Abstract
Inhaled human insulin (Exubera®) is a rapid-acting regular human insulin administered by oral inhalation before meals. It provides a non-invasive alternative to multiple subcutaneous injections for the treatment of hyperglycemia in adult patients with type 1 and type 2 diabetes. Compared with subcutaneous rapid-acting insulin analogs, Exubera provides equivalent HbA1c control. As a monotherapy or in combination with oral agents, Exubera also provides greater glycemic control than oral agents alone, at least in patients with high levels of HbA1c. Exubera demonstrates improved patient satisfaction compared with subcutaneous insulin or oral agents alone. When offered as a treatment option together with standard treatments in uncontrolled patients naïve to insulin, Exubera increases acceptance of insulin therapy three-fold compared with patients offered standard regimens only. Exubera is well tolerated in comparison to subcutaneous insulin, with a similar incidence of mild to moderate hypoglycemia. Although cough is a common adverse effect early in therapy, this leads to treatment discontinuations in less than 1% of patients. Despite an increased incidence of insulin antibodies compared with subcutaneous administration, and a consistent but minor impact on pulmonary function, long-term safety data of up to 4 years continue to support the safety profile of Exubera.
Introduction
Diabetes mellitus is a major contributor to the global disease burden and is currently experiencing a dramatic rise in prevalence. The World Health Organization forecasts a virtual doubling in number of those currently affected to more than 350 million cases by 2030 (CitationWHO 2006). Type 2 diabetes will account for most of the projected increase, which reflects not only the demographics of an aging population, but also increasing numbers of overweight and obese people who are at increased risk of diabetes (CitationGungor and Arslanian 2002).
Compared with the healthy population, diabetes sufferers are at considerable increased risk of morbidity from cardiovascular, cerebrovascular, and peripheral vascular disease, leading to outcomes such as myocardial infarction, stroke and limb amputation (CitationKhaw et al 2004). Individuals with diabetes have a two- to four-fold increased risk of experiencing a cardiovascular event compared with age-matched individuals without diabetes, while the risk of mortality following myocardial infarction is approximately two- to three-fold greater (CitationAmerican Diabetes Association 1998). Cardiac risk factors, such as smoking, hypertension, high cholesterol and excessive weight take on increased significance in the patient with diabetes, and should be treated as aggressively as for the non-diabetic individual with a prior myocardial infarction (CitationGoldfine and Goldfine 2003). In addition to life-threatening macrovascular complications typical of type 2 diabetes, serious complications of the microvasculature, such as neuropathy, nephropathy, and retinopathy, can adversely affect quality of life while imposing a heavy burden on healthcare systems (CitationStratton et al 2000; CitationKhaw et al 2004).
Improved blood glucose control is an important therapeutic goal in diabetes, with intensive control known to be important for reducing the risk of microvascular disease (CitationReichard et al 1991; CitationUKPDS 1996) (). Initial therapy with oral antidiabetic agents can be effective at achieving glycemic control in type 2 diabetes, but as a chronic disease marked by a progressive course, most patients eventually require insulin therapy for effective control (CitationTurner et al 1999; CitationCook et al 2005). Physicians typically rely on a stepwise approach to achieving glycemic control, beginning with diet and exercise, followed by initiation of single agent oral antidiabetic therapy, and progressing to combination oral agents and subsequently insulin (CitationCampbell 2000) (). However, with this approach, patients may be receiving suboptimal glycemic control for many months and even years before they are progressed to the next level of treatment (CitationCampbell 2000; CitationBrown and Nichols 2003). A more proactive approach to glucose management is needed to assist not only the estimated 60% of patients currently failing to reach recommended glycemic targets, but also to more rapidly achieve glycemic targets in all patients (CitationCampbell 2000; CitationDel Prato et al 2005).
Figure 1 Conservative versus proactive management of type 2 diabetes: (A) traditional stepwise approach to long-term glycemic control and (B) early combination approach. Reproduced with permission from Campbell IW. 2000. Need for intensive early glycaemic control in patients with type 2 diabetes. Br J Cardiol, 7:625–31.
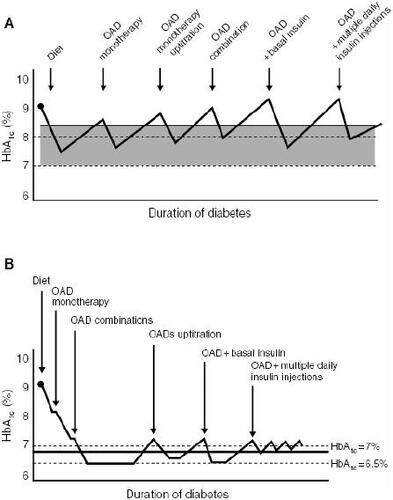
Table 1 Recommended targets for glycemic control
Importance of insulin therapy
Lifestyle intervention in the form of diet and exercise regimens is an integral component of diabetes management, but adherence to such regimens is often difficult to achieve and maintain, and most patients with type 2 diabetes will require pharmacologic intervention for glycemic control (CitationMudaliar and Henry 1999).
