Abstract
Hypertension occurs frequently among hemodialysis (HD) patients and can be due to many factors, such as salt intake, elevated sympathetic tone, and uremic toxins. It is responsible for the high cardiovascular risk associated with renal disease. Generally, in HD patients, while there is an elevation of systolic blood pressure (BP), diastolic BP seems to decrease, and the resultant effect is high pulse pressure, which can have a deleterious effect on the cardiovascular system. Although controversial, in the HD population the relationship between BP and risk of death seems to be U shaped, probably because of pre-existing cardiac disease in patients with the lowest BP. In chronic kidney disease, BP lower than 130/80 mmHg is recommended, but an appropriate target for BP in the HD population remains to be established. Moreover, there is no consensus regarding which routine peridialysis BP (pre- or post-dialysis BP, or both) can ensure the diagnosis of hypertension in this population. Ambulatory BP monitoring remains the gold standard to quantify the integrated BP load applied to the cardiovascular system. As well, home BP assessment could contribute to improve the definition of an optimal BP in the HD population. An ideal goal for post-dialysis systolic BP seems to be a value higher than 110 mmHg and lower than 150 mmHg. However, HD patients are generally old and often have cardiac complications, so a reasonable pre-dialysis target systolic BP could be 150 mmHg. It is prudent to suggest that an improvement in BP control is necessary in the HD population, first by slow and smooth removal of extracellular volume (dry weight) and thereafter by the use of appropriate antihypertensive medication.
Introduction
It is well recognized that arterial hypertension is a powerful predictor of cardiovascular disease, including stroke, coronary heart disease, and peripheral arterial disease (CitationMacMahon et al 1990). Its prevalence of 20% in the general population (CitationESH–ESC 2003) increases with age, but in hemodialysis (HD) patients and those with endstage renal disease (ESRD) the prevalence is much higher, reaching 70%–80% (office pre-dialysis blood pressure [BP]) (CitationCharra et al 2004; CitationKiss et al 2004), and is associated with a very high cardiovascular risk (CitationFoley et al 1998). This high rate of hypertension is already observed at earlier stages of chronic kidney disease and prior to dialysis (CitationLevin 2003). For the NHANES III (National Health and Nutrition Examination Survey), the prevalence strongly increases from a glomerular filtration rate lower than 60 mL/min (CitationNational Kidney Foundation 2002).
Moreover, uncontrolled hypertension in renal patients before ESRD has been identified as a major risk factor for damaging kidney function and also increasing the rate of cardiovascular complications. At the stage of dialysis, this hemodynamic abnormality can increase cardiovascular mortality by 3-fold (CitationLucas et al 2003). A high proportion of cardiovascular morbidity and mortality related to coronary artery disease and left ventricular hypertrophy occurs in patients with chronic renal disease. The increase in risk is partly determined by classical risk factors observed in the general population, such as increased age, hypertension, smoking, diabetes, and hyperlipidemia (CitationLevey and Eknoyan 1999). Pathogenesis of hypertension in patients with renal disease as in dialysis patients is multifactorial but may also vary according to the underlying renal disease () (CitationCampese 2000).
Table 1 Pathophysiological factors playing a role in high blood pressure in end-stage renal disease
Besides the classical risk factors, uremic specific risks such as anemia, hyperphosphatemia, and inflammation can also contribute to the very high cardiovascular mortality rates, accounting for about 50% of the death rate; this is approximately 20–30 times the risk observed in the general population (CitationKimura et al 1996; CitationFoley et al 1998; CitationHorl and Horl 2002; CitationLocatelli et al 2004). In summary, hypertension is a common occurrence in patients with uremia, and this is especially so in ESRD.
This review is devoted to hypertension and HD and attempts to address three important issues regarding BP in this population: (1) What is the target BP level associated with the lowest cardiovascular risk in HD patients? (2) What is the most appropriate procedure and timeline to measure BP in the particular population of patients submitted three times a week for dialysis treatment? (3) How can high BP best be managed in this population?
BP level and cardiovascular risk
In the general population a linear relationship has been demonstrated between elevation of BP and cardiovascular mortality (CitationMacMahon et al 1990; CitationProspective Studies Collaboration 2002), but in HD patients that association remains controversial (CitationSchömig et al 2001). A systematic review of the evidence does not readily reveal the so-called linear relationship between elevated BP and cardiovascular mortality in this population. Indeed, in HD patients, hypotension, frequently induced by fluid removal, could affect coronary perfusion. The associated decrease of BP during the session could involve a cardiovascular risk. CitationZager et al (1998) have observed a U-shaped relationship between post-dialysis systolic BP and cardiac and vascular (peripheral and cerebral) mortality in HD patients (n = 5433). A significant increase in cardiovascular risk was noted when systolic BP was either higher than 180 mmHg or below 110 mmHg. These cut-off points were also mentioned for pre-dialysis systolic BP by CitationPort et al (1999), studying HD patients (n = 4839) from the US Renal Data System. This differs from the classical J-shaped curve relating BP levels to cardiovascular mortality in patients with essential hypertension (CitationCruickshank 1988). However, the association between low BP and increasing mortality could be attributed either to the poor overall health status of patients (CitationZager et al 1998) or to cardiac failure as a consequence of the presence of long-term hypertension (CitationD'Amico and Locatelli 2002).
