Abstract
Lower extremity complications in persons with diabetes have become an increasingly significant public health concern in both the developed and developing world. These complications, beginning with neuropathy and subsequent diabetic foot wounds frequently lead to infection and lower extremity amputation even in the absence of critical limb ischemia. In order to diminish the detrimental consequences associated with diabetic foot ulcers, a com-mon-sense-based treatment approach must be implemented. Many of the etiological factors contributing to the formation of diabetic foot ulceration may be identified using simple, inexpensive equipment in a clinical setting. Prevention of diabetic foot ulcers can be accomplished in a primary care setting with a brief history and screening for loss of protective sensation via the Semmes-Weinstein monofilament. Specialist clinics may quantify neuropathy, plantar foot pressure, and assess vascular status with Doppler ultrasound and ankle-brachial blood pressure indices. These measurements, in conjunction with other findings from the history and physical examination, may enable clinicians to stratify patients based on risk and help determine the type of intervention. Other effective clinical interventions may include patient education, optimizing glycemic control, smoking cessation, and diligent foot care. Recent technological advanced combined with better understanding of the wound healing process have resulted in a myriad of advanced wound healing modalities in the treatment of diabetic foot ulcers. However, it is imperative to remember the fundamental basics in the healing of diabetic foot ulcers: adequate perfusion, debridement, infection control, and pressure mitigation. Early recognition of the etiological factors along with prompt management of diabetic foot ulcers is essential for successful outcome.
Introduction
The rapid rise in the incidence of diabetes, a serious life-long condition, is of alarming concern to health care professionals. Recent data from the Center of Disease Control and Prevention approximate that 20.8 million people, roughly 7% of the United States population, have diabetes (2005). In 2005 alone, 1.5 million new cases of diabetes were diagnosed in people aged 20 years or older (2005). Diabetes mellitus is a disease known for its multifaceted complications, and foot ulceration, which often results in lower extremity amputations, is one of the most common complications associated with the disease (CitationBoulton and Vileikyte 2000; CitationReiber 2001; CitationDang and Boulton 2003; CitationPinzur et al 2005). The prevalence of foot ulcers ranges from 4% to 10% among persons diagnosed with diabetes mellitus (CitationSingh et al 2005). This translates to an annual population-based incidence of 1.0% to 4.1%, and the lifetime incidence may be as high as 25% (CitationSingh et al 2005). Diabetic foot ulcers frequently become infected and are a major cause of hospital admissions (CitationDang and Boulton 2003; CitationPinzur et al 2005). They also account for more than half of non-traumatic lower limb amputations in this patient population (CitationDang and Boulton 2003). Diabetic foot ulcers impose tremendous medical and financial burden on our healthcare system with costs conservatively estimated as high as $45000 per patient (CitationStockl et al 2004). These estimations, however, do not include the deleterious psychosocial effects on the patient’s quality of life because of impaired mobility and substantial loss of productivity (CitationRagnarson Tennvall and Apelqvist 2004). Ulcerations are pivotal events in limb loss for two important reasons. They allow an avenue for infection (CitationArmstrong and Lipsky 2004), and can cause progressive tissue necrosis and poor wound healing in the presence of critical ischemia. Approximately 56% of diabetic foot ulcerations become infected (CitationBlock 1981; CitationGibbons and Eliopoulos 1984; CitationSmith et al 1987) and 20% of these patients with infected foot wounds end up with some type of lower extremity amputation. Therefore the timely prevention and healing of diabetic ulcerations are fundamental for amputation prevention (CitationSchwegler et al 2002; CitationWu et al 2005). This manuscript will focus on the prevention and treatment of diabetic foot ulcerations.
Prevention
Prevention and prophylactic foot care have been advocated to decrease patient morbidity, the utilization of expensive resources, as well as the risk for amputations (CitationPinzur et al 2005). These interventions, which include the identification of risk factors, patient education, and intensive podiatric care (CitationMoreland et al 2004; CitationSingh et al 2005), have been shown to be cost effective or even cost saving (CitationRagnarson Tennvall and Apelqvist 2004). Increased awareness of the potential influence of reimbursement systems on prevention, management, and outcomes of diabetic foot lesions has improved in recent years (CitationRagnarson Tennvall and Apelqvist 2004).
Diabetes is a multifactoral disease and the multidisciplinary approach has been advocated for the comprehensive treatment of diabetes and prevention of its complications (CitationRonnemaa et al 1997; CitationPlank et al 2003). Patients with diabetes who present for care should be under the concomitant management of a primary care physician with appropriate referrals to an endocrinologist, ophthalmologist, nephrologists, vascular surgeon, podiatrist, physical therapist, nutritionist, and a diabetic educator to help ensure adequate care (CitationDang and Boulton 2003; CitationSchaper et al 2003; CitationSingh et al 2005; CitationVan Damme and Limet 2005). These different perspectives and approaches were the basis for the American Diabetes Association Position Statements and the International Consensus on the Diabetic Foot, resulting in a worldwide network of professionals involved in the management of diabetic patients with foot problems (CitationSchaper et al 2003). Applying evidence-based multidisciplinary treatment has been shown to result in a 50% reduction of major lower-limb amputation in this high risk group (CitationVan Damme and Limet 2005).
Lavery et al implemented a lower extremity disease management program consisting of screening and treatment protocols for the diabetic foot in a managed care organization and noted its effectiveness to reduce hospitalizations and amputations (CitationLavery et al 2005). Based on the presence or absence of diabetic neuropathy, peripheral vascular disease, foot deformities and pressures, as well as the history of lower extremity pathology, the authors stratified patients into low and high-risk groups, and implemented preventive or acute care protocols (CitationLavery et al 2005). They noted a 47.4% decrease in the incidence of amputations from 12.89 per 1000 diabetics per year to 6.18 (p < 0.05), and a 37.8% decrease in foot related hospital admissions, from 22.86 per 1000 members per year to 14.23 (37.8%), after implementation of the disease management program (CitationLavery et al 2005). They further noted a 21.7% reduction in the average patient length of stay from 4.75 to 3.72 days (p < 0.05), a 69.8% reduction in the number of skilled nursing facility admissions per 1000 members per year, and a 38.2% reduction in the average length of stay in a skilled nursing facility 8.72 to 6.52 days (p < 0.05) (CitationLavery et al 2005).
