Abstract
Blockade of the renin-angiotensin system with selective AT1 receptor antagonists is recognized as an effective mean to lower blood pressure in hypertensive patients. Among the class of AT1 receptor antagonists, telmisartan offers the advantage of a very long half-life. This enables blood pressure control over 24 hours using once-daily administration. The combination of telmisartan with hydrochlorothiazide is a logical step because numerous previous studies have demonstrated that sodium depletion enhances the antihypertensive efficacy of drugs interfering with the activity of the renin-angiotensin system (RAS). In accordance with past experience using similar compounds blocking the RAS, several controlled studies have now demonstrated that the fixed-dose combination of telmisartan/hydrochlorothiazide is superior in lowering blood pressure than either telmisartan or hydrochlorothiazide alone. Of clinical interest also is the observation that the excellent clinical tolerance of the angiotensin II receptor antagonist is not affected by the association of the low-dose thiazide. Thus telmisartan/hydrochlorothiazide is an effective and well-tolerated antihypertensive combination. Finally, the development of fixed-dose combinations should improve drug adherence because of the one-pill-a-day regimen.
Introduction
Cardiovascular diseases (CVD) are the leading causes of death among adults in the industrialized world (29.2% of total global deaths) and in developing countries, and will probably remain so in the near future (CitationWHO 2003). Hypertension is one of the most prevalent risk factors for the development of cardiovascular complications such as left ventricular hypertrophy, myocardial infarction, stroke, and renal diseases. However, if blood pressure (BP) is effectively controlled, target-organ damage can be prevented and, in the long term, the likelihood of these complications can be reduced (CitationChobanian et al 2003; CitationCifkova et al 2003). Its treatment is also one of the most effective ways to retard the progression of diabetic and non-diabetic renal diseases (CitationChobanian et al 2003; CitationCifkova et al 2003). Unfortunately, reports from several countries around the world have shown that hypertensive patients with well-controlled BP represent only a small percentage of the hypertensive population (usually less than 30%). This contrasts with the rather high response rate obtained in clinical trials investigating new antihypertensive drugs or therapeutic strategies. For example, in the Hypertension Optimal Treatment (HOT) study, 88% of patients assigned to a target diastolic BP ≤90 mmHg achieved this goal after 12 months of antihypertensive treatment (CitationHansson et al 1998). In the ALLHAT study, >60% of the enrolled patients achieved a BP goal <140/90 mmHg at 5 years (CitationThe ALLHAT Officers and Coordinators for the ALHAT Collaborative Research Group 2002). This apparent discrepancy between the results obtained in the general hypertensive population and those of large clinical trials is perhaps explained by the experimental conditions in which clinical trials are conducted and by the selection of patients and physicians both being more motivated or willing to achieve target BP levels when engaged in clinical studies (CitationResnick 2003). Nevertheless, it strongly suggests that it should be possible to increase the overall percentage of patients reaching a satisfactory BP control.
Today, when the patient fails to respond to treatment, the most common medical response is to increase the dose of the antihypertensive agent, to add another drug, or eventually to change the therapeutic agent (CitationWaeber 2003). In some cases, clinical investigations looking for a secondary form of hypertension will be conducted. Thus, physicians have a systematic bias considering that the patient is basically a non-responder or that the pharmacological regimen is inadequate. This “pharmacological” attitude leads either to the use of high doses of antihypertensive agents which are very likely to produce side-effects, or to the prescription of several antihypertensive compounds according to the classical step-care therapy scheme. However, both the occurrence of side-effects and the increased complexity of the regimen have been shown to reduce drug adherence and the persistence of treatment (CitationWuerzner et al 2003).
Treating hypertension with a combination of different drugs has multiple rationale and advantages and might offer the possibility to reduce the number of non-responders. The first advantage is obviously to associate drugs with different mechanisms of action leading to an increased efficacy of each individual drug. Another potential clinical interest of drug combinations is to blunt the activation of physiological compensatory feed-back mechanisms which could either interfere with the activity of a drug or generate side-effects. Thus, combining two agents that may mutually interfere with compensatory responses is more likely to increase the BP control rate (CitationWaeber 2003) and may even prevent side-effects. Studies have clearly demonstrated that BP can be readily controlled in almost two-thirds of the cases by a carefully selected prescription of two drugs (CitationSica 1994; CitationEpstein and Bakris 1996).
In this context, the association of a blocker of the renin-angiotensin system (RAS) with a diuretic is a very logical combination. The natriuretic effect of the diuretic which leads to a decrease in plasma volume stimulates renin secretion and thereby increases angiotensin II and aldosterone production, which in turn causes vasoconstriction and promotes water and sodium retention. The direct consequence of these physiological responses is a limitation of the antihypertensive effect of the diuretic. Blockade of the RAS in such high-renin conditions prevents the angiotensin II-dependent BP maintenance (CitationBrunner et al 1988) and thus enhances the effect of the diuretic (CitationNeutel et al 1999b). Conversely, the antihypertensive efficacy of blockers of the RAS is blunted when patients are salt-loaded because BP becomes volume- rather than renin-dependent. With the addition of a diuretic, BP becomes more renin-dependent and the antihypertensive efficacy of the blocker of the RAS is increased by the diuretic. This explains why a large number of fixed-dose combinations containing RAS blocker and a diuretic have been developed in the past. Clinical as well as experimental studies have demonstrated that when combined with hydrochlorothiazide, angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) lower BP in low-, normal-, and high-renin hypertension (CitationWaeber 2003). The interest in these combinations is particularly evident in black populations, in whom a monotherapy with conventional doses of an ACEI or an ARB is often considered less effective unless patients are maintained on a low-sodium intake (CitationFalconnet et al 2004). Another advantage of such a combination is also its excellent tolerability profile with a very low incidence of adverse effects, as will be discussed below.
Telmisartan/hydrochlorothiazide: fixed-dose combination
Introduction to the compounds
The ARB telmisartan and the thiazide diuretic hydrochlorothiazide are two antihypertensive agents that have a well-recognized clinical efficacy in the treatment of hypertension (CitationVan Brummelen et al 1980; CitationAlmansa et al 1996; CitationNeutel et al 1999a, Citationb; CitationMcGill and Reilly 2001b; CitationNeutel et al 2003). However, as generally observed in the treatment of essential hypertension, many patients are either from the time of diagnosis or later in their follow-up, insufficiently controlled with telmisartan or hydrochlorothiazide alone (CitationLacourciére et al 2001; CitationLacourciére 2002; CitationLacourciére and Martin 2002; CitationFreytag et al 2002). Therefore, fixed-doses combination of telmisartan and hydrochlorothiazide (Micardis-Plus® and Micardis/HCT®, Boehringer Ingelheim, or PritorPlus®, GlaxoSmithKline) have been developed in order to further improve the efficacy of telmisartan and to promote compliance in patient who otherwise would require the administration of two drugs. Combined therapy is available in three combinations of 40 mg/12.5 mg, 80 mg/12.5 mg, and 80 mg/25 mg telmisartan and hydrochlorothiazide, respectively.
Chemistry, pharmacology, pharmacodynamics, and pharmacokinetics of the compounds
Telmisartan is a long-acting, nonpeptide angiotensin II receptor-subtype 1 (AT1) antagonist, which is orally active, highly selective, and potent. It is chemically described as 4′-[(1,4′-dimethyl–2′-propyl[2,6′-bi-1H-benzimidazol] 1′-yl)methyl]-[1,1′-bipheny]-2-carboxylic acid. Telmisartan has the molecular formula C33H30N4O2; its molecular weight is 514.6 g/mol. It is a relatively lipophilic compound and has a higher n-octanol/buffer partition coefficient (log p = 3.20) than candesartan (−0.96), irbesartan (1.48), valsartan (−0.95), or EXP-3174 (−2.45), the active metabolite of losartan (CitationWienen et al 2000). This high lipophilicity that is also reflected in its high volume of distribution (c. 500 L) (CitationStangier et al 2000) enhances tissue penetration, intracellular absorption, and bioavailability (CitationWienen et al 2000).