Evidence presented in a study by CitationMazzuchi et al (2000) seems to suggest that a distinction could be made between the early and late mortality among HD patients (n = 405) who survived at least 2 years. Thus a U-shaped curve in HD patients during the first year of treatment (early mortality) was confirmed, while in the longer term (ie, from the fifth year) the relationship between mortality and BP control appeared to show an increased risk only for the higher BP values (eg, systolic/diastolic BP > 160/90 mmHg). This has led to the suggestion that the association between hypotension and mortality in HD patients may be related not to cardiovascular causes but to other medical conditions (CitationMazzuchi et al 2000).
It is clear that specific optimal systolic and diastolic BP targets for HD patients are needed; however, the BP guidelines as set by the JNC 7 (Joint National Committee 7th Report) and European Society of Hypertension–European Society of Cardiology (ESH–ESC) (CitationChobanian et al 2003; CitationESH–ESC 2003) are probably not applicable for such patients (CitationLondon 2001). Moreover, establishing a precise definition of the lowest acceptable limit of BP, especially for pre-dialysis systolic BP (in older patients), would be of great clinical interest. In summary, although controversial, the relationship between BP and cardiovascular risk in the HD population appears to be characterized by a U-shaped curve, probably due to the presence of pre-existing cardiac disease or other severe medical conditions in patients with the lowest BP levels.
Which target BP should be used in HD?
The latest JNC 7 and ESH–ESC guidelines defined the optimal BP by systolic and diastolic levels lower than 120/80 mmHg and hypertension as levels of 140/90 mmHg or higher. Thus, in patients with hypertension the goal of treatment is to reduce BP to below 140/90 mmHg and even lower in patients with diabetes or renal disease (CitationChobanian 2003; CitationESH–ESC 2003). In these groups, a target lower than 130/80 mmHg has been proposed, which is in total agreement with the target BP set by the CitationNational Kidney Foundation (2004) guidelines. However, these guidelines are unfortunately not applicable to the HD population. Moreover, it is a fact that BP is not well controlled in hypertensive HD patients. For instance, in these patients, CitationAgarwal et al (2003) reported a rate of 70% of uncontrolled BP (pre-dialysis systolic BP > 150 or diastolic BP > 85mmHg). Furthermore, among this uncontrolled population 12% were untreated and 58% were inadequately treated (CitationAgarwal et al 2003).
The question is clearly: what is the ideal in BP management in HD patients? Surprisingly, there is no consensus as to normal BP limits in HD patients. Simply put, the basis for the lack of insight appears to be the difficultly in identifying the best BP measurement time in patients with HD, and the reason for the lack of this information is hemodynamic instability of these patients. To summarize, in chronic kidney disease, BP lower than 130/80 mmHg is recommended, but the optimal BP target in such patients is yet be fully established.
HD and high BP
Normally, patients undergo HD treatment three times a week; this results in alteration in extracellular fluid volume, and it is during these sessions that many patients experience large fluctuations in BP. In HD patients, the basis for the occurrence of high pre-dialysis BP can result from a number of factors: changes in extracellular volume (ie, expansion); low compliance due to restricted salt intake (CitationKhosla and Johnson 2004); sympathetic and renin–angiotensin over-activation (CitationBlankestijn 2004); retention of uremic toxins, which can cause vasoconstriction; accumulation of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase (CitationZoccali et al 2003); and other factors such as parathyroid hormone secretion, erythropoietin treatment, endothelial dysfunction, and obesity associated insulin resistance (CitationMailloux and Levey 1998; CitationCampese 2000).
It has also been noted that shortening of the dialysis session results in difficulty reaching the optimal dry weight (CitationFlanigan 2000; CitationPérez-Garcia et al 2001). This is believed to be related to insufficient removal of volume during the HD session, which seems to occur in spite of increasing sodium concentration in the dialysis bath. The resultant effect appears to be the maintenance of high extracellular volume and pre-dialysis BP (CitationCharra et al 2004; CitationKhosla and Johnson 2004). Achieving a lower dry weight, based on clinician best judgment, and lengthening of HD session time have proved beneficial in the management of hypertension without the use of antihypertensive medications. However, the decrease in BP by such an approach is not immediate, and it could take weeks or months before a stable reduction in BP is observed; thus, this has been labeled “the lag phenomenon” (CitationChazot et al 1999). It has been recently proposed that other types of dialysis procedure, such as slow but long duration dialysis (3 times 8 hours per week), short daily dialysis (2–3 hours daily, 6 times per week), or even nocturnal dialysis (6–7 overnight sessions per week), can improve the management of hypertension in HD patients (CitationKiss et al 2004). Reduction of dietary salt intake, fluid restriction, and decreasing sodium concentration in the dialysis bath have also proved effective and viable methods of lowering BP in HD patients (CitationKooman et al 2004).
It seems that during the dialysis session the removal of fluids results in a progressive decline in stroke volume and cardiac output and a concomitant increase in systemic vascular resistance (CitationDaugirdas 2001). These modifications are associated with a reduction of vascular compliance in response to dialysis which may also contribute to the increased cardiovascular risk (CitationGadegbeku et al 2003). However, one method of dialysis that may be beneficial in decreasing BP in patients is the use of biocompatible dialysis membranes. It has been reported that the use of this technique over a course of 6 weeks decreased the mean 24-hour BP in diabetic patients (CitationSchröder et al 2001). It was further postulated that one contributing factor for the antihypertensive effect in these patients was the removal of ADMA and changes in ADMA/arginine ratio resulting in “upregulation” of the nitric oxide/arginine pathway.