Singh et al conducted a literature review of the efficacy of various diabetic foot ulcer prevention methods in the primary care setting from articles published between January 1980 and April 2004 available through EBSCO, MEDLINE, the National Guideline Clearinghouse databases, Cochrane Library, and relevant Web sites (CitationSingh et al 2005). The authors noted substantial evidence to support screening of all patients with diabetes to identify those at risk for foot ulceration (CitationSingh et al 2005). The authors further noted that patients may benefit from certain prophylactic interventions, including patient education, prescription footwear, intensive podiatric care, and evaluation for surgical interventions (CitationSingh et al 2005). However, the patient and their health care professional must be fully informed of their problems, understand the management process, and be willing to make the necessary lifestyle changes to minimize complications (CitationHelfand 2003).
A thorough history and physical is fundamental to identify risk factors for the development of diabetic foot ulcers. This includes an assessment of loss of protective sensation, foot structure, limited joint mobility, vascular status, and a history of previous foot ulceration, amputation or Charcot neuroarthropathy (CitationLavery et al 1998; CitationMayfield et al 2003).
History
Known factors for foot ulcerations include a current ulcer, past history of previous ulceration, prior lower extremity amputation, or the presence of neuropathic fractures (CitationLavery et al 1998; CitationBoyko et al 1999; CitationAbbott et al 2002), which increase the risk for further ulceration, infection and subsequent amputation (CitationGoldner 1960; CitationPecoraro zet al 1990; CitationLavery et al 1998). Within one year of wound healing following ulceration, up to 60% of patients with a positive ulceration history will develop another because the skin plantar to that site may be less resilient and less well fortified to accept repetitive stress and therefore more prone to subsequent breakdown (CitationHelm et al 1991; CitationUccioli et al 1995). This population segment has the highest risk of developing subsequent foot ulceration (CitationLavery et al 1998; CitationPeters and Lavery 2001) and is the easiest risk group to identify. This patient population is also the group most in need of frequent foot assessment, intensive education, therapeutic shoes, padded stockings and rigorous blood glucose control.
Foot exam
Annual foot examinations are recommended for all individuals with diabetes to identify high-risk foot conditions including peripheral vascular insufficiency, structural foot deformities, and loss of protective sensation for which specific interventions have been shown to be effective in reducing amputation risk (CitationMayfield et al 2000).
Vascular exam
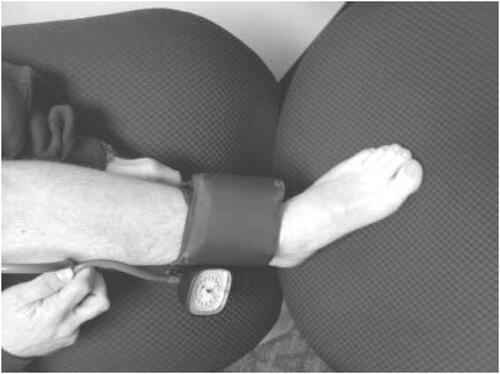
Peripheral arterial disease (PAD) is twice as common in persons with diabetes as in persons without (CitationGregg et al 2004) and is also a major risk factor for lower extremity amputation (2003). However, PAD is not the most common cause of foot ulceration, and is a component factor in only about a quarter of all cases (CitationEdmonds 1987; CitationThompson et al 1991). In a recent two-center study PAD partially contributed to 30% of all foot ulcers (CitationReiber et al 1999). Vascular assessment should include palpation of all lower extremity pulses, including femoral, popliteal, posterior tibial, and dorsalis pedis pulses. The palpation of pulses is a learned skill with a high degree of inter-observer variability and high false positive and false negative rates (2003). The dorsalis pedis pulse has been reported to be absent in 8.1% of healthy individuals and the posterior tibial pulse is absent in 2.0% (2003). Although the absence of both pedal pulses when assessed by a person experienced in this technique, strongly suggests the presence of vascular disease, the presence of palpable pulses cannot exclude peripheral vascular disease. Ankle brachial pressure index (ABPI) (), in contrast to the variability of pulse assessment and the often nonspecific nature of information obtained via history, is an easily reproducible and reasonably accurate method of diagnosing vascular insufficiency in the lower limbs (2003). However, a normal ABPI may be deceiving, as medial arterial calcification of the foot vessels results in hardening of the arteries thereby falsely elevating the ankle brachial pressure index (CitationBrooks et al 2001; CitationBonham 2006). CitationParameswaran et al (2005) assessed doppler waveform of lower extremity arteries, ABI and pulse oximetry in 57 consecutive patients with type 2 diabetes and no symptoms of PAD and found that 31% of the patients had PAD in the lower extremity (CitationParameswaran et al 2005). The authors noted that ABPI only had a sensitivity of 63% (95% CI, 46%–77%) and a specificity of 97% (95% CI, 91%–99%) (CitationParameswaran et al 2005).
In addition, clinical evidence of dependent rubor, pallor on elevation, absence of hair growth, dystrophic toenails, and cool, dry, fissured skin should also be noted as they may be concomitant signs of vascular insufficiency (2003).
Due to the high estimated prevalence of PAD in patients with diabetes, the ADA consensus statement issued the following recommendations (2003).
A screening ABPI be performed in all diabetic patients >50 years of age; if the results are normal, the test should be repeated every 5 years.