Telmisartan lacks the tetrazole unit which is usually present in the structure of sartans, but has a common benzimidazole group with candesartan. The substitution of this benzimidazole moiety with a basic heterocycle results in potent AT1 antagonism and good absorption after oral administration (CitationRies et al 1993). Telmisartan selectively and insurmountably inhibits stimulation of the AT1 receptor by angiotensin II without affecting other receptor systems involved in cardiovascular regulation. It inhibits the binding of labeled angiotensin II to AT1 receptors in rat lung with a Ki of 3.7 nmol/L (CitationWienen et al 1993). The drug dissociates slowly from AT1 receptors with a dissociation half-life of about 75 minutes compared with 14.5 minutes for angiotensin II, and there appears to be no re-association of telmisartan with the receptor (CitationMaillard et al 2002). This feature may explain why telmisartan has the longest half-life (approximately 24 hours) of all commercially available ARBs (CitationMaillard et al 2001), making it suitable for once-daily dosing (CitationNeutel et al 1998). Telmisartan blunts all the AT1-mediated effect of angiotensin II, including the vasoconstrictor and aldosterone-secreting effect of angiotensin II. It also inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increases in plasma renin activity and angiotensin II circulating levels do not overcome the blockade exerted by telmisartan (CitationStangier et al 2001; Citationde Gasparo 2004). In addition to its vasodilatory properties, telmisartan also appears to modulate renal function, an effect which may contribute to its antihypertensive efficacy and has been demonstrated in vitro on isolated perfused kidneys (CitationWienen et al 1993) as well as in vivo in rats and dogs (CitationWienen and Entzeroth 1994; CitationSchierok et al 2001). Finally, In addition to its AT1 receptor antagonism, telmisartan has also been identified as an activator of the peroxisome proliferator-activated receptor γ (PPAR-γ) (CitationBenson et al 2004; CitationSchupp et al 2004). The transcription factor PPAR-γ is a nuclear hormone receptor, which acts as a central regulator of insulin and glucose metabolism, improving insulin sensitivity. Full receptor agonists such as the thiazolidinidiones pioglitazone (Actos®; Eli Lilly) or rosiglitazone (Avendia®; GlaxoSmithKline) are approved for the indication of diabetes mellitus (CitationPittas and Greenberg 2002). Unfortunately, besides their insulin-sensitizing effect these drugs also showed some adverse effects like weight gain, edema and fluid retention (CitationZanchi et al 2004). In contrast, telmisartan showed only a partial agonistic activity for the PPAR-γ receptor and thus modulates the activity of PPAR-γ, retaining the metabolic efficacy of PPAR-γ activation with reduction in adverse effects exerting in parallel with AT1 receptor blockade (CitationSchupp et al 2005). This special feature (also present though in a smaller extend in other ARBs such as irbesartan (CitationSchupp et al 2004) and losartan (CitationMarshall et al 2006)) may provide a new therapeutic option for better cardiovascular risk management in metabolic diseases. Indeed, telmisartan that combines antihypertensive and antidiabetic actions has been found in some preclinical and clinical studies to improve carbohydrate and lipid metabolisms without causing the side effects that accompany full PPAR-γ activators (CitationKurtz 2005). Yet, the clinical relevance of these effects of telmisartan on PPAR-γ receptors remains to be demonstrated on a large group of hypertensive patients.
Hydrochlorothiazide (HCTZ) is a sulfonamide derivative thiazide diuretic. Chemically it is described as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadizine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2; and its molecular weight is 297.7 g/mol. Thiazide diuretics such as hydrochlorthiazide are widely used for the treatment of hypertension. Though the mechanism of the antihypertensive effect of thiazides is still not fully understood, it is obvious that these agents act by promoting sodium excretion leading to a transitory negative sodium balance. Thiazides affect the renal tubular mechanisms of electrolytes reabsorption in the distal segment of the nephron, directly increasing the excretion of sodium and chloride in approximately equivalent amounts by blocking the Na+Cl− co-transporter. Following oral administration of hydrochlorthiazide, urinary electrolytes and water excretion increase within 2 hours, peaks at 4 hours, and the effect lasts for up to 24 hours.
Unfortunately, the drawback of such compounds is, as already mentioned, the secondary stimulation of the RAS resulting in an increase in aldosterone secretion which in turn causes a loss of potassium and a compensatory increase in proximal re-absorption of uric acid leading to hyperuricemia. These effects are responsible for two of the major side-effects of thiazide diuretics, ie, hypokalemia and gout (CitationReyes 2003). Of note, thiazide-induced hypokalemia can potentially trigger adverse cardiac events (CitationBeermann and Groschinsky-Grind 1977; CitationSiscovick et al 1994).
The pharmacokinetics and pharmacodynamic properties of the telmisartan/hydrochlorothiazide combination have not been studied extensively in patients or healthy volunteers. For example, to date, only one study has been designed to compare the steady-state pharmacokinetics of a high-dose telmisartan with and without a high-dose of hydrochlorothiazide in order to compare the steady-state pharmacokinetics of hydrochlorothiazide and telmisartan given alone or in combination. This open-label, crossover study was done in 13 healthy volunteers. All subjects received telmisartan 160 mg qd, hydrochlorothiazide 25 mg qd and telmisartan/hydrochlorothiazide 160/25 mg qd for 7 days. Each treatment phase was separated by a 14-day washout period (CitationYoung et al 2000). The results showed that there were no appreciable differences in trough plasma concentrations of hydrochlorothiazide and telmisartan, given alone or in combination. Mean values of the primary end points (Cmax and AUC0-24) and secondary end points (Cmin and t1/2) for both telmisartan and hydrochlorothiazide were unaffected when administered simultaneously. Moreover, concurrent telmisartan had no effect on urinary excretion of hydrochlorothiazide. The absence of any significant effect on the pharmacokinetics of either hydrochlorothiazide or telmisartan demonstrates that no dose adjustment is required if the two agents are given together for the management of hypertension.
In contrast to other ARBs, some drug interactions have been described with telmisartan. In particular, telmisartan increases (49% at peak and 30% at trough) serum digoxin concentration, and may also decrease plasma warfarin levels (CitationSharpe et al 2001). The administration of telmisartan did not result in clinically significant interaction with acetaminophen, amlodipine, glibenclamide, simvastatin, ibuprofen, or hydrochlorothiazide. Since telmisartan had no effects in vitro on cytochrome P450 enzymes, except for some minimal inhibition of CYP2C19, it is not expected to interact with drugs that inhibit cytochrome P450 enzymes nor with drugs that are metabolized by cytochrome P450, except for possible inhibition for drugs metabolized by CYP2C19 (CitationUnger and Kaschina 2003). On the other hand, hydrochlorothiazide may potentially interact with a large variety of drugs. Thus, the absorption of hydrochlorothiazide is impaired in the presence of anionic exchange resins. Single doses of either cholestyramine or colestipol resins bind the hydrochlorothiazide and reduce its absorption from the gastrointestinal tract by up to 85% and 43%, respectively. Thiazide diuretics reduce the renal clearance of lithium and lead to a high risk of lithium toxicity.