It is recognized that the relationship between BP and fluid removal during dialysis is influenced by the cardiac status of the patient. For example, lowering of an equal intradialytic plasma volume caused a more substantial decrease of BP in patients with cardiac failure when compared with those free of heart failure (CitationVan der Sande et al 1998). However, it must be also stated that the relationship between lowering of intradialytic plasma volume and changes in BP is a controversial topic. For instance, in a subset of patients from the HEMO Study, pre- and post-dialysis BP were differently influenced by acute decrease in weight (an indicator of interdialytic fluid gain) and plasma volume (an indicator of post-dialysis volume status) (CitationLeypoldt et al 2002). In this study, the pre- and post-dialysis BP were associated with larger intradialytic decreases in bodyweight but smaller intradialytic reductions in plasma volume. Each kilogram reduction in bodyweight during HD was associated with 2.95 and 1.65 mmHg higher pre-dialysis and post-dialysis systolic BP, respectively. In contrast, each 5% greater contraction of plasma volume during HD was associated with 1.5 and 2.56 mmHg lower pre-dialysis and post-dialysis systolic BP. It seems that weight and plasma volume reductions were weak determinants of the peridialysis BP. This suggests that other factors most likely contribute to the control of BP in HD patients, such as effective dry weight and cardiac status (CitationLeypoldt et al 2002).
Another problem in the HD population is sleep apnea, which independently increases the prevalence of systemic hypertension as well as cardiac and vascular (cerebral and peripheral) diseases (CitationZoccali et al 2001). This problem seems to occur in 2%–4% of the general population (CitationSharabi et al 2004) but is more commonly observed in obese individuals and diabetic patients, while in ESRD patients the prevalence exceeds 50% (CitationZoccali et al 2001). It appears that pathophysiology of sleep apnea could be more linked to uremia itself rather than to the dialysis treatment or the mode of dialysis (CitationHanly 2004).
In summary, hypertension in HD is influenced by many factors associated with uremia but also by the amount of hydrosaline removal during the session, as well as cardiovascular system adaptability.
Assessment of BP profile in HD patients
HD patients are more characterized by isolated systolic hypertension than by elevations in both systolic and diastolic BP, which seem to occur in fewer than 20% of that population (CitationMailloux and Haley 1998). Numerous observational epidemiological studies and randomized, controlled trials have demonstrated that elevated systolic BP is an independent and powerful predictor of the high incidence of cardiovascular and renal diseases (CitationHe and Whelton 1999).
Systolic hypertension is mainly due to a decrease in arterial compliance and is a characteristic of aging in the non-uremic population (CitationFarsang and Sleight 2001; CitationFranklin 2005). ESRD patients seem thus to have an accelerated vascular aging process, especially in the large arteries (CitationLondon et al 1990; CitationAgarwal 2003). When assessed by the pulse wave velocity measurement, increased aortic stiffness has been considered as a strong determinant of all-cause and cardiovascular mortality in ESRD patients (CitationBlacher et al 1999). At any mean arterial pressure level, most HD patients have higher systolic BP and lower diastolic BP than control subjects with normal renal function (CitationAgarwal 2003).
Thus, the majority of HD patients exhibit high pulse pressure, which has also been prospectively identified as a predictor of cardiovascular and total mortality (CitationAmar et al 2000; CitationSafar et al 2002). CitationKlassen and colleagues (2002) have suggested that even an elevation of 10 mmHg in pulse pressure is associated with a 12% increase in risk of death. A similar view has been expressed by CitationZoccali (2003), who revealed that patients with high pulse pressure (>70 mmHg) had a risk of cardiovascular events more than twice that of patients with lower pulse pressure. Therefore, it seems that pulse pressure plays a more important role in cardiovascular risk in uremic patients than in the general population (CitationPastor-Barriuso et al 2003). However, in essential hypertension with preserved normal renal function, cardiovascular mortality after adjustment for confounding variables also increased with higher aortic pulse wave velocity as noted in the uremic population (CitationAnderson et al 1991). The increasing evidence that raised pulse pressure and arterial stiffening are independent predictors of cardiovascular mortality in hypertensive and ESRD patients has brought new challenges in pharmacotherapy of this population of patients (CitationVan Bortel et al 2001).
It must be recognized that controlling BP in HD is not an easy task, given that the extracellular volume modifications during and between the dialysis sessions can have a considerable and unpredictable effect on BP. So it would be of great interest to know which BP will be more representative of the true BP status applied to the cardiovascular system. On the basis of this background, CitationSantos et al (2003) randomly studied the BP pattern of HD patients (n = 71). This observation revealed that systolic and diastolic BP, as well as pulse pressure, increased during the interdialytic period (44-hour ambulatory BP monitoring; ABPM), but this was not related to the weight gain (hydrosaline accumulation) noticed during that period. Although hypertension (defined by average 44-hour BP ≥ 135/85 mmHg) was diagnosed in 55% of the patients while on antihypertensive medications, the Kt/V, hematocrit, or the weekly erythropoietin dose could not explain why BP remained uncontrolled. High rates of non-dipping (night BP fall <10% or night/day ratio >0.90) were observed in 77% on day 1 and in 83% on day 2, illustrating the lack of normal nocturnal BP fall pattern in the majority of HD patients (CitationSantos et al 2003).