A screening ABPI should be considered in patients with diabetes <50 years of age who have other peripheral arterial disease risk factors. These risk factors include smoking, hypertension, hyperlipidemia, or duration of diabetes >10 years.
A diagnostic ABPI should be performed in any patient with symptoms of PAD.
Protective sensation
Protective sensation, a level of sensory loss that allows patients to hurt themselves without recognizing injury, is a major component of nearly all diabetic ulcerations (CitationReiber et al 1999). The consequent vulnerability to physical and thermal trauma increases the risk of foot ulceration sevenfold (CitationSingh et al 2005). All patients with diabetes should be screened for loss of protective sensation to identify those at risk for foot ulceration (CitationOlaleye et al 2001; CitationSingh et al 2005). The absence of protective sensation may be determined using simple, non-invasive instruments such as a 128 Hz tuning fork, a Semmes-Weinstein 5.07/10 gram monofilament nylon wire, a calibrated vibration perception threshold (VPT) meter, or by a comprehensive physical examination (CitationAbbott et al 2002).
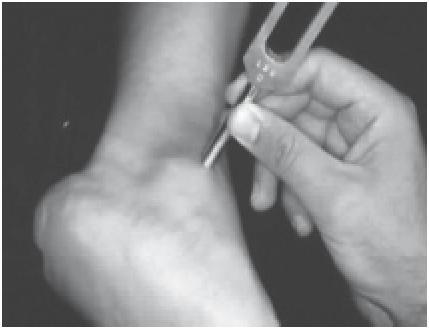
The conventional 128 Hz tuning fork test is an easy and inexpensive tool to assess vibratory sensation. The tuning fork is held over bony prominences such as the first metatarsal head and the lateral malleolus (), and the test is considered positive when the patient is unable to perceive any vibration that the examiner can perceive (CitationSingh et al 2005). The 5.07/10 g Semmes Weinstein monofilament consists of a plastic handle supporting a nylon filament () and is one of the most frequently utilized screening tools to identify loss of protective sensation in the United States (CitationArmstrong 2000; CitationSingh et al 2005). Testing via the Semmes Weinstein monofilament is administrated with the patient sitting supine in the examination chair with both feet level. The monofilament is applied perpendicular to the skin until it bends or buckles from the pressure, left in place for approximately one second and then released (CitationSingh et al 2005). The patient with his or her eyes closed responds “yes” each time he or she perceives the application of the monofilament.
Figure 3 Semmes Weinstein monofilament. The monofilament is applied perpendicular to the skin until it bends or buckles from the pressure, left in place for approximately one second and then released.
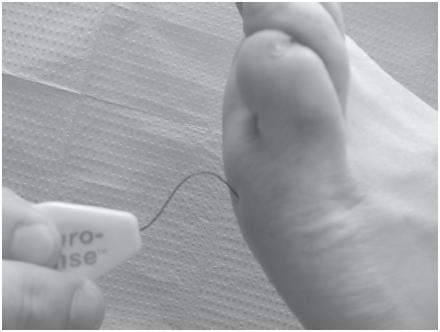
The patient’s inability to perceive 10 g of force applied by the 5.07 monofilament is clinically significant for large-fiber neuropathy. Although vibratory testing has demonstrated greater sensitivity (CitationSorman and Edwall 2002), the Semmes Weinstein monofilament test is sensitive enough to identify patients with the highest risk of foot complications (CitationGin et al 2002; CitationSorman and Edwall 2002).
The VPT meter, also known as Biothesiometer or Neurothesiometer, is a hand-held device with a rubber tactor that vibrates at 100 Hz. The hand-held device is connected to a base unit displaying a linear scale of applied voltage, ranging from 0 to 100 v (CitationArmstrong 1999; CitationPham et al 2000). The device is generally held with the rubber tactor balanced vertically on the pulp of the big toe. The voltage is increased until the patient perceives a vibration. A mean of three readings measured in Volts is generally used to determine the vibration perception threshold for each foot. In a prospective four year study, a vibration perception threshold greater than 25 v had a sensitivity of 83%, a specificity of 63%, a positive likelihood ratio of 2.2, and a negative likelihood ratio of 0.27 for predicting foot ulceration (CitationYoung et al 1994; CitationMason et al 1999). A comparison of the screening methods is illustrated in .
Table 1 Comparison of screening methods to help identify persons with diabetes at increased risk for foot ulceration
Foot deformities and biomechanics
Foot deformities and limitedjoint mobility impose excessive pressure on the plantar aspect of the foot. This limitation in joint mobility is secondary to non-enzymatic glycosylation of periarticular soft tissues and reduces the foot’s ability to accommodate for ambulatory ground reactive force to increase plantar pressure (CitationFernando et al 1991; CitationBirke et al 1995; CitationLavery et al 1995; CitationFrykberg et al 1998; CitationArmstrong et al 1999; CitationVan Damme and Limet 2005). This excessive pressure combined with the repetitive or constant stress from daily ambulation along with neuropathy will ultimately lead to failure of the protective integument and ulceration (). Although the precise pathophysiological mechanisms underlying the development of diabetic foot ulcerations are complex (CitationVan Damme and Limet 2005), it is generally associated with the presence of peripheral neuropathy and repetitive trauma due to normal walking activities which exposes the foot to moderate or high pressure and shear forces (CitationBrand 1991; CitationCavanagh et al 2005; CitationWu et al 2005). CitationBrand (1983) theorized that local inflammatory response, focal tissue ischemia, tissue destruction, and ulceration may occur when these types of forces are applied to a specific area over an extended period of time. Ulceration sites correlate with the highest plantar pressure points (CitationDuckworth et al 1982; CitationBoulton 1987; CitationBirke et al 1991; CitationCavanagh et al 1996; CitationArmstrong et al 1998). Foot deformities, limited joint mobility, partial foot amputations and other structural deformities often predispose diabetic persons with peripheral neuropathy to abnormal weight bearing areas of concentrated pressure that significantly increase their risk of ulceration (CitationLavery et al 1995; CitationBoulton 1996; CitationLavery et al 1996). In one study of patients with peripheral neuropathy, 28% with high plantar pressure developed a foot ulcer during a 2.5 year follow-up compared with none with normal pressure (CitationVeves et al 1992).