We have summarized in the main pharmacodynamics of telmisartan and hydrochlorothiazide separately. Indeed, apart from its antihypertensive action in rats (CitationWienen and Schierok 2001), no special report has been published on the pharmacodynamic properties of the telmisartan/hydrochlorothiazide combination itself. Therefore, the properties described in this table are from studies in animals, healthy volunteers, and patients receiving telmisartan or hydrochlorothiazide as monotherapy.
Table 1a Pharmacodynamic properties of telmisartan
Table 1b Overview of the pharmacodynamic properties of hydrochlorothiazide
Clinical efficacy of telmisartan/hydrochlorothiazide
Numerous reviews dealing with the clinical antihypertensive efficacy of telmisartan/hydrochlorothiazide have been recently published (CitationFenton et al 2003; CitationSchmieder 2004; CitationMaillard and Burnier 2005; CitationMeredith 2005; CitationBattershill and Scott 2006). A series of large, multicenter, randomized, controlled clinical trials has been performed recently in order to evaluate the clinical efficacy of this therapy (see ). Different primary end points were defined in these studies: the reduction in DBP, either from baseline (CitationKarlberg et al 1999; CitationLacourciére et al 2001; CitationMcGill and Reilly 2001b; CitationLacourciére and Martin 2002; CitationFreytag et al 2002) or to values inferior to 90 mmHg (CitationNeutel et al 2002; CitationFreytag et al 2002); changes in ambulatory DBP and SBP (CitationLacourciére et al 2003); and decreases in supine SBP (CitationKarlberg et al 1999; CitationFreytag et al 2001). In these controlled clinical trials with over 2500 patients, patients were exposed to telmisartan (20–160 mg) and concomitant hydrochlorothiazide (6.25–25 mg). These trials included one factorial design trial with combinations of telmisartan (20, 40, 80, 160 mg, or placebo) and hydrochlorothiazide (6.25, 13.5, 25 mg, or placebo) (CitationMcGill and Reilly 2001b). Four other studies of at least 6 months’ duration allowed the addition of hydrochlorothiazide for patients who either were not adequately controlled on the randomized monotherapy dose or had not achieved adequate response after a complete up-titration of telmisartan (CitationKarlberg et al 1999; CitationFreytag et al 2001, Citation2002; CitationNeutel et al 2002). All these studies proved this combination both as fixed-dose combination (CitationLacourciére et al 2001, Citation2003; CitationMcGill and Reilly 2001b; CitationLacourciére and Martin 2002) or as an add-on (CitationFreytag et al 2001, Citation2002; CitationNeutel et al 2002), to be highly effective in reducing BP (CitationMaillard and Burnier 2005), particularly in the early morning period (CitationLacourciere et al 2005). Combining telmisartan with hydrochlorothiazide enhances the antihypertensive activity of telmisartan in almost two-thirds of hypertensive patients with mild to moderate hypertension. Moreover, because of their synergism, BP control can sometimes be achieved with lower doses of each component (CitationSchmieder 2004; CitationMaillard and Burnier 2005). This is particularly interesting since the metabolic disturbances associated with high dose hydrochlorothiazide were avoided by combining low doses of this drug with telmisartan (CitationSchmieder 2004).
Table 2 Efficacy of the telmisartan/hydrochlorothiazide (HCTZ) combination compared with that of placebo or other antihypertensive agents: changes in blood pressure in patients with mild to moderate or severe hypertension in multicenter randomized, double bind studies
When compared with other antihypertensive such as atenolol (CitationFreytag et al 2001), amlodipine (CitationNeldam and Edwards 2006), or enalapril (CitationKarlberg et al 1999) given alone or in combination with hydrochlorothiazide, long-term studies demonstrated that telmisartan alone or combined with hydrochlorothiazide is at least as effective in treating high BP in patients with mild to moderate hypertension as these other drugs.
Thus, for example, the antihypertensive efficacy and tolerability of two doses of telmisartan and of the beta-blocker atenolol were compared in patients with mild to moderate hypertension in a 26-week multicenter, randomized, double-blind, double-dummy, parallel-group trial (CitationFreytag et al 2001). This study was conducted in 520 men and women aged 22–79 years with morning mean supine DBP between 95 and 114 mmHg and SBP <210 mmHg after a 2- to 3-week placebo run-in period. These patients were randomized in a 2:1 ratio to telmisartan (40 mg titrated to 80 mg or to 120 mg) or atenolol (50 mg titrated to 100 mg) in order to obtain a DBP ≤90 mmHg or a decrease of DBP from baseline of ≥10 mmHg (primary efficacy end point). Open-label hydrochlorothiazide 12.5 or 25 mg was added if needed according to a predefined titration rule. At the end of the study, 84% of the telmisartan and 78% of the atenolol-treated patients had achieved the primary end point (difference not statistically significant), The final reduction in SBP/DBP was 20.9/14.4 mmHg with telmisartan and 16.7/13.3 mmHg with atenolol; only the difference in SBP between both regimen was significant (p = 0.005). By week 26, 32% of the telmisartan and 28% of the atenolol patients required hydrochlorothiazide. For these patients, additional mean reductions in supine SBP/SDP of 7.2/6.0 mmHg were observed when the diuretic was added to telmisartan therapy, and 6.6/3.5 mmHg when hydrochlorothiazide was added to atenolol therapy (CitationFreytag et al 2001).
Some studies have compared the efficacy of the ARB-diuretic therapy with other combinations in older patients. The ATHOS study has been designed to compare the changes in 24-hour ambulatory BP in 872 elderly with predominantly systolic hypertension receiving either telmisartan 80 mg/hydrochlorothiazide 12.5 mg or amlodipine 10 mg/hydrochlorothiazide 12.5 mg in a prospective, randomized, open-label, blinded-end point trial. The results of this study (CitationNeldam and Edwards 2006) showed that telmisartan/hydrochlorothiazide and amlodipine/hydrochlorothiazide changed SBP for the last 6 hours of the dosing interval by −18.3 and −17.4 mmHg, respectively (p = 0.2520). However, telmisartan/hydrochlorothiazide provided significantly greater reductions compared than amlodipine/hydrochlorothiazide over the 24-hour period (−19.3 and −17.2 mmHg, respectively; p = 0.001) and provided higher SBP control rates (65.9% and 58.3%, respectively; p = 0.0175). Treatment effects were similar across age groups and in both men and women (CitationNeldam and Edwards 2006).
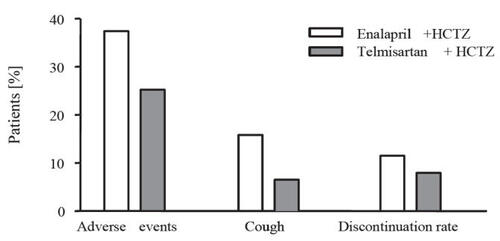
On other hand, when compared with enalapril in elderly patients (65 years and over) in a 26-week, multicenter, double-blind, parallel-group, dose-titration study (CitationKarlberg et al 1999), no significant difference were observed in mean changes from baseline both in supine DBP (−12.8 mmHg for telmisartan and −11.4 mmHg for enalapril) or SBP (−22.1 mmHg and −20.1 mmHg, respectively) at trough, and both regimens provided effective BP lowering over the 24-hour dosing interval, as determined by ambulatory BP monitoring. Both regimens were well tolerated; however, the incidence of treatment-related cough was significantly higher with enalapril than with telmisartan (16 vs 6.5%).