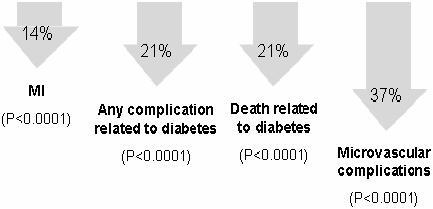
Randomized, controlled trials have provided compelling evidence that achieving strict glycemic control can reduce the long-term complications of diabetes (CitationDCCT 1993; CitationMalmberg 1997; CitationUKPDS 1998; CitationShichiri et al 2000). In the Diabetes Control and Complications Trial (DCCT), patients with type 1 diabetes with and without mild retinopathy at baseline were assigned to either intensive insulin treatment involving frequent blood glucose monitoring or conventional therapy. In the primary prevention cohort, there was a strong relationship between risk of retinopathy and mean glycosylated hemoglobin (HbA1c) levels. For each 10% decrease in HbA1c, such as from 8.0% to 7.2%, there was a 39% decrease in risk over the range of HbA1c values. Furthermore, intensive insulin therapy delivered by external pump or by three-times-daily injections was associated with a 47% reduction in the development of severe retinopathy in patients with prior retinopathy at baseline and a 76% reduction in risk of developing retinopathy in the primary prevention cohort, as compared with conventional therapy involving once- or twice-daily insulin injections (CitationDCCT 1993). The UK Prospective Diabetes Study (UKPDS) evaluated the impact of intensive insulin or oral antidiabetic therapy versus diet alone on microvascular and macrovascular complications in patients with newly diagnosed type 2 diabetes. Projections suggested that for each 1% reduction in mean HbA1c there would be a corresponding 37% reduction in the risk of microvascular complications, a 14% lower rate of myocardial infarction, and 21% fewer deaths related to diabetes (CitationStratton et al 2000) ().
Figure 2 In the UK Prospective Diabetes Study (UKPDS), each 1% reduction in HbA1c was projected to produce significant reductions in the risk of diabetesrelated complications (CitationStratton et al 2000).
While DCCT, UKPDS and other studies have been useful in demonstrating the overall benefit of insulin treatment in preventing the onset and/or progression of complications in patients with both type 1 and type 2 diabetes, the same studies also lend support to early implementation of insulin (CitationDCCT 1993; CitationUKPDS 1998; CitationShichiri et al 2000; CitationDCCT/EDIC 2002). Early and intensive pharmacologic intervention takes on increased significance given that postprandial excursions, which appear to be more adequately controlled by insulin compared with oral antidiabetic agents, have been linked to increased cardiovascular risk (CitationMalone et al 2003; CitationCeriello et al 2004; CitationEsposito et al 2004). Increasingly, evidence suggests that insulin has a protective role against endothelial dysfunction, which in the context of postprandial hyperglycemia may prevent progression of atherosclerosis (CitationCeriello et al 2004; CitationEsposito et al 2004). Studies of intensive insulin therapy versus conventional treatment for tight glycemic control in high-risk patients with or without diabetes have demonstrated a survival benefit for intensive insulin therapy, which is putatively related to anti-inflammatory, anti-thrombogenic and anabolic effects of insulin (CitationMalmberg et al 1999; CitationVan den Berghe et al 2001; CitationLazar et al 2004).
Achievement of glycemic targets
One of the main goals of diabetes management is to achieve blood glucose levels that are as close to the normal range as possible in order to prevent the development of diabetic complications (CitationTurner et al 1999). In contrast to microvascular disease, evidence suggests there is a continuous relationship between blood glucose concentrations and macrovascular disease, which continues even below diagnostic threshold levels for diabetes (CitationKhaw et al 2004). However, despite increasingly stringent guidelines, over 60% of patients throughout the world are currently not reaching glycemic targets (Del Prato et al 2005). In a 6-month study evaluating physician records of 7000 patients with type 2 diabetes from 8 European countries, just 31% of patients achieved good glycemic control defined as HbA1c ≤6.5%, while the mean HbA1c value across the entire study population was 7.5% (CitationLiebl et al 2002). Similarly, in a US study sample derived from the National Health and Nutrition Examination Survey (NHANES), glycemic control rates defined as HbA1c level <7% declined from 44.5% between 1988 and 1994 to 35.8% between 1999 and 2000 (CitationKoro et al 2004). A further worrying trend to have emerged from this study was the decline in insulin usage from 24.2% to 16.4% of those surveyed over the same period (CitationKoro et al 2004).
Physicians often wait too long to move patients from oral antidiabetic therapy to insulin (CitationHayward et al 1997; CitationNathan 2002). In addition to a lack of consensus among physicians as to when and how intensive insulin therapy should be initiated, the delay in using insulin may also relate to concerns that insulin therapy promotes insulin resistance, increases the risk of cardiovascular events, is not effective at controlling hyperglycemia, and is associated with dramatic weight gain (CitationRiddle 2002). However, such concerns are unfounded, with recent findings demonstrating that insulin safely improves glycemic control without promoting increased hypoglycemia or weight gain (CitationHayward et al 1997; CitationWright et al 2002; CitationRiddle 2002). Moreover, the tendency among physicians to reserve insulin therapy for those patients who are inadequately controlled with diet and oral antidiabetic agents means that a high proportion of patients receiving insulin will have pre-existing co-morbidities and complications due to longstanding disease (CitationLiebl et al 2002). In recognizing the barriers to achieving current glycemic targets, the Global Partnership for Effective Diabetes Management now recommends that combination therapy or insulin should be initiated immediately for all patients with HbA1c ≥9% at diagnosis (CitationDel Prato et al 2005). In addition, most patients with established diabetes who are unable to achieve recommended glycemic goals using oral antidiabetic agents are candidates for insulin therapy.
