Abstract
Lowering elevated blood pressure (BP) with drug therapy reduces the risk for catastrophic fatal and nonfatal cardiovascular events such as stroke and myocardial infarction. Given the heterogeneity of hypertension as a disease, the marked variability in an individual patient’s BP response, and low response rates with monotherapy, expert groups such as the Joint National Committee (JNC) emphasize the value of combination antihypertensive regimens, noting that combinations, usually of different classes, have additive antihypertensive effects. Metoprolol succinate extended-release tablet is a beta-1 (cardio-selective) adrenoceptor-blocking agent formulated to provide controlled and predictable release of metoprolol. Hydrochlorothiazide (HCT) is a well-established diuretic and antihypertensive agent, which promotes natruresis by acting on the distal renal tubule. The pharmacokinetics, efficacy, and safety/tolerability of the antihypertensive combination tablet, metoprolol extended release hydrochlorothiazide, essentially reflect the well-described independent characteristics of each of the component agents. Not only is the combination product more effective than monotherapy with the individual components but the combination product allows a low-dose multidrug regimen as an alternative to high-dose monotherapy, thereby, minimizing the likelihood of dose-related side-effects.
By treating hypertensive patients with metoprolol succinate extended release in combination with hydrochlorothiazide (metoprolol ER/HCT), the prescribing physician can expect additive antihypertensive effects. As will be illustrated in this review, the pharmacokinetics, safety, and tolerability of this antihypertensive combination essentially reflect the well-described independent characteristics of each of the component agents.
Background
For over a decade, developed counties have been facing a persistent cardiovascular public health dilemma – a substantial proportion of hypertensives remain unaware of their elevated blood pressure and of those diagnosed, many are not treated to optimal, cardiovascular risk lowering target blood pressure levels (CitationHajjar and Kotchen 2003; CitationJNC-7 2003). Repeatedly, treatment guidelines and clinical trials underscore the fact that many patients require a multi-drug regimen (CitationALLHAT 2002; CitationEuropean Society of Hypertension 2003; CitationJNC-7 2003; CitationWHO 2003; CitationMourad et al 2004). Expert panels such as the Joint National Committee point out that when used in combination, antihypertensive drugs additively lower blood pressure and can often do so employing low doses of the individual agents, thereby minimizing dose-related adverse effects (CitationJNC-7 2003; CitationOfili 2006). Furthermore, many antihypertensive agents complement the actions of others, particularly when they are of different classes, eg, calcium channel blockers (CCBs) and beta-blockers; and, some combinations exhibit an off-setting impact on adverse effects, eg, diuretic vs angiotensin receptor blocker effects on serum potassium (CitationJNC-7 2003). Combination antihypertensive regimens can also “override” population-specific effects. For example, while Blacks tend to respond to renin-angiotensin system (RAS) blockade to a lesser degree than Whites, both Blacks and Whites respond equally well to diuretic/RAS inhibitor combinations (CitationHolland and Fairchild 1982; CitationHawkins et al 1988; The Association of Black Cardiologists (ABC) CitationCandesartan Study Group 2000; CitationPapademetriou et al 2000). Moreover, the likelihood that a patient with more severely elevated blood pressure (>20/10 mmHg above goal) will attain goal with single agent treatment is so low that JNC-7 suggests initiating antihypertensive treatment for these patients with a drug-combination. The JNC panel also notes that combining anti-hypertensive drugs into a single fixed combination tablet can simplify multi-drug treatment regimens, an important consideration to facilitate patient compliance, and that fixed combinations can also be less expensive (CitationJNC-7 2003).