In another prospective, double-blind, randomized study (CitationBarnett et al 2004), enalapril and telmisartan were compared in patients with type 2 diabetes and early nephropathy. Two hundred and fifty patients with normal levels of glomerular filtration rate (>90 mL/min per 1.73 m2 of body-surface area) were enrolled and receive either telmisartan 80 mg or enalapril 20 mg. Hydrochlorothiazide was given as concomitant therapy in more than 50% of patients in each group. After 5 years of treatment, the adjusted mean reduction in SBP was 6.9 mmHg with telmisartan and 2.9 mmHg with enalapril (95% CIl, −8.5 to 0.5 mmHg, not significant). There was also no significant difference between groups in DBP mean difference. Of note, the primary end point of this study was the change in glomerular filtration rate (GFR) from baseline to the last available value during the five years of treatment. GFR decreased by 17.9 mL/min per 1.73 m2 of body-surface area with telmisartan compared with 15.0 mL/min per 1.73 m2 of body-surface area with enalapril, with a treatment difference of 2.6 mL/min. This difference was not significant (CitationBarnett et al 2004).
Several other clinical studies have assessed the renal protective effect of telmisartan (CitationBattershill and Scott 2006). CitationVogt et al (2005) recently published a substudy of the Angiotensin II Receptor Antagonist Micardis in Isolated Systolic hypertension (ARAMIS) Study in which they investigated the reduction in urinary albumin excretion in patients with isolated systolic hypertension (ISH) unselected for albuminuria. This study showed that telmisartan 20–80 mg induced a significantly greater decrease in urinary albumin excretion than hydrochlorothiazide 12.5 mg, irrespective of the baseline UAE, and despite comparable reductions in SBP with both drugs.
Of note, based on the results of these studies and on the previous data from the RENAAL (CitationBrenner et al 2001), IDNT (CitationLewis et al 2001), and IRMA II studies (CitationParving et al 2001), the telmisartan renoprotective study from incipient nephropathy to overt nephropathy (INNOVATION) has been initiated (CitationMakino et al 2005). This prospective, randomized, double-blind forced-titration, parallel-group, placebo-controlled study compares a high and low dose of telmisartan in 1800 hypertensive or normotensive Japanese patients. It will assess whether telmisartan provides clinical benefits in hypertensive or normotensive patients with diabetes mellitus and diabetic nephropathy. This study has recently been completed and should be presented in 2006. In the same group of clinical studies (the PROTECTION trial program [CitationWeber 2003]), two sister trials, VIVALDI in Europe and AMADEO in North America, will compare the potential benefits of telmisartan and valsartan (VIVALDI) (CitationBoger et al 2005) or losartan (AMADEO) in the progression of renal disease in patients with diabetes and nephropathy. These two studies will be completed in early 2007.
Besides the antihypertensive effect of telmisartan and its potential ability to protect the kidney, telmisartan was also recently shown to be active in improving metabolic parameters, such as insulin resistance in hypertensive patients with coexisting type 2 diabetes or metabolic syndrome (CitationBattershill and Scott 2006). However, none of these studies (CitationDerosa et al 2004a, Citationb; CitationMiura et al 2005; CitationVitale et al 2005; CitationSengul et al 2006) involved patients under concomitant therapy with hydrochlorothiazide.
Finally, the ability of telmisartan to reduce left ventricular hypertrophy (LVH) has been shown by several studies assessing left ventricular thickness or left ventricular mass as primary end point in mild to moderate hypertensive patients (CitationBattershill and Scott 2006; Goebel et al 2006). A meta-analysis has identified differences in the ability of different classes of anti-hypertensive agents to prevent or revert LVH, with agents that target the RAS appearing superior to other agents, such as beta-blockers and diuretics (CitationDiez et al 2001). In this context, in a recent 12-month, double-blind study in 69 patients with mild to moderate hypertension, left ventricular mass index (LVMI) was significantly reduced from baseline with once-daily telmisartan 80 mg (141 vs 125 g/m2; p < 0.001), whereas there was little change in patients receiving once-daily hydrochlorothiazide 25 mg (139 vs 135 g/m2) (CitationGalzerano et al 2004). The additional activity of telmisartan may probably be explained by the sustained BP control and the non-hemodynamic effects of targeting the RAS. The ultimate proof of the clinical value of telmisartan on reducing LVH will be provided by the outcome trials ONTARGET/TRANSCEND (CitationTeo et al 2004) and PROTECTION (CitationWeber 2003) which will clarify the cardioprotective role of telmisartan in in high-risk patients whose BP is well controlled.
Long-term exposure to telmisartan/hydrochlorothiazide
Two open-label extension studies have been performed to assess the long-term efficacy and tolerability of telmisartan 40–80 mg administered once daily alone or in association with hydrochlorothiazide (12.5–25 mg). One study provided results over a 1-year treatment period (CitationNeutel et al 2002) and in the second study, data were gathered for up to 4 years (CitationFreytag et al 2002).
In Neutel’s study, 483 patients had their treatment with telmisartan 80 mg/day titrated over 1 year with stepwise addition of hydrochlorothiazide 12.5 mg or 25 mg, and/or other antihypertensive drugs to achieve a DBP <90 mmHg (CitationNeutel et al 2002). At the final visit, DBP control was achieved in 70.0% (194/277) of patients in the telmisartan monotherapy group and by 56%, 55%, and 65% of the patients receiving telmisartan/hydrochlorothiazide 80/12.5 mg/day, 80/25 mg/day, or telmisartan 80 mg plus another antihypertensive with or without the hydrochlorothiazide (12.5 or 25 mg). Progressively greater BP reductions occurred with the sequential addition of hydrochlorothiazide and other antihypertensives. All treatments were well tolerated (CitationNeutel et al 2002).
The second long-term study assessing the efficacy and tolerability of telmisartan combined with hydrochlorothiazide lasted 4 years (CitationFreytag et al 2002). This open-label extension study of 4 controlled trials involved 888 patients with mild to moderate hypertension. Patients received telmisartan 40–80 mg once daily with add-on hydrochlorothiazide (12.5–25 mg) if necessary and/or other antihypertensives excluding other ARBs. The main goal was to lower supine DBP below 90 mmHg. Almost 80% of the patients (701/888) completed the trial with a median exposure of 1184 days (>3 years). The proportion of responders was 89% in the telmisartan monotherapy (40 or 80 mg) treatment category after 8 weeks of treatment. However, this percentage dropped years to 59% after 3 years, indicating that about one-third of patients initially responsive to telmisartan alone required additional therapy over time. With the addition of hydrochlorothiazide, in most cases 25 mg qd, the primary end point was reached in 75% of the patients who became unresponsive to the monotherapy. Most adverse events were of mild to moderate intensity and unrelated to treatment (CitationFreytag et al 2002).
Safety and tolerability
With an adverse-event profile closely resembling that of placebo, telmisartan, like other ARBs, is characterized by a very high safety and tolerability, which has been confirmed in several clinical trials (CitationFenton et al 2003; CitationBattershill and Scott 2006) but also in a large, 6-month, post-marketing surveillance study enrolling 19,870 patients (CitationMichel et al 2004). The profiles of adverse events observed in these studies were similar in frequency and severity. Most adverse effects were mild and transient and occurred with a similar incidence in the placebo groups. Headache and dizziness were the most frequent adverse events. The tolerability and safety record of telmisartan is consistent across a wide range of patient demographic variables such as age, sex, and ethnic group (CitationBattershill and Scott 2006). Neither the dry cough associated with ACE inhibitors nor the edema associated with calcium channel blockers (CCBs) has been observed with telmisartan treatment (CitationWhite 2002).
