating the cardiac efficacy of an iron chelator. It was evaluated in the EPIC and ESCALATOR trials, which enrolled patients with baseline LVEF within the reference range for healthy adults (ie, ≥56%). LVEF, as measured by echocardiogram or cardiovascular magnetic resonance, was maintained at approximately 67% in the EPIC substudy,Citation41 and improved significantly from 65.1% to 66.8% (P = 0.0002) in the 1-year core ESCALATOR study.Citation24 As well as removing iron from the heart, deferasirox may also prevent the accumulation of cardiac iron in iron-overloaded patients with normal cardiac iron levels ().Citation42 This ability may potentially help in preventing future cardiac failure associated with myocardial siderosis in patients with β-thalassemia major.
Figure 4 The effect of deferasirox on the mean cardiac T2* by baseline T2* in patients A) with cardiac iron overload; B) with normal cardiac levels.Citation41,Citation42
β-thalassemia intermedia
Although patients with β-thalassemia intermedia are rarely transfused they are still at risk for developing iron overload due to increased intestinal iron absorption secondary to chronic anemia. Few studies with chelation therapy have been conducted in this population to date. However, data are emerging from small numbers of patients demonstrating that deferasirox can significantly decrease mean serum ferritin levels over 1 year of treatment (1356 to 914 ng/mL, P < 0.05;Citation43 2030 to 1165 ng/mL, P = 0.02,Citation44 at doses of 10 to 20 mg/kg/day, respectively). A large prospective study is underway to fully evaluate the use of deferasirox in patients with thalassemia intermedia.
Myelodysplastic syndromes
Several studies have demonstrated the efficacy of deferasirox in maintaining or reducing body iron burden in patients with MDS.Citation22,Citation31,Citation45–Citation48 As in the β-thalassemia population, deferasirox has dose-dependent efficacy in patients with MDS.Citation22 The largest cohort of MDS patients (n = 341) ever assessed with iron chelation therapy were enrolled in the EPIC study; with appropriate dose adjustments, significant reductions in serum ferritin were noted after 1 year of deferasirox treatment ().Citation26,Citation31 Another large study in 176 heavily iron-overloaded MDS patients also showed decreases in serum ferritin.Citation46 Preliminary data suggest that in addition to decreases in serum ferritin, improvements in hematological parameters can occur with deferasirox treatment in patients with MDS.Citation49 Serum ferritin decreases during deferasirox treatment in patients with MDS have been shown to be associated with significant improvements in alanine aminotransferase (ALT), which is an indicator of hepatocellular injury.Citation47,Citation50 This is of importance given that liver dysfunction is a common complication in MDS;Citation51,Citation52 prospective studies are warranted to further investigate this observation. MDS patients who are most likely to benefit from receiving chelation therapy are transfusion-dependent patients with lower-risk MDS (ie, Low or Int-1 International Prognostic Scoring System) and life expectancy >1 year, who have serum ferritin levels of >1000 ng/mL.Citation53,Citation54
Sickle cell disease
Deferasirox has also demonstrated dose-dependent efficacy in the SCD population.Citation23 In one Phase II study in adult and pediatric patients, LIC was significantly reduced from baseline after 1 year of deferasirox treatment (P < 0.05).Citation23 Serum ferritin levels were also decreased, although there was substantial intrapatient variability. Deferasirox doses of 10 to 30 mg/kg/day were shown to have similar efficacy to DFO. Of 195 patients enrolled in this 1-year study, 132 entered the ongoing extension phase. To date, these patients have received deferasirox for a median treatment period of 3.1 years and have shown continued reduction in serum ferritin levels (−651 ng/mL from baseline; P = 0.0533).Citation55 Further evidence for the efficacy of deferasirox in SCD is emerging from smaller studies that have demonstrated substantial reductions in serum ferritin and liver iron levels over 1 year.Citation56,Citation57
Aplastic anemia, Diamond-Blackfan anemia, and other rare anemias
Patients with AA, DBA and other types of rare anemia often require blood transfusions as supportive therapy; however, the efficacy of chelation therapy has rarely been evaluated in these populations. A 1-year prospective Phase II trial with deferasirox included 30 patients with DBA and 22 patients with other rare anemias (including AA, α-thalassemia and sideroblastic anemia).Citation22 Significant, dose-dependent effects on LIC and serum ferritin were observed in both groups over the 1-year treatment period.Citation22,Citation58,Citation59 Patients with AA (n = 116), DBA (n = 14) and rare anemias (n = 43) were also enrolled in the EPIC study and significant overall reductions in serum ferritin from baseline to 1 year were observed in each of the disease groups ().Citation32–Citation34 As with the overall EPIC population, changes in serum ferritin were reflective of dose adjustments and iron intake during the study. A similar observation has been made based on interim data from 50 patients with AA in Korea, where significant reductions in mean LIC (P = 0.01) and serum ferritin (4185 to 2913 ng/mL, P < 0.01) were noted over the 1-year treatment period.Citation48
Pediatric patients