Encouragingly, in recent years there has been some improvement in hypertension control rates and this has been paralleled by an increased reliance upon combination therapy. Using the National Health and Nutrition Examination Survey (NHANES), CitationGu et al (2006) reported that during the years 1999–2002, 62.9% of US hypertensive adults took a prescription antihypertensive medication, compared with 57.3% for the years 1988–1994. During this same time period, multiple drug use increased from 29.1% to 35.8%. Of those patients on multiple drug treatment, about one-fourth were taking a diuretic/beta-blocker combination and about one-fourth a diuretic/angiotensin converting enzyme inhibitor (ACEI) combination, while somewhat lesser proportions were taking other combinations (diuretics/CCBs, beta-blockers/CCBs, ACEIs/CCBs, etc.) (CitationGu et al 2006). The relatively popular use of a diuretic/beta-blocker combination is not too surprising given the established benefit of thiazides in averting the cardiovascular complications of hypertension and the potential benefit of including a beta blocker in antihypertensive regimens for patients with important comorbid illnesses such as coronary artery disease (CitationALLHAT 2002; CitationJNC-7 2003; CitationPsaty et al 2003; CitationTurnbull et al 2003).
Formulation
Metoprolol is a beta-1 (cardioselective) adrenoreceptor-blocking agent. It was first introduced as a tartrate salt and had pharmacokinetic/pharmacodynamic properties that necessitated twice- to thrice-daily dosing. This formulation is commonly referred to as “immediate release”. Metoprolol was subsequently formulated as an extended release tablet (metoprolol ER) using the succinate salt such that 95 mg is equivalent to 100 mg of the metoprolol tartrate salt (TOPROL-XL® 2005; CitationSandberg et al 1988). This formulation difference resulting in different pharmacokinetic and pharmacodynamic characteristics and dosing regimens is an important distinction, as many research reports fail to specify the metoprolol formulation studied; approved indications for the formulations differ in some countries and clinical study findings have differed, depending on the formulation (TOPROL-XL® 2005).
The metoprolol ER properties are achieved by encapsulation of the succinate salt with a polymeric coating to form micro-beads, which are then embedded in a tablet matrix. In the gastrointestinal tract the beads are released from the matrix and each bead, upon exposure to fluid, allows outward diffusion of metoprolol over a period of about 20 hours (CitationPlosker and Clissold 1992; CitationSandberg et al 1988; TOPROL-XL® 2005). Hydrochlorothiazide is a thiazide diuretic, typically available as a tablet formulation (CitationBeermann et al 1976). By modifying the matrix of the metoprolol ER tablet to incorporate HCT it has been possible to develop a fixed combination tablet that allows immediate release of HCT from the matrix while retaining the extended release properties for metoprolol (data on file; Sunzel Study D4026C00005 2004).
Pharmacokinetic/pharmacodynamic properties
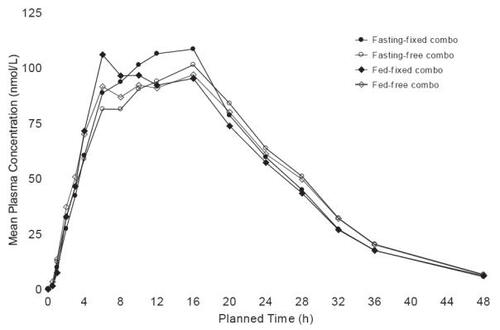
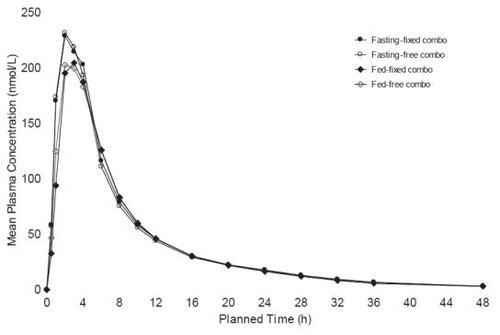
The metoprolol ER/HCT fixed combination tablet proved bioequivalent to the concomitant administration of the individual agents in single dose, cross-over studies at both metoprolol ER/HCT; 23.75/6.25 mg (low dose) and 95/12.5 mg (high dose) based both on Cmax and AUC (90% confidence intervals for the ratios within 80%–125%) (data on file: Sunzel Study D4026C00006 2005; Sunzel Study D4026C00005 2004). The high dose study was conducted with subjects fasting and fed; the pharmacokinetic parameters were nearly identical under both conditions ( and ). The fixed tablet bioequivalence with concomitantly administered components was confirmed in a multiple dose (once daily for 5 days) study