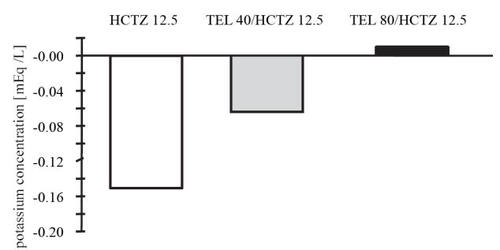
The safety and tolerability of telmisartan/hydrochlorothiazide was investigated in most of the above-mentioned studies (CitationKarlberg et al 1999; CitationLacourciére et al 2001; CitationFreytag et al 2001; CitationMcGill and Reilly 2001a, Citationb; CitationNeutel et al 2002; CitationLacourciére and Martin 2002; CitationLacourciére et al 2003). The overall tolerability profile was good, the incidence of adverse effects in the combination groups being similar to that observed in placebo-treated groups. For example, in the factorial design trial with combinations of telmisartan, hydrochlorothiazide, and placebo (CitationMcGill and Reilly 2001b), where all the 818 randomized patients were included in the safety analysis, adverse events were reported in 96 of 209 (45.9%) patients receiving telmisartan monotherapy, 61 of 121 (50.4%) receiving hydrochlorothiazide monotherapy, 199 of 414 (48.1%) receiving combination therapy, and 31 of 74 (41.9%) receiving placebo. Most adverse effects were mild in intensity and transient and did not require discontinuation of the therapy. No unexpected serious adverse events have been observed and adverse experiences have been limited to those that have been previously reported with telmisartan and/or hydrochlorothiazide. Thus, adverse events occurring in more than 2% of telmisartan/hydrochlorothiazide-treated patients were dizziness (5%), sinusitis and upper respiratory tract infection (4%–8%), fatigue (3%), influenza-like symptoms (2%), and gastrointestinal problems (nausea 2%, diarrhea 3%). In the same study, hydrochlorothiazide caused a dose-related decrease in serum potassium. Combination with telmisartan tended to offset the effects of hydrochlorothiazide on serum potassium levels (CitationMcGill and Reilly 2001b) ().
Figure 1 Telmisartan plus hydrochlorothiazide (HTCZ) combined therapy offsets the effects of hydrochlorothiazide on serum potassium levels (derived from data CitationMcGill et al 2001b).
Finally, a meta-analysis of data collected from 34 randomized trials also concluded that the incidence of treatment-related adverse events is similar in placebo-treated (10.5% n = 819) patients and patients receiving either telmisartan 10–60 mg/day (12%, n = 6575) or telmisartan/hydrochlorothiazide 10–160/6.25–25 mg/day (12.8%, n = 2180) (CitationMancia 2002). Of note, the incidence of treatment-related adverse events per patient per treatment year was higher in patients receiving placebo (1.02) than in patients on telmisartan monotherapy (0.20) or on combined telmisartan/hydrochlorothiazide therapy (0.18) (statistical analysis not reported) (CitationMancia 2002). As with telmisartan alone, the most commonly reported adverse events in patients receiving telmisartan and hydrochlorothiazide were dizziness and headache and fatigue. In the same study, serious adverse events occurred less frequently in placebo recipients (1.1%) than in telmisartan-treated patients (5.6 % with hydrochlorothiazide and 4.3% without hydrochlorothiazide, respectively). However, per patient per treatment year, the incidence was similar in all groups (CitationMancia 2002). Treatment-related hyperuricemia and hypokalemia occurred only in 5 and 6 patients, respectively, receiving combination therapy (CitationMancia 2002).
Unfortunately, we have to emphasize that most of studies that have investigated the effect and the safety of the telmisartan/hydrochlorothiazide association have been short and medium term, and results of the longest ONTARGET and PROTECTION studies are not yet available. Generally, most of the longest prospective randomized trials in hypertension last only 4–6 years and therefore provide little if any information on long-term safety, particularly for diuretics with or without combination with other antihypertensive drugs. Many patients are exposed to BP-lowering drugs for many decades, and drug-induced changes could be cumulative (Grosmann and Messerli 2006). This is potentially true with the adverse metabolic effects that are seen with the diuretics. However, a combination with hydrochlorothiazide blunted the deleterious effect of diuretics decreasing the development of new-onset diabetes in shorter-term experiments as observed in LIFE, SCOPE, and VALUE trials (CitationElliot 2005). Therefore we have to wait for cohort studies investigating morbidity and mortality of hypertensive patients which require an intervention on the RAS system to fully characterize the long-term protective effect of combining an ARB with a thiazide diuretic.
In all studies, adverse events occurred at approximately the same rate in men and women, older and younger patients (CitationKarlberg et al 1999; CitationNeldam and Edwards 2006), black and non-black patients (CitationMcGill and Reilly 2001a), and diabetic or non-diabetic (CitationFenton et al 2003). Long-term combination therapy is also well-tolerated as already mentioned in the 4-year extension trial published by Freytag et al (CitationFreytag et al 2002).
Compared with other combined therapy, patients treated with telmisartan plus hydrochlorothiazide in the ATHOS study experienced fewer treatment-related adverse events than patients receiving amlodipine plus hydrochlorothiazide (8% vs 33%; p < 0.0001), with the incidence of peripheral edema being significantly lower in the telmisartan combination therapy (1.2% vs 24.3%; p < 0.0001) (CitationNeldam and Edwards 2006) (). Relative to ACEIs, the incidence of treatment-related adverse events was similar for telmisartan and enalapril (CitationKarlberg et al 1999). However, as already mentioned above, more than twice as many patients taking enalapril experienced cough (16 vs 6.5%), which is believed to be caused by an accumulation of bradykinin that is not degraded in patients treated with an ACE inhibitor (CitationIsraili and Hall 1992) ().
Figure 2 Drug-related adverse events, incidence of peripheral edema, and discontinuation rates in the ATHOS Study (derived from data of CitationNeldam et al 2006).
Abbreviations: HTCZ, hydrochlorothiazide.
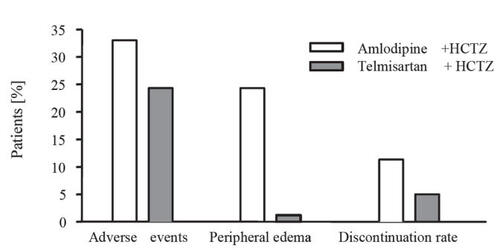
Figure 3 Drug-related adverse events, incidence of cough, and discontinuation rates in a study comparing telmisartan plus hydrochlorothiazide (HTCZ) with enalapril plus hydrochlorothiazide in patients with mild to moderate hypertension (derived from data of CitationKarlberg et al 1999).
Persistence on treatment
The more active drug will never be effective if it is not taken by the patient, and poor compliance is certainly an important cause of inadequate BP control. Several patient-related and physician-related parameters can affect drug adherence. There is, however, good evidence that drug-related issues can also interfere with the adherence to treatment. Thus the treatment complexity, the frequency of dosing, as well as the tolerability profile of the drugs are important determinants of drug adherence.
One very common reason why patients do not comply with the prescription is the fact that they have to take several different medications with varying frequencies of dosing (CitationResnick 2003). This is an important contributory factor to poor compliance particularly in elderly patients (CitationWuerzner et al 2003). Therefore, efforts should been made to simplify drug regimens, and one appropriate intervention is to reduce the frequency of the drug administration as well as the number of agents to be taken. Indeed, a once-daily regimen induces a better compliance than a twice-daily regimen. This can be achieved using long-acting agents and fixed-dose combinations which reduce the number of daily tablets. Several studies performed using ambulatory BP monitoring have shown that telmisartan shifts the entire BP profile to lower levels including in the early morning period (CitationNeutel et al 1998; CitationLacourciere et al 2005). Moreover, the use of long-acting antihypertensive drugs such as telmisartan may prevent the rise in BP induced by a missed dose and may improve the 24-hour BP control even in the early morning hours before the next dose is taken (CitationWillich et al 1987).