Figure 4 Neuropathic foot ulceration secondary to excessive pressure (from foot deformity) in combination with the repetitive stress from daily ambulation.
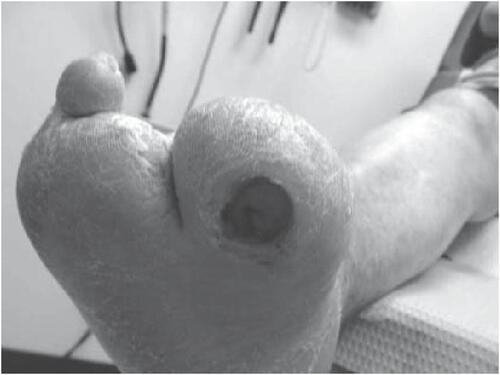
Lavery et al reported that patients with neuropathy but no deformity or history of ulcer or amputation are at 1.7 times greater risk for ulceration compared with patients without neuropathy (CitationLavery et al 1998). Neuropathy with concomitant deformity or limitedjoint mobility yields a 12.1 times greater risk, and patients with a history of previous ulceration or amputation have a 36.4 times greater risk for presenting with another ulcer. Additional studies by Peters and Lavery, and Mayfield and co-workers (2001) by and large support these findings (CitationMayfield et al 1996; CitationPeters and Lavery 2001).
Other contributing factors
Clinicians should also examine the patient for other contributing risk factors. Cutaneous manifestations associated with diabetes such as dry or fissured skin, calluses, tinea, or onychomycosis should all be noted.
Patient education
Educating patients at risk for diabetic foot ulceration have been shown to be beneficial (CitationMalone et al 1989; CitationLitzelman et al 1993; CitationSingh et al 2005). CitationMalone et al (1989) assessed the effectiveness of diabetic foot education by randomizing 103 patients (203 limbs) to receiving an hour foot care education and 100 patients (193 limbs) to receiving an hour of general diabetes mellitus education for 24 months total. The authors noted a lower incidence of foot ulcers in the group that received an hour of foot care education (4.5% vs 14.7%; RR, 0.31 [95% CI, 0.14 to 0.66]; ARR, −0.10 [95% CI, −0.16 to −0.04]; p = .002) (CitationMalone et al 1989). Litzelman and coworkers (1993) conducted sessions on foot care, telephone reminders, and postcard reminders in 191 patients and gave standard care to 205 patients for a period of 12 months and noted fewer serious foot lesions in the group that received sessions on foot care and telephone/postcard reminders (OR, 0.41 [95% CI, 0.16–1.00]; p =.05) (CitationLitzelman et al 1993).
Patients along with their family members or care takers should understand the implications of the loss of protective sensation, and the importance of daily foot examinations and the proper foot care (CitationMayfield et al 2003).
Treatment
There have been advances in managing diabetic foot ulceration with the development of new dressings, growth factors, bioengineered skin and tissue substitutes, hyperbaric oxygen, negative pressure wound therapy, and other novel approaches to stimulate wound healing (CitationSteed 1995; CitationSteed et al 1996; CitationGough et al 1997; CitationDonaghue et al 1998; Citation2000; CitationHopf et al 2001). Allogeneic bi-layered cultured skin equivalent (Apligraf, Organogenesis Inc., Canton, MA) is a living, biological dressing developed from neonatal foreskin and consists of living cells and structural proteins. It is FDA approved for the treatment of diabetic foot ulcers (2000) and was shown to heal more noninfected, nonischemic chronic plantar diabetic foot ulcers faster and in more patients than conventional therapy in a large-scale multi-center randomized prospective clinical trial (CitationVeves et al 2001). Becaplermin (Regranex, Johnson & Johnson, New Brunswick, NJ) is a hydrogel that contains 0.01% platelet derived growth factor-BB (rhPDGF-BB) and is currently the only commercially available topical growth factor for use in cutaneous wound healing. Although the efficacy becaplermin has been demonstrated in several studies (CitationRees et al 1999; CitationEmbil et al 2000) this has not been translated equivocally onto daily practice. It has been suggested that bercaplermin’s lack of clinical success may be secondary to the imbalance between levels of matrix metalloproteases and their inhibitors in the fluids of ulcers causes elevated levels of proteases (CitationYager et al 1996). The proteases in turn destroy essential growth factors, extracellular matrix proteins, and receptors, including the ones specific for PDGFBB to ultimately prevent wounds from healing (CitationLadwig et al 2002). Many suggest combining bercaplermin gel with collagen and oxidized regenerated cellulose to help bind the matrix metalloproteases and potentiate becaplermin’s effects on wound healing.
Collagen and oxidized regenerated cellulose (Promogran, Johnson & Johnson, New Brunswick, NJ) is a sterile, freeze dried matrix sheet. ORC absorbs wound exudate and forms a soft, biodegradable gel that binds and inactivates matrix metalloproteases (MMP) which have been shown to have a detrimental effect on wound healing when present in excessive quantities (CitationWysocki et al 1993; CitationLadwig et al 2002). In addition, ORC binds growth factors within the wound, protects them from degradation and releases them back into the wound in an active form as the matrix is slowly broken down. The effectiveness of ORC has been demonstrated in several studies (CitationVin et al 2002; CitationOmugha and Jones 2003).
