Abstract
Angiotensin-converting enzyme (ACE) inhibitors today are the standard therapy of patients with myocardial infarction and heart failure due to their proven beneficial effects in left ventricular remodeling and left ventricular function. ACE inhibitors have also been demonstrated to lead to regression of left ventricular hypertrophy (LVH). It is believed that the mechanism of action of LVH regression with ACE inhibitors arises from more than simple blood pressure reduction. LVH is an important risk factor for cardiovascular disease morbidity and mortality independent of blood pressure. Moexipril hydrochloride is a long-acting, non-sulfhydryl ACE inhibitor that can be taken once daily for the treatment of hypertension. Moexipril has now also been demonstrated to have beneficial effects on LVH and can lead to LVH regression.
Background
Moexipril hydrochloride is a long acting, non-sulfhydryl angiotensin-converting enzyme (ACE) inhibitor that can be taken once daily for the treatment of hypertension (HTN) (CitationWhite et al 1994; CitationStimpel et al 1995). It is a pro-drug that needs to be hydrolyzed in the liver into its active carboxylic metabolite, moexiprilat, in order to be effective (CitationStimpel et al 1995). Moexipril’s synthesis has been reported previously in 1982 and 1986 (CitationHoefle et al 1982; CitationKlucthko et al 1986). It is incompletely absorbed after oral administration, and its bioavailability is low, accounting for 22% of unchanged drug. This is similar in comparison with other ACE inhibitors, such as benazepril, fosinopril, and trandolapril, which have bioavailability of 37%, 32%, and 30%, respectively. Cilazapril, enalapril, quinapril, and ramipril have higher bioavailability () (CitationGrass and Morehead 1986; CitationBarfour and Gos 1995; CitationLancaster and Todd 1998; CitationSinghvi et al 1998; CitationSong and White 2002).
Table 1 Pharmacokinetic characteristics of ACE inhibitors (Froshlich et al 1991; Edling et al 1995; Stimpel et al 1995)
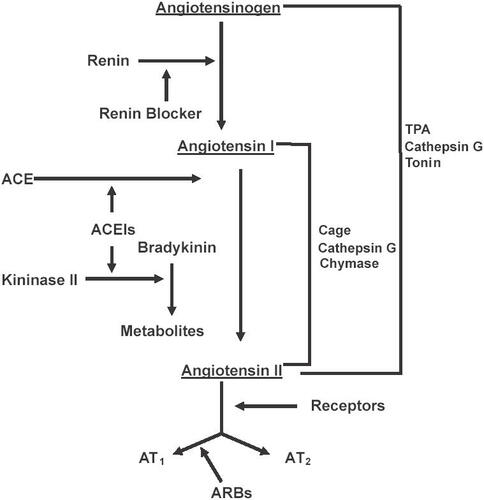
Moexipril exerts its biological and antihypertensive effects after its metabolism in the liver into its active metabolite, moexiprilat, by blocking the conversion of angiotensin I to angiotensin II (). Additionally, it blocks the degradation of bradykinin, which causes a hypotensive effect because of the potent vasodilation caused by the production of prostaglandin E2 and nitric oxide. Animal studies comparing moexipril to captopril have demonstrated equivalency in their antihypertensive effects. When compared with enalapril in spontaneously hypertensive rats, both moexipril and enalapril reduced the mean blood pressure by 24% at 28 days (CitationEdling et al 1995). In clinical studies, moexipril produced significant reduction in both systolic and diastolic blood pressure with its maximum effect seen at 6 hours post-administration (CitationStrauss et al 1994; CitationLucas et al 1995). When administered in a dose between 7.5 mg and 15 mg daily, the blood pressure effects have been shown to last 24 hours.