Development of inhaled insulins
The potential benefits of offering subcutaneous insulin therapy in patients with diabetes are frequently limited due to injection aversion, psychological resistance to another therapy following prior failure to control glycemia, concerns over complications and pain, and fear of disease progression, among others (CitationHunt et al 1997; CitationKorytkowski 2002; CitationFunnell et al 2004; CitationHeinemann 2004; CitationFreemantle et al 2005). Moreover, reluctance to initiate insulin therapy is often shared by patients and physicians alike (CitationKorytkowski 2002; CitationFunnell et al 2004). As a result, adherence to an insulin regimen can be difficult to achieve and maintain, thereby compromising optimal glycemic control (CitationRoyle et al 2003).
The successful development of an inhalable, rapid-acting insulin that represents a noninvasive alternative to multiple daily subcutaneous insulin injections promises to change the management of diabetes. Made possible by advances in inhaler devices and insulin formulation technology, there are now several insulin inhalation systems at varying stages of clinical development (CitationPatton et al 2004). Inhaled human insulin (Exubera® (insulin human [rDNA origin]) Inhalation Powder), developed by Pfizer Inc in collaboration with Nektar Therapeutics, has received approval in both the US and the European Union for the control of hyperglycemia in adult patients with type 1 or type 2 diabetes. Exubera consists of a fine, dry-powder formulation of regular human insulin packaged in unit doses of 1 or 3 mg for inhalation in blister packs, which are administered via a unique and reusable mechanical pulmonary inhaler. Clinical experience to date indicates that Exubera has the potential to offer treatment and quality of life benefits to patients with diabetes, which are achieved through the delivery of a systemic dose of insulin via the pulmonary route while fulfilling the appropriate pharmacokinetic and pharmacodynamic profiles to effectively control hyperglycemia (CitationPatton et al 2004).
Clinical pharmacology
The pharmacokinetic profile of Exubera closely mimics the natural pattern of postprandial insulin secretion that is also achieved with rapid-acting subcutaneous insulin analogs, but not regular human insulin (CitationRave et al 2005). Whereas regular insulin has a relatively slow onset of action and a prolonged duration of action when injected subcutaneously, resulting in suboptimal control of postprandial hyperglycemia, Exubera is associated with an onset of action that is at least as fast as the subcutaneously injected rapid-acting insulin analog, insulin lispro (CitationRave et al 2005). Peak serum insulin concentrations following inhalation of Exubera, or subcutaneous administration of insulin lispro or regular insulin reflect glucose consumption rates, with Exubera attaining peak serum levels at a faster rate than either insulin lispro or regular insulin (CitationRave et al 2005).
In addition to offering a rapid onset of action, the longer duration of action of Exubera relative to insulin lispro may be better suited to postprandial glucose control. Clinical studies have suggested that the duration of action of subcutaneously administered insulin analogs is too short to provide adequate postprandial control, as indicated by rising glucose levels post-absorption (CitationDel Sindaco et al 1998; CitationCiofetta et al 1999; CitationRave et al 2005). The reason for the prolonged metabolic action of inhaled insulin relative to subcutaneously administered rapid-acting insulin analogs is unclear, but may relate to the size-dependent absorption and dissociation characteristics of inhaled insulin particles (CitationRave et al 2005).
Smoking has a significant impact on the absorption of Exubera, with pharmacokinetic analysis indicating that absorption of inhaled insulin is increased in smokers relative to non-smokers (CitationBecker et al 2006). This effect is partly reversed after only 1 week of smoking cessation, but reverts back to absorption levels typical of chronic smokers within a couple of days of smoking resumption. Due to the increased risk of hypoglycemia in smokers as a consequence of short-term changes in insulin availability, patients with diabetes should abstain from smoking before and during treatment with Exubera (CitationBecker et al 2006).
Variable response to insulin among patients with diabetes is an important aspect of insulin delivery in the context of clinical practice. In a comparison of Exubera and subcutaneous insulin in obese, elderly patients with type 2 diabetes, within-subject variability at doses producing comparable systemic insulin exposure over 6 hours was at least as good for inhaled insulin as for subcutaneous administration (CitationHenry et al 2003). Thus, inhaled insulin offers the benefits of non-invasive administration and a pharmacokinetic-pharmacodynamic profile that combines the relative advantages of rapid-acting insulin analogs (rapid onset of action) and regular insulin (prolonged metabolic action), while also offering consistent absorption in diverse patient groups making it suitable as an insulin replacement therapy (CitationHenry et al 2003; CitationRave et al 2005).