To summarize, in HD patients, the systolic component of BP is increased while the diastolic seems to be decreased, resulting in high pulse pressure which appears to have a negative influence on the arterial tree. This characteristic, at the functional level, occurs as a result of the accelerated aging process. To this end, control of BP is also made difficult by the cyclical variations of volume status.
Peridialysis BP (pre- or post-dialysis): which predicts the best interdialytic level?
BP seems to be acutely influenced by the dialysis session, speed of volume removal, and counter-regulation response. It is pertinent to examine whether one should consider the pre-dialysis or post-dialysis BP, or both, as reliable predictors of interdialytic BP.
Several studies assessed the prediction that can be attributed to BP levels measured before or after the dialysis session (CitationFoley et al 2002; CitationMendes et al 2003). Post-dialysis rather than pre-dialysis BP was found to be independently related to increased mortality. This evidence confirmed the superiority of post-dialysis BP to predict mortality, although this relationship was found to be weak by others. In contrast, it has been proposed that the average of pre- and post-dialysis BP is a better representation of pre-dialysis BP (CitationMendes et al 2003). According to CitationMitra et al (1999), who compared conventional BP measurement, self-measured BP (Dinamap), and interdialytic BP (ABPM), the 20-minute post-dialysis BP reading was the best representative BP parameter of the average ambulatory interdialytic BP. It seems that pre-dialysis BP is indeed too much influenced by a white-coat phenomenon (CitationMitra et al 1999).
It appears that errors in BP measurements (accuracy of the devices, observer bias, patient behavior) coupled with the variability of BP patterns among individuals during dialysis could considerably limit the use of peridialysis BP (CitationSankaranarayanan et al 2004). However, that opinion is not shared by everyone. For instance, according to CitationZoccali (2003), pre-dialysis BP was a better predictor of left ventricular mass index than post-dialysis BP.
ABPM has been generally considered as the most accurate method for evaluating BP load in the general population as well as in HD patients (CitationPeixoto et al 2000). Moreover, it is a useful tool for effective management of antihypertensive therapy. One main advantage of using ABPM to monitor BP is the identification of altered nycthemeral BP rhythm, which seems prevalent in HD patients. Indeed, such diagnosis has been performed in some mild renal disease patients with near-normal renal function (CitationValero et al 1999). A blunted rhythm is common among HD patients (eg, in 55%–75%) (CitationCovic and Goldsmith 1999; CitationChughtai and Peixoto 2003). Evidently, a complete reversal of diurnal rhythm has been observed in fewer than 10% (CitationRedon 1998). One explanation that has been offered to account for this reversal is over-activation of the sympathetic nervous system during sleep in response to hypoxemia, an occurrence that has been observed in the non-renal disease, non-dipper population (CitationArita et al 1996). Other significant factors that have been suggested to affect BP pattern are decreased arterial distensibility and erythropoietin therapy in treated hypertensive HD patients (CitationAmar et al 1997). Moreover, as already mentioned, a high proportion of HD patients seem to experience sleep apnea, an abnormality responsible for elevated nocturnal BP as described in obese individuals and patients with essential hypertension (CitationBarenbrock et al 1996).
While there is solid evidence to indicate that ABPM monitoring of BP is more reproducible than isolated measurement of BP at pre- or post-dialysis (CitationPeixoto et al 2000), it has also been shown that the reproducibility of ABPM recordings poorly documents nocturnal decreases in BP (CitationPeixoto et al 2000). However, the non-dipping phenomenon (CitationVerdecchia et al 1994) in stable HD patients has been prospectively related to a deterioration of left ventricular function as compared with that in patients who had a satisfactory nocturnal fall in BP (CitationCovic et al 1997). Non-dipping has been frequently associated to left ventricular hypertrophy (LVH) in essential hypertension but it is also observed in the absence of hypertension (CitationRedon et al 1999).
In a follow-up study of HD patients (n = 80), it was shown that non-dippers had an impaired circadian rhythm of autonomic function and a 9 times higher incidence than dippers of death due to cardiovascular failure (CitationLiu et al 2003). However, when results were adjusted for age, sex, and history of cardiovascular disease, it was suggested that night-time systolic BP more than dipper status was associated with cardiovascular prognosis (CitationAmar et al 2000). Clearly, more clinical studies are needed in this area before this issue can be fully resolved. It is also clear that most observations have focused on the real importance of the BP curve analysis during the interdialytic period and not only on the pre- or post-dialysis BP levels in assessment of the relationship between BP and cardiovascular risk (CitationZoccali 2003). However, CitationZoccali (2003) observed that when left ventricular mass is considered as a surrogate end point, pre-dialysis systolic BP seems superior to post-dialysis BP and equally informative as 24-hour ABPM when the effects of “integrated” pressure load on the heart have to be assessed.
In summary, it is difficult to define hypertension in dialysis because of the variation of total bodyweight during repetitive dialysis sessions. There is no consensus regarding which peridialysis BP can best ensure the diagnosis of hypertension. ABPM adds important information on nocturnal BP pattern, but more studies are needed to integrate that technology into the assessment of cardiovascular risk in HD patients.
