Abstract
Fondaparinux is the first selective inhibitor of the coagulation factor Xa which is commercially avaliable for clinical use. It has been approved for the prevention of venous thromboembolism in patients undergoing orthopedic surgery and for the initial therapy of venous thromboembolism. In randomized clinical trials the value of fondaparinux in the treatment of ST-elevation myocardial infarction (STEMI) has been investigated. The PENTALYSE study showed that fondaparinux was at least as effective and safe as unfractionated heparin in 333 patients with STEMI undergoing fibrinolysis with t-PA. In the recent large OASIS-6 trial with 12,092 patients the treatment with 2.5 mg fondaparinux daily significantly reduced death and reinfarctions until day 30 compared with guideline recommended usual care and compared with unfractionated heparin (9.7% vs 11.2%, p = 0.008) without increasing major bleedings (1.0% vs 1.3%, p = 0.13). This advantage was predominantly seen in the subgroups of patients with fibrinolysis and without early reperfusion therapy. However, in the subgroup of primary percutaneous coronary interventions (PCIs) no clinical benefit of fondaparinux was found, but there were more catheter thrombosis and acute thrombotic complications. In summary, fondaparinux is a new antithrombin that is an efficient, safe, and easy to use in treatment for STEMI patients, particularly those not undergoing primary PCI.
Introduction
Antithrombin therapy in ST-elevation myocardial infarction
The pathophysiological mechanism of acute ST-elevation myocardial infarction (STEMI) is the rupture of a vulnerable or high-risk plaque and exposition of its contents to the passing bloodstream, resulting in activation of the coagulation cascade, which ultimately results in the deposition of fibrin strands. In addition, platelets are activated and aggregate. The cornerstone of acute management for STEMI is early reperfusion therapy with primary percutaneous coronary intervention (PCI) or fibrinolysis. Due to the pathophysiological mechanisms there is a rationale for adjunctive medical therapy with anticoagulants to inhibit the coagulation cascade in patients with STEMI. In addition to establishing and maintaining patency of the infarct-related artery, antithrombins are given in STEMI patients for prevention of left ventricular thrombus, cerebral embolization, deep-vein thrombosis, and pulmonary embolism (CitationAntman et al 2004).
Unfractionated heparin
The traditional anticoagulant in treatment of STEMI patients is unfractionated heparin. Heparins are naturally occurring glycosaminoglycans acting as cofactors of endogenous inhibitors of coagulation (antithrombin, heparin cofactor II). The heparin effect has multiple targets in the coagulation cascade (CitationFreedman 1992). In clinical practice unfractionated heparin has been used in patients with myocardial infarction for over 40 years.
The ACC/AHA guidelines recommend unfractionated heparin in STEMI patients undergoing percutaneous or surgical revascularization and in patients undergoing reperfusion therapy with fibrin-specific thrombolytic agents (Class I recommendation). In reperfusion therapy with non-specific fibrinolytic agents, which themselves produce a systemic coagulopathy, unfractionated heparin is recommended in patients at high risk of systemic emboli, and patients with large myocardial infarction or known left ventricular thrombus (Class I recommendation) (CitationAntman et al 2004).
In patients treated with fibrin specific fibrinolytics such as t-PA, reteplase or tenecteplase effective anticoagulation (aPTT 2-3 times control) is associated with an improved patency rate of the infarct vessel and a reduction in the rate of reinfarctions.
However, the current role of unfractionated heparin in the treatment of STEMI patients is controversial and the search for better alternatives in the anticoagulative treatment began years ago. Major bleedings, especially hemorrhagic stroke, are the most important complications of antithrombin therapy. The correct dose of unfractionated heparin to achieve beneficial effects and avoid bleeding complications depends on other adjunctive treatments of STEMI patients, body weight, and concomitant diseases. The anticoagulant effect of unfractionated heparin can be measured by the activated clotting time (ACT) in the setting of primary angioplasty or by the partial thromboplastin time (PTT) in thrombolysis patients. However, a limitation in predictable bioavailability and maintenance of the therapeutic range of unfractionated heparin has been criticized by different authors (CitationGurfinkel et al 1998). A further complication of treatment with unfractionated heparin is heparin-induced thrombocytopenia (HIT). The incidence of HIT in therapy with unfractionated heparin is 3% and it is associated with a substantial risk of prothrombotic events and a worse prognosis. The ACC/AHA guidelines therefore recommend daily platelet counts in patients receiving unfractionated heparin (CitationAntman et al 2004).