The deferasirox clinical trial program included patients with a wide range of ages. Pediatric patients (aged 2 to 16 years) were well represented, comprising approximately 40% of all patients enrolled in the five pivotal studies. When used at appropriate doses for the degree of iron burden and the ongoing transfusional iron intake, deferasirox provides dose-dependent efficacy in pediatric patients for up to 5 years of treatment;Citation29,Citation60 these effects are similar to those observed in adult patients.Citation28 Efficacy data have been confirmed by the large number of pediatric patients (n = 166) enrolled in the ESCALATOR study.Citation24 In this heavily iron-overloaded pediatric population, significant decreases in mean LIC (−7.9 ± 8.7 mg Fe/g dw; P < 0.0001) and median serum ferritin (−1126 ng/mL; P < 0.0001) were observed after a median of 2.8 years’ treatment.Citation61
Effect of deferasirox on labile plasma Iron
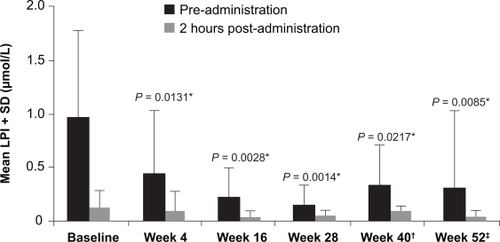
Excess iron in the blood saturates the iron-binding protein transferrin leading to non-transferrin-bound iron (NTBI) in the plasma. The cellular uptake of NTBI is uncontrolled, which can potentially lead to excessive accumulation of labile iron in tissues such as the heart, liver and endocrine system. This labile iron may be a key mediator of iron toxicity due to its ability to catalyze production of reactive oxygen species.Citation62 Control of labile plasma iron (LPI) is therefore an important aim of chelation therapy. In the ESCALATOR study in patients with β-thalassemia, LPI levels were analyzed using an assay that measured iron-specific redox cycling capacity in the presence of low ascorbate concentrations.Citation63 Redox reactions were detected by the oxidation of a fluorogenic probe to its fluorescent form, which allowed distinction of chelator-bound from chelator-free LPI. Data from this study demonstrated that daily trough levels of deferasirox are sufficient to maintain suppression of LPI ().Citation64 After 4 weeks of treatment and throughout the remainder of the 1-year treatment period, peak LPI levels observed just before deferasirox dosing were significantly decreased compared with baseline and remained within normal values. Similar sustained reductions in LPI with deferasirox have been observed in other disease cohorts, including MDS and AA.Citation46,Citation65,Citation66 These findings support the concept that once-daily deferasirox therapy may decrease unregulated tissue iron loading and prevent further end-organ damage across various transfusion-dependent anemias.
Figure 5 Mean labile plasma iron (LPI) taken pre-administration and 2 hours post-administration of deferasirox reproduced with permission. Daar S, Pathare A, Nick H, et al. Reduction in labile plasma iron during treatment with deferasirox, a once-daily oral iron chelator, in heavily iron-overloaded patients with β-thalassaemia. Eur J Haematol. 2009;82(5):454 457.Citation64 © Wiley-Blackwell 2009.
*Versus pre-administration at baseline.
LPI data are from 13 patients except †n = 11 ‡n = 12 due to lost samples.
Safety and tolerability of deferasirox
Deferasirox has a well characterized and manageable safety profile in adult and pediatric patients as young as 2 years with various transfusion-dependent anemias.Citation20–Citation23 Most patients remain on deferasirox therapy and adverse events are not a common reason for study discontinuation; eg, only 74 of >1000 patients enrolled in the core clinical studies discontinued treatment with deferasirox due to the occurrence of adverse events.Citation16,Citation20–Citation23 The most common drug-related adverse events reported during deferasirox treatment include transient, mild-to-moderate gastrointestinal disturbances (such as nausea, vomiting, abdominal pain and diarrhea) and skin rash; these events generally resolved spontaneously. For severe skin rash deferasirox should be interrupted until the rash has resolved; reintroduction at a lower dose with subsequent gradual escalation may then be considered in combination with a short period of oral steroid administration. Mild, non-progressive increases in serum creatinine, generally within the upper limit of normal (ULN), were observed in approximately one-third of patients in the pivotal 1-year clinical trials.Citation16,Citation20–Citation23 Serum creatinine levels spontaneously returned to baseline in approximately two-thirds of patients who experienced these increases.Citation67 There were no cases of moderate-to-severe renal insufficiency or renal failure and no patients permanently discontinued treatment due to creatinine increases. If there is an increase in serum creatinine beyond the age-appropriate ULN, deferasirox should be interrupted until levels have returned to the normal range. Treatment may then be restarted at a lower dose with gradual escalation. In the 1-year studies elevations of liver transaminases were reported in about 2% of patients; these were not dependent on dose and most patients had elevated baseline levels. Elevations greater than 10 × ULN, suggestive of hepatitis, were uncommon (0.3%).Citation10 Following any unexplained, persistent, or progressive increases in serum transaminases, deferasirox treatment should be interrupted. Once the cause of the transaminase increases has been established or when levels have returned to normal, deferasirox may be restarted at a lower dose followed by gradual escalation.