ABPM and hypertension criteria
As already mentioned, defining the true BP status of HD patients is quite difficult owing to a lack of agreement between BP measurements made just before and after a dialysis session. Indeed, it seems that routine BP measurement results in an overestimation of the real BP (CitationRahman et al 2002). In contrast, ABPM during the interdialytic period has proved to be superior to the classical sphygmomanometer method to assess the high prevalence (about 70%) of uncontrolled hypertension among HD patients, even those being treated with antihypertensive medication (CitationCovic and Goldsmith 2002; CitationAgarwal et al 2003; CitationCovic et al 2003). A criterion for the classification of hypertension in chronic HD patients has been proposed on the basis of comparisons between 44-hour ABPM interdialytic BP and conventional measurements, namely systolic/diastolic BP of >150/85 and >130/75 mmHg, for pre-dialysis and post-dialysis, respectively. This criterion seems to offer a very sensitive assessment of overall BP in these patients. However, pre- and post-dialysis BP measurements cannot be relied upon to predict an absolute level of mean ambulatory BP with confidence (CitationAgarwal and Lewis 2001; CitationAgarwal et al 2003). Needless to say, a more accurate assessment of target BP would be determined by prospectively linking BP measured by ABPM to cardiac function.
CitationAgarwal (2002) has critically assessed measurement of BP in HD patients. He suggested that a comparison and subsequent analysis for agreement between two methods of BP measurement; ie, ABPM and conventional BP measurement analysis should be performed in the HD unit. Indeed, there is evidence to indicate that determination of BP in the HD unit is not a reliable predictor of the interdialytic level and/or cardiac complications in these patients (CitationAgarwal 2002). The best correlation with left ventricular mass index seems to come from ABPM, especially the systolic BP load component, which appears to be an independent predictor of LVH. On the other hand, night-time ABPM was the best predictor of left ventricular posterior wall thickness (CitationErtürk et al 1996). When comparing ABPM with 2-week averaged peridialysis unit BP measurements in 70 patients, no close relationship between the two methods was found (CitationAgarwal and Lewis 2001). Indeed, pre-dialysis systolic BP measurement using conventional methods can overestimate or underestimate by 50 mmHg and 20 mmHg, respectively, when compared with ABPM (CitationAgarwal and Lewis 2001). Thus it seems that BP measurement in the HD unit can be considered only as a qualitative but not quantitative indicator of the control (or lack of control) of BP and that an accurate measurement can be guaranteed only by ABPM. However, this view is not universally accepted (CitationNystrom et al 2002).
To summarize, ABPM remains the best technique to calculate the integrated BP load applied to the cardiovascular system. However, an active debate persists on the subject of whether peridialytic BP measurement provides consistent information in these HD patients.
Home BP
The use of automatic devices to measure BP at home has become quite common. This approach appears to be beneficial and more effective than office BP measurements. In addition, the monitoring of BP at home is a cost-effective means that could help diagnosis of undiagnosed hypertension in dialysis patients (CitationAgarwal 1999).
However, there are likely to be substantial errors associated with the measurement of BP at home, although this may be less than with office measurements. In addition, this approach is interesting because it involves participation of patients in the management of their own BP measurements. There is evidence, at least in the non-renal disease hypertensive population, to indicate that this is both helpful and beneficial to patients in terms of compliance with medication (CitationYarows et al 2000). It may also provide better information for predicting cardiovascular risk (CitationBobrie et al 2004). Nevertheless, the evidence that such an approach is beneficial in the management of BP as well as cardiovascular risk assessment in HD patients is yet to be validated. In summary, controlled studies need to be designed and carried out to determine whether monitoring of BP at home in HD patients is of benefit.
Which BP thresholds to use for definition and control of hypertension in the HD population
To our knowledge there is no consensus on a definition of normal pre-dialysis BP. According to CitationRitz (2000) and CitationLocatelli et al (2004), a target pre-dialysis BP below 140/90 mmHg should perhaps be considered as normal in the HD population. This definition should be modified according to age (<60 or ≥60 years) as proposed by the British Renal Association (CitationThe Renal Association 1995). As the cardiovascular risk associated with systolic BP is minimized between 100 and 150 mmHg for pre- and post-dialysis (CitationZoccali et al 2002), it has been proposed that a reasonable treatment goal would be to stabilize the pressure at a maximum of 150 mmHg, while an attempt should be made to achieve a lower level in patients with normal pulse pressure (CitationZoccali 2003).
However, as previously mentioned, control of hypertension in HD patients is a challenge, despite the use of many effective antihypertensive drugs, especially in patients on short HD treatment time. CitationAgarwal et al (2003), using a less restrictive systolic BP limit (ie, target BP < 150/85 mmHg), reported that in fewer than 30% of hypertensive HD patients BP is adequately controlled (this seems also to apply to the general population). Improving control of hypertension often needs more hydrosaline removal and knowledge of the patient's clinical characteristics (anephric, LVH, malignant hypertension of long duration), which could provide information on the different mechanisms or severity of hypertension. Prolongation of the dialysis time could thus be beneficial but, unfortunately, this is unpopular among HD patients. Moreover, inconsistent use of antihypertensive therapy (ie, only on days without dialysis to avoid hypotension during dialysis sessions) and the use of submaximal drug dosage are significant barriers in the control of BP.
In summary, to prevent cardiovascular mortality, an ideal goal of systolic BP during HD appears to be at levels above 100 and below 150 mmHg when other cardiovascular risk factors and cardiac function are controlled. Since HD patients are generally older and cardiac complications are frequent, a more reasonable target could be 150 mmHg; however, lower targets should be attempted in patients with normal pulse pressure.
