Abstract
Normalization of blood glucose is essential for the prevention of diabetes mellitus (DM)-related microvascular and macrovascular complications. Despite substantial literature to support the benefits of glucose lowering and clear treatment targets, glycemic control remains suboptimal for most people with DM in the United States. Pharmacokinetic limitations of conventional insulins have been a barrier to achieving treatment targets secondary to adverse effects such as hypoglycemia and weight gain. Recombinant DNA technology has allowed modification of the insulin molecule to produce insulin analogues that overcome these pharmacokinetic limitations. With time action profiles that more closely mimic physiologic insulin secretion, rapid acting insulin analogues (RAAs) reduce post-prandial glucose excursions and hypoglycemia when compared to regular human insulin (RHI). Insulin glulisine (Apidra®) is a rapid-acting insulin analogue created by substituting lysine for asparagine at position B3 and glutamic acid for lysine at position B29 on the B chain of human insulin. The quick absorption of insulin glulisine more closely reproduces physiologic first-phase insulin secretion and its rapid acting profile is maintained across patient subtypes. Clinical trials have demonstrated comparable or greater efficacy of insulin glulisine versus insulin lispro or RHI, respectively. Efficacy is maintained even when insulin glulisine is administered post-meal. In addition, glulisine appears to have a more rapid time action profile compared with insulin lispro across various body mass indexes (BMIs). The safety and tolerability profile of insulin glulisine is also comparable to that of insulin lispro or RHI in type 1 or 2 DM and it has been shown to be as safe and effective when used in a continuous subcutaneous insulin infusion (CSII). In summary, insulin glulisine is a safe, effective, and well tolerated rapid-acting insulin analogue across all BMIs and a worthy option for prandial glucose control in type 1 or 2 DM.
Introduction
The prevalence of diabetes mellitus (DM) is increasing at an alarming rate largely due to an aging population coupled with high rates of obesity, unhealthy diet, and sedentary lifestyle (CitationIDF 2006). An estimated 7% of the United States population, or >20.8 million Americans, have type 1 or type 2 DM, representing a 14% increase in prevalence over the last 2 years (CitationADA 2006). Furthermore, >41 million Americans have impaired glucose tolerance (IGT) or impaired fasting glucose (IFG), conditions classified as pre-diabetes because they significantly increase an individual’s risk for the development of diabetes; the expected conversion rate to diabetes for such persons is 7% per year (CitationADA 2006). Statistics are equally sobering for the European population. The current prevalence of 7.8% is expected to increase to 9.1%, roughly 60 million Europeans, by the year 2025 (CitationIDF 2006).
The impact of DM on society and the persons affected is profound. Both type 1 and 2 DM are associated with microvascular and macrovascular complications that result in considerable morbidity and an estimated 3.2 million deaths per year worldwide (CitationWorld Health Organization 2006). Although there has been an overall decrease in mortality from coronary artery disease in patients without DM over the last 10 years, there has been a paradoxical increase in DM-related mortality (CitationSobel et al 2003). Despite these statistics, this disease can be well managed with individualized intensive treatment regimens.
Blood glucose lowering is essential for the prevention of DM-related microvascular and macrovascular complications. The Diabetes Control and Complications Trial (DCCT) found that intensive insulin therapy to improve glycemic control, as measured by glycosylated hemoglobin (A1c), significantly lowered the risk for retinopathy by 47%, for nephropathy by 54%, and for neuropathy by 60% in patients with type 1 DM followed for an average of 6.5 years (CitationThe Diabetes Control and Complications Trial Research Group 1993). Similar findings with intensive treatment were seen with large cohorts of patients with type 2 DM followed in the United Kingdom Prospective Diabetes Study (UKPDS) and the Kumamoto studies (CitationUKPDS 1998; CitationShichiri et al 2000). It is estimated that for every percentage point A1c is lowered, the risk of microvascular complications is decreased by 37% and the risk of macrovascular complications is decreased 14% (CitationStratton et al 2000).
More recent data comes from the Epidemiology of Diabetes and Interventions and Complications (EDIC) trial, a follow-up study of the DCCT that analyzed study participant data for up to 18 years (CitationNathan et al 2005). In addition to observing continued reductions in microvascular complications, researchers found that intensive therapy during the DCCT years was associated with a 42% reduction in the risk of cardiovascular disease and a 57% reduction in cardiovascular events, including myocardial infarction, stroke, and death. Overall, every 10% reduction in A1c (ie, a decrease from an A1c of 8.0% to 7.2%) was associated with a 21% decrease in cardiovascular disease.