Negative pressure wound therapy (Vacuum Assisted Closure, Kinetic Concepts Inc., San Antonio, TX) is a noninvasive wound closure system that uses controlled, localized sub-atmospheric pressure to help promote healing in chronic and acute wounds. This sub-atmospheric or negative pressure can be conveyed either continuously or intermittently though a sterile, latex free polyurethane or polyvinyl alcohol foam dressing. Negative Pressure Wound Therapy has been advocated my numerous authors as a safe and effective adjunctive modality in the treatment of diabetic foot wounds (CitationArmstrong et al 2002; CitationEginton et al 2003; CitationArmstrong and Lavery 2005; CitationMendonca et al 2005).
Despite these advances, it is imperative to remember the fundamental basics in the healing of diabetic foot ulcers: adequate perfusion, debridement, infection control, and pressure mitigation.
Vascularity
Adequate vascular perfusion is of utmost importance in wound healing (CitationDang and Boulton 2003). If pulses are not palpable, doppler ultrasound, ankle-brachial blood pressure indices as well as other non-invasive vascular studies such as segmental pressures, pulse volume recordings, and transcutaneous oxygen tension are warranted. A prompt vascular surgery consult and possible intervention such as angioplasty, stenting, or femorodistal bypass to improve perfusion and thereby effect healing is also indicated (CitationCavanagh et al 2005).
Infection control
Since infection is not well defined, the genesis, diagnosis and resolution of infection remains a clinical endeavor (CitationCavanagh et al 2005). Cultures, laboratory results, and subjective symptoms are helpful adjuncts. Wound cultures reveal the causative pathogens, however, tissue specimens are strongly preferred over wound swabs for wound cultures (CitationCavanagh et al 2005). While the diagnostic criteria for infection are imprecise, there is little doubt that infection is a major cause of lower extremity morbidity that frequently eventuates into wet gangrene and subsequent amputation. Antimicrobial therapy should be guided by culture results, focused at curing the infection rather than healing the wound (CitationCavanagh et al 2005).
Debridement
Debridement, the removal of hyperkeratotic and devitalized tissue, foreign materials, and particulate matter from a wound, is often the key first step of effective wound care (CitationLewis et al 2001; CitationArmstrong et al. 2004; CitationSteed 2004). Debridement helps reduce the rate of infection and provides an ideal healing environment by converting chronic wounds into acute (CitationFalanga 2004). Wound debridement helps reduce chronic inflammatory byproducts (CitationNwomeh et al 1999; CitationJude et al 2001; CitationArmstrong and Jude 2002) and may be accomplished surgically, chemically, mechanically, biologically, or by autolysis (CitationLewis et al 2001). Sharp or surgical debridement, the most direct and efficient method to clean the woundbed (CitationBlanke and Hallern 2003), is generally considered the gold standard.
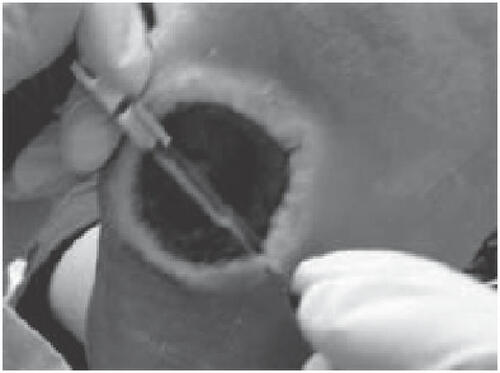
In addition to the wound base, it is also important to debride wound margins to mitigate the “edge effect” (CitationArmstrong and Athanasiou 1998; CitationArmstrong et al. 2004) (). The edge effect is secondary to the skin interruption which increases both vertical and shear stresses on the edges of that interruption (CitationArmstrong and Athanasiou 1998). The vertical force progressively deepens the wound, while the shear force from the underlying epithelium widens the wound via undermining (CitationArmstrong and Athanasiou 1998).
Pressure mitigation
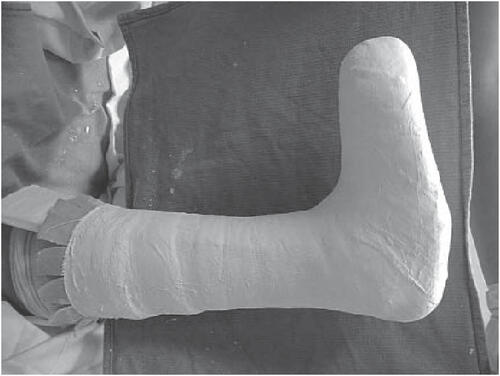
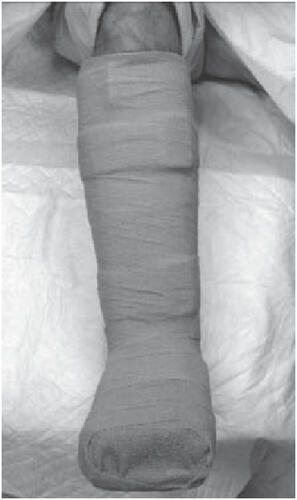
With sufficient vascular supply, appropriate debridement, moist wound environment, and infection control, the primary mode of healing a diabetic neuropathic foot ulcer is pressure dispersion (CitationArmstrong et al 2004; CitationWu et al 2005). Of the plethora of offloading modalities including bed rest, wheel chairs, crutches, total contact casts, felted foam, half shoes, therapeutic shoes, custom splints, and removable cast walkers, total contact casting (TCC) () is considered by many to be the “gold standard” in achieving pressure redistribution (CitationColeman et al 1984; CitationWalker et al 1985; CitationBoulton et al 1986; CitationSinacore et al 1987; CitationWalker et al 1987; CitationKominsky 1991; CitationLavery et al 1997). TCC has been shown to reduce pressure at the site of ulceration by 84%–92% (CitationLavery et al 1996) and is quite effective with healing rates ranging from 72% to 100% over a course of 5–7 weeks (CitationHelm et al 1984; CitationWalker et al 1985; CitationSinacore et al 1987; CitationWalker et al 1987; CitationMyerson et al. 1992). However, the application of TCC is time consuming and often associated with a learning curve. Most centers do not have a physician or cast technician available with adequate training or experience to safely apply a TCC; improper cast application can cause skin irritation and in some cases even frank ulceration. In addition TCCs do not allow daily assessment of the foot or wound and are therefore often contraindicated in cases of soft tissue or bone infections. Recent studies have shown that an instant total contact cast (iTCC), made by simply wrapping a removable cast walker with a single layer of cohesive bandage, elastoplast, or casting tape (, , ), may bejust as effective as a TCC in pressure mitigation and the healing of diabetic foot ulcers (CitationWu et al 2005). A randomized controlled study compared the standard TCC with an iTCC (CitationKatz et al 2005) and found no differences in healing rates and mean healing time between patients who received the TCC versus the iTCC. The proportions of patients that healed within 12 weeks in the iTCC and TCC groups were 80% and 74%, respectively (CitationKatz et al 2005). There were no differences in complications between the two groups, however, the cost in materials and personnel was much lower for the iTCC compared with the TCC. The study concluded that the iTCC is not only equally efficacious in healing diabetic foot ulcers, when compared with the TCC but is quicker, easier, and more cost effective than the TCC (CitationKatz et al 2005).