The tolerability and safety profile of deferasirox in pediatric patients is similar to that observed in adults.Citation29 As growth retardation and hypogonadism remain significant clinical problems in pediatric patients with β-thalassemia, it is important to note that growth and sexual development proceed normally during deferasirox therapy.Citation24,Citation29 In the ESCALATOR study, growth in pediatric patients was assessed based on height standard deviation score (h-SDS).Citation68 At baseline, both boys and girls were initially smaller than the reference group across all ages (2 to <6, 6 to <12 and 12 to <16 years).Citation24 Over the 1-year study period, the observed growth as evaluated by change from baseline in h-SDS showed growth improvements (>0.5 SDS) in 18.1% of patients, worsening in growth in 9.4% of patients, and no change from baseline in 72.5% of patients. In this population, girls aged 12 to <16 years showed a notable improvement in growth, with 66.7% of this cohort exhibiting a net increase in h-SDS (25% percentile or first quartile = −0.06).Citation24
Long-term safety of deferasirox
Safety data with deferasirox has now been reported for up to 5 years of treatment ().Citation28,Citation60,Citation69 Based on 472 patients with β-thalassemia who received deferasirox for a median of 4.5 years, 50 (10.6%) discontinued due to adverse events. The types of drug-related adverse events reported in the extension studies were similar to those reported in the initial 1-year treatment period, and the annual frequency generally decreased from year to year, ranging from 0% to 2.3% in years 2 to 5.Citation28 In addition, there were no progressive increases in serum creatinine over longer-term deferasirox treatment.Citation28 The long-term safety profile of deferasirox in diseases other than β-thalassemia is also available. Deferasirox has a well characterized and manageable safety profile in SCD patients for up to 3.5 years of treatment and no progressive increases in serum creatinine were observed, demonstrating a favorable renal safety profile in these patients who are at risk of progressive renal disease.Citation69 In MDS patients the most common adverse events are gastrointestinal disturbances and skin rash, similar to that observed with β-thalassemia patients.Citation31
Table 3 Most common drug-related adverse events during a median 4.5-year treatment period with deferasirox in 472 patients with β-thalassemiaCitation28Table Footnote*
Drug interactions and post-marketing surveillance
Deferasirox inhibits CYP3A4 and CYP2C8 in vitro, therefore caution should be used when administering deferasirox with drugs metabolized by these enzymes. Deferasirox also inhibits CYP1A2, CYP2A6, CYP2D6, and CYP2C19, however the clinical significance of this is unknown. The concomitant use of deferasirox with potent UDP-glucuronosyltransferase inducers (eg, rifampicin, phenytoin, phenobarbital, ritonavir) may result in a decrease in deferasirox efficacy. The concomitant administration of deferasirox with other iron chelators, aluminum-containing antacid preparations, vitamin C or hydroxyurea has not been formally studied. There have been post-marketing reports of cytopenias (both spontaneous and from clinical trials) in patients treated with deferasirox, although all of these patients had either pre-existing hematologic disorders that are commonly associated with bone-marrow failure or complications of the underlying disease that are associated with cytopenias (eg, hypersplenism, sickle cell crisis, administration of chemotherapy). The relationship of these episodes to treatment with deferasirox is uncertain. As with the standard clinical management of such hematological disorders, blood counts should be monitored regularly. Post-marketing cases of acute renal failure, some with a fatal outcome, have also been reported. Most of the fatalities occurred in patients with severe complications related to the underlying disease (eg, patients with multiple co-morbidities who were in advanced stages of disease). It is therefore recommended that particular attention is given to monitoring serum creatinine levels in patients who have preexisting renal conditions, are elderly, have co-morbid conditions that may affect renal function, or are receiving medicinal products that depress renal function. There have also been post-marketing reports of hepatic failure, some with a fatal outcome, in patients treated with deferasirox. Most of these events occurred in patients with significant comorbidities, including liver cirrhosis and multi-organ failure. In addition, upper gastrointestinal ulceration and hemorrhage have been reported in some patients, including children and adolescents, receiving deferasirox. Physicians and patients should remain alert for signs and symptoms of these events and promptly initiate additional evaluation and treatment if a serious gastrointestinal adverse event is suspected.