In a recent meta-analysis presented at the European Meeting of Hypertension, the efficacy of fixed-dose combinations to improve patient compliance therapy was compared with free-drug component regimens in patients with chronic disease (CitationBangalore et al 2006). Nine studies that reported medication compliance/adherence or persistence when using fixed-dose combinations versus free-drug combinations were selected, of which 4 dealt with antihypertensive agents. In the hypertension studies, the authors showed that fixed-dose combinations of antihypertensive drugs (amlodipine plus benazepril (CitationTaylor and Shoheiber 2003), lisinopril plus hydrochlorothiazide or enalapril plus hydrochlorothiazide (CitationDezii 2000), and ARBs plus diuretic (CitationBangalore et al 2006)) improve compliance to medication by more than 50% when compared with free-drug combinations ((OR 1.54, 95% CI 1.42–1.66). Such improvement in drug adherence should have an important impact on clinical outcomes.
Another reason why hypertensive patients do not take their pills regurlarly is that they develop side-effects while the hypertension is usually asymptomatic (CitationBrondolo et al 1999). In this context, the excellent tolerability profile of ARBs including telmisartan combined with hydrochlorothiazide is a potential advantage. Indeed, studies that have analyzed patterns of compliance and persistence (defined as continuing therapy with the originally prescribed antihypertensive drug) have shown that both are intimately linked to tolerability (CitationKjellgren et al 1998; CitationConlin et al 2001; CitationWogen et al 2003). In a study of persistence that evaluated prescription refill data from 21,723 patients enrolled in a large pharmaceutical management organization (CitationBloom 1998), the percentage of patients continuing initial ARB therapy was substantially higher at 12 months’ follow-up than the percentage continuing therapy with ACEIs, calcium antagonists, beta-blockers, or thiazide diuretics (64% vs 58%, 50%, 43%, and 38%, respectively); and patients taking an ARB were more likely to continue with their initial therapy than those taking an ACEI, a calcium antagonist, a beta-blocker, or a thiazide diuretic (CitationBloom 1998). Another study determined whether the stay-on-therapy (persistence) patterns observed in a previous analysis at one year were maintained over a 4-year period. Significantly more patients whose initial therapy was based on an ARB (losartan in this case) had continued with this treatment at 24 months (p < 0.007) and at 36 months (p < 0.01) compared with patients initially given drugs from other antihypertensive class. At 48 months, half (50.9%) of the original losartan-prescribed group was still taking the initial agents whereas 36.5% of the other antihypertensive cohorts remained on their initial drug class (CitationConlin et al 2001). In addition another study assessing persistence with antihypertensive therapy and discontinuation patterns in patients newly dispensed different antihypertensive drug classes in a natural Canadian population-based setting showed that compared with ARBs, the likelihood of discontinuing therapy over the 39-month study period was significantly higher for ACEIs (HR 1.29; 95% CI 1.16–1.43), calcium antagonists (HR 1.42; 95% CI 1.27–1.60), beta blockers (HR 1.62; 95% CI 1.45–1.80), and diuretics (HR 1.92; 95% CI 1.73–2.14). In the year following treatment discontinuation, between 54% and 75% of patients initiated a second course of treatment. Patients initiated on an ARB had a significantly higher likelihood of starting a new course of therapy after a first treatment discontinuation, compared with all other agents (CitationBourgault et al 2005). In the ATHOS study discussed before, the discontinuation rate was two-fold higher in the amlodipine-hydrochlorothiazide group than in the telmisartan/hydrochlorothiazide group (11.3 compared with 5.0%; p < 0.001), mainly due to peripheral edema (CitationNeldam and Edwards 2006).
Conclusion
Blockade of the RAS with selective AT1 receptor antagonists is recognized as an effective mean to lower BP in hypertensive patients. Among AT1 receptor antagonists, telmisartan is characterized by a very long half-life. This enables smooth BP control over 24 hours using a once-daily administration and might result in potential clinical advantages such as greater compliance, sustained BP control, maintenance of control regardless of missing a dose, and reduced risk of cardiovascular events during the early morning hours. The preclinical data demonstrating the partial PPAR-γ agonistic activity of telmisartan are of great interest considering the actual epidemiological trends of diabetes and obesity. However, whether these in vitro properties translate into real clinical benefits still needs clinical confirmation in large groups of patients.
The combination of telmisartan with hydrochlorothiazide is logical and also very effective. In accordance with past experiences using other compounds, several controlled studies have demonstrated that combining telmisartan with a low-dose thiazide is superior in lowering BP than either telmisartan or hydrochlorothiazide alone. Moreover, the excellent tolerability profile of the ARB is not affected by the addition of a low-dose thiazide.
Finally, fixed-dose combinations are increasingly used in clinical practice in order to achieve the therapeutic goals defined by most national and international guidelines. The use of drugs combinations will probably increase further in future with the introduction of fixed-dose combinations as possible first-step therapies for hypertension according to European guidelines (CitationCifkova et al 2003). American guidelines proposed the use of fixed-dose combination as initial therapy in patients with stage 2 and 3 hypertension (CitationChobanian et al 2003). Since the AT1 receptor antagonist/thiazide diuretic combination is an effective and well-tolerated drug association, one might expect that the use of these combinations including that of telmisartan and hydrochlorothiazide will increase steadily over the next 5 years.
References
- AlmansaCGomezLAde ArribaAFDiphenypropionic acids as new AT-1 selective angiotensin II antagonistsJ Med Chem19963921972068667363
- BangaloreKamalakkannanPanjrathFixed-dose combination improves medication compliance: a meta analysis [abstract]J Clin Hyper20068A71
- BarnettAHBainSCBouterPAngiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathyN Engl J Med200435119526115516696
- BattershillAJScottLJTelmisartan: a review of its use in the management of hypertensionDrugs200666518316398568
- BeermannBGroschinsky-GrindMPharmacokinetics of hydrochlorothiazide in manEur J Clin Pharmacol197712297303590315
- BensonSCPershadsinghHAHoCIIdentification of telmisartan as a unique angiotensin II receptor antagonist with selective PPAR gamma-modulating activityHypertension200443993100215007034
- BloomBSContinuation of initial antihypertensive medication after 1 year of therapyClin Ther199820671819737827
- BogerRHSchwedhelmEMaasRADMA and oxidative stress may relate to the progression of renal disease: rationale and design of the VIVALDI studyVasc Med200510Suppl 1S9710216444875
- BöhmMLeeMKreutzRAngiotensin II receptor blockade in TGR(mREN2)27: effects of renin-angiotensin system gene expression and cardiovascular functionsJ Hypertens19951389198557967
- BöhmMLippoldtAWienenWreduction of cardiac hypertrophy in TGR(mREN2)97 by angiotensin II receptor blockadeMol Cell Biochem19961634217218974060
- BourgaultCSenecalMBrissonMPersistence and discontinuation patterns of antihypertensive therapy among newly treated patients: a population-based studyJ Hum Hypertens2005196071315920457