Cardiovascular effects
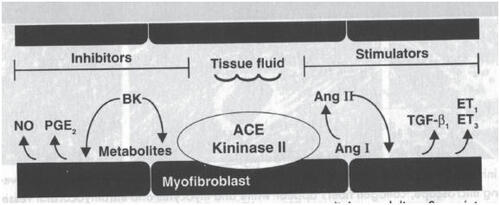
Moexipril has been demonstrated in in vitro and in vivo studies to possess cardioprotective properties. In rats, administration of 10 mg moexipril either alone or in combination with losartan, one week prior to induction of myocardial infarction, decreased the infarct size. These beneficial effects of moexipril were negated by the bradykinin b2 receptor antagonist icatibant. Administration of losartan alone did not demonstrate any significant effect on infarct size (CitationRosendorff 1996). Although these findings suggest that these beneficial effects of moexipril were mediated exclusively through inhibition of the breakdown of bradykinin, other studies have shown that the cardioprotective effects of ACE inhibitors are mediated through a combination of inhibition of angiotensin II production and bradykinin degradation (CitationFroshlich and Horinak 1991; CitationBrilla et al 1996; CitationRumble et al 1996; CitationGrohe et al 1997; CitationChrysant 1998a). Angiotensin II exerts its remodeling effects on the cardiovascular system through its direct proliferative actions and also indirectly through its stimulation of the production of endothelin 1 and 3 (ET1, ET3) and the transforming growth factor 1(TGF-b1), all of which have tissue proliferative effects (). Bradykinin itself, and through its stimulation in the production of prostaglandin E2 (PGE2) and nitric oxide (NO), exerts anti-proliferative effects (CitationChrysant 1998a). In addition, angiotensin II stimulates the production of various protooncogenes, such as c-fos, c-jum and c-myc which all have cellular proliferative actions (CitationFroshlich and Horinak 1991). The antiproliferative effects of moexipril have been demonstrated in vitro studies where moexipril inhibited the estrogen-stimulated growth of neonatal cardiac fibroblasts in rats (CitationPfeffer et al 1992). It should be stated here that these effects of moexipril on cardiovascular remodeling have been demonstrated with other ACE inhibitors and they appear to be a class effect (CitationGrohe et al 1997).
ACE inhibitors today are the standard therapy of patients with myocardial infarction and heart failure due to their proven beneficial effects in left ventricular remodeling and left ventricular function (CitationSOLVD 1991; CitationDahlof et al 2002).
Clinical experience with moexipril in hypertension
Moexipril has been extensively studied in patients with mild, moderate, or severe hypertension, compared with placebo and other antihypertensive drugs. It was also studied in fixed combinations with low dose hydrochlorothiazide (HTZ). Most of the studies were double-blind, randomized, multicenter short-term and a few were open label long-term studies. They were based on sitting diastolic blood pressure (SDBP) of 95–120 mmHg, of 8–24 weeks’ or 2 years’ duration. In all these studies, moexipril was dispensed once daily due to its long elimination half-life of 10 hours, in doses ranging from 7.5–30 mg. The results of the most pertinent studies are listed in .
Table 2 Summary of clinical trails with moexipril
Placebo-controlled studies
In placebo-controlled multicenter trials in Europe and the United States of 8–12 weeks’ duration in patients with mild to moderate essential hypertension (SDBP 95–114 mmHg), moexipril was more effective than placebo. Given in single daily doses of 7.5–30 mg, moexipril produced a sustained blood-pressure control over 24 hours and decreased the SDBP from 4 to 11 mmHg over placebo with a response rate of 48%–61% for those receiving moexipril 7.5 and 15 mg/day, vs 29% and 34% for those receiving placebo, respectively (CitationStrauss et al 1994; CitationLucas et al 1995; CitationStimpel and Koch 1996, Citation1997).
Comparative studies
The antihypertensive effectiveness and safety of moexipril has also been compared with other antihypetensive drugs in patients with mild to moderately severe hypertension (SDBP 95–114 mmHg). In comparison with HTZ 25 mg once daily, atenolol 25–50 mg once daily, metoprolol 100 mg once daily, verapamil-SR 120–240 mg once daily, nitrendipine 20 mg once daily, and captopril 25–50 mg twice daily, moexipril given in single daily doses of 7.5–15 mg was as effective in lowering the blood pressure as the other antihypertensive drugs (CitationDickstein et al 1994; CitationAbernethy et al 1995; CitationChrysant et al 1995; CitationStimpel et al 1996a, b; CitationWhite et al 1997).
Add-on studies
Moexipril was also studied in patients with moderate to severe hypertension as add-on therapy to pre-existing drugs. In one study, moexipril 7.5 once daily, or verapamil-SR 180 mg once daily, were added to pretreatment with HCTZ 25 mg once daily if the SDBP was 100–114 mmHg inclusive (CitationChrysant and Simpel 1998). If after 4 weeks of treatment the SDBP was ≥90 mmHg, the dose of moexipril was increased to 15 mg once daily and that of verapamil-SR to 240 mg once daily. This resulted in further, but similar decrease of SDBP by week 8. Similar results were reported by other investigators when moexipril 7.5 or 15 mg once daily were added to HCTZ 25 mg once daily, or to nifedipine sustained release 20 mg twice daily (CitationChrysant 1994; CitationChrysant et al 1996).