Efficacy and tolerability
Efficacy
Randomized clinical trials in patients with type 1 and type 2 diabetes have shown that Exubera achieves and maintains effective glycemic control that is comparable to subcutaneously administered regular and NPH insulin (CitationCefalu et al 2001; CitationSkyler et al 2001; CitationHollander et al 2004; CitationQuattrin et al 2004; CitationDumas et al 2005; CitationSkyler et al 2005). In the original proof-of-concept study, 53 patients with type 2 diabetes for a mean duration of 11 years were randomized in an open-label manner to either continue to receive subcutaneous insulin therapy or to switch to inhaled insulin for a period of 12 weeks (CitationCefalu 2001; CitationCefalu et al 2001; CitationCappelleri et al, 2002). Patients in the experimental group received preprandial inhaled insulin plus a bedtime subcutaneous ultralente insulin injection. Inhaled insulin treatment significantly improved HbA1c compared with baseline, achieving glycemic control that was at least as good as conventional treatment. In a similar study conducted in patients with type 1 diabetes, changes in HbA1c and glycemic control were indistinguishable for inhaled versus conventional insulin treatment (CitationSkyler et al 2001). In Phase III randomized controlled trials of patients with type 1 and type 2 diabetes, Exubera achieved similar reductions in HbA1c as subcutaneous insulin. However, in type 1 diabetes, Exubera was more effective than subcutaneous insulin in reducing fasting and postprandial plasma glucose, while in type 2 diabetes, more patients treated with Exubera compared with subcutaneous insulin achieved an HbA1c level of <7.0% after 6 months (CitationHollander et al 2004; CitationQuattrin et al 2004).
In clinical trials of patients with type 2 diabetes inadequately controlled with oral antidiabetic agents alone, patients assigned to Exubera monotherapy or in combination with oral agents had greater improvement in HbA1c compared with patients treated with oral agents alone (CitationWeiss et al 2003; CitationRosenstock et al 2005; CitationBarnett et al 2006a, Citationb). One of these trials, a 6-month randomized open-label study comparing inhaled insulin with metformin as adjunctive therapy, recruited patients typically seen in clinical practice with a range of BMI values and a baseline HbA1c value of at least 8.0% (CitationBarnett et al 2006a). Compared with metformin plus a sulfonylurea, inhaled insulin in combination with a sulfonylurea produced a significantly greater reduction in HbA1c in patients with a baseline HbA1c value >9.5%. Two 3-month investigations provided further demonstration of a benefit for inhaled insulin (CitationWeiss et al 2003; CitationRosenstock et al 2005). In both these studies the percentage of patients achieving HbA1c <7.0% was higher for those assigned to a regimen that included inhaled insulin compared with oral antidiabetic agents alone.
Physicians continue to place their greatest emphasis on fasting plasma glucose and HbA1c levels in the management of type 2 diabetes. However, the disease is characterized by a gradual decline in insulin secretion in response to nutrient loading, making the third component of the glucose triad – postprandial plasma glucose – an important consideration for effective disease management. Evidence is increasingly supporting a more prominent role for postprandial plasma glucose regulation, with postprandial hyperglycemia contributing at least 70% of the overall glycemic load in patients with an HbA1c level of around 7.0% (CitationLeiter et al 2005). In clinical trials, Exubera has been shown to effectively control HbA1c concentrations in patients with type 1 or type 2 diabetes, while there is also evidence that it improves postprandial plasma glucose and fasting plasma glucose levels compared with oral agents alone or subcutaneous insulin regimens (CitationHollander et al 2004; CitationQuattrin et al 2004; CitationWeiss et al 2003; CitationRosenstock et al 2005; CitationSkyler et al 2005).
Tolerability
Hypoglycemia and cough are the main adverse effects reported in clinical trials of Exubera (CitationOdegard and Capoccia 2005). Consistent with the incidence of hypoglycemia with subcutaneous insulin use, hypoglycemia is the most frequent adverse effect for Exubera with an event rate of around 0.3 to 1.4 events per patient-month in type 2 diabetes, and 5.5 to 9.3 events per patient-month in type 1 diabetes (CitationCefalu et al 2001; CitationSkyler et al 2001; CitationHollander et al 2004; CitationQuattrin et al 2004; CitationWeiss et al 2003; CitationSkyler et al 2005; CitationBarnett et al 2006). However, both the frequency and nature of hypoglycemia with Exubera use are comparable to those with subcutaneous insulin, with most events being mild to moderate in severity (CitationOdegard and Capoccia 2005). Mild cough is not an unexpected finding with inhaled insulin and occurs more frequently than with subcutaneous insulin, although symptoms decrease over time (CitationHollander et al 2004; CitationQuattrin et al 2004). Fewer than 1% of patients discontinue therapy due to cough (CitationQuattrin et al 2004; CitationBarnett et al 2006).
As for any therapeutic protein, anti-insulin antibodies may develop during treatment with Exubera, with the primary concern being the potential for effects on insulin resistance (CitationOdegard and Capoccia 2005). In clinical trials, anti-insulin antibodies developed more frequently and mean titers were higher in patients who switched from subcutaneous treatment to Exubera compared with those who remained on subcutaneous insulin (CitationHollander et al 2004; CitationQuattrin et al 2004; CitationRosenstock et al 2005; CitationSkyler et al 2005). Antibody titers were higher in patients with type 1 diabetes compared with type 2 diabetes, and reached a plateau within 6–12 months of exposure (CitationFineberg et al 2005; CitationBarnett et al 2006). However, in clinical trials to date there is no evidence of a relationship between the presence of anti-insulin antibodies and HbA1c, hypoglycemia, or hyperglycemia, nor is there evidence of any other adverse clinical consequences (CitationFineberg et al 2005).