Management of hypertensive patients treated by HD
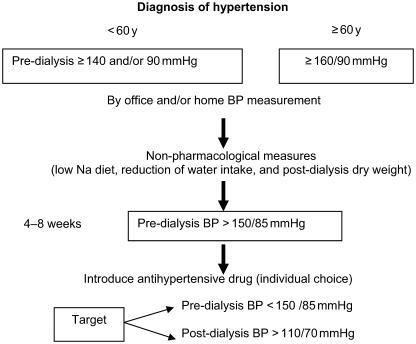
In the management of hypertension the goal is, at least, to reach pre-dialysis BP ≤ 150/85 mmHg without any excessive BP decrease during and just after the dialysis session (BP must remain ≥110/70 mmHg) in order to avoid coronary ischemia ().
First, a smooth reduction in post-dialysis weight must be reached over the course of a few weeks. In parallel, sodium load during dialysis treatment should be limited as required.
If necessary, prolongation of the dialysis treatment time and/or an extra dialysis session must be implemented. It should be recognized that the BP lowering effect is significantly observed only after a few weeks, owing to the lag phenomenon, and this requires patience from both physician and patient.
Second, if target BP is not achieved after a few weeks, antihypertensive drugs can be introduced. The choice of antihypertensive medication needs to be tailored in accordance with underlying pathology in the patient. β-Blockers are an excellent choice in case of angina pectoris, after myocardial infarction, and in tachyarrhythmia. Angiotensin-converting enzyme inhibitors could be proposed in the presence of congestive heart failure and after myocardial infarction or in diabetic patients. Calcium channel blockers, which have been widely prescribed in HD patients, are useful when there is angina pectoris or systolic hypertension or in older patients. However, there is no evidence from randomized trials to demonstrate superiority of one class over the others in dialysis patients (CitationGriffith et al 2003). There is a need for such trials to be conducted. Recent evidence based on a retrospective study showed that the use of calcium channel blockers could be associated with a lower risk of mortality among ESRD patients (CitationKestenbaum et al 2002). The evidence provided from the VALUE clinical trial seems to suggest that in patients with a high cardiovascular risk, such as the HD population, amlodipine provided greater protection in patients with chronic kidney disease when compared with patients receiving valsartan. Amlodipine was even superior in preventing myocardial infarction when compared with valsartan (CitationJulius et al 2004). This could be due to more rigorous control of BP in patients treated with calcium channel blockers. In summary, an improvement in the control of BP is necessary in the HD population, first by a slow and smooth removal of extracellular volume (dry weight) and thereafter by use of antihypertensive medication on a case-by-case basis.
Guidelines to improve BP control in HD
The HD population is highly exposed to cardiovascular disease. The risk is strongly influenced by hypertension, which is present in the majority of patients. Currently, despite the various means available to decrease BP, control of hypertension in HD patients remains unsatisfactory (as in the general population).
To improve the management of high BP, we agree with CitationAgarwal's proposals (2003), which are as follows:
Make an accurate diagnosis of hypertension (repetitive office BP measurements, self-home BP determination, or, even better, by using ABPM).
Institute non-pharmacological measures, ie, salt and water restrictions, physical exercise, decrease in dry weight, and use of an optimal composition of the dialysate, which respects the sodium balance (to remove, by HD, the exact amount of sodium that has accumulated during the interdialytic period).
Prescribe, as a last resort, antihypertensive agents, the choice dictated by the characteristics of the patient (see above).
In some patients, modification of the dialysis time and frequency should be implemented with the goal of reaching a target pre-dialysis BP below 150/85 mmHg and a post-dialysis BP above 110/70 mmHg.
References
- AgarwalRRole of home blood pressure monitoring in hemodialysis patientsAm J Kidney Dis199933682710196009
- AgarwalRAssessment of blood pressure in hemodialysis patientsSemin Dial20021529930412358628
- AgarwalRSystolic hypertension in hemodialysis patientsSemin Dial2003162081312753679
- AgarwalRLewisRPrediction of hypertension in chronic hemodialysis patientsKidney Int2001601982911703618
- AgarwalRNissensonABatlleDPrevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United StatesAm J Med2003115291712967694
- AmarJVernierIRossignolVInfluence of nycthemeral blood pressure pattern in treated hypertensive patients on hemodialysisKidney Int199751186369186876
- AmarJVernierIRossignolVNocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patientsKidney Int20005724859110844617
- AndersonKMOdellPMWilsonPWFCardiovascular disease risk profilesAm Heart J199112129381985385
- AritaMMinamiENahamuraCRole of the sympathetic nervous system in the nocturnal fall in blood pressureHypertens Res1996191952008891748
- BarenbrockMZidekWWinterbergBSleep apnoea in long-term haemodialysis patientsJ Am Soc Nephrol199671438
- BlacherJGuerinAPannierBImpact of aortic stiffness on survival in end-stage renal diseaseCirculation1999992434910318666
- BlankestijnPSympathetic hyperactivity in chronic kidney diseaseNephrol Dial Transplant2004191354715069180