The American Diabetes Association (ADA), the American Association of Clinical Endocrinologists (AACE), and the International Diabetes Federation (IDF) recommend near-normalization of plasma glucose levels with target A1c values of <7.0%, ≤6.5%, and ≤6.5%, respectively (CitationIDF 1999; CitationAACE 2002; CitationADA 2006). Although the ADA recommends an A1c goal of <7.0% for patients in general, the goal for individual patients should be set as close to normal (<6.0%) as possible without significant hypoglycemia (CitationADA 2006). Each organization also provides specific goals for fasting plasma glucose (FPG) and postprandial glucose (PPG) (see ) (CitationADA 2006; CitationAACE 2002; CitationIDF 1999).
Table 1 Treatment goals for glycemia (% of patients to targets)
Despite substantial literature to support the benefits of glucose lowering, clear treatment targets, and the availability of effective treatments, the average A1c in the United States remains above 9% (CitationHirsch 2004). For several decades, pharmacokinetic limitations of conventional insulins made treatment goals difficult to achieve for many patients. Studies have shown that intensive regimens with these older insulins often results in frequent hypoglycemia, weight gain, glucose variability, and wide glucose fluctuations (CitationDiabetes Control and Complications Trial Research Group 1993; CitationUKPDS 1998). However, the development of recombinant DNA technology has allowed modification of the insulin molecule to produce insulin analogues with more physiologic time-action profiles (see ) (CitationGarg and Ulrich 2006). This article discusses the benefits of intensive insulin regimens designed to mimic physiological basal/bolus insulin secretion, with a focus on the new rapid-acting insulin analogue, glulisine.
Table 2 Pharmacokinetic parameters of available insulins
Background
Type 1 DM, which constitutes 5%–10% of the diabetic population, occurs when autoimmune destruction of the β-cells leads to absolute insulin deficiency. Patients with type 1 DM are therefore dependent on exogenous insulin replacement for survival. Type 2 DM, the more common form of the disease, results from a combination of relative insulin deficiency and a defect in insulin action, or insulin resistance, which is frequently associated with obesity. Initially, patients with insulin resistance may have sufficient β-cell function to compensate for the increased peripheral insulin demand (CitationPlonsky et al 1996). However, once β-cell failure begins, abnormalities in glycemia occur.
Patients with type 2 DM have a loss of first phase insulin release from pancreatic β-cells, which results in PPG excursions (CitationPlonsky et al 1996). Furthermore, as the disease progresses, the β-cells lose the ability to maintain sufficient basal insulin secretion leading to increased FPG (CitationPlonsky et al 1996). In type 2 DM, there is an approximate 50% loss of β-cell function at the time of diagnosis, followed by a gradual, yet progressive decline (CitationUKPDS 1995). Chronic hyperglycemia may further impair β-cell function and tissue sensitivity to insulin, resulting in an even greater deficit in insulin secretion and action (CitationRossetti et al 1990).
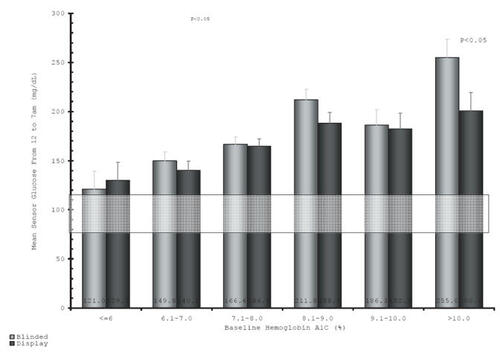
Excessive post-prandial glucose excursions may elevate A1c levels even when FPG remains in goal range (CitationMonnier et al 2003). Although A1c is determined by a combination of fasting and PPG levels, the exact contribution of each is not known. One study done using intermittent glucose monitoring demonstrated that at higher A1c values (A1c >8%), FPG had a more significant influence on overall control, however as patients got closer to target A1c, the post-prandial component became the predominant factor. A more recent trial conducted using continuous glucose monitoring showed that FPG was normal only for patients with an A1c <6% and that PPG continued to influence average glycemic control even at higher A1c values () (CitationGarg 2006). Regardless of the exact role of FPG vs PPG, it is evident that regimens must lower both fasting and post-prandial hyperglycemia to achieve therapeutic goals.
Figure 1 Fasting glucose (12 fasting glucose (12–7 am) vs A1c. Reproduced with permission from CitationGarg S. 2006. Safety, accuracy, and improvement in glucose profiles observed using a 7-day continuous glucose sensor. Diabetes Care®, 29:2644-9. Copyright © 2006 American Diabetes Association.
Basal/bolus therapy
Intensive insulin therapy for patients with type 1 DM should consist of a basal/bolus regimen which can be achieved with a combination of long/intermediate and short- or rapid-acting insulins or a continuous infusion of a short- or rapid-acting insulin via an insulin pump. Unless a patient presents with profound hyperglycemia, type 2 DM can and should be initially managed with diet and exercise with or without the addition of oral glucose-lowering medications. Lifestyle modifications centered around diet, exercise, and weight loss can have a profound effect on insulin sensitivity and should be the first step in the management of type 2 DM (CitationADA 2006). Specifically, overweight patients should be encouraged to achieve and maintain a weight loss of at least 7% of total body weight through a healthy diet and 150 minutes per week of moderate intensity exercise. Furthermore, the Diabetes Prevention Program Research Study showed that if these interventions are initiated early enough, they can prevent or delay the progression of pre-diabetes to diabetes (CitationDiabetes Prevention Program Research Group 2002).