Small but consistent treatment group differences in pulmonary function tests have been reported with inhaled insulin, with pulmonary function reduced slightly in patients assigned to Exubera (CitationHollander et al 2004; CitationQuattrin et al 2004; CitationSkyler et al 2005; CitationRosenstock et al 2005; CitationBarnett et al 2006). These differences occur early after treatment initiation and are typically <1%–2% lower than those for the comparator group, non-progressive in nature with safety data now extending out to 4 years of therapy, not driven by outliers, and are reversible following treatment discontinuation (CitationDreyer et al 2004; CitationSkyler et al 2004; CitationOdegard and Capoccia 2005; CitationRiese et al 2005).
Patient-reported outcomes
Barriers to insulin therapy
Barriers to starting and maintaining treatment with subcutaneous insulin have a major impact on patients’ abilities to self-manage their disease, and have resulted in insulin being portrayed as a treatment of last resort (CitationHunt et al 1997; CitationZambanini et al 1999; CitationKorytkowski 2002; CitationHeinemann 2004; CitationHauber et al 2005). Typical barriers to administering subcutaneous treatment arise due to both injection-related and experiential concerns, with the latter commonly described as psychological insulin resistance (CitationHunt et al 1997; CitationFunnell et al 2004). Thus, negative attitudes relating to injection pain, concerns over correct technique and inconvenience can lead to injection aversion (CitationHunt et al 1997). Similarly, psychological insulin resistance may arise from insulin therapy being perceived as a threat or failure on a prior regimen, concerns about hypoglycemia and other adverse effects, concerns over disease progression, prior mention of insulin by the physician as a threat to encourage compliance to oral therapies, and fear of treatment failure (CitationHunt et al 1997; CitationKorytkowski 2002; CitationFunnell et al 2004; CitationHeinemann 2004). In type 2 diabetes, an estimated one-quarter of patients progressing to subcutaneous insulin therapy refuse treatment once it has been prescribed (CitationPolonsky et al 2005). The presence of these barriers may influence compliance, glycemic control and quality of life (CitationZambanini et al 1999; CitationRoyle et al 2003).
Evidence for greater patient acceptance of inhaled insulin
Encouraging positive patient attitudes and beliefs is essential in diabetes management where patients require lifelong treatment (CitationPolonsky et al 2005). Noninvasive methods of insulin delivery promise to return greater patient satisfaction and treatment acceptance (CitationTesta 2003). This in turn will potentially see more patients achieving glycemic targets, with consequent improvement in health outcomes, such as those relating to microvascular and macrovascular complications (CitationFreemantle et al 2005).
Available patient satisfaction data have shown that Exubera is associated with greater treatment satisfaction relative to subcutaneous insulin in patients with type 1 or type 2 diabetes. A 15-item questionnaire was developed to gauge the level of patient satisfaction between Exubera and conventional subcutaneous insulin therapy in two similar, open-label, 3-month Phase II trials in patients with type 1 and type 2 diabetes. In both trials, subjects randomized to Exubera also received a bedtime Ultralente injection, while those randomized to subcutaneous insulin continued their pre-study regimen (2–3 injections/day). In both studies, the mean percentage improvement in overall patient satisfaction score with Exubera was markedly greater than that with subcutaneous insulin: 35% vs 12% (p=0.01) in subjects with type 1 diabetes (CitationGerber et al 2001) and 38% vs 14% (p<0.05) in subjects with type 2 diabetes (CitationCappelleri et al, 2002). To evaluate long-term treatment satisfaction with Exubera a 1year extension was offered to subjects who had completed the above 3-month studies (CitationRosenstock et al 2004). From baseline to the end of the 1-year extension, subjects on Exubera had a greater improvement than those on subcutaneous insulin in global satisfaction (38.8% versus 4.0%, p<0.01), convenience/ease of use (42.4% vs −1.7%, p<0.01), and social comfort (43.3% vs 12.7%, p=0.11). Together, these data suggest that Exubera was preferred over subcutaneous insulin and resulted in better patient satisfaction in the short and longer term (at least 1 year).
In a 12-week study of 309 patients with type 2 diabetes inadequately controlled by a sulfonylurea and either metformin or a thiazolidinedione, those who were randomized to Exubera either alone or in addition to existing therapy reported improved overall satisfaction relative to baseline (CitationSimonson et al 2001). In contrast, overall satisfaction was unchanged in patients who continued to receive oral antidiabetic agents alone. Changes from baseline for subscales of advocacy, efficacy, general satisfaction and preference were more favorable for Exubera compared with oral agents alone while subscales of convenience, burden, flexibility, hassle, life interference, social limitations, and pain were not significantly different among treatments. The only significant difference between Exubera in addition to existing therapy and continued oral antidiabetic agents alone was in the side-effects (weight gain and hypoglycemia) satisfaction scale, which favored continued oral agents alone. Improved endpoint HbA1c values as well as reduced symptom interference were correlated with more favorable satisfaction scores (CitationSimonson et al 2001).