Low-molecular-weight heparins
Low-molecular-weight heparins have the pharmacological advantages of better bioavailability and minimal protein binding compared with unfractionated heparin, and they are associated with a lower rate of heparin-induced thrombocytopenia. Different recent studies investigated the value of low-molecular-weight heparins in STEMI and fibrinolysis. By far best studied low-molecular-weight heparin is enoxaparin.
In the ASSENT-3 and ASSENT-3 PLUS trials a superiority of enoxaparin compared with unfractionated heparin was found in patients receiving tenecteplase for the combined end point of efficiacy and safety (a composite of 30-day mortality, reinfarction, refractory ischemia, and bleeding). An excess of intracranial hemorrhage was observed in elderly women >75 years (CitationArmstrong et al 2006).
The ExTRACT-TIMI 25 trial randomly assigned 20506 patients with STEMI who were scheduled to undergo fibrinolysis to receive enoxaparin throughout hospitalization or weight-based unfractionated heparin for at least 48 hours. The enoxaparin dosing strategy was adjusted according to patient`s age and renal function. A reduced dosage for patients at least 75 years of age or with an estimated creatinine clearance of less than 30 mL/minute omitted the intravenous bolus and reduced the s.c. dose to 75%. Unfractionated heparin was to be administered beginning with an intravenous bolus of 60 U/kg body weight (maximum 4000 U, the bolus was to be omitted for patients who received open-label unfractionated heparin within three hours before randomization), followed by an infusion of 12 U/kg/hour (maximum 1000 U/hour).
The ExTRACT-TIMI 25 investigators found a superiority of enoxaparin compared with unfractionated heparin in STEMI patients undergoing thrombolysis for death and reinfarctions after 30 days (12.0% vs 9.9%, p < 0.001); however more major bleedings were observed in the enoxaparin group (1.4 vs 2.1%, p < 0.001) (CitationAntman et al 2006).
Despite the pharmacological advantages of low-molecular-weight heparins compared with unfractionated heparin – better bioavailability, minimal protein binding, and lower rate of heparin-induced thrombocytopenia – therapy with low-molecular-weight heparins still has some problems.
The risk of bleedings is the major problem of this treatment. Low-molecular weight heparins have to be dosed carefully in the elderly and in patients with renal function to avoid cumulation and major bleedings. In the bleeding situation an antagonism with protamin is not as helpful as with unfractionated heparin. Because low-molecular-weight heparins have a cross-reactivity with HIT antibodies, they should not be used in HIT patients.
For these reasons new antithrombins were sought with a specific mode of action, an easy and safe way of use, and high efficacy in the treatment of acute coronary syndromes.
Thrombin inhibitors
Anticoagulants may inhibit the coagulation cascade at multiple positions (as heparins) or selectively. In the search for an optimal anticoagulant, selective inhibition of coagulation factors is an attractive strategy to produce predictable anticoagulant response (CitationBauer 2006).
The first selective inhibitors of a specific coagulation factor were the recombinant hirudins, which target thrombin (= factor II). Hirudins are direct thrombin inhibitors, which inhibit thrombin by directly binding to specific surface binding sites. Hirudins have been shown to have a rather narrow therapeutic window, limiting their use in the general population.
It was hypothesized that selective inhibitors of coagulation factors located further upstream in the coagulation pathway might be safer with respect to bleeding by not inhibiting thrombin activity directly. Factor Xa has been identified as a potential target for the design of new anticoagulants, as selective inhibitors of factor Xa would effectively block coagulation (CitationBauer 2006).
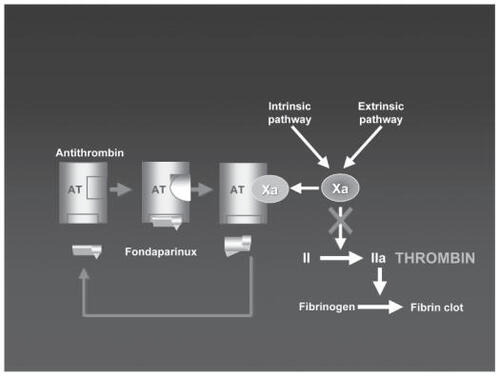
Fondaparinux is the first of the selective Xa inhibitors with clinical importance. It is an indirect factor Xa inhibitor (). It is a pentasaccharide designed specifically to bind to plasma antithrombin. This binding induces a conformational change in antithrombin which increases the affinity of antithrombin for factor Xa, potentiating the natural inhibitory effect of antithrombin against factor Xa.