Fixed combination studies
Moexipril has been thoroughly investigated in the treatment of hypertension as a low-dose combination with HCTZ. In multi-factorial design studies, the combination of moexipril 3.75 mg to 30 mg with HCTZ 3.125 to 50 mg were more efficacious in lowering the blood pressure than the individual components alone (CitationChrysant 1997). In another study, 223 patients with SDBP of 95–114 mmHg and SSBP ≤200 mmHg, were treated with a fixed very low dose combination of moexipril/HCTZ 3.75/6.25 mg once daily, and demonstrated a reduction of their SSDP/SDBP by −7.6/–7.6 mmHg compared with placebo of +0.2/–3.9 mmHg (CitationChrysant 1998b). The fixed combinations of HCTZ 12.5 mg with moexipril 7.5 mg or with metoprolol 100 mg given once daily were studied in 140 hypertensive patients (SDBP 95–114 mmHg), for 12 weeks (CitationChrysant et al 1983). In this study both combination treatments reduced the SSBP/SDBP by 17.6/12.8 and 17.2/13.9 mmHg for the moexirpil-HCTZ and metoprolol-HCTZ combinations, respectively ().
Left ventricular hypertrophy (LVH)
LVH, determined by echocardiography, is a left ventricular mas (LVM) or LVM index (LVMI); LVM/body surface area in the upper 2.5%–5.0% of the adult population (CitationLevy et al 1988; CitationPhillips and Diamond 1999). An LVMI of ≥25 g/m2 is considered to be approximately at the 95th percentile. A direct and progressive relationship exists between LVM and cardiovascular disease (CVD) risk, including risk of coronary heart disease (CHD), heart failure (HF), stroke, and sudden death (CitationFrolich et al 1992; CitationDevereux and Roman 1993; CitationHaider et al 1998). Increased LVM has been shown to predict the risk of CV events, especially fatal events, independent of BP or CHD (CitationKoren et al 1991; CitationGhali et al 1992; CitationLevy et al 1994). Conversely, in persons with CHD, the relative risk of mortality is increased two-fold by LVH (CitationPhillips and Diamond 1999). In the Framingham cohort, each increase in LVM of 50 g/m2 was associated with an increase in relative risk of CVD to 1.49 in men, and 1.57 in women (CitationLevy et al 1988).
LVH is an important risk factor for CVD morbidity and mortality independent of BP. The prognostic importance of LVH has been shown in population studies such as the Framingham Heart Study and the Honolulu Heart Program, as well as in clinical studies of patients with essential HTN, secondary HTN and CHD (CitationKoren et al 1991; CitationGhali et al 1992; CitationLevy et al 1994). LVH found on resting ECG has been associated with the highest risk of fatal CHD, conferring a relative risk of 11.4 in one cohort of 7682 men followed for 12 years (CitationKnutsen et al 1988). In the Framingham cohort, follow-up was extended until a subject developed CVD, died or attended clinic two years later. The increased risk of CV events was related to baseline electrocardiogram (ECG) voltage by quartile (sum of R wave in lead AVL and the S wave in lead V3 ≥1.3 mV [25th percentile], 1.8 mV [50th percentile], and 2.3 mV [75th percentile]) and repolarization abnormalities (classified as normal, mildly abnormal [ST-T flattening, isolated ST depression, T-wave inversion], or severely abnormal [ST by as much as 50%. Conversely, if voltage increased serially depression in association with inverted or biphasic T waves]). over two years of follow-up, risk doubled (CitationLevy et al 1994). Persons in the highest quartile of voltage (sum of R wave in The prevalence of ECG evidence of LVH declined from 4.5% lead AVL and the S wave in lead V3 ≥2.3 mV) were at highest to 2.5% in men and 3.6% to 1.1% in women over 38 years of risk. When voltage decreased or repolarization abnormalities follow-up. Mean BP reduction was greater in women than in improved (in men only), risk declined and prognosis improved men (–15/–8 mmHg vs −4/–3 mmHg) and the prevalence of severe HTN (SBP ≥160 mmHg or DBP ≥100 mmHg) declined to a greater extent in women than men (28% and 18.5% to 7.7% and 9.2% respectively) over this period (CitationDevereux 1995). The reduction in BP, particularly in severe HTN, is thought to correlate with the increased use of pharmacologic agents over the last several decades.