- BobrieGChatellierGGenesNCardiovascular prognosis of “masked hypertension” detected by blood pressure self-measurement in elderly treated hypertensive patientsJAMA2004171342915026401
- CampeseVNeurogenic factors and hypertension in renal diseaseKidney Int200057Suppl 75S26
- CharraBJeanGChazotCIntensive dialysis and blood pressure control: a reviewHemodial Int200485160
- ChazotCCharraBVo VanCThe Janus-faced aspect of “dry weight”Nephrol Dial Transplant199914121410052490
- ChobanianAVBakrisGLBlackHRThe seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 reportJAMA2003290197
- ChughtaiIPeixotoAAmbulatory blood pressure monitoring: a review of its clinical and prognostic relevanceHosp Physician200362March4756
- CovicAGoldsmithDAmbulatory blood pressure monitoring in nephrology: focus on BP variabilityJ Nephrol199912220910493565
- CovicAGoldsmithDAmbulatory blood pressure monitoring: an essential tool for blood pressure assessment in uraemic patientsNephrol Dial Transplant20021717374112270978
- CovicAGoldsmithDMititiucIAbnormal circadian blood pressure (BP) profile and not BP levels are associated with left ventricular dilatation and systolic dysfunctionNephrology19973SIS157
- CovicAHaydarAGoldsmithDRecent insights from studies using ambulatory blood pressure monitoring in patients with renal diseaseCurr Opin Nephrol Hypertens200312645814564203
- CruickshankJMCoronary flow reserve and the J curve relation between diastolic blood pressure and myocardial infarctionBMJ19882971227303145062
- D'AmicoMLocatelliFHypertension in dialysis: pathophysiology and treatmentJ Nephrol2002154384512243377
- DaugirdasJTPathophysiology of dialysis hypotension: an updateAm J Kidney Dis200138Suppl 4S111711602456
- ErtürkSErturgAAtesKRelationship of ambulatory blood pressure monitoring data to echocardiographic findings in haemodialysis patientsNephrol Dial Transplant199611205048918721
- [ESH–ESC] European Society of Hypertension–European Society of Cardiology. Guidelines CommitteeGuidelines for the management of arterial hypertensionJ Hypertens20032110115312777938
- FarsangCSleightPIsolated systolic hypertension: cardiovascular risk and treatment benefitsEur Soc Hypertens Sci Newsletter200126
- FlaniganMJRole of sodium in hemodialysisKidney Int200058Suppl 76S728
- FoleyRHerzogCCollinsABlood pressure and long-term mortality in United States hemodialysis patients: ESRD waves 3 and 4 studyKidney Int20026217849012371980
- FoleyRNParfreyPSSarnakMJClinical epidemiology of cardiovascular disease in chronic renal diseaseAm J Kidney Dis199832Suppl 3S112199820470
- FranklinSArterial stiffness and hypertension: a two-way street?Hypertension2005453495115710783
- GadegbekuCAShrayyefMZUllianMEHemodynamic effects of chronic hemodialysis therapy assessed by pulse waveform analysisAm J Hypertens2003168141714553959
- GriffithTChuaBAllenACharacteristics of treated hypertension in incident hemodialysis and peritoneal dialysis patientsAm J Kidney Dis2003421260914655199
- HanlyPSleep apnea and daytime sleepiness in end-stage renal diseaseSemin Dial2004171091415043611
- HeJWheltonPElevated systolic blood pressure as a risk factor for cardiovascular and renal diseaseJ Hypertens199917Suppl 2S713
- HorlMPHorlWHHemodialysis-associated hypertension: pathophysiology and therapyAm J Kidney Dis2002392274411840363
- JuliusSKjeldsenSEWeberMOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet20041920223115207952
- KestenbaumBGillenDSherrardDCalcium channel blocker use and mortality among patients with end-stage renal diseaseKidney Int20026121576412028456
- KhoslaUJohnsonRHypertension in the hemodialysis patient and the “lag phenomenon”: insights into pathophysiology and clinical managementAm J Kidney Dis2004437395115042553
- KimuraGTomitaJNakamuraSInteraction between hypertension and other cardiovascular risk factors in survival of hemodialyzed patientsAm J Hypertens199691006128896653
- KissIFarsangCRodicioJTreatment of hypertension in dialysed patientsEur Soc Hypertens Sci Newsletter2004521
- KlassenPSLowrieEGReddanDNAssociation between pulse pressure and mortality in patients undergoing maintenance hemodialysisJAMA200228715485511911757
- KoomanJvan der SandeFLeunissenKRole of sodium and volume in the pathogenesis of hypertension in dialysis patientsBlood Purif20042255914732812
- LeveyAEknoyanGCardiovascular disease in chronic renal diseaseNephrol Dial Transplant1999148288310328452
- LevinAClinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysisSemin Dial200316101512641872
- LeypoldtJCheungADelmezJRelationship between volume status and blood pressure during chronic hemodialysisKidney Int2002612667511786109
- LiuMTakahashiHMoritaYNon-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patientsNephrol Dial Transplant200318563912584280
- LocatelliFCovicAChazotCHypertension and cardiovascular risk assessment in dialysis patientsNephrol Dial Transplant20041910586815004266
- LondonGControversy on optimal blood pressure on haemodialysis: lower is not always betterNephrol Dial Transplant200116475811239018
- LondonGMMarchaisSJSafarMEAortic and large artery compliance in end-stage renal failureKidney Int199037137422299800
- LucasMFQueredaCTeruelJEffect of hypertension before beginning dialysis on survival of hemodialysis patientsAm J Kidney Dis2003418142112666068