However, because type 2 DM is a progressive disease, lifestyle interventions alone are often not sufficient to maintain glycemic control over time (CitationTurner et al 1999). Most patients will require multiple pharmacological agents to achieve glucose targets and eventually, as further β-cell deterioration results in failure of the oral agents to control hyperglycemia, many patients need insulin introduced (CitationTurner et al 1999; CitationUKPDS 1995).
When utilized in patients with either type 1 or 2 DM, an ideal intensive insulin regimen mimics endogenous basal and prandial insulin secretion as closely as possible. Basal insulin release regulates hepatic glucose homeostasis in the fasting state and supplies approximately 50% of an individual’s overall insulin requirement (CitationWittlin et al 2002). The bolus component of endogenous insulin secretion consists of rapidly released short-lived bursts of insulin in response to prandial stimuli. Postprandial insulin is released in a 2- to 5-minute burst (first phase) followed by a slow increase in insulin release lasting 5–52 minutes (second phase) (CitationWittlin et al 2002). The first-phase insulin secretion at mealtime inhibits endogenous gluconeogenesis in the liver and disposal of the carbohydrate load, thereby limiting PPG excursions. Early normalization of glucose after a meal is important, especially in type 2 DM to prevent late hyperinsulinemia and prolonged stress on β-cells (CitationRossetti et al 1990).
Conventional insulin vs rapid acting analogues
Regular human insulin (RHI) was the only short-acting bolus insulin available for many years. Because it requires the addition of zinc for stability, RHI exists as a molecular hexamer that, once injected, must break down into dimers and ultimately monomers to be absorbed and biologically active (CitationWittlin et al 2002). Although RHI has advantages over previously used pork and bovine insulins, its slower onset and longer duration of action do not completely mimic endogenous insulin secretion after a carbohydrate load (CitationWittlin et al 2002). Patients should take injections 30–45 minutes prior to a meal to prevent extended postprandial hyperglycemia and subsequent hypoglycemia during its longer duration of action. However, most patients do not follow this recommendation (CitationOvermann and Heinemann 1999). Furthermore, peak concentrations may not occur until 2–4 hours after injection, requiring frequent snacks to avoid hypoglycemia and thereby increasing the potential for weight gain.
The advent of recombinant DNA technology using a non-pathogenic strain of Escherichia coli (K12) has facilitated the development of insulin analogues that overcome the pharmacokinetic limitations of RHI. Amino acid substitutions in the insulin molecule allows weak dimeric and hexamer formation and thereby rapid disassociation of the dimers and hexamers after subcutaneous injection, resulting in rapid absorption and onset of action (CitationHowey et al 1994). Currently available rapid acting analogues (RAAs), which include insulin lispro, insulin aspart, and insulin glulisine, demonstrate less variability in absorption at the injection site and potentially less variability in and among patients (CitationHowey et al 1994). RAAs have shown greater efficacy in reducing post-prandial glucose excursions and hypoglycemia when compared with RHI (CitationAnderson et al 1997; CitationHome et al 1998; CitationGarg et al 1999). A meta-analysis of comparative trials with insulin lispro and RHI reported a 25% reduction in the frequency of severe hypoglycemia (CitationBrunelle et al 1998). Some, but not all trials have shown modest reductions in A1c with these analogues. One meta-analysis noted a small but significant decrease of −0.12% in A1c with RAAs vs RHI in patients with type 1 DM; however, this benefit was not seen with the type 2 DM population (CitationPlank et al 2005).
Insulin glulisine
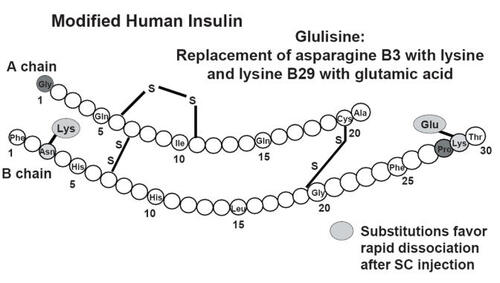
Insulin glulisine (Apidra®) is a rapid-acting insulin analogue created by making two amino acid substitutions in the B chain of human insulin: lysine for asparagine at position B3 and glutamic acid for lysine at position B29 (see ) (CitationGarg et al 2005). As with other RAAs these modifications to the insulin molecule reduce its tendency to aggregate as hexamers, allowing rapid dissociation and absorption following a subcutaneous injection. The quick absorption of insulin glulisine more closely reproduces physiologic first-phase insulin secretion.