In addition to LVM or LVMI, the geometric pattern of the hypertrophied LV has been shown to relate to CV events. Four geometric patterns have been described: 1) concentric LVH (increased LVM and increased relative wall thickness, RWT) 2) eccentric LVH (increased LVM and normal RWT); 3) concentric remodeling (normal LVM and increased RWT); 4) normal geometry (normal LVM and RWT) (CitationKrumholz et al 1995; CitationVerdecchia et al 1995; CitationGhali et al 1998). The pattern associated with the highest incidence of morbidity and mortality is concentric LVH (CitationVerdecchia et al 1995, Citation1998). The incidence of CV events, in one study, was 30% in those with concentric LVH, 25% in those with eccentric LVH, 15% in those with concentric remodeling, and 9% in those with normal geometry (CitationKoren et al 1991). In another study, subjects with LVH (LVMI of >125 g/m2) at baseline experienced a 47% lower event rate when LVMI was reduced to <125 g/m2 at follow-up (mean follow-up was 9 years) (p = 0.002) (CitationSchlaich and Schmeider 1998). The prognostic importance of LV geometry independent of CHD risk factors and LVM/LVMI is controversial. A study of 694 hypertensive patients with normal LVMI (<125 g/m2) showed that concentric remodeling of the LV was an important predictor of CV mortality, independent of conventional risk factors for CHD (CitationVerdecchia et al 1998). However, other studies demonstrated that when traditional risk factors for CHD and LVM are taken into account, the geometric pattern of the LV is less predictive of CV events (CitationKrumholz et al 1995; CitationPhillips and Diamond 1999).
Pathophysiologic mechanisms for the development of LVH include both hemodynamic (increased BP, wall stress, and increasing arterial stiffness of central arteries) and non-hemodynamic factors (genetics, activation of the sympathetic nervous system, and the RAAS) (CitationGottdiener et al 1997). LVM has been shown to be closely related to SBP, while RWT appears to be more closely related to DBP. A large body of evidence relates the development of LVH to increased RAAS activity, in particular angiotensin II. Angiotensin II has been shown to stimulate fibroblast activity, synthesis, and release of cytokines and growth factors and myocardial fibrosis. Aldosterone has been associated with an increase in collagen in the myocardium, leading to interstitial fibrosis in ventricular tissue.
Angiotensin-converting enzyme inhibitors and left ventricular hypertrophy
Antihypertensive therapy is effective in producing regression of LVH (CitationLiebson et al 1995; CitationMoser and Herbert 1996; CitationSchmieder et al 1996; CitationThurmann et al 1998). An analysis of six large HTN trials involving a total of 26 741 patients showed that long-term use of any antihypertensive medication except direct vasodilators leads to regression of LVH (CitationThurmann et al 1998). Further, a large meta-analysis of 39 trials of diuretics, beta-blockers (BBs), calcium channel blockers (CCBs), and ACE inhibitors (ACEIs) in hypertensive subjects showed that LVM was related to the treat-ment-induced decline in BP, particularly SBP (p < 0.001 vs p = 0.08 for DBP). Reductions in LVM of 13%, 9%, 6%, and 7% were demonstrated with ACEIs, CCBs, BBs, and diuretics, respectively (CitationMoser and Herbert 1996). Diuretics have been shown in several trials to be effective in reducing LVM (CitationNeaton et al 1993; CitationMoser and Herbert 1996; CitationSchmieder et al 1996; CitationThurmann et al 1998). Results of studies that have examined the effects of BBs and CCBs on regression of LVH have been mixed, with some studies suggesting that one or both of these agents are not effective (CitationNeaton et al 1993; CitationMoser and Herbert 1996; CitationSchmieder et al 1996).
Agents that interrupt the RAAS, including ACEIs and antiotensin II receptor blockers (ARBs), may be more effective than other classes of antihypertensive drugs in reducing LVM. Whether reduced synthesis of angiotensin II, with its cytokine/growth factor-stimulating effects, potentiation of bradykinin with resultant stimulation of NO synthesis and release, and/or inhibition of aldosterone synthesis are salient factors in LVH reversal related to use of these drugs is a matter of conjecture. Further, whether, in fact, these agents effect reversal of LVH via mechanisms other than BP lowering is a matter of active debate.