The selective action of fondaparinux towards factor Xa contrasts with the action of unfractionated heparin, which has activity against a number of coagulation factors and equipotent activitity against factor IIa and Xa, and it contrasts with low-molecular-weight heparin which is also non-specific and has more activity against factor Xa than against factor IIa (CitationBauer 2006).
Pharmacological properties of fondaparinux
Fondaparinux contrasts with the previously described pharmacological characteristics of heparins (low bioavailability in unfractionated heparins, heterogenous structure and multiple targets in the coagulation pathway in all heparins, and heparin-induced thrombocytopenia especially in unfractionated heparins).
The main pharmacological properties of fondaparinux are listed in . Due to the unchanged elimination in urine the dose must be reduced in elderly patients, and patients with impaired renal function.
Table 1 Main pharmacological properties of fondaparinux
Following the product information of fondaparinux sodium (Arixtra®, GlaxoSmithKline, NC, USA), therapy is contraindicated in patients with severe renal impairment (creatinine clearance <30 mL/minute).
In patients with body weight <50 kg prophylactic fondaparinux therapy in surgery patients is contraindicated due to higher bleeding complications. In the therapy of deep vein thrombosis and pulmonary embolism a weight-depending dosage is recommended (5 mg daily in patients with body weight <50 kg, 7.5 mg in patients with 50–100 kg, 10 mg in patients with >100 kg). It is recommended to use fondaparinux with caution in elderly patients.
In patients with active major bleeding or bacterial endocarditis, fondaparinux is contraindicated. Therapy should be used with care in patients with a bleeding diathesis, uncontrolled arterial hypertension or history of gastrointestinal ulceration, diabetic retinopathy, and hemorrhage.
In the large clinical studies on fondaparinux in acute coronary syndromes (OASIS 5 and 6), patients with creatinine levels greater than 3.0 mg/dL, contraindications to anticoagulation, including high risk of bleeding, recent hemorrhagic stroke, and receiving oral anticoagulants were excluded.
Fondaparinux – clinical studies
Studies in prevention and treatment of venous thromboembolism
In four multicenter randomized trials (EPHESUS, PENTATHLON, PENTHIFRA, PENTAMAKS) the thromboprophylactic efficacy and safety of fondaparinux in patients undergoing orthopedic surgery was evaluated.
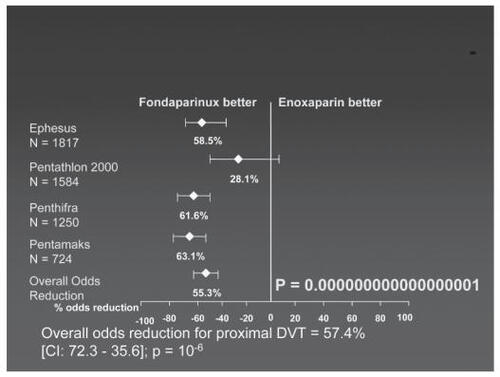
A meta-analysis of the four studies showed that fondaparinux is more effective than enoxaparin in preventing venous thrombosis in patients undergoing orthopedic surgery (). The incidence of pulmonary embolism did not differ between fondaparinux and enoxaparin. The incidence of major bleedings under fondaparinux was significantly lower when the first injection was given at 6 hours or more after skin closure compared with patients with earlier fondaparinux injection after skin closure with comparable incidence of thromboembolism (CitationBauer 2006).
Figure 2 Meta-analysis of fondaparinux versus enoxaparin in prevention of deep venous thrombosis prevention in randomized clinical trials. Compiled from data of CitationTurpie et al (2002)
The MATISSE-DVT and MATISSE-PE trials showed that initial therapy of deep-vein thrombosis or pulmonary embolism with fondaparinux was at least as efficacious and safe as low-molecular-weight heparin or intravenous unfractionated heparin (CitationBauersachs 2005).
After these studies were published, fondaparinux was FDA approved for prevention of venous thromboembolism following hip surgery, total knee replacement, and major abdominal surgery and was also FDA approved for the initial treatment of patients with deep-vein thrombosis and pulmonary embolism.