A recent study has demonstrated that inhaled insulin promotes greater acceptance of insulin therapy in general when inhaled insulin is available as a treatment option along with oral antidiabetic agents and/or subcutaneous insulin (CitationFreemantle et al 2005). This was a randomized controlled study of 779 patients with type 2 diabetes who were inadequately controlled by dietary measures and/or oral antidiabetic agents. Subjects were assigned to educational information about the potential risks and benefits of either standard treatment options of oral antidiabetic agents and/or subcutaneous insulin, or inhaled insulin in addition to standard treatment options. In the group offered information about inhaled insulin as a treatment option, 43.2% opted for a treatment that included insulin at a follow-up physician consultation compared with just 15.5% of patients who were informed about standard therapies only (CitationFreemantle et al 2005) (). Significantly fewer patients offered inhaled insulin chose to make no change to their therapy compared with patients offered standard treatments only, while fewer patients in the former group opted for regimens containing oral antidiabetic agents or subcutaneous insulin. This finding suggests that the increased willingness of patients to adopt an insulin-containing regimen when offered inhaled insulin as a treatment option may increase the potential for glycemic control with consequent reductions in diabetic complications.
Figure 3 Proportion of patients choosing indicated treatment based on theoretical availability of standard therapy only or inhaled insulin in addition to standard therapy. Patients with type 2 diabetes currently managed by dietary measures and/or oral antidiabetic drugs were randomized to receive educational information about the potential risks and benefits of standard therapy alone (oral antidiabetic drugs and/or subcutaneous insulin, n=388) or inhaled insulin in addition to standard therapy (n=391). In the group offered inhaled insulin as an option, 43.2% of patients opted for a treatment that included insulin during a patient-physician consultation compared with 15.5% of patients who were offered standard therapy only (odds ratio 4.16 [95% CI, 2.93–5.95], p<0.0001). Reprinted with permission from CitationFreemantle N, et al. 2005. Availability of inhaled insulin promotes greater perceived acceptance of insulin therapy in patients with type 2 diabetes. Diabetes Care, 28:427–8. Copyright © American Diabetes Association.
![Figure 3 Proportion of patients choosing indicated treatment based on theoretical availability of standard therapy only or inhaled insulin in addition to standard therapy. Patients with type 2 diabetes currently managed by dietary measures and/or oral antidiabetic drugs were randomized to receive educational information about the potential risks and benefits of standard therapy alone (oral antidiabetic drugs and/or subcutaneous insulin, n=388) or inhaled insulin in addition to standard therapy (n=391). In the group offered inhaled insulin as an option, 43.2% of patients opted for a treatment that included insulin during a patient-physician consultation compared with 15.5% of patients who were offered standard therapy only (odds ratio 4.16 [95% CI, 2.93–5.95], p<0.0001). Reprinted with permission from CitationFreemantle N, et al. 2005. Availability of inhaled insulin promotes greater perceived acceptance of insulin therapy in patients with type 2 diabetes. Diabetes Care, 28:427–8. Copyright © American Diabetes Association.](/cms/asset/ce5b1005-c17a-4510-b1b5-e08d44c9e3ad/dvhr_a_12187347_f0003_b.jpg)
One final interesting observation has come from a systematic review of clinical studies that investigated patient acceptability for inhaled insulin versus injected insulin (CitationRoyle et al 2003). The review included 6 open-label randomized controlled trials lasting at least 12 weeks, which enrolled a total of 1191 patients with type 1 or type 2 diabetes. Despite similar glycemic control achieved for inhaled insulin and subcutaneous insulin, patient satisfaction and quality of life measures were significantly greater in the inhaled insulin group (five of the six studies were with Exubera).
Conclusions
Large-scale studies such as UKPDS and DCCT have demonstrated the importance of achieving strict glycemic control in type 1 and type 2 diabetes in order to reduce the likelihood of diabetes-related complications. Despite guidelines recommending aggressive treatment to achieve normal or near-normal blood glucose levels, most patients remain inadequately controlled. Clearly, conventional treatment options, particularly in relation to type 2 diabetes in which insulin therapy is normally reserved as a last resort, are inadequate for optimal disease management. The perceived need among physicians for delaying insulin therapy is of potential interest in the context of type 2 diabetes management and appears to be related to negative attitudes held by many patients that are often exacerbated by physicians, leading to injection aversion and psychological insulin resistance. Conversely, it may be that positive attitudes associated with early acceptance of insulin therapy among patients and physicians alike may assist with glycemic control and its likely benefits of minimizing or preventing diabetes-related complications. Thus, early insulin intervention is an important consideration in tomorrow’s management guidelines, particularly as a high proportion of patients will be increasingly younger at diagnosis and will face living longer with the disease and an earlier recourse to insulin therapy than a typical patient currently living with the disease.
Inhaled insulin has been developed to address some of the fundamental deficits of conventional subcutaneous rapid-acting insulin analogs: namely, a lack of patient satisfaction and convenience, needle aversion, and a propensity for psychological insulin resistance. Inhaled insulin products such as Exubera are an exciting development in the management of diabetes since they potentially avoid and certainly reduce the need for subcutaneous injections while providing a physiologic response to postprandial glucose. Compared with subcutaneous insulin, Exubera demonstrates equivalent efficacy in terms of HbA1c control, superior efficacy in terms of fasting plasma glucose, and is well tolerated. In addition, offering Exubera as an alternative treatment option to subcutaneous insulin increases the number of patients inadequately controlled on oral agents who are likely to accept insulin as a therapy. This has the potential to improve glycemic control, reduce diabetes-related complications and decrease complication-related costs.