- BrennerBMCooperMEDe ZeeuwDEffects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathyNew Engl J Med2001345861911565518
- BrondoloERosenRCKostisJBRelationship of physical symptoms and mood to perceived and actual blood pressure in hypertensive men: a repeated-measures designPsychosom Med1999613111810367611
- BrunnerHRWaeberBNussbergerJResponsiveness of renin secretion: The key to understanding the efficacy of inhibition of the renin-angiotensin systemKidney Int198834805
- ChenLingCuddyPharmacological characterization of two new angiotensin II receptor antagonists, WAY-126277 and WAY-126756 [abstract]Circ Res199388I2330 I-433, 2330
- ChobanianAVBakrisGLBlackHRSeventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureHypertension20034212065214656957
- CifkovaRErdineSFagardRPractice guidelines for primary care physicians: 2003 ESH/ESC hypertension guidelinesJ Hypertens20032117798614508180
- ConlinPRGerthWCFoxJFour-year persistence patterns among patients initiating therapy with the angiotensin II receptor antagonist losartan versus other artihypertensive drug classesClin Ther2001231999201011813934
- de GasparoMUngerTSchölkensBAAT-1 Receptor Antagonists: PharmacologyAngiotensin - Vol II - Part 5. Inhibition of the Renin-Angiotensin System. Series: Handbook of Experimental Pharmacology (Vol 163)2004Springer Verlag41752
- DerosaGCiceroAFBertoneGComparison of the effects of telmisartan and nifedipine gastrointestinal therapeutic system on blood pressure control, glucose metabolism, and the lipid profile in patients with type 2 diabetes mellitus and mild hypertension: a 12-month, randomized, double-blind studyClin Ther2004a2612283615476904
- DerosaGRagonesiPDMugelliniAEffects of telmisartan compared with eprosartan on blood pressure control, glucose metabolism and lipid profile in hypertensive, type 2 diabetic patients: a randomized, double-blind, placebo-controlled 12-month studyHypertens Res2004b274576415302981
- DeziiCMA retrospective study of persistence with single-pill combination therapy vs. concurrent two-pill therapy in patients with hypertensionManag Care200092611729417
- DiezJGonzalezALopezBRavassaSFortunoMAEffects of antihypertensive agents on the left ventricle: clinical implicationsAm J Cardiovasc Drugs200112637914728026
- ElliotWJDifferential effects of antihypertensive drugs on new-onset diabetes?Curr Hypertens Rep200572495616061042
- EpsteinMBakrisGNewer approaches to antihypertensive therapyArch Intern Med19961561969788823150
- FalconnetCBochudMBovetPGender difference in the response to an angiotensin-converting enzyme inhibitor and a diuretic in hypertensive patients of Africa descentJ Hypertens20042212132015167457
- FentonCKeatingGMScottLJTelmisartan/hydrochlorothiazide. In the treatment of essential hypertensionDrugs20036320132612962518
- FreytagFHolwerdaNJKarlbergBELong-term exposure to telmisartan as monotherapy or combination therapy: efficacy and safetyBlood Press2002111738112126264
- FreytagFSchellingAMeinickeTComparison of 26-week efficacy and tolerability of telmisartan and atenolol, in combination with hydrochlorothiazide as required, in the treatment of mild to moderate hypertension: a randomized, multicenter studyClin Ther2001231082311219471
- GalzeranoDTammaroPCercielloAFreehand three-dimensional echocardiographic evaluation of the effect of telmisartan compared with hydrochlorothiazide on left ventricular mass in hypertensive patients with mild-to-moderate hypertension: a multicentre studyJ Hum Hypertens20041853914688811
- GohlkePWeissSJansenAAT1 receptor antagonist telmisartan administered peripherally inhibits central responses to angiotensin ii in conscious ratsJ Pharmacol Exp Ther2001298627011408526
- GrossmanEMesserliFHLong term safety of antihypertensive therapyProg Cardiovasc Dis200649162516867847
- HanssonLZanchettiACarruthersSGEffects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomized trialThe Lancet1998351175562
- IsrailiZHHallWDCough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiologyAnn Intern Med1992117234421616218
- KarlbergBELinsLEHermanssonKEfficacy and safety of telmisartan, a selective AT1 receptor antagonist, compared with enalapril in elderly patients with primary hypertension. TEES Study GroupJ Hypertens19991729330210067800
- KjellgrenKIAhlnerJDahlofBPatients’ and physicians’ assessment of risks associated with hypertension and benefits from treatmentJ Cardiovasc Risk19985161610201552
- KurtzTWTreating the metabolic syndrome: telmisartan as a peroxisome proliferator-activated receptor-gamma activator2Acta Diabetol200542Suppl 1S91615868121
- LacourciéreYA new fixed-dose combination for added blood pressure control: telmisartan plus hydrochlorothiazideJ Int Med Res2002303667912235918
- LacourciéreYGil-ExtremeraBMuellerOEfficacy and tolerability of fixed-dose combinations of telmisartan plus HCTZ compared with losartan plus HCTZ in patients with essential hypertensionInt J Clin Pract20031756975
- LacourciéreYMartinKComparison of a fixed-dose combination of 40 mg telmisartan plus 12.5 mg hydrochlorothiazide with 40 mg telmisartan in the control of mild to moderate hypertensionAm J Ther200291111711897925
- LacourciereYNeutelJMSchumacherHComparison of fixed-dose combinations of telmisartan/hydrochlorothiazide 40/12.5 mg and 80/12.5 mg and a fixed-dose combination of losartan/hydrochlorothiazide 50/12.5 mg in mild to moderate essential hypertension: pooled analysis of two multicenter, prospective, randomized, open-label, blinded-end point (PROBE) trials1Clin Ther200527179580516368450
- LacourciéreYTytusRO’KeefeDEfficacy and tolerability of a fixed-dose combination of telmisartan plus hydrochlorothiazide in patients uncontrolled with telmisartan monotherapyJ Hum Hypertens2001157637011687919
- LewisEJHunsickerLGClarkeWRRenoprotective effect of the angiotensin-receptor antagonist Irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med20013458516011565517
- MaillardMBurnierMTelmisartan/hydrochlorothiazide: a new fixed dose combinationExpert Rev Cardiovasc Ther200533758615889965
- MaillardCentenoWienenMolecular basis for the insurmountable AT-1 receptor antagonism of telmisartan [abstract]Am J Hypertens20011412A
- MaillardMPerregauxCCentenoCIn vitro and in vivo characterization of telmisartan: an insurmountable angiotensin II receptor antagonistJ Pharmacol Exp Ther200230210899512183667
- MakinoHHanedaMBabazonoTThe telmisartan renoprotective study from incipient nephropathy to overt nephropathy—rationale, study design, treatment plan and baseline characteristics of the incipient to overt: angiotensin II receptor blocker, telmisartan, Investigation on Type 2 Diabetic Nephropathy (INNOVATION) StudyJ Int Med Res2005336778616372586
- ManciaTolerability and safety of telmisartan as monotherapy or combined with hydrochlorothiazide compared with placebo [abstract]J Hypertens Suppl200220S377
- MarshallTGLeeREMarshallFECommon angiotensin receptor blockers may directly modulate the immune system via VDR, PPAR and CCR2bTheor Biol Med Model20063116403216
- MattioliAVZennaroMBonattiSRegression of left ventricular hypertrophy and improvement of diastolic function in hypertensive patients treated with telmisartanInt J Cardiol200497383815561322
- McGillJBReillyPACombination treatment with telmisartan and hydrochlorothiazide in black patients with mild to moderate hypertensionClin Cardiol2001a24667211195609
- McGillJBReillyPATelmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group trialClin Ther2001b238335011440284
- MeredithPAAngiotensin II receptor antagonists alone and combined with hydrochlorothiazide: potential benefits beyond the antihypertensive effectAm J Cardiovasc Drugs200551718315901205
- MichelMCBohnerHKosterJSafety of telmisartan in patients with arterial hypertension: an open-label observational studyDrug Saf2004273354415061687