with a 100/12.5 mg tablet (data on file: Abrahamsson and Häggström Study 895 1987). In addition, the absence of a metoprolol/HCT pharmacokinetic interaction was demonstrated in a multiple dose study in which a 50/12.5 mg tablet was compared with each component administered individually, a finding that was consistent with older studies conducted with metoprolol tartrate (CitationJordo et al 1979). Relative to immediate release metoprolol, the extended release formulation gives plasma metoprolol concentrations that tend to be smoother, exhibiting less peak-to-trough variation. This is reflected in less variability in inhibition of exercise-induced increase in heart rate, a measure of beta-1 blockade (CitationDarmansjah et al 1990; CitationSandberg et al 1990; CitationPolsker and Clissold 1992). These extended release properties are maintained in the combination as illustrated by a study that compared the metoprolol ER/HCT combination with an immediate release combination (100/12.5 mg), ie, there was less peak to trough variability in metoprolol plasma levels with the extended release combination and the extended release formulation elicited a smoother and more sustained beta-blockade over the inter-dosing interval (90% confidence interval for the ratio of the area under the 24 hour effect curve: 105%–129%) (data on file: Abrahamsson and Häggström Study 896 1987).
Antihypertensive effects
Although first introduced as a fixed combination tablet of 100/12.5 mg (metoprolol succinate ER/HCT) in 1989 (Denmark), the antihypertensive activity of the combination over a wide dose range was only subsequently detailed in the Assessment of Toprol-XL Taken in Combination with Hydrochlorothiazide (ATTACH) trial. This was a US multicenter, randomized, double-blind, placebo-controlled, parallel group, unbalanced factorial study of 3 dose levels of hydrochlorothiazide (6.25, 12.5, and 25 mg), 4 dose levels of metoprolol ER (25, 50, 100, and 200 mg), and 9 of the 12 potential combinations (metoprolol ER/HCT: 200/6.25 mg, 25/25 mg, and 50/25 mg were not included). The design was structured to test the hypothesis that at least 1 metoprolol ER/HCT combination was superior to its components with regard to change from baseline in trough (24 hours post dosing) sitting diastolic blood pressure (SiDBP). The study enrolled men and women 18–80 years of age with SiDBP 95–114 mmHg and SiSBP <180 mm Hg (following a placebo run-in period).
The trial randomized a total of 1571 patients and met its primary objective, illustrating that the combination represented contributions from both components for both SiDBP and SiSBP, ie, the antihypertensive effects were additive. Blood pressure declined with the combination in a dose-related fashion as it did with the component agents and these declines were well described, fitted to quadratic regression dose-response surface models ( and ). The model-derived, placebo-corrected reductions with blood pressure ranged from (SiSBP/SiDBP) 5.5/3.3 mmHg to 14.7/10.4 mmHg for the combinations (). As is also apparent in , blood pressure reductions achieved with the highest doses of the individual agents can be attained with much lower doses in combination, eg, metoprolol ER 50/HCT 6.25 mg or 12.5 mg (CitationPapademetriou et al 2006).
Figure 3 Dose response surface from polynomial regression of changes from baseline to week 8/LOCF in trough sitting diastolic blood pressure (intent-to-treat population) (ATTACH Trial).
Regression equation: DBP: y = −5.34392 −0.06023*Toprol-XL −0.34772*HCT + 0.00015*Toprol-XL2 + 0.00703*HCT2.
Reprinted with permission from Papademetriou V, Hainer JW, Sugg J, et al, and ATTACH Study Group. 2006. Factorial antihypertensive study of an extended-release metoprolol and hydrochlorothiazide combination. Am J Hypertens, 19:1217–25. Copyright © 2006 American Journal of Hypertension, Ltd.