Safety at low iron burden and high deferasirox doses
Maintaining serum ferritin levels below 1000 ng/mL is known to be associated with a reduced risk of iron-overload-related complications, such as heart failure, in patients with thalassemia.Citation5 However, as the use of DFO has been associated with increased toxicity at low serum ferritin levels, it is of interest to assess the adverse event profile of patients enrolled in the deferasirox studies who achieved serum ferritin levels of <1000 ng/mL.Citation5,Citation70,Citation71 An analysis of 174 patients demonstrated a similar safety profile to that observed in patients who did not achieve serum ferritin levels of <1000 ng/mL (n = 300).Citation72 For example, the frequency of drug-related adverse events such as nausea (14.9% vs 12.7%), vomiting (8.0% vs 8.3%) and skin rash (5.2% vs 5.3%), were comparable. In addition, similar proportions of patients experienced increases in serum creatinine >33% above baseline and ULN (14.9% vs 12.0%) and increases in ALT > 10 × ULN (6.9% vs 6.7%). These data suggest that when appropriately dosed, serum ferritin can be maintained at levels lower than 1000 ng/mL during deferasirox treatment without increases in the frequency or type of adverse event reported.Citation73
Data from patients who have received deferasirox doses of >30 mg/kg/day demonstrate that the safety profile is consistent with that observed at doses of <30 mg/kg/day.Citation35 The most common drug-related adverse events were gastrointestinal events such as vomiting (n = 7, 3.1%), abdominal pain and nausea (n = 4, 1.8% for both).Citation35
Health economics of deferasirox
It is well established that compliance is a key factor determining outcome of iron chelation therapy,Citation3,Citation74 and the route of administration has an impact on compliance. Due to the need for 8- to 12-hour infusions, 5 to 7 times per week, compliance with DFO is suboptimal, ranging from 59% to 78%.Citation6 Poor compliance with treatment can lead to serious morbidities and have a significant impact on cost, for example complications of iron overload such as heart disease or diabetes may increase annual costs by US$40,000.Citation75 As deferasirox is a once-daily oral treatment, improvements in compliance compared with DFO may lead to improved patient outcomes and lower treatment costs. These have been evaluated in a number of analyses.
Cost effectiveness of deferasirox
Two separate studies have compared the cost-effectiveness of DFO and deferasirox.Citation76,Citation77 In a US model, treatment with deferasirox resulted in an additional 4.5 quality adjusted life years (QALYs) per patient at an additional lifetime cost of US$126,018 per patient, translating into a cost-effectiveness ratio of US$28,255 per QALY gained.Citation76 Deferasirox was also associated with lower net costs and higher QALYs than DFO in a UK-based model.Citation77 In this analysis drug dose and cost was relayed to patient weight; for patients with a mean weight of 62 kg the incremental cost per QALY gained was £7775.Citation77
Effect of deferasirox on patient-reported outcomes
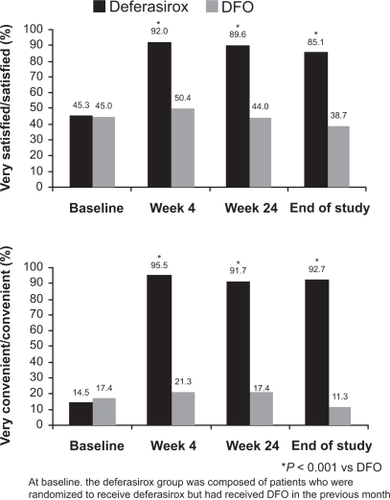
A number of deferasirox studies have evaluated patient-reported satisfaction, convenience and treatment preference, which may provide surrogate measures for treatment compliance, in patients with β-thalassemia, SCD and MDS.Citation78–Citation81 In one study, 97% of patients with β-thalassemia who switched from DFO to deferasirox reported that they preferred deferasirox to DFO, primarily due to convenience (37%), no injection-site soreness (25%) and less daily disruption (23%).Citation78 Most patients were more satisfied with deferasirox therapy and found it to be more convenient than DFO ().Citation78 Similarly, another study found that 91% of 252 patients with β-thalassemia were ‘satisfied/very satisfied’ with treatment after 1 year compared with 23% at baseline (all patients had received DFO and/or deferiprone prior to initiation of deferasirox).Citation80 Time lost for normal activities due to chelation therapy was substantially reduced with deferasirox treatment compared with prior treatments (from 28.8 hours/month at baseline to 3.0 hours/month after 1 year).Citation80 Similar results have been observed in patients with SCD.Citation81 Improvements in health-related quality of life, based on SF-36 domain scores (eg, physical functioning and general health), were observed during 1 year of deferasirox treatment in patients with β-thalassemia (n = 274), MDS (n = 168) and SCD (n = 50) who were enrolled in the EPIC study.Citation82
Figure 6 Overall treatment satisfaction with iron chelation therapy in patients previously treated with deferoxamine (DFO). Reprinted from Clinical Therapeutics. Cappellini MD, Bejaoui M, Agaoglu L, et al. Prospective evaluation of patient-reported outcomes during treatment with deferasirox or deferoxamine for iron overload in patients with β-thalassemia. Clin Ther. 2007;29(5):909 917Citation78 with permission from Excerpta Medica, Inc.