References
- American Diabetes AssociationDiabetes Care199821Consensus Development Conference on the diagnosis of coronary heart disease in people with diabetes10-11 February 1998Miami, Florida155199727908
- BarnettAHDreyerMLangePAn open, randomized, parallel group study to compare the efficacy and safety profile of inhaled human insulin (Exubera®) with metformin as adjunctive therapy in patients with type 2 diabetes poorly controlled on a sulfonylureaDiabetes Care2006a291282716732009
- BarnettAHDreyerMLangePAn open, randomized, parallel-group study to compare the efficacy and safety profile of inhaled human insulin (Exubera) with glibenclamide as adjunctive therapy in patients with type 2 diabetes poorly controlled on metforminDiabetes Care20062918182516873786
- BeckerRHAShaSFrickADThe effect of smoking cessation and subsequent resumption on absorption of inhaled insulinDiabetes Care2006292778216443873
- BrownJBNicholsGASlow response to loss of glycemic control in type 2 diabetes mellitusAm J Manag Care20039213712643338
- CampbellIWNeed for intensive, early glycaemic control in patients with type 2 diabetesBr J Cardiol2000762531
- CappelleriJCCefaluWTRosenstockJKouridesIAGerberRATreatment satisfaction with inhaled insulin in patients with type 2 diabetes: a randomized controlled trialClin Ther2002245526412017400
- CefaluWTInhaled insulin: a proof-of-concept studyAnn Intern Med2001134795611329241
- CefaluWTSkylerJSKouridesIAInhaled human insulin treatment in patients with type 2 diabetes mellitusAnn Intern Med2001134203711177333
- CerielloACavarapeAMartinelliLThe post-prandial state in type 2 diabetes and endothelial dysfunction: effects of insulin aspartDiabet Med200421171514984453
- CiofettaMLalliCDel SindacoPContribution of postprandial versus interprandial blood glucose to HbA1c in type 1 diabetes on physiologic intensive therapy with lispro insulin at mealtimeDiabetes Care19992279580010332684
- CookMNGirmanCJSteinPPGlycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetesDiabetes Care200528995100015855556
- DCCTThe effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research GroupN Engl J Med1993329977868366922
- DCCT/EDICEffect of intensive therapy on the microvascular complications of type 1 diabetes mellitusJAMA20022872563912020338
- Del PratoSFeltonA-MMunroNImproving glucose management: ten steps to get more patients with type 2 diabetes to glycaemic goalInt J Clin Pract20055913455516236091
- Del SindacoPCiofettaMLalliCUse of the short-acting insulin analogue lispro in intensive treatment of type 1 diabetes mellitus: importance of appropriate replacement of basal insulin and time-interval injection-mealDiabet Med1998155926009686700
- DreyerMfor the Exubera® Phase III Study GroupEfficacy and two-year pulmonary safety of inhaled insulin as adjunctive therapy with metformin or glibenclamide in type 2 diabetes patients poorly controlled with oral monotherapy [abstract]Diabetologia200447Suppl 1A44
- DumasREnglandRDRieseRJExubera® is well tolerated and achieves tight glycemic control in patients with type 1 diabetes [abstract]Diabetes200554Suppl 1A87
- EspositoKGiuglianoDNappoFRegression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitusCirculation2004110214915197140
- FinebergSEKawabataTFinco-KentDAntibody response to inhaled insulin in patients with type 1 or type 2 diabetes. An analysis of initial phase II and III inhaled insulin (Exubera) trials and a two-year extension trialEndocrinol Metab200590328794
- FreemantleNBlondeLDuhotDAvailability of inhaled insulin promotes greater perceived acceptance of insulin therapy in patients with type 2 diabetesDiabetes Care200528427815677807
- FunnellMMKrugerDFSpencerMSelf-management support for insulin therapy in type 2 diabetesDiabetes Educ2004302748015095517
- GerberRACappelleriJCKouridesIAGelfandRATreatment satisfaction with inhaled insulin in patients with type 1 diabetes: a randomized controlled trialDiabetes Care2001241556911522698
- GoldfineABGoldfineHLCardiovascular disease in the diabetic patientCirculation2003107e141612538441
- GungorNArslanianSPathophysiology of type 2 diabetes mellitus in children and adolescents: treatment implicationsTreat Endocrinol200213597115832489
- HauberABJohnsonFRSauriolLRisking health to avoid injections: preferences of Canadians with type 2 diabetesDiabetes Care2005282243516123499
- HaywardRAManningWGKaplanSHStarting insulin therapy in patients with type 2 diabetes: effectiveness, complications, and resource utilizationJAMA1997278166399388085
- HeinemannLOvercoming obstacles: new management optionsEur J Endocrinol2004151Suppl 2T23T715487981
- HenryRMudaliarSFryburgDAWithin-subject variability of inhaled insulin (Exubera®) versus subcutaneous regular insulin in elderly obese patients with type 2 diabetes mellitus [abstract]Diabetes200352Suppl 1A105
- HollanderPABlondeLRoweREfficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 2 diabetesDiabetes Care20042723566215451900
- HuntLMValenzuelaMAPughJANIDDM patients’ fears and hopes about insulin therapy: the basis of patient reluctanceDiabetes Care19972029289051375