Studies in patients with acute coronary syndromes without ST elevations
PENTUA
In PENTUA four different doses of fondaparinux (2.5 mg, 4 mg, 8 mg, 12 mg once daily) and enoxaparin (1 mg twice daily) were compared, both given for 3–7 days in 1138 patients with acute coronary syndromes without ST segment elevations. There was no dose-dependent effect observed for ischemic events until day 9 or severe bleeding complications. The lowest event rates for the combination of death, myocardial infarction, and recurrrent ischemia were observed in the 2.5 mg (30%) fondaparinux group, which was significantly lower than in the enoxaparin group (40.2%, p < 0.05). Therefore this dose regimen was selected for further studies in patients with acute coronary syndromes.
OASIS 5
In the OASIS-5 (Fifth Organization to Assess Strategies in Acute Ischemic Syndromes) trial, 20,078 patients with acute coronary syndromes without ST elevation were randomized to either fondaparinux (2.5 mg daily) or enoxaparin (1 mg/kg twice daily) for a mean of 6 days (CitationYusuf et al 2006). The primary outcome at 9 days (death, myocardial infarction, or refractory ischemia) was similar in the two groups (5.9% vs 5.8%). After 30 days (8.1% vs 8.8%) and 180 days (12.1% vs 13.1%) a non-significant trend towards a lower value was found in the fondaparinux group. The rate of major bleedings at day 9 was significantly lower in the fondaparinux group (2.1% vs 4.0%). Fondaparinux was associated with a significantly reduced number of deaths at 30 days (2.9% vs 3.5%, p = 0.022) and at 180 days (5.6% vs 6.3%, p = 0.037).
In summary, the OASIS-5 trial showed that fondaparinux in acute coronary syndromes without ST elevations is comparable with enoxaparin in preventing ischemic events, is associated with fewer bleedings than enoxaparin, and is associated with a lower mid-term mortality.
Studies in patients with PCI
In the ASPIRE trial 350 patients undergoing elective or urgent PCI were randomized to receive unfractionated heparin, 2.5 mg fondaparinux, or 5 mg fondaparinux intravenously. Fondaparinux was comparable to unfractionated heparin for clinical safety and efficacy outcomes: the incidence of bleeding was 7.7% in the heparin group and 6.4% in the combined fondaparinux group (with fewer bleedings with 2.5 mg than 5 mg fondaparinux); the composite efficacy outcome (mortality, myocardial infarction, urgent revascularization, bailout GPIIb/IIIa antagonist) occurred in 6.0% both in the heparin group and in the fondaparinux group (CitationMehta et al 2005).
Studies in patients with STEMI
The PENTALYSE study
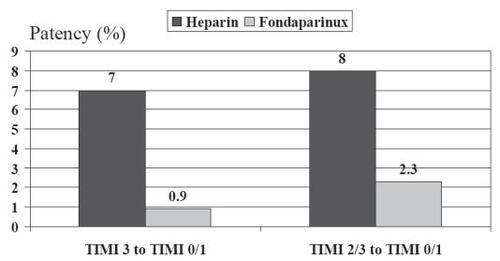
In 2001, first results on fondaparinux (factor Xa inhibitor ORG31540/SR9017A) in patients with STEMI were published by the PENTALYSE study group. In this trial 333 patients with STEMI of less than 6 hours’ duration were treated with aspirin and alteplase and randomized to unfractionated heparin (given intravenously 48–72 hours) or to a low (4 mg), medium (8 mg), or high (12 mg) dose of fondaparinux (given daily for 5–7 days, first intravenously, then subcutaneously). Coronary angiography was performed at 90 minutes after the start of fibrinolysis and on days 5–7. Fondaparinux given together with alteplase was as effective as unfractionated heparin in restoring coronary artery patency and was as safe as unfractionated heparin (identical primary safety end point of intracranial hemorrhage and need for blood transfusion) (CitationCoussement et al 2001). Fondaparinux was associated with a trend towards a reduction in the rate of reocclussions between days 1 and 5 ().
OASIS-6
In the randomized, double-blind OASIS-6 trial 12,092 patients with STEMI presenting within 24 hours after onset of symptoms were included. Exclusion criteria were contraindications to anticoagulation, including those at high risk of bleeding, receiving oral anticoagulants, or with creatinine >3 mmol/L. Randomization was stratified by indication for the use of unfractionated heparin according to the current guidelines as judged by the investigators: stratum 1: no indication for heparin; stratum 2: indication for unfractionated heparin (eg, fibrin-specific fibrinolysis, primary PCI, fibrinolysis but eligible for antithrombotics). In stratum 1 blinded fondaparinux (2.5 mg daily for up to 8 days) or placebo was given; in stratum 2 blinded fondaparinux (2.5 mg daily for up to 8 days) or unfractionated heparin (intravenous for 24–48 hours) was given (CitationYusuf et al 2006).