Conclusions and place in therapy
This article demonstrates the efficacy and safety profile of the oral iron chelator deferasirox, which has specific advantages over the other available chelators due to its formulation and route of administration. The large clinical development program including patients with various underlying anemias has demonstrated the ability of deferasirox to remove iron from the liver and heart. The efficacy of deferasirox is dependent on appropriate dosing according to current iron burden, ongoing transfusional iron intake and safety markers,Citation27,Citation30 and regular monitoring of these parameters is necessary to ensure dose adjustments are made in a timely manner.Citation10 Recent data from a substudy of EPIC have confirmed the findings of previous smaller studies demonstrating that deferasirox can remove cardiac iron and maintain cardiac function based on LVEF.Citation39,Citation41,Citation42 Data from this substudy also show that deferasirox can prevent the accumulation of cardiac iron, highlighting the importance of early intervention to aid in the prevention of future cardiac events resulting from myocardial siderosis.Citation83–Citation85 Data from several studies of up to 5 years’ duration provide support for the long-term efficacy of deferasirox in maintaining or reducing overall iron burden across a range of anemias.Citation28,Citation55 Deferasirox is well tolerated with a manageable safety profile over long-term treatment in both pediatric and adult patients. The frequency of treatment-related adverse events generally decreases over time; the most common adverse events reported are mild-to-moderate gastrointestinal disorders and skin rash.Citation28,Citation29,Citation35 The safety profile is similar at doses above and below 30 mg/kg/day and at serum ferritin levels above and below 1000 ng/mL.Citation72 Improved satisfaction with, and convenience of, deferasirox compared with DFO has also been shown,Citation78–Citation81 which may translate into improved compliance and subsequent cost savings. In conclusion, once-daily oral therapy with deferasirox provides a significant development in the treatment of iron overload in patients with transfusion-dependent anemias.
Acknowledgements
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Andrew Jones, PhD for medical editorial assistance with this manuscript.
Disclosures
Ali Taher has received research grants and honoraria from Novartis Pharmaceuticals. Maria Domenica Cappellini has received honoraria from Novartis Pharmaceuticals, and is a member of their Speaker’s Bureau.
References
- PorterJBPractical management of iron overloadBr J Haematol2001115223925211703317
- PootrakulPKitcharoenKYansukonPThe effect of erythroid hyperplasia on iron balanceBlood1988714112411293355891
- GabuttiVPigaAResults of long-term iron-chelating therapyActa Haematol199695126368604584
- OlivieriNFNathanDGMacMillanJHSurvival in medically treated patients with homozygous β-thalassemiaN Engl J Med199433195745788047081
- OlivieriNFBrittenhamGMIron-chelating therapy and the treatment of thalassemiaBlood19978937397619028304
- DeleaTEEdelsbergJSofryginOConsequences and costs of non compliance with iron chelation therapy in patients with transfusion-dependent thalassemia: a literature reviewTransfusion200747101919192917880620
- Ferriprox. Summary of Product Characteristics. Apotex; 1999
- CeciABaiardiPFelisiMThe safety and effectiveness of deferiprone in a large-scale, 3-year study in Italian patientsBr J Haematol2002118133033612100170
- CohenARGalanelloRPigaADe SanctisVTrictaFSafety and effectiveness of long-term therapy with the oral iron chelator deferiproneBlood200310251583158712763939
- Duffy-WarrenFChesiARigourdSFrederickAExjade® (deferasirox) Basic Prescribing InformationURL http://wwwexjadecom/indexjsp2008
- NickHAcklinPLattmannRDevelopment of tridentate iron chelators: from desferrithiocin to ICL670Curr Med Chem200310121065107612678677
- SteinhauserSHeinzUBartholomaMWeyhermüllerTNickHHegetschweilerKComplex formation of ICL670 and related ligands with FeIII and FeIIEur J Inorg Chem20042141774192
- GlicksteinHElBRLinkGAction of chelators in iron-loaded cardiac cells: accessibility to intracellular labile iron and functional consequencesBlood200610893195320316835377
- WoodJCOtto-DuesselMGonzalesIDeferasirox and deferiprone remove cardiac iron in the iron-overloaded gerbilTransl Res2006148527228017145573
- NickHWongAAcklinPICL670A: preclinical profileAdv Exp Med Biol200250918520312572995