Conclusion
There is a high occurrence of foot ulcers within the population of people with diabetes. Foot ulcerations may lead to infections, lower extremity amputations and are major causes of disability to patients, often resulting in significant morbidity, extensive periods of hospitalization, and mortality. In order to diminish the detrimental consequences associated with diabetic foot ulcers, a high standard of care must be provided. Many of the etiological factors contributing to the formation of diabetic foot ulceration may be identified using simple, inexpensive equipment in a clinical setting, and early recognition of these factors along with prompt management of the ulcers are essential for successful outcome (CitationVan Damme and Limet 2005). Aggressive treatment of infections, correction of vascular occlusive disease, adequate wound care, and appropriate pressure mitigation are essential steps in the treatment protocol (CitationVan Damme and Limet 2005). With the implementation of good prevention and treatment programs, a significant reduction of lower extremity complications well within reach.
References
- Introduction. Healing chronic wounds: technologic solutions for today and tomorrowAdv Skin Wound Care200013Suppl245
- Living skin substitute can heal diabetic foot ulcer woundsFDA Consum2000346
- Peripheral arterial disease in people with diabetesDiabetes Care20032633334114633825
- Incidence of end-stage renal disease among persons with diabetes—United States, 1990–2002MMWR Morb Mortal Wkly Rep200554109710016267496
- AbbottCACarringtonALThe North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohortDiabet Med2002193778412027925
- ArmstrongDGLoss of protective sensation: a practical evidence-based definitionJ Foot Ankle Surg199938798010028476
- ArmstrongDGThe 10-g monofilament: the diagnostic divining rod for the diabetic foot? [editorial] [In Process Citation]Diabetes Care20002388710895835
- ArmstrongDGAthanasiouKAThe edge effect: how and why wounds grow in size and depthClin Podiatr Med Surg19981051089463771
- ArmstrongDGJudeEBThe Role of Matrix Metalloproteinases in Wound HealingJ Amer Podiatr Med Assn2002In Press
- ArmstrongDGLaveryNegative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trialLancet2005366949817041016291063
- ArmstrongDGLaveryLAOutcomes of subatmospheric pressure dressing therapy on wounds of the diabetic footOstomy Wound Manage20024864811993062
- ArmstrongDGLaveryLAIt is not what you put on, but what you take off: techniques for debriding and offloading the diabetic foot woundClin Infect Dis200439S92915306986
- ArmstrongDGLipskyBAAdvances in the treatment of diabetic foot infectionsDiabetes Technol Ther200461677715117583
- ArmstrongDGPetersEJIs there a critical level of plantar foot pressure to identify patients at risk for neuropathic foot ulceration?J Foot Ankle Surg19983730379710782
- ArmstrongDGStacpoole-SheaSLengthening of the achilles tendon in diabetic patients who are at high risk for ulceration of the footJ Bone Joint Surg (Am)199981A535810225799
- BirkeJAFranksDFirst ray joint limitation, pressure, and ulceration of the first metatarsal head in diabetes mellitusFoot Ankle199516277847633584
- BirkeJANovickAMethods of treating plantar ulcersPhys Ther199171116221989007
- BlankeWHallernBVSharp wound debridement in local anaesthesia using EMLA cream: 6 years’ experience in 1084 patientsEur J Emerg Med2003102293112972901
- BlockPThe diabetic foot ulcer: a complex problem with a simple treatment approachMilit Med19811466446
- BonhamPAGet the LEAD out: noninvasive assessment for lower extremity arterial disease using ankle brachial index and toe brachial index measurementsJ Wound Ostomy Continence Nurs200633304116444101
- BoultonAJThe pathogenesis of diabetic foot problems: an overviewDiabet Med199613Suppl 1S12168741822
- BoultonAJVileikyteLThe diabetic foot: the scope of the problem [In Process Citation]J Fam Pract200049Suppl 11S3811093553
- BoultonAJMConnorHBoultonAJMWardJDThe importance of abnormal foot pressure and gait in causation of foot ulcersThe foot in diabetes1987ChilchesterJohn Wiley and Sons1126
- BoultonAJMBowkerJHUse of plaster casts in the management of diabetic neuropathic foot ulcersDiabetes Care19869149523698780
- BoykoEJAhroniJHA prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot StudyDiabetes Care19992210364210388963
- BrandPWEllenbergMRifkinHThe diabetic footDiabetes mellitus, theory and practice1983New YorkMedical Examination Publishing80328
- BrandPWJahssMThe insensitive foot (including leprosy)Disorders of the Foot and Ankle1991PhiladelphiaSaunders21705
- BrooksBDeanRTBI or not TBI: that is the question. Is it better to measure toe pressure than ankle pressure in diabetic patients?Diabet Med2001185283211553180
- CavanaghPRLipskyBATreatment for diabetic foot ulcersLancet2005366949817253516291067
- CavanaghPRUlbrechtJSBiomechanical aspects of diabetic foot disease: aetiology, treatment, and preventionDiabet Med199613Suppl 1S17228741823
- ColemanWBrandPWThe total contact cast, a therapy for plantar ulceration on insensitive feetJ Am Podiatr Med Assoc19847454852
- CoppiniDVYoungPJOutcome on diabetic foot complications in relation to clinical examination and quantitative sensory testing: a case-control studyDiabet Med199815765719737806