In the LVH regression substudy of the HOPE trial, the use of ramipril led to LVH regression in nearly 92% of patients (CitationDevereux et al 2004). This effect was independent of blood pressure control. Consequently, the use of ramipril led to a reduction in death, MI, and stroke. Another interesting result was that the use of ramipril was associated with prevention of new HF.
Moexipril and left ventricular hypertrophy
The use of moexipril in hypertensive subjects has been demonstrated to have beneficial effects on LVH. In one trial of 72 patients with echocardiographic evidence of LVH (defined as a LVMI of >111 g/m2 in men and >106 g/m2 in women), a dose of 15 mg of moexipril daily led to a significant decrease in LVMI. The average LVMI was reduced from 121 +/–20 g/m2 to 103 +/–17 g/m2 (p < 0.001) in a period of 24 weeks (CitationSayegh et al 2005). Another more robust study, the Moexipril and REgression of left ventricular hypertrophy in combination therapy (MORE) trial, examined the effects of moexipril in 426 hypertensive patients (CitationSpinar and Vitovec 2005). While no predefined value was given for LVH, the baseline LVMI in this study was 149 g/m2, which is well above any published accepted value for LVH by LVMI. The presence of LVH was established by echocardiography. Moexipril was added on to the existing therapy and titrated up as needed to achieve blood pressures of <140/90 mmHg. In this trial, BBs were used in over 50% of patients as either monotherapy or in combination with diuretics and/or CCBs. After 6 months of follow-up, the average blood pressure was reduced from 161/97 mmHg to 136/82 mmHg (p < 0.0001), and the LVMI decreased from 149 +/–51 g/m2 to 137 +/–47 g/m2 (p < 0.0001). Several interesting findings were observed. First, an improvement in left atrial size was demonstrated (39.81 mm vs 39.04 mm, p = 0.002) in addition to an improvement in LV size and LVMI. Second, an improvement in LV diastolic function was demonstrated with a significant increase in the E/A ratio from 0.91 to 0.94 (p < 0.0005). Third, there was a significant reduction in pulse pressure (PP) from 12.5 mmHg to 9.8 mmHg (p < 0.01). This is an important finding due to the very well established link between PP and cardiovascular morbidity and mortality. Therefore, it appears that moexipril as added therapy provides benefits beyond simple blood pressure lowering that have been demonstrated with other ACE inhibitors.
Conclusion
Moexipril is a nonsulfhydryl ACE inhibitor with prolonged duration of action and is suitable for once a day administration for the treatment of hypertension. In doses of 7.5, 15, and 30 mg alone or in combination with other antihypertensives, such as HCTZ and calcium channel blockers, moexipril is effective for the treatment of moderate to severe hypertension. Evidence is also emerging that moexipril, like other ACE inhibitors, is effective in reducing left ventricular hypertrophy. This is an extremely important feature as LVH has well established links to cardiovascular morbidity and mortality.
References
- AbernethyDRFoxAALStimpelMMoexipril in the treatment of mild to moderate essential hypertension: comparison with sustained release varapamilJ Clin Pharmacol1995357959
- Agabiti-RoseiEAmbrosioniEPirelliEfficacy and tolerability of moexipril and nitrendipine in postmenopausal women with hypertension: MADAM study groupEur J Clin Pharmacol199955185910379633
- BarfourJAGosKLBenazepril, a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in hypertension and congestive heart failureDrugs19954251139
- BrillaCGMaubaraIWeberKTAdvanced hypertensive heart disease in spontaneously hypertensive rats: lisinopril-medicated regression of myocardial fibrosisHypertension199628269748707393
- ChrysantSGAntihypertensive effectiveness of low dose lisinoprilhydrochlorothiazide combinations: a large multicenter studyArch Intern Med1994154737438147677
- ChrysantSGPerindopril/hydrochlorothiazide dose combination for the treatment of hypertension: a multicenter studyJ Clin Pharmacol19973747529048272
- ChrysantSG.Vascular remodeling: the role of angiostensin-converting enzyme inhibitorsAm Heart J1998a135S21S309488609
- ChrysantSGFixed low-dose drug combination for the treatment of hypertensionArch Fam Med1998b737069682692