Figure 2 Glulisine vs RHI vs lispro: pharmacokinetics in obese individuals. Reproduced with permission from CitationGarg SK, Ellis SL, Ulrich H. 2005. Insulin glulisine: a new rapid-acting insulin analogue for the treatment of diabetes. Expert Opin Pharmacother, 6:643–51. Copyright © 2005 Informa Healthcare.
Insulin glulisine is approved in the United States and Europe for the treatment of type 1 and type 2 DM in adults. It may be administered via subcutaneous injection (abdomen, thigh, or deltoid) or CSII. When utilized as a prandial subcutaneous injection, it should be given 15 minutes pre-meal or within 20 minutes post-meal.
Pharmacodynamics/pharmacokinetics
Insulin glulisine demonstrates equivalent bioefficacy with RHI. In an initial randomized, open-label crossover study with 16 healthy volunteers (mean age = 22 years, mean body mass index [BMI] = 23.9 kg/m2) the euglycemic clamp technique was used to compare the molar efficacy of the two insulins (CitationBecker et al 2005). During a 2-hour continuous infusion, insulin glulisine and RHI had superimposible mean glucose infusion rates (GIR) and area under the glucose infusion rate time-curves at steady state (GIR-AUCSS). Both insulins also presented equal total glucose exposure as measured by the glucose infusion rate time-curve from time 0 to clamp end (GIR-AUC0-clamp end). The overall equivalent bioefficacy demonstrated that patients can switch from RHI to insulin glulisine on a unit-per-unit basis. A randomized, double-blind study also employing the euglycemic clamp technique in healthy volunteers was initiated to compare plasma concentrations and time action profiles of insulin glulisine, insulin lispro, and RHI (CitationBecker et al 2003). Median time to maximum plasma concentration (tmax) and mean residence time (MRT) for insulin glulisine and insulin lispro were approximately half that of RHI (see ) (CitationBecker et al 2003).
Figure 3 Glulisine duration of activity shorter than regular human insulin (RHI)F. Reproduced with permission from CitationBecker RHA, Frick A, Wessels D. 2003. Pharmacodynamics and pharmacokinetics of a new rapidly-acting insulin analog, insulin glulisine [abstract no. 471-P] Diabetes®, 52(Suppl1):A110. Copyright © 2003 American Diabetes Association.
![Figure 3 Glulisine duration of activity shorter than regular human insulin (RHI)F. Reproduced with permission from CitationBecker RHA, Frick A, Wessels D. 2003. Pharmacodynamics and pharmacokinetics of a new rapidly-acting insulin analog, insulin glulisine [abstract no. 471-P] Diabetes®, 52(Suppl1):A110. Copyright © 2003 American Diabetes Association.](/cms/asset/4b921931-b68b-4745-b126-45bcbb355d4c/dvhr_a_12187350_f0003_b.jpg)
More recently, a larger randomized crossover study was conducted by Spitzer et al to evaluate the pharmacokinetics and pharmacodynamics of insulin glulisine versus insulin lispro in lean to obese individuals (CitationSpitzer et al 2006). Eighty healthy subjects were stratified into one of 4 groups based on BMI (<25, 25–<30, 30–<35, and >35 kg/m2) and given insulin glulisine and insulin lispro at doses of 0.2 U/kg and 0.4 U/kg in random order over a 4-day study period with a euglycemic glucose clamp. GIR and insulin concentration (INS) were measured for 10 hours following the dose. Overall, onset of exposure and activity, as measured by INS-t10% and GIR-t10%, was 6 minutes faster with insulin glulisine. Early insulin exposure and early metabolic action were consistently greater by 18%–30% with insulin glulisine than insulin lispro, which was significant in the total population and across a wide range of BMIs.
Insulin glulisine has been shown to maintain its rapid-acting kinetic profile in studies that enrolled patients with type 1 or 2 DM. In a randomized crossover study of 20 patients with type 1 DM, insulin glulisine was absorbed more quickly than RHI, although total availability did not differ between the insulins (CitationNosek et al 2004). Patients received a 0.15 U/kg subcutaneous injection of either RHI 30 minutes pre-meal or insulin glulisine immediately before or 15 minutes post-meal. Mean AUC from 0 to 2 hours (AUC2) was 7278 and 4258 μIU min/ML with insulin glulisine and RHI respectively (p < 0.05). However, mean insulin AUC from 0 to 6 hours (AUC6) did not differ between groups (11,912 vs 11,550 μIU min/mL). Mean Cmax, AUC6, and tmax were equivalent between the pre- and post-meal glulisine injections, indicating that the analogue is safe and effective when administered either before or after a meal. This may provide greater flexibility for patients, especially those prone to forgetting pre-meal injections.