- PigaAGalanelloRForniGLRandomized phase II trial of deferasirox (Exjade®, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overloadHaematologica200691787388016818273
- GalanelloRPigaAAlbertiDRouanMCBiglerHSechaudRSafety, tolerability, and pharmacokinetics of ICL670, a new orally active iron-chelating agent in patients with transfusion-dependent iron overload due to β-thalassemiaJ Clin Pharmacol200343656557212817519
- Nisbet-BrownEOlivieriNFGiardinaPJEffectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: a randomised, double-blind, placebo-controlled, dose-escalation trialLancet200310361(9369):1597160212747879
- GalanelloRPigaACappelliniMDEffect of food, type of food, and time of food intake on deferasirox bioavailability: recommendations for an optimal deferasirox administration regimenJ Clin Pharmacol200848442843518281442
- CappelliniMDCohenAPigaAA phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with β-thalassemiaBlood200610793455346216352812
- GalanelloRPigaAForniGLPhase II clinical evaluation of deferasirox, a once-daily oral chelating agent, in pediatric patients with β-thalassemia majorHaematologica200691101343135117018383
- PorterJGalanelloRSaglioGRelative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective studyEur J Haematol200880216817618028431
- VichinskyEOnyekwereOPorterJA randomized comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell diseaseBr J Haematol2007136350150817233848
- TaherAEl-BeshlawyAElalfyMSEfficacy and safety of deferasirox, an oral iron chelator, in heavily iron-overloaded patients with β-thalassaemia: the ESCALATOR studyEur J Haematol200982645846519187278
- TaherAEl-BeshlawyAElalfyMDeferasirox significantly reduces iron burden in heavily iron-overloaded patients with beta-thalassaemia: 2.7 year results from the ESCALATOR studyHaematologica200994Suppl 2abstr 209.
- CappelliniMDPorterJEl-BeshlawyATailoring iron chelation by iron intake and serum ferritin: Prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemiasHaematologica2009In press.
- CohenARGlimmEPorterJBEffect of transfusional iron intake on response to chelation therapy in β-thalassemia majorBlood2008111258358717951527
- CappelliniMDGalanelloRPigaAEfficacy and safety of deferasirox (Exjade®) with up to 4.5 years of treatment in patients with thalassemia major: a pooled analysisBlood200811211abstr 5411.
- PigaAForniGLKattamisADeferasirox (Exjade®) in pediatric patients with β-thalassemia: update of 4.7-year efficacy and safety from extension studiesBlood200811211abstr 3883.
- CappelliniMDElalfyMSKattamisAEfficacy and safety of once-daily, oral iron chelator deferasirox (Exjade®) in a large group of regularly transfused patients with β-thalassemia majorBlood200811211abstr 3878.
- GattermannNSchmidMDella PortaMEfficacy and safety of deferasirox (Exjade®) during 1 year of treatment in transfusion-dependent patients with myelodysplastic syndromes: results from EPIC trialBlood200811211abstr 633.
- LeeJWYoonSSShenZXIron chelation in regularly transfused patients with aplastic anemia: efficacy and safety results from the large deferasirox EPIC trialBlood200811211abstr 439.
- PorterJBLinKHHabrDDomokosGHmissiATheinSLDeferasirox efficacy and safety for the treatment of transfusion-dependent iron overload in patients with a range of rare anemiasBlood200811211abstr 1419.
- PorterJBForniGLBerisPEfficacy and safety of 1 year’s treatment with deferasirox (Exjade®): assessment of regularly transfused patients with Diamond-Blackfan anemia enrolled in the EPIC studyBlood200811211abstr 1048.
- TaherACappelliniMDVichinskyEEfficacy and safety of deferasirox doses of >30 mg/kg per d in patients with transfusion-dependent anaemia and iron overloadBr J Haematol2009[Epub ahead of print].
- Borgna-PignattiCRugolottoSDe StefanoPSurvival and complications in patients with thalassemia major treated with transfusion and deferoxamineHaematologica200489101187119315477202
- PathareATaherADaarSDeferasirox (Exjade®) significantly improves cardiac T2* in heavily iron-overloaded patients with beta-thalassemia majorAnn Hematol2009[Epub ahead of print].
- RoghiACassinerioEProtoPThe effect of deferasirox on myocardial iron overload and cardiac function: a prospective independent monocentric study using cardiovascular magnetic resonance T2* (MRI T2*)Haematologica200994Suppl 2abstr 762.
- WoodJThompsonAPaleyCDeferasirox reduces cardiac iron burden in chronically transfused β-thalassemia patients with mild-to-moderate cardiac siderosis as demonstrated by MRI T2*Haematologica200994Suppl 2abstr 763.