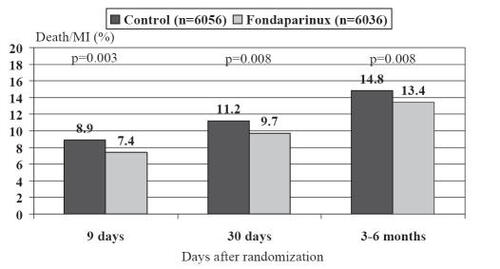
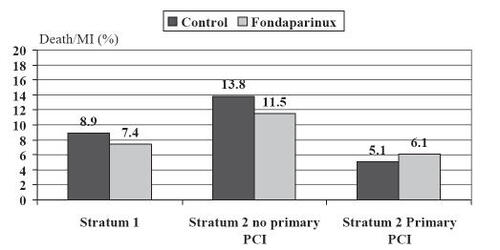
The primary end point of a composite of death or reinfarction at 30 days was significantly reduced by fondaparinux (9.7% fondaparinux group vs 11.2% control group, hazard ratio (HR) 0.86, confidence interval [CI] 0.77–0.96; p = 0.008) (). These benefits were also observed after 9 days and at the final follow-up (3–6 months) and were not heterogenous in the two strata.
Figure 4 Incidence of the combined endpoint of death and myocardial infaction at different times points after randomization in the OASIS-6 trial.
However, in patients with primary PCI no benefit by fondaparinux was found. If primary PCI was performed without addition of a small amount of unfractionated heparin, a higher rate of coronary complications – mainly due to guiding catheter thrombosis – was found (), without a significant increase in myocardial infarction or death or a negation of the overall benefits. In the 496 patients receiving heparin prior to primary PCI, no differences in catheter thrombosis (0 vs 2), coronary complications (24 vs 24), and bleeding complications (1 vs 4) between heparin and fondaparinunx were observed.
Table 2 Outcomes/complications at 30 days in patients undergoing PCI in the OASIS-6 trial
In patients with fibrinolysis and in patients without reperfusion therapy, fondaparinux was superior to unfractionated heparin ().
Figure 5 Combined endpoint according to the randomization strata in the OASIS-6 study.
In the fondaparinux group a non-significant trend of fewer severe bleedings and a significantly lower rate of cardiac tamponade was found (). Interestingly the incidence of bleeding complications in stratum 1 with fondaparinux was lower than placebo (1.0% vs 1.6%).
Table 3 Major bleeding complications until day 9 in the OASIS-6 trial
In summary, the OASIS-6 trial showed a moderate but significant reduction in mortality and reinfarctions by fondaparinux compared with usual care and unfractionated heparin, without increasing bleeding or hemorrhagic stroke in STEMI patients.
In patients undergoing primary PCI this benefit was not found and the patients with fondaparinux showed a higher rate of guiding catheter thrombosis and coronary complications during PCI, if PCI was performed without unfractionated heparin. Among the 496 patients who received heparin prior to primary PCI, no differences in the rate of death and myocardial infarction, coronary complications, catheter thrombus, and severe bleedings were observed. These data indicate that the additional use of heparin with fondaparinux during PCI is safe and avoids thrombotic complications.
Summary
After the OASIS-5 trial had shown that fondaparinux is an efficient and safe anticoagulant in the treatment of acute coronary syndromes without ST elevations, the OASIS-6 trial demonstrated a reduction in mortality and reinfarctions by fondaparinux compared with usual care and unfractionated heparin in more than 10,000 patients with STEMI. Treatment of STEMI patients with fondaparinux was safe and not associated with an increase in bleedings or hemorrhagic strokes.
However, some points and possible disadvantages of fondaparinux have to be discussed.
For primary PCI in STEMI patients, the actual data of the OASIS-6 trial suggest that at least during the intervention, unfractionated heparin is necessary in addition to fondaparinux to avoid catheter thrombosis and ischemic complications. However, in the ASPIRE trial performed on patients with elective PCI, no difference in coronary complications was observed between heparin and fondaparinux (Metha 2005). The data of the OASIS trials suggest that a periprocedural combined use of unfractionated heparin and fondaparinux is safe; nonetheless, further prospective evaluation seems to be required analyzing fondaparinux/unfractionated heparin and the periprocedural antithrombotic strategy in PCI patients. The reasons for the insufficient antithrombotic effect of fondaparinux in foreign materials such as catheters should be evaluated. Based on available data, fondaparinux should be used in primary PCI with the addition of unfractionated heparin.