In a comparison study of patients with type 2 DM, AUC2 was again significantly greater with insulin glulisine compared with RHI (7661 vs 4221), but at the end of the 10 hour clamp, AUC10 was comparable between these two insulins (18,408 vs 19,731 μIU min/mL) (CitationBecker et al 2004). This study, which also used insulin lispro as a comparator, confirmed that both of these rapid-acting insulin analogues have equivalent pharmacokinetic and pharmacodyamic profiles, which more closely mimic physiologic prandial insulin secretion compared with RHI.
The volume of distribution for insulin glulisine following intravenous administration is 13 L (vs 21 L for RHI) (Gabry 2004). Half-lives for insulin glulisine and RHI were measured at 13 and 17 minutes (intravenous administration) and 42 and 86 minutes (subcutaneous injection) respectively (Gabry 2004). The pharmacokinetics of insulin glulisine were comparable between various sites of subcutaneous injection (CitationFrick et al 2003). Following injection into abdominal, deltoid, or femoral sites in healthy volunteers, insulin glulisine demonstrated consistent pharmacokinetic parameters, including absolute bioavailability. It was noted that the use of the abdominal route led to slightly more rapid insulin delivery.
Special populations
The rapid-acting pharmacokinetic properties of insulin glulisine have been consistent among various patient subtypes including: obesity, renal impairment, different ethnicity, and childhood.
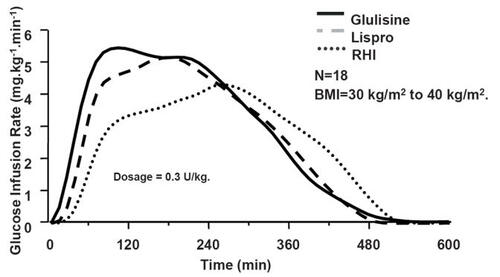
The pharmcodynamic and pharmacokinetic properties for insulin glulisine were initially compared to RHI and insulin lispro in a randomized, double-blind, crossover study in obese non-diabetic individuals (n = 18, BMI = 30–40 kg/m2) (CitationFrick et al 2004). Patients received single doses of insulin (0.3 IU/kg) administered subcutaneously in the abdomen. The Cmax for RHI, insulin lispro, and insulin glulisine was 203, 133, and 77 μIU/mL, respectively. Tmax (144, 99, and 76 minutes) and MRT (229, 166, and 149 minutes) were shortest with insulin glulisine, and the difference between the two analogues was statistically significant over the first 2 hours (). Total glucose disposal was equivalent for all insulins studied. Of note, there was a correlation found between the thickness of the abdominal fat area and the time action profiles for RHI and insulin lispro. This correlation was not found for insulin glulisine, suggesting that this analogue may better maintain its rapid-acting kinetics over a range of subcutaneous fat thickness. This finding was supported by the more recent data from a larger trial by Spitzer et al, described previously (CitationSpitzer et al 2006). The trial demonstrated advantages of early action of insulin glulisine versus insulin lispro in individuals who were lean, overweight, obese, or severely obese.
A single dose study was conducted to evaluate insulin glulisine in non-diabetic patients (n = 24) with moderate (creatinine clearance = 30–50 mL/min), severe (<30 mL/min), or no (>80 ml/min) renal impairment (CitationJaros et al 2004). No differences in absorption, as measured by Cmax, tmax, and AUC2, were noted between groups. There was, however, a correlation between renal impairment and total exposure and total clearance, which were increased by 29%–47%.
Insulin glulisine was compared with RHI and insulin lispro in a randomized, double-blind, crossover study that enrolled Japanese and Caucasian males matched for BMI (CitationRave et al 2004). Insulin glulisine demonstrated a more rapid-acting time profile and greater glucose lowering at 2 hours compared with RHI. Slight, but non-significant, differences were noted for onset of activity and metabolic effect of all insulins in Japanese versus Caucasian individuals.
Likewise, the rapid-acting pharmacokinetic profile of insulin glulisine was maintained in pediatric and adolescent patients with type 1 DM (CitationDanne et al 2005). Absorption was more rapid (tmax 54 vs 66 minutes) and blood glucose excursions were lower with the insulin analogue compared to RHI. Overall, the pharmacokinetic properties of insulin glulisine in pediatric patients were similar to those seen in healthy and diabetic adult patients.
Efficacy and tolerability
Type 1 DM
A multinational, randomized, controlled, open-label parallel group study compared insulin glulisine and insulin lispro administered subcutaneously 0–15 minutes pre-meal in adults with type 1 DM (n = 672) (CitationDreyer et al 2005). Prior to randomization, a 4-week run-in phase was initiated with insulin glargine for basal coverage and RHI for bolus insulin. Patients were then randomized to one of the two rapid-acting analogues for bolus control over a 26-week treatment phase. The primary outcome was the change in A1c from baseline to endpoint (subject’s last available measurement).