- KhawK-TWarehamNBinghamSAssociation of hemoglobin 1c with cardiovascular disease and mortality in adults: the European Prospective Investigation into Cancer in NorfolkAnn Intern Med20041414132015381514
- KoroCEBowlinSJBourgeoisNGlycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetesDiabetes Care200427172014693960
- KorytkowskiMWhen oral agents fail: practical barriers to starting insulinInt J Obes200226Suppl 3S18S24
- LazarHLChipkinSRFitzgeraldCATight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic eventsCirculation2004109149750215006999
- LeiterLACerielloADavidsonJAPostprandial glucose regulation: new data and new implicationsClin Ther200527Suppl BS42S5616519037
- LieblAMataMEschwegeEEvaluation of risk factors for development of complications in type II diabetes in EuropeDiabetologia200245S23S2812136408
- MalmbergKProspective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study GroupBr Med J1997314151259169397
- MaloneJKBeattieSDCampaigneBNTherapy after single oral agent failure: adding a second oral agent or an insulin mixture?Diabetes Res Clin Pract2003621879514625133
- MalmbergKNorhammarAWedelHGlycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction. Long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) StudyCirculation19999926263210338454
- MudaliarSHenryRRCombination therapy for type 2 diabetesEndocr Pract199952081915251678
- NathanDMClinical practice. Initial management of glycemia in type 2 diabetes mellitusN Engl J Med20023471342912397193
- OdegardPSCapocciaKLInhaled insulin: ExuberaAnn Pharmacother2005398435315827072
- PattonJSBukarJGEldonMAClinical pharmacokinetics and pharmacodynamics of inhaled insulinClin Pharmacokinet20044378180115355125
- PolonskyWHFisherLGuzmanSPsychological insulin resistance in patients with type 2 diabetesDiabetes Care2005282543516186296
- QuattrinTBelangerABohannonNJVEfficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 1 diabetesDiabetes Care2004272622715504996
- RaveKBottSHeinemannLTime-action profile of inhaled insulin in comparison with subcutaneously injected insulin lispro and regular human insulinDiabetes Care20052810778215855570
- ReichardPBerglundBBritzAIntensified conventional insulin treatment retards the microvascular complications of insulin-dependent diabetes mellitus (IDDM): the Stockholm Diabetes Intervention Study (SDIS) after 5 yearsJ Intern Med199123010181865159
- RiddleMCThe underuse of insulin therapy in North AmericaDiabetes Metab Res Rev200218Suppl 3S42S4912324985
- RieseRJTeeterJGEnglandRDInhaled insulin does not induce immediate airway responsiveness in patients with type 1 diabetes mellitus [abstract]Eur Respir J200526Suppl 49582S
- RosenstockJCappelleriJCBolinderBGerberRAPatient satisfaction and glycemic control after 1 year with inhaled insulin (Exubera) in patients with type 1 or type 2 diabetesDiabetes Care20042713182315161782
- RosenstockJZinmanBMurphyLJInhaled insulin improves glycemic control when substituted for or added to oral combination therapy in type 2 diabetesAnn Intern Med20051435495816230721
- RoylePWaughNMcAuleyLInhaled insulin in diabetes mellitusCochrane Database Syst Rev2003 CD003890
- ShichiriMKishikawaHOhkuboYLong-term results of the Kumamoto Study on Optimal Diabetes Control in type 2 diabetic patientsDiabetes Care200023Suppl 2B21910860187
- SimonsonDCHayesJFTurnerRRTreatment satisfaction and preferences in type 2 diabetes: a randomized trial of oral agents vs. inhaled insulin [abstract]Diabetes200150Suppl 2A131
- SkylerJSCefaluWTKouridesIAEfficacy of inhaled human insulin in type 1 diabetes mellitus: a randomised proof-of-concept studyLancet2001357331511210993
- SkylerJfor the Exubera® Phase II Study GroupSustained long-term efficacy and safety of inhaled insulin after 4 years of continuous therapy [abstract]Diabetologia200447Suppl 1A311
- SkylerJSWeinstockRSRaskinPUse of inhaled insulin in a basal/bolus insulin regimen in type 1 diabetic subjectsDiabetes Care2005281630515983312
- StrattonIMAdlerAINeilHAWAssociation of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational studyBr Med J20003214051210938048
- TestaMAImproving diabetes therapy: improving satisfactionDiabetes Voice200348235
- TurnerRCCullCAFrighiVGlycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49)JAMA199928120051210359389
- UKPDS GroupUK Prospective Diabetes Study 17: A nine-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitusAnn Intern Med1996124136458554206
- UKPDS GroupIntensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33)Lancet1998352837539742976
- Van den BergheGWoutersPWeekersFIntensive insulin therapy in critically ill patientsN Engl J Med200134513596711794168
- WeissSRChengS-LKouridesIAInhaled insulin provides improved glycemic control in patients with type 2 diabetes mellitus inadequately controlled with oral agentsArch Intern Med200316322778214581245
- [WHO]World Health Organization [online] Accessed 28 February 2006. URL: http://www.who.int/diabetes/facts/world_figures/en
- WrightABurdenACFPaiseyRBfor the UK Prospective Diabetes Study GroupSulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57)Diabetes Care200225330611815505
- ZambaniniANewsonRBMaiseyMInjection related anxiety in insulin-treated diabetesDiab Res Clin Pract19994623946