- MacMahonSPetoRCutlerJBlood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution biasLancet1990335765741969518
- MaillouxLHaleyWHypertension in the ESRD patient: pathophysiology, therapy, outcomes, and future directionsAm J Kidney Dis199832705199820438
- MaillouxLLeveyAHypertension in patients with chronic renal diseaseAm J Kidney Dis1998325 Suppl 3S120419820471
- MazzuchiNCarbonellEFernandez-CeanJImportance of blood pressure control in hemodialysis patient survivalKidney Int20005821475411044236
- MendesRSantosSDorigoDThe use of peridialysis blood pressure and intradialytic blood pressure changes in the prediction of interdialytic blood pressure in haemodialysis patientsBlood Press Monit20038243814688554
- MitraSChandnaSFarringtonKWhat is hypertension in chronic haemodialysis? The role of interdialytic blood pressure monitoringNephrol Dial Transplant19991429152110570097
- National Kidney FoundationK/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratificationAm J Kidney Dis2002392 Suppl 1S126611904577
- National Kidney FoundationK/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney diseaseAm J Kidney Dis200443Suppl 2129014712421
- NystromFMalmqvistKOhmanKPNurse-recorded and ambulatory blood pressure predicts treatment-induced reduction of left ventricular hypertrophy equally well in hypertension: results from the Swedish irbesartan left ventricular hypertrophy investigation versus atenolol (SILVHIA) studyJ Hypertens20022015273312172314
- Pastor-BarriusoRBanegasJDamiánJSystolic blood pressure, diastolic blood pressure, and pulse pressure: an evaluation of their joint effect on mortalityAnn Intern Med2003139731914597457
- PeixotoASantosSMendesRReproducibility of ambulatory blood pressure monitoring in hemodialysis patientsAm J Kidney Dis2000369839011054355
- Pérez-GarciaRLópez-GómezMJuncoRHaemodialysis dose, extracellular volume control and arterial hypertensionNephrol Dial Transplant200116Suppl 198101
- PortFKHulbert-ShearonTEWolfeRAPre-dialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patientsAm J Kidney Dis1999335071710070915
- Prospective Studies CollaborationAge-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studiesLancet200236019031312493255
- RahmanMGriffinVKumarAA comparison of standardized versus “usual” blood pressure measurements in hemodialysis patientsAm J Kidney Dis20023912263012046035
- RedonJAmbulatory blood pressure and the kidneyBlood Press Monit199831576110212347
- RedonJOliverVZaragozaMDAmbulatory blood pressure during diseases of the kidneyBlood Press Monit199942677410547649
- Ritz E for the Medical Expert GroupClinical algorithms on cardiovascular risk factors in renal patientsNephrol Dial Transplant200015134510607792
- SafarMBlacherJPannierBCentral pulse pressure and mortality in end-stage renal diseaseHypertension200239735811897754
- SankaranarayananNSantosSFPeixotoAJBlood pressure measurement in dialysis patientsAdv Chronic Kidney Dis2004111344215216485
- SantosSMendesRSantosCProfile of interdialytic blood pressure in hemodialysis patientsAm J Nephrol2003239610512481148
- SchömigMEisenhardtARitzEControversy on optimal blood pressure on haemodialysis: normotensive blood pressure values are essential for survivalNephrol Dial Transplant2001164697411239017
- SchröderMRiedelEBeckWIncreased reduction of dimethylarginines and lowered interdialytic blood pressure by the use of biocompatible membranesKidney Int200159Suppl 78S1924
- SharabiYDaganYGrossmanESleep apnea as a risk factor for hypertensionCurr Opin Nephrol Hypertens2004133596415073496
- The Renal Association, on behalf of the Renal Association and the Royal College of PhysiciansTreatment of adult patients with renal failure: recommended standards and audit measuresJ R Coll Physicians Lond199529319017658412
- ValeroFAMartinez-VeaABardajiAAmbulatory blood pressure and left ventricular mass in normotensive patients with autosomal dominant polycystic kidney diseaseJ Am Soc Nephrol1999101020610232688
- Van BortelLStruijker-BoudierASafarMPulse pressure, arterial stiffness, and drug treatment of hypertensionHypertension2001389142111641309
- Van der SandeFMMulderAWHoorntjeSJThe hemodynamic effect of different ultrafiltration rates in patients with cardiac failure and patients without cardiac failure: comparison between isolated ultrafiltration and ultrafiltration with dialysisClin Nephrol19985030189840318
- VerdecchiaPPorcellatiCSchillaciGAmbulatory blood pressure. An independent predictor of prognosis in essential hypertensionHypertension1994247938017995639
- YarowsSAJuliusSPickeringTGHome blood pressure monitoringArch Intern Med20001601251710809027
- ZagerPNikolicJBrownR“U” curve association of blood pressure and mortality in hemodialysis patientsKidney Int19985456199690224
- ZoccaliCArterial pressure components and cardiovascular risk in end-stage renal diseaseNephrol Dial Transplant2003182495212543876
- ZoccaliCMallamaciFTripepiGSleep apnea in renal patientsJ Am Soc Nephrol2001122854911729258
- ZoccaliCMallamaciFTripepiGHypertension as a cardiovascular risk factor in end-stage renal failureCurr Hypertens Rep20024381612217257
- ZoccaliCMallamaciFTripepiGTraditional and emerging cardiovascular risk factors in end-stage renal diseaseKidney Int200363Suppl 85S10510