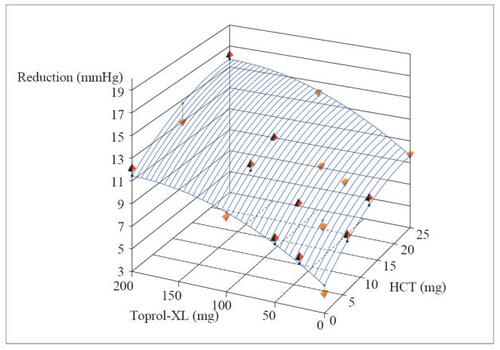
Figure 4 Dose response surface from polynomial regression of changes from baseline to Week 8/LOCF in trough sitting diastolic blood pressure (intent-to-treat population) (ATTACH Trial).
Regression equation: SBP: y = −4.20691 −0.08645*Toprol-XL −0.63844*HCT + 0.00026*Toprol-XL2 + 0.01324*HCT2.
Reprinted with permission from Papademetriou V, Hainer JW, Sugg J, et al, and ATTACH Study Group. 2006. Factorial antihypertensive study of an extended-release metoprolol and hydrochlorothiazide combination. Am J Hypertens, 19:1217–25. Copyright © 2006 American Journal of Hypertension, Ltd.
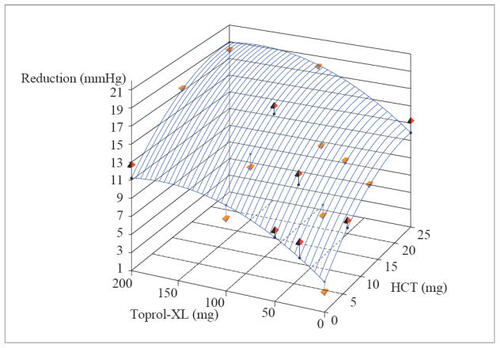
Table 1 Placebo-corrected predicted valuesTable Footnoteafor change from baseline in SBP/DBP
When translated into hypertension control rates (<140/90 mmHg or 130/80 mmHg if diabetic) the combination rendered 31%–65% (based on dose) of patients controlled. The highest control rates attainable with the monotherapies at their highest doses were 38% for HCT and 47% for metoprolol ER. It is noted that about half the patients entered the trial with a baseline blood pressure >20/10 mmHg above their JNC goal (<140/90 mmHg or 130/80 mmHg if diabetic) and that 15%–50% of these Stage 2 hypertensive patients attained their goal with a combination regimen (CitationPapademetriou et al 2006).
Subgroup analyses in ATTACH based on age (≥65 vs <65 years), sex, and ethnicity (Blacks vs others) identified little difference in overall antihypertensive responsiveness. Consistent with other reports, in ATTACH Blacks tended to respond to a lesser degree to metoprolol ER, and their response to diuretics was more pronounced, so that the combination was effective in both ethnic groups (CitationHolland and Fairchild 1982; CitationHawkins et al 1988; CitationPapademetriou et al 2006).
In ATTACH all patients received treatment as “initial” therapy. The effect of the metoprolol ER/HCT combination employed as an “add-on” treatment was evaluated in a smaller (n = 47) study of patients who remained hypertensive in spite of receiving HCT 12.5 mg per day. In this crossover study once daily metoprolol ER/HCT 100/12.5 mg was compared with an older metoprolol tartrate/HCT combination tablet. Blood pressure declined by approximately 10/10 mmHg with the metoprolol ER combination and 8/8 mmHg with the tartrate combination (differences not statistically significant) (data on file: Stenström Study S902 1988).