Similar reductions in A1c occurred in both study groups at 12 and 26 weeks and at study endpoint (mean change from baseline = −0.14% in both groups), demonstrating non-inferiority. There was no significant difference in the proportion of patients achieving target A1c. Furthermore, self-monitored 7-point blood glucose profiles were comparable between the insulin glulisine and insulin lispro groups. Basal insulin doses increased for the patients taking insulin lispro group but not for patients taking insulin glulisine, which led to a reduced total daily dose for the insulin glulisine group and an increased total daily dose for insulin lispro. Hypoglycemic events (symptomatic, nocturnal, and severe) were comparable between treatments and no appreciable differences were observed in safety variables: systemic hypersensitivity reactions, injection site reactions, insulin or Escherichia coli antibody formation, hematology or clinical chemistry changes, lipid profiles, body weight, or blood pressure. Similar results were noted in a 26-week clinical extension study.
A second large clinical trial compared pre- and post-meal insulin glulisine injections versus pre-meal RHI injections in adult type 1 patients (CitationGarg et al 2005). Again, the subjects completed a 4-week run-in period using insulin glargine and RHI, then they were subsequently randomized to one of the three treatment groups for a 12-week period. At 8 and 12 weeks, A1c was significantly reduced for the pre-meal glulisine subjects compared to the post-meal and RHI groups (p < 0.01). There was no statistically significant difference in A1c lowering between insulin post-meal insulin glulisine and RHI. Total insulin dose was decreased significantly in both of the insulin glulisine groups, secondary to decreases in basal insulin requirements. There was a 0.6 kg difference in weight at the end of the study between the post-meal glulisine subjects and the other groups (−0.3 kg with post-meal, +0.3 kg with pre-meal and RHI). Similar rates of hypoglycemia and adverse events, including hypersensitivity and injection site reactions, were observed.
Given the proportion of patients with type 1 DM currently treated with a continuous subcutaneous insulin infusion (CSII), or insulin pump, it is important to assess efficacy and safety of insulin glulisine for this patient population. This was achieved during a multinational, randomized controlled 12-week trial comparing insulin glulisine with insulin aspart used in CSII (CitationHoogma and Schumicki 2006). There were no statistically significant differences between the two insulins with respect to A1c lowering, self-monitored blood glucose profiles, catheter occlusions, mean total insulin dose, and hypoglycemia. Seven patients (20.7%) and 14 patients (40.0%) reported at least one episode of unexplained hyperglycemia in the insulin glulisine and insulin aspart groups, respectively. However, this difference was not statistically significant. Four patients (13.8%) in the insulin glulisine group versus 8 patients (26.7%) in the insulin aspart group experienced at least one catheter occlusion. Again, this difference was not statistically significant. The catheter change rate and time between catheter changes were comparable between groups. Glulisine is a safe an effective option for type 1 patients on insulin pump therapy.
Type 2 DM
Insulin glulisine is the first of the rapid-acting analogues to exhibit statistically significant A1c lowering in comparison with RHI in type 2 DM. 876 patients with type 2 DM, under relatively good control (mean baseline A1c = 7.55%) were randomized to receive NPH + insulin glulisine or NPH + RHI for 26 weeks (CitationDailey et al 2004). Pre-study oral diabetes medications were continued, unless hypoglycemia necessitated a dose reduction. A statistically significant reduction in A1c occurred with insulin glulisine (−0.46% vs −0.30% with RHI) at study endpoint. Statistical significance in favor of insulin glulisine was also achieved for differences between post-dinner and post-breakfast blood glucose levels. There were no between group differences with respect to insulin dose changes, hypoglycemia, weight gain, and adverse events. A 26-week extension trial was conducted; however the results have not been published. Data on file indicate that A1c reductions at the end of the 52 week period were −0.23% and −0.13% for insulin glulisine and RHI, respectively (Gabry 2004). The significant reductions in self-monitored blood glucose profiles persisted during this extension period.
Safety of insulin glulisine
It is known that amino acid changes of the insulin molecule may change binding with the insulin and insulin-like growth factor 1 (IGF-1) receptors, thereby potentially increasing the risk of mitogenesis, or increased cell growth (CitationBerti et al 1998). It is therefore necessary to evaluate insulin and IGF-1 receptor binding for any analogue prior to approval for use. A comprehensive preclinical evaluation that compared insulin glulisine with RHI concluded that receptor binding was similar between the two agents and that insulin glulisine did not promote excessive mitogenic activity in vitro or in vivo (CitationStammberger et al 2006).