- DangCNBoultonAJChanging perspectives in diabetic foot ulcer managementInt J Low Extrem Wounds2003241215866821
- DonaghueVMChrzanJSEvaluation of a collagen-alginate wound dressing in the management of diabetic foot ulcersAdv Wound Care199811114199729942
- DuckworthTBettsRPThe measurement of pressure under the footFoot and Ankle198231307152398
- EdmondsMEConnorHBoultonAJMWardJDExperience in a multidisciplinary diabetic foot clinicThe foot in diabetes1987ChichesterJohn Wiley and Sons12131
- EgintonMTBrownKRA prospective randomized evaluation of negative-pressure wound dressings for diabetic foot woundsAnn Vasc Surg200317645914534844
- EmbilJMPappKRecombinant human platelet-derived growth factor-BB (becaplermin) for healing chronic lower extremity diabetic ulcers: an open-label clinical evaluation of efficacyWound Repair Regen20008162810886806
- FalangaVThe chronic wound: impaired healing and solutions in the context of wound bed preparationBlood Cells Mol Dis200432889414757419
- FernandoDJSMassonEARelationship of limitedjoint mobility to abnormal foot pressures and diabetic foot ulcerationDiabetes Care1991148111991444
- FrykbergRGLaveryLARole of neuropathy and high foot pressures in diabetic foot ulceration [In Process Citation]Diabetes Care1998211714199773736
- GibbonsG.EliopoulosG. M.KozakGPHoarCSRowbothamJLInfection of the Diabetic FootManagement of Diabetic Foot Problems1984PhiladelphiaWB Saunders97102
- GinHRigalleauVComparison between monofilament, tuning fork and vibration perception tests for screening patients at risk of foot complicationDiabetes Metab2002284576112522325
- GoldnerMGThe fate of the second leg in the diabetic amputeeDiabetes19609100313850722
- GoughAClappertonMRandomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infectionLancet1997350908185599310604
- GreggEWSorliePPrevalence of lower-extremity disease in the US adult population ≥40 years of age with and without diabetes: 1999-2000 national health and nutrition examination surveyDiabetes Care2004271591715220233
- HelfandAEAssessing and preventing foot problems in older patients who have diabetes mellitusClin Podiatr Med Surg2003205738212952054
- HelmPAWalkerSCTotal contact casting in diabetic patients with neuropathic foot ulcerationsArch Phys Med Rehabil19846569136497615
- HelmPAWalkerSCRecurrence of neuropathic ulcerations following healing in a total contact castArch Phys Med Rehabil199172967701953319
- HopfHWHumphreyLMAdjuncts to preparing wounds for closure: hyperbaric oxygen, growth factors, skin substitutes, negative pressure wound therapy (vacuum-assisted closure)Foot Ankle Clin200166618212134577
- JudeEBRogersAAMatrix metalloproteinase and tissue inhibitor of metalloproteinase expression in diabetic and venous ulcersDiabetologia200144Suppl 1A3
- KatzIAHarlanAA randomized trial of two irremovable off-loading devices in the management of plantar neuropathic diabetic foot ulcersDiabetes Care200528555915735187
- KominskySJThe ambulatory total contact cast. The high risk foot in diabetes mellitus1991F. R. New YorkChurchill Livingstone44955
- LadwigGPRobsonMCRatios of activated matrix metallo-proteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcersWound Repair Regen200210263711983004
- LaveryLAArmstrongDGPractical criteria for screening patients at high risk for diabetic foot ulcerationArch Intern Med199815815862
- LaveryLAArmstrongDGHealing Rates of Diabetic Foot Ulcers Associated with Midfoot Fracture Due to Charcot’s Arthropathy.”Diabetic Medicine19971446499017353
- LaveryLAArmstrongDGPredictive value of foot pressure assessment as part of a population-based diabetes disease management programDiabetes Care20032610697312663575
- LaveryLALaveryDCIncreased foot pressures after great toe amputation in diabetesDiabetes Care199518146028722070
- LaveryLAVelaSAReducing dynamic foot pressures in high-risk diabetic subjects with foot ulcerations. A comparison of treatmentsDiabetes Care199619818218842597
- LaveryLAWunderlichRPDisease management for the diabetic foot: effectiveness of a diabetic foot prevention program to reduce amputations and hospitalizationsDiabetes Res Clin Pract20057031716126121
- LewisRWhitingPA rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intentionHealth Technol Assess20015113111399237
- LitzelmanDKSlemendaCWReduction of lower extremity clinical abnormalities in patients with non-insulin-dependent diabetes mellitus. A randomized, controlled trialAnn Intern Med199311936418498761
- MaloneJMSnyderMPrevention of amputation by diabetic educationAm J Surg19891585203 discussion 523–42589581
- MasonJO’KeeffeCA systematic review of foot ulcer in patients with Type 2 diabetes mellitus. II: treatmentDiabet Med19991688990910588519
- MayfieldJAReiberGEA foot risk classification system to predict diabetic amputation in pima indiansDiabetes Care19961970498799623
- MayfieldJAReiberGEDo foot examinations reduce the risk of diabetic amputation?J Fam Pract20004949950410923547
- MayfieldJAReiberGEPreventive foot care in people with diabetesDiabetes Care200326Suppl 1S78912502623
- MendoncaDACoskerTVacuum-assisted closure to aid wound healing in foot and ankle surgeryFoot Ankle Int200526761616174508