In addition to these controlled clinical trials, a number of large clinical experience trials evaluated metoprolol ER in combination with HCT (). Furthermore, 2 large hypertension clinical outcome trials (HOT and STOP-hypertension 2) allowed for the use of metoprolol ER in combination with diuretics (). In general, the experience described in these programs is consistent with the findings in the ATTACH trial and support the use of diuretic/beta-blocker combination treatment to avert the cardiovascular consequences of hypertension.
Table 2 Clinical studies of metoprolol/hydrochlorothiazide combination tablets
Table 3 Clinical outcome trials in support of safety and tolerability with combination treatment
Safety and tolerability
From a safety/tolerability perspective, prescribing physicians should view metoprolol ER/HCT as “inheriting” all the potential safety/tolerability characteristics of the individual agents, including the admonition (“black box warning”) that abrupt cessation of beta-blocker therapy can exacerbate angina pectoris or even precipitate acute myocardial infarction (TOPROL-XL® 2005). A key question for the combination is whether there are any safety/tolerability findings that are either unique to the combination or which represent an additive effect. Examples might include fatigue or metabolic abnormalities, both of which are common to each component agent. In the ATTACH trial of 1564 patients, the incidence of fatigue was 2.6% with metoprolol ER/HCT (across all doses) compared with 0.7% with placebo but at the highest dose (200/25 mg; metoprolol ER/HCT) the rate was 10.2% which compared with 2.0% and 6.3% for the individual agents, implying a possible additive effect for fatigue with high dose combinations. Relative to placebo, the rates for other common adverse effects were low: nasopharyngitis (3.4% vs 1.3%), dizziness (2.6% vs 2.6%), and nausea (1.4% vs 0.7%); adverse event discontinuation rates were 2.7% and 2.6% for combination and placebo, respectively (CitationPapademetriou et al 2006).
The effects of thiazide diuretics and beta-blocking agents on glucose metabolism are also well described and the metoprolol ER/HCT combination would also be expected to induce similar metabolic effects (CitationDornhorst et al 1985; CitationSamuelsson et al 1994; CitationHydroDiuril 1998; TOPROL-XL® 2005). With regard to metoprolol, the large GEMENI trial noted an increase in hemoglobin A1C with immediate release metoprolol tartrate relative to the alpha-beta blocking drug, carvedilol in hypertensive type II diabetic patients already receiving a RAS blocking agent (ACE inhibitor or ARB). At the end of the trial, over 40% of the patients in both treatment groups were also receiving a thiazide diuretic (CitationBakris et al 2004). While the ATTACH trial noted no change in fasting glucose levels with metoprolol ER/HCT, the study was not of adequate size to critically assess an impact on glucose control in patients with diabetes as only 10% of the study subjects were diabetic (CitationPapademetriou et al 2006). Falkner et al looked specifically at insulin sensitivity (insulin clamp method) and found that metoprolol ER did not alter insulin sensitivity when added to HCT treatment in hypertensive patients. On average, the patients were already “insulin resistant” prior to adding metoprolol ER (CitationFalkner et al 2006). She repeated the experiment in hypertensive patients who also had diabetes but, again, found that metoprolol ER did not significantly alter insulin sensitivity (CitationFalkner B pers comm 2007).
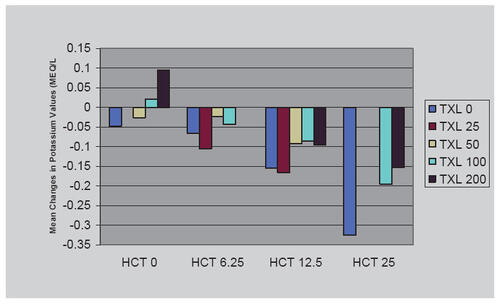
As one would expect, serum potassium declined with metoprolol ER/HCT in the ATTACH trial but somewhat less so than with HCT mono-therapy, perhaps attributable to renin-inhibition consequences of beta-adrenergic blockade () (CitationPapademetriou et al 2006). Also as expected, the ATTACH trial noted changes in lipids with metoprolol ER. There was a small decline in mean HDL cholesterol. The combination treatments tended to reflect the influence of the effects of the individual agents: there was a small increase in total cholesterol with HCT as monotherapy and a small decline in total cholesterol with TOPROL-XL®, ie, no consistent change); and while generally small, the increases in triglyceride levels tended to be additive (CitationPapademetriou et al 2006).