An analysis of safety data was compiled from the previously discussed trials for the purpose of FDA review (n = 1833 for insulin glulisine, n = 1524 for RHI or insulin lispro) (Gabry 2004). Overall, no significant difference in the adverse event rate was noted with insulin glulisine versus the comparator insulins (66.2% vs 66.0% for type 1 patients, 82.3% vs 79.6% for type 2 patients). The most common serious side-effect was severe hypoglycemia, the rate of which did not differ between treatments. The incidence of non-hypoglycemic adverse events was also comparable (12.8% vs 12.1%) with no differences in cardiac disorders, ketoacidosis, hypersensitivity, or injection site reactions. In total, 10 deaths (5 with insulin glulisine, 5 with comparator treatment) occurred throughout the clinical trials, none of which were attributed to the study treatment. In conclusion, compiled data on the safety of glulisine indicate that the analogue is as safe as human insulin.
Conclusion
All patients with type 1 DM and many patients with type 2 DM require intensive insulin therapy to lower A1c to treatment goals and prevent long-term complications of the disease. When intensive insulin treatment is called for, a combination of long/intermediate and short acting insulins can be employed to provide both basal and bolus insulin needs. New insulin analogues, such as insulin glulisine, have been synthesized by recombinant DNA technology to provide more physiologic bolus insulin. Insulin glulisine, which has a two amino acid substitution of the B chain of human insulin, has a more rapid absorption and shorter duration of action than RHI exhibited across a wide range of patient subtypes (obese, renally impaired, different ethnic background, and pediatric clinical trials have demonstrated comparable or greater efficacy of insulin glulisine versus insulin lispro or RHI, respectively). In addition, glulisine appears to have a more rapid time action profile compared to insulin lispro across various BMIs. In patients with type 2 DM, insulin glulisine lowered A1c to a greater extent than RHI. The safety and tolerability profile of insulin glulisine is also comparable to that of insulin lispro or RHI in type 1 or 2 DM and it has been shown to be as safe and effective as insulin aspart when used in CSII therapy. In summary, insulin glulisine provides a worthy option for use as prandial insulin in a basal/bolus intensive insulin regimen.
References
- [AACE] American Association of Clinical Endocrinologists, American College of EndocrinologyMedical guidelines for the management of diabetes mellitus: The AACE system of intensive diabetes self-management—2002 updateEndocr Pract20028Suppl 1408211939758
- [ADA] American Diabetes AssociationStandards of medical care in diabetes -2006Diabetes Care200629Suppl 1S4S4216373931
- American Diabetes AssociationNational Diabetes Fact Sheet [online]2005 Accessed September 28, 2006. URL: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf
- AndersonJBrunelleRLKoivistaVAReduction of postprandial hyperglycemia and frequency of hypoglycemia in IDDM patients on insulin-analog treatmentDiabetes199746265709000704
- BeckerRHFrickADBurgerFA comparison of the steady-state pharmacokinetics and pharmacodynamics of a novel rapid-acting insulin analog, insulin glulisine, and regular human insulin in healthy volunteers using the euglycemic clamp techniqueExp Clin Endocrinol Diabetes2005113292715926116
- BeckerRHAFrickADKapizitaCKPharmacodynamics (PD) and pharmacokinetics (PK) of insulin glulisine compared with insulin lispro (IL) and regular human insulin (RHI) in patients with type 2 diabetes [abstract no. 503-P]Diabetes200453Suppl 2A119
- BeckerRHAFrickAWesselsDPharmacodynamics and pharmacokinetics of a new rapidly-acting insulin analog, insulin glulisine [abstract no. 471-P]Diabetes200352Suppl 1A110
- BertiLKellererMBossenmaierBThe long-acting human insulin analog HOE901: characteristics of insulin signalling in comparison to Asp(B10) and regular insulinHorm Metab Res199830123299566852
- BrunelleRLLlewelynJAndersonJMeta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetesDiabetes Care1998211726319773738
- DaileyGRosenstockJMosesRGInsulin glulisine provides improved glycemic control in patients with type 2 diabetesDiabetes Care2004272363815451901
- DanneTBeckerRHHeiseTPharmacokinetics, prandial glucose control, and safety of insulin glulisine in children and adolescents with type 1 diabetesDiabetes Care2005282100516123473
- The Diabetes Control and Complications Trial Research GroupThe effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitusN Engl J Med1993329977868366922
- Diabetes Prevention Program Research GroupReduction in the incidence of type 2 diabetes with lifestyle intervention or metforminN Engl J Med200234639340311832527
- DreyerMPragerRRobinsonAEfficacy and safety of insulin glulisine in patients with type 1 diabetesHorm Metab Res200537702716308840