- EleftheriouPTannerMPennellDPorterJBResponse of myocardial T2* to oral deferasirox monotherapy for 1 year in 29 patients with transfusion-dependent anaemias; a subgroup analysisHaematologica200691Suppl 1abstr 999.
- PennellDPorterJBCappelliniMDEfficacy and safety of deferasirox (Exjade®) in reducing cardiac iron in patients with β-thalassemia major: results from the cardiac substudy of the EPIC trialBlood200811211abstr 3873.
- PennellDSutcharitchanPEl-BeshlawyAEfficacy and safety of deferasirox (Exjade®) in preventing cardiac iron overload in β-thalassemia patients with normal baseline cardiac iron: results from the cardiac substudy of the EPIC trialBlood200811211abstr 3874.
- LadisVBerdoussiHKattamisATreatment with deferasirox for non-transfusional iron overload in patients with thalassemia intermediaHaematologica200994Suppl 2abstr 1279.
- VoskaridouEKonstantinidouMDouskouMTreatment with deferasirox effectively decreases iron burden in patients with thalas-semia intermediaHaematologica200994Suppl 2abstr 204.
- GreenbergPLSchifferCKollerCAGlynosTPaleyCChange in liver iron concentration (LIC), serum ferritin (SF) and labile plasma iron (LPI) over 1 year of deferasirox (DFX/Exjade®) therapy in a cohort of myelodysplastic patientsBlood200811211abstr 5083.
- ListAFBaerMRSteensmaDIron chelation with deferasirox (Exjade®) improves iron burden in patients with myelodysplastic syndromes (MDS)Blood200811211abstr 634.
- WimazalFNosslingerTBaumgartnerCSperrWRPfeilstockerMValentPDeferasirox induces regression of iron overload in patients with myelodysplastic syndromesEur J Clin Invest20093940641119320908
- MinYHKimHJLeeKHA multi-center, open label study evaluating the efficacy of iron chelation therapy with deferasirox in transfusional iron overload patients with myelodysplastic syndromes or aplastic anemia using quantitative R2 MRIBlood200811211abstr 3649.
- MessaECilloniDMessaFDeferasirox treatment improved the hemoglobin level and decreased transfusion requirements in four patients with the myelodysplastic syndrome and primary myelofibrosisActa Haematol20081202707418827475
- GattermannNSchmidMGuerci-BreslerAReduction in serum ferritin (SF) is associated with improvement in liver transaminase levels during treatment with deferasirox (Exjade®) in iron overloaded patients with myelodysplastic syndromes (MDS)Leuk Res200933Suppl 1S140S141(abstr P140).
- DeleaTEHagiwaraMPhatakPDRetrospective study of the association between transfusion frequency and potential complications of iron overload in patients with myelodysplastic syndrome and other acquired hematopoietic disordersCurr Med Res Opin200925113914719210147
- SchaferAICheronRGDluhyRClinical consequences of acquired transfusional iron overload in adultsN Engl J Med198130463193246777701
- BennettJMConsensus statement on iron overload in myelodysplastic syndromesAm J Hematol20088385886118767130
- GattermannNPorterJLopesLFConsensus statement on iron overload in myelodysplastic syndromesHematol Oncol Clin North Am200519Suppl 11825
- VichinskyECoatesTThompsonAADeferasirox (Exjade®), the once-daily oral iron chelator, demonstrates safety and efficacy in patients with sickle cell disease (SCD): 3.5-year follow-upBlood200811211abstr 1420.
- CancadoROlivatoMCBrunieraPChiattoneCDeferasirox for the treatment of transfusional iron overload in sickle cell anemia: a 1-yr prospective studyHaematologica200994Suppl 2abstr 210.
- VoskaridouEDouskouMPlataETreatment with deferasirox effectively decreases iron burden in patients with sickle cell syndromesHaematologica200994Suppl 2abstr 215.
- RoseCGattermannNGlimmERabaultBDeferasirox (Exjade, ICL670), the novel, once-daily oral iron chelator, is well tolerated and effective in treating transfusional iron overload in patients with a range of rare anaemiasHaematologica200691Suppl 1abstr 22.
- TcherniaGVichinskyEJengMThe once-daily oral iron chelator ICL670 is well tolerated and effective in treating transfusional iron overload in Diamond-Blackfan anaemia patientsHaematologica200590Suppl 2192
- PigaAKebailiKGalanelloRCumulative efficacy and safety of 5-year deferasirox (Exjade®) treatment in pediatric patients with thalassemia major: a Phase II multicenter prospective trialBlood200811211abstr 5413.
- TaherAAl JefriAElalfyMSDeferasirox (Exjade®) treatment in pediatric β-thalassemia patients with high iron burden: 2.8 years results from ESCALATOR trialBlood200811211abstr 3879.