Dosing and administration
A combination metoprolol succinate ER/HCT tablet has been available in a number of countries (first approved in Denmark in 1989) as Selocomb ZOK® prolonged release tablet. In the US the FDA granted approval for the combination on 28 August 2006 as Dutoprol® – indicated for the management of hypertension. The fixed-dose combination is not indicated for initial therapy. The dosing and administration labeling cites the findings from the ATTACH trial, noting that HCT was effective in doses of 6.25–25 mg and metoprolol ER in doses of 25–200 mg and recommends that dosing be individualized considering baseline and target blood pressure as well as experience with the individual agents. Dutoprol tablet strengths (metoprolol ER/HCT) are 25/12.5 mg, 50/12.5 mg, and 100/12.5 mg, which can be divided to give half tablets of 50/6.25 mg (FDA website: http://clinicaltrials.gov).
Summary
Metoprolol succinate extended release/hydrochlorothiazide (metoprolol ER/HCT) tablets lower blood pressure in hypertensive patients. The blood pressure reductions are dose related and represent additive antihypertensive contributions from the component agents. The likelihood of controlling elevated blood pressure with the combination product is greater than with the component agents employed as monotherapies, even in patients with more severe levels of hypertension. The combination also provides an option to treat with lower doses of the individual agents. Metoprolol ER/HCT is generally well tolerated, reflecting the characteristics of the components, but it also reflects the same safety profiles including the admonition to avoid abrupt cessation of treatment in patients with or at risk for underlying coronary artery disease.
Physicians might also consider that low dose combination products provide an option to intervene with well-tolerated treatments early in the course of hypertension and might well help patients avert more intractable hypertension (CitationJulius et al 2006). Certainly, this combination approach expands the population likely to respond to antihypertensive therapy compared with single agent treatment.
Acknowledgements
We gratefully acknowledge the assistance of Judith Borak, MS, MBA with manuscript preparation.
References
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research GroupMajor outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diureticJAMA200228829819712479763
- BakrisGLFonsecaVKatholiREMetabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertensionJAMA200429222273615536109
- BeermannBGroschinsky-GrindMRosenAAbsorption, metabolism, and excretion of hydrochlorothiazideClin Pharmacol and Thera1976195317
- DahlofBLindholmLHHanssonLMorbidity and mortality in the Swedish trial in old patients with hypertension (STOP-hypertension)Lancet1991338128151682683
- DarmansjahIWongESetiawatiAPharmacokinetic and pharmacodynamic properties of controlled release (CR/ZOK) metoprolol in healthy Oriental subjects: a comparison with conventional formulations of metoprolol and atenololJ Clin Pharmacol199030S39S452312778
- DornhorstAPowellSHPenskyJAggravation by propranolol of hyperglycaemic effect of hydrochlorothiazide in type II diabetics without alteration of insulin secretionLancet1985112362857210
- European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertensionJ Hypertension200321101153
- FalknerBFrancosGKushnerHMetoprolol succinate, a selective ß-adrenergic blocker, has no effect on insulin sensitivityJ Clin Hypertension2006833643
- GeislerLSHypertension therapy in the elderlyMedWelt19813291014
- GoodfellowRWestbergBThe treatment of high blood pressure in the elderly: a multi-center evaluation of a fixed combination of metoprolol and hydrochlorothiazide (Co-Betaloc) in general practiceCurr Med Res Opin19817536427307545
- GuQPaulose-RamRDillonCBurtVAntihypertensive medication use among US adults with hypertensionCirculation20061132132116391156