Compared with the large clinical experience, which physicians have achieved with respect to heparin treatment in the past years, little is known about treatment with fondaparinux in clinical practice. No data exist on fondaparinux treatment in patients, who escape the inclusion criteria of randomized trials. However, the OASIS-6 trial included a wide variety of patients, assuring the applicability of the results to a general population of STEMI patients.
No data are available that compare fondaparinux and low-molecular-weight heparins in the treatment of STEMI. Both the PENTALYSE and OASIS-6 trials compared fondaparinux and unfractionated heparin. The OASIS-5 trial compared fondaparinux and enoxaparin, but was performed in acute coronary syndromes without ST elevations. With respect to the recent investigations of low-molecular-weight heparins in STEMI patients (ASSENT-3, ExTRACT TIMI 25), a comparison of fondaparinux and enoxaparin seems to be warranted.
In OASIS 6 in both groups only ~2% of the patients received GP IIb/IIIa-antagonists (~16% thienopyridine respectively) within 7 days of randomization (during the hospital course after randomization ~16% IIb/IIIa-antagonists and ~58% thienopyridine). Further studies investigating the efficacy and safety of fondaparinux in patients receiving high-dose antithrombotic therapy with GP IIb/IIIa-antagonists and high-dose clopidogrel therefore seem warranted.
In the bleeding situation there is no known antidote for fondaparinux.
On the other hand, the actual studies present fondaparinux as a safe and efficient new antithrombotic agent in the treatment of acute coronary syndromes. A major advantage of fondaparinux is its ease of use. A single daily subcutaneous administration of 2.5 mg fondaparinux provides a stable and predictable anti-coagulation without the need for laboratory control of coagulation parameters. In addition it is not associated with the risk of heparin associated thrombocytopenia. Therefore it can be used in a wide range of patients and settings. The data of the OASIS-6 trial suggest that selective factor Xa inhibition with fondaparinux is an attractive, easy to use, and therefore widely applicable new antithrombotic strategy in the treatment of STEMI.
References
- AntmanEMAnbeDTArmstrongPWACC/AHA guidelines for the management of patients with ST-elevation myocardial infarctionJ Am Coll Cardiol20044467171915358045
- AntmanEMMorrowDAMcCabeCHExTRACT-TIMI 25 investigators. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarctionN Engl J Med200635414778816537665
- ArmstrongPWChangWCWallentinLASSENT-3 and ASSENT-3 PLUS investigators. Efficacy and safety of unfractionated heparin versus enoxaparin: a pooled analysis of ASSENT-3 and -3 PLUS dataCMAJ20061741421616682709
- BauerKANew anticoagulants: Anti IIa vs Anti Xa – Is one better?J Thromb Thrombolysis200621677216475045
- BauersachsRMFondaparinux: An update on new study resultsEur J Clin Invest200535Suppl 1273215701145
- CoussementPKBassandJPConvensCA synthetic factor-Xa inhibitor (ORG31540/SR9017A) as an adjunct to fibrinolysis in acute myocardial infarction. The PENTALYSE studyEur Heart J20012217162411511121
- FreedmanMDPharmacodynamics, clinical indications, and adverse effects of heparinJ Clin Pharmacol199232584961322434
- GurfinkelEFareedJAntmanERationale for the management of coronary syndromes with low-molecular-weight heparinsAm J Cardiol19988215L18L
- MehtaSRStegPGGrangerCBRandomized, blinded trial comparing fondaparinux with unfractionated heparin in patients undergoing contemporary percutaneous coronary interventionCirculation200511113909715781750
- TurpieAGBauerKAErikssonBIFondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studiesArch Intern Med200216218334012196081
- YusufSMehtaSRChrolaviciusSThe Fifth Organization to Access Strategies in Acute Ischemic Syndromes InvestigatorsComparison of fondaparinux and enoxaparin in acute coronary syndromesN Engl J Med200635414647616537663
- YusufSMehtaSRChrolaviciusSEffects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction. The OASIS-6 randomized trialJAMA29515193016537725