- MorelandMEKilbourneAMDiabetes preventive care and non-traumatic lower extremity amputation ratesJ Healthc Qual20042612715468650
- MyersonMPapaJThe total contact cast for management of neuropathic plantar ulceration of the footJ Bone Joint Surg199274A26191311710
- NwomehBCLiangHXMMP-8 is the predominant collagenase in healing wounds and nonhealing ulcersJ Surg Res199981189959927539
- OlaleyeDPerkinsBAEvaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinicDiabetes Res Clin Pract2001541152811640995
- OmughaNJonesAMThe management of hard-to-heal necrobiosis with PROMOGRANBr J Nurs200312Suppl 15S142012937381
- ParameswaranGIBrandKPulse oximetry as a potential screening tool for lower extremity arterial disease in asymptomatic patients with diabetes mellitusArch Intern Med2005165442615738375
- PecoraroREReiberGEPathways to diabetic limb amputation: basis for preventionDiabetes Care199013513212351029
- PetersEJLaveryLAEffectiveness of the diabetic foot risk classification system of the International Working Group on the Diabetic FootDiabetes Care2001241442711473084
- PhamHTArmstrongDGScreening techniques to identify the at risk patients for developing diabetic foot ulcers in a prospective multicenter trialDiabetes Care2000236061110834417
- PhamHTEconomidesPAThe role of endothelial function on the foot. Microcirculation and wound healing in patients with diabetesClin Podiatr Med Surg19981585939463769
- PinzurMSSlovenkaiMPGuidelines for diabetic foot care: recommendations endorsed by the Diabetes Committee of the American Orthopaedic Foot and Ankle SocietyFoot Ankle Int2005261131915680122
- PlankJHaasWEvaluation of the impact of chiropodist care in the secondary prevention of foot ulcerations in diabetic subjectsDiabetes Care2003261691512766095
- Ragnarson TennvallGApelqvistJHealth-economic consequences of diabetic foot lesionsClin Infect Dis200439Suppl 2S132915306992
- ReesRSRobsonMCBecaplermin gel in the treatment of pressure ulcers: a phase II randomized, double-blind, placebo-controlled studyWound Repair Regen19997141710417749
- ReiberGEJ. BowkerHPfeiferMAEpidemiology of foot ulcers and amputations in the diabetic footThe Diabetic Foot2001St. LouisMosby1332
- ReiberGEVileikyteLCausal pathways for incident lower-extremity ulcers in patients with diabetes from two settingsDiabetes Care1999221576210333919
- Rith-NajarianSJStoluskyTIdentifying diabetic patients at risk for lower extremity amputation in a primary health care settingDiabetes Care199215138691425105
- RonnemaaTHamalainenHEvaluation of the impact of podiatrist care in the primary prevention of foot problems in diabetic subjectsDiabetes Care199720183379405902
- SchaperNCApelqvistJThe international consensus and practical guidelines on the management and prevention of the diabetic footCurr Diab Rep20033475914611743
- SchweglerBBoniT[Practical management of diabetic foot]Ther Umsch2002594354212235737
- SinacoreDRMuellerMJDiabetic plantar ulcers treated by total contact castingPhys Ther198767154373310052
- SinghNArmstrongDGPreventing foot ulcers in patients with diabetesJAMA20052932172815644549
- SmithDWeinbergerMA controlled trial to increase office visits and reduce hospitalization in diabetic patientsJ General Int Med198722328
- SormanEEdwallLL[Examination of peripheral sensibility. Vibration test is more sensitive than monofilament test]Lakartidningen20029913394011998167
- SteedDLClinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study GroupJ Vasc Surg1995217187823364
- SteedDLDebridementAm J Surg200418771S74S15147995
- SteedDLDonohoeDEffect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study GroupJ Am Coll Surg19961836148673309
- StocklKVanderplasACosts of lower-extremity ulcers among patients with diabetesDiabetes Care20042721293415333473
- ThompsonFJVevesAA team approach to diabetic foot care- the Manchester experienceFoot199117582
- UccioliLFagliaEManufactured shoes in the prevention of diabetic foot ulcersDiabetes Care199518137688721941
- Van DammeHLimetR[The diabetic foot]Rev Med Liege2005605162516035320
- VevesAFalangaVGraftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Apligraf Diabetic Foot Ulcer StudyDiabetes Care200124290511213881
- VevesAMurrayHJThe risk of foot ulceration in diabetic patients with high foot pressure: a prospective studyDiabetolgica1992356603
- VinFTeotThe healing properties of Promogran in venous leg ulcersJ Wound Care2002113354112430368
- WalkerSCHelmPAChronic diabetic neuropathic foot ulcerations and total contact casting: healing effectiveness and outcome probability [abstract]Arch Phys Med Rehabil198566574
- WalkerSCHelmPATotal contact casting and chronic diabetic neuropathic foot ulcerations: healing rates by wound locationArch Phys Med Rehabil198768217213566513
- WuSCCrewsRTThe pivotal role of offloading in the management of neuropathic foot ulcerationCurr Diab Rep20055423916316592
- WysockiABStaiano-CoicoLWound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9J Invest Dermatol19931016488392530
- YagerDRZhangLYWound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluidsJ Invest Dermatol199610774388875960
- YoungMJBreddyJLThe prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective studyDiabetes Care199417557608082524