- FrickABeckerRHAWesselsDPharmacokinetic and glucodynamic profiles of insulin glulisine following subcutaneous administration at various injection sites [abstract no. 471-P]Diabetes200352Suppl 1A110
- FrickABurgerFScholtzHTime-action profile of insulin glulisine versus regular human insulin and insulin lispro in obese subjects [abstract no. 526-P]Diabetes200452Suppl 2A124
- GabryKEApplication number: 21–629 (insulin glulisine)Medical Review [online]2006 Accessed September 9, 2006. URL: http://www.fda.gov/cder/foi/nda/2004/21–629_Apidra_Medr_P1.pdf
- GargSAndersonJPerrySLong-term efficacy of humalog in subjects with type 1 diabetes mellitusDiabet Med1999163848710342337
- GargSKEllisSLUlrichHInsulin glulisine: a new rapid-acting insulin analogue for the treatment of diabetesExpert Opin Pharmacother200566435115934890
- GargSSafety, accuracy, and improvement in glucose profiles observed using a 7-day continuous glucose sensorDiabetes Care2006292644917130198
- GargSRosenstockJWaysKOptimized basal-bolus insulin regimens in type 1 diabetes: insulin glulisine versus regular human insulin in combination with basal insulin glargineEndocr Pract20051111716033730
- GargSUlrichHAchieving goal glycosylated hemoglobin levels in type 2 diabetes mellitus: practical strategies for success with insulin therapyInsulin2006110921
- HirschIBEffect of insulin therapy on nonglycemic variables during acute illnessEndocr Pract200410Suppl 2637015251643
- HomePDLindholmAHyllebergBImproved glycemic control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patientsDiabetes Care1998211904099802741
- HoogmaRPSchumickiDSafety of insulin glulisine when given by continuous subcutaneous infusion using an external pump in patients with type 1 diabetesHorm Metab Res2006384293316823727
- HoweyDCBowsherRRBrunelleRL[Lys(B28),Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulinDiabetes1994433964028314011
- [IDF] International Diabetes FederationA desktop guide to type 2 diabetes mellitus. European Diabetes Policy Group 1999Diabet Med1999167163010510947
- [IDF] International Diabetes FederationExecutive Summary of the Diabetes Atlas, second addition [online]2006 Accessed September 28, 2006. URL: http://www.eatlas.idf.org/
- JarosMMartinekVPiechatzekRPharmacokinetics of insulin glulisine in non-diabetic renally impaired patients [abstract no. 1330-P]Diabetes200453Suppl 2A32122
- MonnierLLapinskiHColetteCContributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c)Diabetes Care200326881512610053
- NathanDMClearyPABacklundJYThe Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetesN Engl J Med200535326435316371630
- NosekLBeckerRHAFrickADPrandial blood glucose control with pre- and post-meal insulin glulisine versus regular human insulin [abstract no.588-P]Diabetes200453Suppl 2A13940
- OvermannHHeinemannLInection-meal interval: recommendations of diabetologists and how patients handle itDiab Res Clin Pract19994313742
- PlankJSiebenhoferABergholdASystematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitusArch Intern Med200516513374415983281
- PolonskyKSSturisJBellGNon-insulin dependent diabetes mellitus: a genitically programmed failure of the beta cell to compensate for insulin resistanceN Engl J Med1996334777838592553
- RaveKNosekLHeinemannLInsulin glulisine: pharmacokinetic and pharmacodynamic properties in comparison with insulin lispro and regular human insulin in Japanese and caucasian volunteers [abstract no. 603-P]Diabetes200453Suppl 2A143
- RossettiLGiaccariADeFronzoRAGlucose toxicityDiabetes Care199013610302192847
- ShichiriMKishikawaHOhkuboYLong term results of the Kumamoto study on optimal diabetes control in type 2 diabetic patientsDiabetes Care200023Suppl 2B21910860187
- SobelBEFryeRDetreKMRationale for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D)Trial Circulation200310763642
- SpitzerHHeiseTHeinemannLInsulin glulisine: faster onset of absorption and action than insulin lispro in lean to obese subjects [abstract no. 0148]Diabetologia200649Suppl 1945
- StammbergerISeipkeGBartelsTInsulin glulisine--a comprehensive preclinical evaluationInt J Toxicol200625253316510354
- StrattonIMAdlerAINeilHAWon behalf of the UK Prospective Diabetes Study GroupAssociation of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational studyBMJ20003214051210938048
- TurnerRCCullCAFrighiVfor the UK Prospective Diabetes Study (UKPDS) GroupGlycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49)JAMA199928120051210359389
- [UKPDS] UK Prospective Diabetes Study GroupIntensive blood glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33)Lancet1998352837539742976
- [UKPDS] UK Prospective Diabetes Study GroupUK Prospective Diabetes Study 16. Overview of 6 years’ therapy of type 2 diabetes: a progressive diseaseDiabetes1995441249587589820
- WittlinSDWoehrleHJGerichJEInsulin pharmacokineticsInsulin Therapy2002New York, NYMarcel Dekker Inc
- World Health OrganizationDiabetes action now [online]2006 Accessed September 9, 2006. URL: http://www.who.int/diabetes/actionnow/en/DANbooklet.pdf