- ChrysantSGDunnMMarplesDSevere reversible azortemia from captopril therapy: report of three cases and review of the literatureArch Intern Med1983143437416338847
- ChrysantSGFoxAALStimpelMComparison of moexipril, a new ACE inhibitor, to varapamil-SR as add-on therapy to low dose hydrochlorothiazide in hypertensive patientsAm J Hypertens19958418217619356
- ChrysantSGFaganTGlazerREffects of benazepril and hydrochlorothiadize, given alone and in low and high-dose combinations, on blood pressure in patients with hypertensionArch Fam Med1996517248542050
- ChrysantSGStimpelMAntihypertensive effectiveness of a very low fixed-dose comination of moexipril and hydrochlorothiazideJ Cardiovasc Pharmacol199831384909514183
- DahlofBDevereuxRBKjeldsenSECardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomized trial against atenololLancet2002359995100311937178
- DevereuxRBLeft ventricular geometry, pathophysiology and prognosisJ Am Coll Cardiol19952588577884092
- DevereuxRBDahlofBGerdtsERegression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the losartan intervention for endpoint reduction in hypertension (LIFE) trialCirculation200411014566215326072
- DevereuxRBRomanMJInter-relationships between hypertension, left ventricular hypertrophy and coronary heart diseaseJ Hypertens19931139
- DicksteinKAaslandTFerrariPComparison of the efficacy of three dose levels of moexipril versus placebo as add-on therapy to hydrochlorothiazide in patients with moderate hypertensionJ Cardiovasc Pharmacol199424247557526056
- EdlingOBaoGFeelischMMoexipril, a new angiotensin converting enzyme (ACE) inhibitor: pharmacological characterization and comparison with enalaprilJ Pharmacol Exp Ther1995275854637473177
- FrohlichEDApsteinCChobanianAVThe heart in hypertensionN Engl J Med199232799810081518549
- FroshlichEDHorinakSCardiac and aortic effects of angiotensin converting enzyme inhibitorsHypertension199118Suppl II27
- GhaliJKLiaoYCooperRSInfluence of left ventricular geometric patterns on prognosis in patients with or without coronary artery diseaseJ Am Coll Cardiol1998311635409626845
- GhaliJKLiaoYSimmonsBThe prognostic role of left ventricular hypertrophy in patients with or without coronary artery diseaseAnn Intern Med199211783161416558
- GottdienerJSRedaDJMassieBMEffect of single-drug therapy on reduction of left ventricular mass in mild to moderate hypertensionCirculation1997952007149133508
- GrassGMMoreheadWEvidence for site specific absorption of a nove ACE inhibitorPharm Res19896759652554270
- GroheCKahlertSLobbertKEffects of moexiprilat on estrogen-stimulated cardiac fibroblast growthBr J Pharmacol1997121159146879
- HaiderAWLarsonMGBenjaminEJIncreased left ventricular mass and hypertrophy are associated with increased risk for sudden deathJ Am Coll Cardiol1998321454629809962
- HoefleMLKlutchkoS.Substituted acyl derivatives of 1, 2, 3, 4 tetrahydroquinoline 3-carboxylic acidsUS Pat19829449
- KnutsenRKnutsenSCurbJDThe predictive value of resting electrocardiograms for 12-year incidence of coronary heart disease in the Honolulu Heart ProgramJ Clin Epidemiol1988412933023339383
- KorenMJDevereuxRBCasalePNRelation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertensionAnn Intern Med1991114345521825164
- KrumholzHMLarsonMLevyDPrognosis of left ventricular geometric patterns in the Framingham Heart StudyJ Am Coll Cardiol199525879847884091
- KlucthkoSBlankelyCJFlemingRWSynthesis of the novel angiotensin converting enzyme inhibitor quinapril and related compounds. A divergence of structure-activity relationship for non-sulfhydryl and sulfhydryl typesJ Med. Chem19862919539613020249
- LancasterSGToddPAMoexipril: a preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in hypertension and congestive heart failureDrugs199835646672844497
- LevyDAndersonKMSavageDDEchocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart StudyAnn Intern Med19881087132962527
- LevyDSalomonMD’AgostinoRBPrognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophyCirculation1994901786937923663
- LiebsonPRGranditsGADianzumbaSComparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS)Circulation1995916987067828296
- LucasCDDargaLLFoxAALA study of the efficacy and safety of moexipril in mild to moderate hypertensionAm J Ther199528869211854803
- MoserMHebertPRPrevention of disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trialsJ Am Coll Cardiol1996271214188609345