- MiuraYYamamotoNTsunekawaSReplacement of valsartan and candesartan by telmisartan in hypertensive patients with type 2 diabetes: metabolic and antiatherogenic consequencesDiabetes Care200528757815735228
- NeldamSEdwardsCTelmisartan plus HCTZ vs. amlodipine plus HCTZ in older patients with systolic hypertension: results from a large ambulatory blood pressure monitoring studyAm J Geriatr Cardiol2006151516016687967
- NeutelJMFrishmanWHOparilSComparison of telmisartan with lisinopril in patients with mild-to-moderate hypertensionAm J Ther1999a6161610423659
- NeutelJMKleinCMeinickeTWLong-term efficacy and tolerability of telmisartan as monotherapy and in combination with other antihypertensive medicationsBlood Press200211302912458653
- NeutelJMKollochREPlouinPFTelmisartan vs losartan plus hydrochlorothiazide in the treatment of mild-to-moderate essential hypertension--a randomized ABPM studyJ Hum Hypertens2003175697512874615
- NeutelJMSmithDHGReillyPAThe efficacy and safety of telmisartan compared to enalapril in patients with severe hypertensionInt J Clin Pract1999b53175810665127
- NeutelJMSmithDHGTelmisartan US Study GroupDose response and antihypertensive efficacy of the AT1 receptor antagonist telmisartan in patients with mild-to-moderate hypertensionAdv Therapy19981520617
- ParvingHHLehnertHBrochner-MortensenJThe effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetesN Engl J Med2001345870811565519
- PickkersPGarchaRSSchachterMInhibition of carbonic anhydrase accounts for the direct vascular effects of hydrochlorothiazideHypertension1999331043104810205245
- PittasAGGreenbergASThiazolidinediones in the treatment of type 2 diabetesExpert Opin Pharmacother200235294011996632
- ResnickLMWhy we can’t translate clinical trials into clinical practice in hypertensionAm J Hypertens200316421512745206
- ReyesAJCardiovascular drugs and serum uric acidCardiovasc Drugs Ther20031739741415107595
- RiesUMihmGNarrB6-Substituted benzimidazoles as new nonpeptide angiotensin II receptor antagonists: synthesis, biological activity, and structure-activity relationshipsJ Med Chem1993364040518258826
- SchierokHJPairetMHauelNEffects of telmisartan on renal excretory function in conscious dogsJ Intern Med2001291319
- SchmiederRETelmisartan/hydrochlorothiazide combination therapy in the treatment of essential hypertensionExpert Opin Pharmacother2004523031015500377
- SchuppMClemenzMGinesteRMolecular characterization of new selective peroxisome proliferator-activated receptor {gamma} modulators with angiotensin receptor blocking activityDiabetes2005543442345216306360
- SchuppMJankeJClasenRAngiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activityCirculation20041092054715117841
- SengulAMAltuntasYKurkluABeneficial effect of lisinopril plus telmisartan in patients with type 2 diabetes, microalbuminuria and hypertensionDiabetes Res Clin Pract2006712101916112244
- SharpeMJarvisBGoaKLTelmisartan: A review of its use in hypertensionDrugs20016115012911558835
- SicaDAFixed-dose combination antihypertensive drugs. Do they have a role in rational therapyDrugs19944816247525192
- SiscovickDSRaghunathanTEPsatyBMDiuretic therapy for hypertension and the risk of primary cardiac arrest 1N Engl J Med1994330185278196728
- StangierJSchmidJTurkDAbsorption, metabolism, and excretion of intravenously and orally administered [14C]telmisartan in healthy volunteersJ Clin Pharmacol20004013122211185629
- StangierJSuCAPvan HeiningenPNMInhibitory effect of telmisartan on the blood pressure response to angiotensin II challengeJ Cardiovasc Pharmacol2001386728511602814
- TaylorAAShoheiberOAdherence to antihypertensive therapy with fixed-dose amlodipine besylate/benazepril HCl versus comparable component-based therapyCongest Heart Fail200393243214688505
- TeoKYusufSSleightPRationale, design, and baseline characteristics of two large, simple, randomized trials evaluating telmisartan, ramipiril and their combination in high-risk patients: the ONgoing Telmisartan Alone and combination with Ramipiril Global Endpoint Trial / Telmisartan Randomized Assessment study in ACE intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trialsAm J Heart20041485261
- The ALLHAT Officers and Coordinators for the ALHAT Collaborative Research GroupMajor outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT)JAMA200228829819712479763
- UngerTKaschinaEDrug interactions with angiotensin receptor blockers: a comparison with other antihypertensivesDrug Safety2003267072012862505
- Van BrummelenPMan in’t VeldAJSchalekampMAHaemodynamic changes during long term thiazide treatment of essential hypertension in responders and non-respondersClin Pharmacol Ther198027328366987024
- van Heiningenvan Lierde BruinSingle dose study on the pharmacodynamics and pharmacokinetics of the angiotensin II receptor antagonist BIBR0277SE [abstract]Pharm World Sci1994164
- Van MeelJCARedemannNHaighRMhypotensive effects of the angiotensin II antagonist telmisartan in conscious chronically-instrumented transgenic ratsArzneim-Forsh /Drug Res19964675599125273
- VitaleCMercuroGCastiglioniCMetabolic effect of telmisartan and losartan in hypertensive patients with metabolic syndromeCardiovasc Diabetol20054615892894
- VogtLNavisGKosterJThe angiotensin II receptor antagonist telmisartan reduces urinary albumin excretion in patients with isolated systolic hypertension: results of a randomized, double-blind, placebo-controlled trialJ Hypertens20052320556116208149
- WaeberBCombination therapy with ACE inhibitors/angiotensin II receptor antagonists and diuretic in hypertensionExpert Rev Cardiovasc Ther20031435015030296
- WeberMThe telmisartan Programme of Research tO show Telmisartan End-organ proteCTION (PROTECTION) programmeJ Hypertens200321S37S46
- WhiteWBComparative effects of telmisartan in the treatment of hypertensionJ Clin Hypertens (Greenwich)2002420512147925
- [WHO] World Health OrganizationCardiovascular disease: prevention and control. [online]2003 Accessed 18 June 2006. URL: http://www.who.int/dietphysicalactivity/publications/facts/cvd/en
- WienenWEntzerothMEffects on binding chracteristics and renal function of the novel, non-peptide angiotensin II antagonist BIBR-277 in the ratJ Hypertens199412119288021462
- WienenWEntzerothMVan MeelJCAA review on telmisartan: a novel, long-acting angiotensin II receptor antagonistCardiovasc Drug Rev20001812756
- WienenWHauelNVan MeelJCAPharmacological characterization of the novel nonpeptide angiotensin II receptor antagonist, BIBR 277Br J Pharmacol1993110245528220885
- WienenWRichardSChamperouxPComparative antihypertensive and renoprotective effects of telmisartan and lisinopril after long-term treatment in hypertensive diabetic ratsJRAAS20012313611881063
- WienenWSchierokHJEffects of telmisartan, hydrochlorothiazide and their combination on blood pressure and renal excretory parameters in sponeneously hypertensive ratsJ Renin Angiotensin Aldosterone Syst20012123811881111
- WillichSNLevyDRoccoMBCircadian variation in the incidence of sudden cardiac death in the Framingham Heart Study populationAm J Cardiol19876080163661393
- WogenJKreilickCALivorneseRCPatient adherence with amlodipine, lisinopril, or valsartan therapy in a usual-care settingJ Manag Care Pharm20039424914613440
- WuerznerKHasslerCBurnierMDifficult blood pressure control: watch out for non-compliance!Nephrol Dial Transplant20031819697313679466
- YoungCLDiasVCStangierJMultiple-dose pharmacokinetics of telmisartan and of hydrochlorothiazide following concurrent administration in healthy subjectsJ Clin Pharmacol20004013233011185630
- ZanchiAChioléroAMaillardMEffects of the Peroxisomal Proliferator-Activated Receptor-gamma agonist pioglitazone on renal and hormonal responses to salt in healthy menJ Clin Endocr Metab2004891140515001599