- HajjarIKotchenTTrends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000JAMA200329019920612851274
- HanssonLLindholmLHEkbomTRandomized trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish trial in old patients with hypertension-2 studyLancet19993541751610577635
- HawkinsDWDieckmannMRHornerRDDiuretics and hypertension in black adultsArch Intern Med198814880353281620
- HildemannSKFischerHPittrowDMetoprolol succinate SR plus hydrochlorothiazide (Beloc-Zok® Comp) in patients with essential hypertension in general practice. A prospective, observational trial in 14,964 patientsClin Drug Invest20022271929
- HollandOBFairchildCRenin classification for diuretic and beta-blocker treatment of black and hypertensive patientsJ Chron Dis198235179826120945
- HydroDiuril® (hydrochlorothiazide) professional information brochure1998Merck & Co., Inc
- JNC 7 reportChobanianAVBakrisGLBlackHRThe seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressureJAMA200328925607112748199
- JordoLJohnssonGLundborgPBioavailability and disposition of metoprolol and hydrochlorothiazide combined in one tablet and of separate doses of hydrochlorothiazideBr J Clin Pharmacol197975637465277
- JuliusSNesbittSDEaganBMFeasibility of treating prehypertension with an angiotensin-receptor blockerN Engl J Med200635416859716537662
- LaPalioLSchorkAGlasserSSafety and efficacy of metoprolol in the treatment of hypertension in the elderlyJ Am Geriatr Soc19924035481556363
- MouradJ-JWaeberBZannadFon behalf of the investigators of the STRATHE trialComparison of different therapeutic strategies in hypertension: a low-dose combination of perindopril/indapamide versus a sequential monotherapy or a stepped-care approachJournal of Hypertension20042223798615614033
- OfiliEDispelling the myth of “aggressive” antihypertensive therapyJ Clin Hypertens20068Suppl 1411
- PapademetriouVHainerJWSuggJATTACH Study GroupFactorial antihypertensive study of an extended-release metoprolol and hydrochlorothiazide combinationAm J Hypertens20061912172517161766
- PapademetriouVReifMHenryDCombination therapy with candesartan cilexetil and hydrochlorothiazide in patients with systemic hypertensionJ Clin Hypertens200023728
- PloskerGLClissoldSPControlled release metoprolol formulations: a review of their pharmacodynamic and pharmacokinetic properties, and therapeutic use in hypertension and ischaemic heart diseaseDrugs1992433824141374320
- PsatyBMLumleyTFurbergCHealth outcomes associated with various antihypertensive therapies used as first-line agentsJAMA200328925344412759325
- SamuelssonOHednerTBerglundGDiabetes mellitus in treated hypertension: incidence, predictive factors and the impact of non-selective beta-blockers and thiazide diuretics during 15 years treatment of middle-aged hypertensive men in the primary prevention trial Goteborg, SwedenJ Hum Hypertens19948257637912734
- SandbergARagnarssonGJonssonUEDesign of a new multiple-unit controlled-release formulation of metoprolol – metoprolol CREur J Clin Pharmacol198833S3S73371391
- SandbergAAbrahamssonBRegârdhC-GPharmacokinetic and biopharmaceutic aspects of once daily treatment with metoprolol CR/ZOK: a review articleJ Clin Pharmacol199030S2S162179280
- The Association of Black Cardiologists (ABC) Candesartan Study GroupEvaluation of candesartan cilexetil in black patients with systemic hypertension: The ABC TrialHeart Dis20002392911728289
- TOPROL-XL® (metoprolol succinate) Extended Release Tablets professional information brochure2005AstraZeneca LP
- TurnbullFNealBAlgertCEffects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trialsLancet200336215273514615107
- WikstrandJWestergrenGBerglundGAntihypertensive treatment with metoprolol or hydrochlorothiazide in patients aged 60 to 75 yearsJAMA19862551304103511308
- [WHO] World Health Organization, International Society of Hypertension Writing GroupWorld Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertensionJ Hypertens20032119839214597836