- NeatonJDGrimmRJJrStamlerJTreatment of Mild Hypertension Study Research Group. Treatment of Mild Hypertension Study: final resultsJAMA1993270713248336373
- PerssonBStimpelMEvaluation of the antihypertensive efficacy and tolerability of moexipril, a new ACE inhibitor, compared to hydrochlorothiazide in elderly patientsEur J Clin Pharmacol199650259648803515
- PfefferMABraunwaldEMoyeLAEffect of captopril on morality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE InvestigatorsN Engl J Med1992327669771386652
- PhillipsRADiamondJAOparilSWeberMALeft ventricular hypertrophy, congestive heart failure, and coronary flow reserve abnormalities in hypertensionHypertension: A Companion to Brenner and Rector’s The Kidney1999PhiladelphiaW.B. Saunders Company24477
- RosendorffCThe rennin-angiotensin system and vascular hypertrophyJ Am Coll Cardiol199628803128837552
- RumbleJRKomersRCooperMEKinins or nitric oxide, or both are involved in the antitrophic effects of angiotensin converting enzyme inhibitors on diabetes-associated mesenteric vascular hypertrophy in the ratJ Hypertens19961460178762203
- The SOLVD InvestigatorsEffects on enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failureN Engl J Med19913252933022057034
- SayeghFTopouchianJHlawatyMRegression of left ventricular hypertrophy with moexipril, an angiotensin-converting enzyme inhibitor, in hypertensive patientsAm J Ther2005123815662286
- SchlaichMPSchmiederRELeft ventricular hypertrophy and its regression: pathophysiology and therapeutic approachJ Hypertens1998111394404
- SchmiederREMartusPKlingbeilAReversal of left ventricular hypertrophy in essential hypertensionJAMA19962751507138622227
- SinghviSMDuchinKCMorrisonRADisposition of fosinopril sodium in healthy subjectsBr J Clin Pharmacol1998259152967089
- SongJCWhiteCMClinical pharmacokinetics and selective pharmacodynamics of new angiotensin converting enzyme inhibitors. An updateClin Pharmacokinet2002412072411929321
- SpinarJVitovecJMORE-moexipril and regression of left ventricular hypertrophy in combination therapy. A multicentric open label clinical trialInt J Cardiol200510019920615823625
- StimpelMBonnRKochBKPharmacology and clinical use of the new ACE inhibitor moexiprilCardiovasc Drug Rev19951321129
- StimpelMKochBAntihypertensive treatment in postmenopausal women with hypertension and obesity: moexipril versus atenolol [abstract]Am J Hypertens19969175A
- StimpelMKochBJansenTMoexrpril versus captopril in patients with mild to moderate hypertensionJ Cardiovasc Pharmacol1996a28769738961074
- StimpelMKochBDicksteinKMoexipril as add-on therapy to hydrochlorothiazide in moderate to severe hypertensionCardiology1996b87313188793166
- StimpelMKochBAntihypertensive treatment with moexipril plus HCTZ vs. metrprolol plus HCTZ in patients with mild to moderate hypertensionJ Hum Hypertens19971113379140801
- StimpelMKochBOparilSAntihypertensive treatment in postmenopausal women: results from a prospective, randomized, double blind, controlled study comparing an ACE inhibitor (moexipril) with a diuretic (hydrochlorothiazide)Cardiology19988927169643274
- StimpelMKochBWeberMAComparison between moexipril and atenolol in obese postmenopausal women with hypertensionMaturitas19983069779819786
- StraussHMZhuYCRedlichTAngiotensin-converting enzyme inhibition in infarct-induced heart failure in rats: bradykinin versus angiotensin IIJ Cardiovasc Risk19941255627621306
- ThurmannPAKenediPSchmidtAInfluence of the angiotensin II antagonist valsartan on left ventricular hypertrophy in patients with essential hypertensionCirculation1998982037429808602
- VerdecchiaPSchillaciGBorgioniCAdverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular massJ Am Coll Cardiol199525879847884091
- VerdecchiaPSchillaciGBorgioniCPrognostic significance of serial changes in left ventricular mass in essential hypertensionCirculation19989748549443431
- WhiteWBFoxAALStimpelMLong term efficacy and safety of moexipril in the treatment of hypertensionJ Hum Hypertens199489179217884791
- WhiteWBKochBStimpelMUsefulness of moexipril and hydrochlorothiazide in moderately severe essential hypertensionAm J Ther19974123910423601
- WhiteWBStimpelMLong-term efficacy and safety of moexipril alone and in combination with hydrochlorothiazide in elderly patients with hypertensionJ Hum Hypertens19959879848583466