- KruszewskiMThe role of labile iron pool in cardiovascular diseasesActa Biochim Pol200451247148015218543
- EspositoBPBreuerWSirankaprachaPLabile plasma iron in iron overload: redox activity and susceptibility to chelationBlood20031022670267712805056
- DaarSPathareANickHReduction in labile plasma iron during treatment with deferasirox, a once-daily oral iron chelator, in heavily iron-overloaded patients with β-thalassaemiaEur J Haematol200982545445719191863
- PorterJBCappelliniMDEl-BeshlawyAEffect of deferasirox (Exjade®) on labile plasma iron levels in heavily iron-overloaded patients with transfusion-dependent anemias enrolled in the large-scale, prospective 1-year EPIC trialBlood200811211abstr 3881.
- GhotiHFibachEMerkelDDecrease in intra- and extra-cellular free iron species and oxidative stress parameters and increase in serum and urinary hepcidin during treatment with deferasirox in iron-loaded patients with MDSHaematologica200994Suppl 2abstr 797.
- BennettWPonticelliCPigaAKattamisAGlimmEFordJSummary of long-term renal safety data in transfused patients with secondary iron overload receiving deferasirox (Exjade®, ICL670)Blood200610811abstr 3816.
- KuczmarskiRJOgdenCLGrummer-StrawnLMCDC growth charts: United StatesAdv Data200031412711183293
- VichinskyECoatesTThompsonASafety and efficacy of iron chelation therapy with deferasirox in patients with sickle cell disease (SCD): 3.5-year follow-upHaematologica200994Suppl 2abstr 200.
- OlivieriNFBuncicJRChewEVisual and auditory neurotoxicity in patients receiving subcutaneous deferoxamine infusionsN Engl J Med1986314148698733485251
- PorterJBJaswonMSHuehnsEREastCAHazellJWDesferrioxamine ototoxicity: evaluation of risk factors in thalassaemic patients and guidelines for safe dosageBr J Haematol19897334034092605127
- PorterJBPigaACohenAFordJMBodnerJCappelliniMDAssessment of safety in patients receiving longer-term iron chelation therapy with deferasirox who had achieved serum ferritin levels of <1000 ng/mL during the study courseHaematologica200994Suppl 2abstr 199.
- PorterJBPigaACohenASafety of deferasirox (Exjade®) in patients with transfusion-dependent anemias and iron overload who achieve serum ferritin levels <1000 ng/mL during long-term treatmentBlood200811211abstr 5423.
- BrittenhamGMGriffithPMNienhuisAWEfficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia majorN Engl J Med199433195675738047080
- RenfroeJLForbesPBraunsteinJNeufeldEJRelationship of transfusion and iron-related complications to cost of care in thalassemiaBlood200510611abstr 2240.
- DeleaTESofryginOThomasSKBaladiJFPhatakPDCoatesTDCost effectiveness of once-daily oral chelation therapy with deferasirox versus infusional deferoxamine in transfusion-dependent thalassaemia patients. US healthcare system perspectivePharmacoeconomics200725432934217402805
- KarnonJTolleyKOyeeJJewittKOssaDAkehurstRCost-utility analysis of deferasirox compared to standard therapy with desferrioxamine for patients requiring iron chelation therapy in the United KingdomCurr Med Res Opin20082461609162118439348
- CappelliniMDBejaouiMAgaogluLProspective evaluation of patient-reported outcomes during treatment with deferasirox or deferoxamine for iron overload in patients with β-thalassemiaClin Ther200729590991717697909
- PorterJBBowdenDGanserASatisfaction and adherence significantly improves in patients with β-thalassemia and myelodysplastic syndromes treated with deferasirox (Exjade®)Blood200811211abstr 1306.
- TaherAAl JefriAElalfyMImproved treatment satisfaction and convenience with deferasirox in iron-overloaded patients with β-thalassemia: Results from ESCALATOR trialHaematologica200893Suppl 1abstr 799.
- VichinskyEPakbazZOnyekwereOPatient-reported outcomes of deferasirox (Exjade®, ICL670) versus deferoxamine in sickle cell disease patients with transfusional hemosiderosis: substudy of a randomized open-label Phase II trialActa Haematol2008119313314118408362
- PorterJBBowdenDGanserAImproved health-related quality of life in patients with hematological disorders receiving deferasirox (Exjade®)Blood200811211abstr 1307.
- AldouriMAWonkeBHoffbrandAVHigh incidence of cardiomyopathy in beta-thalassaemia patients receiving regular transfusion and iron chelation: reversal by intensified chelationActa Haematol19908431131172123060
- AndersonLJWestwoodMAHoldenSMyocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonanceBr J Haematol2004127334835515491298
- DavisBAPorterJBLong-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk β-thalassemiaBlood20009541229123610666195