Abstract
Patients with type 2 diabetes face an increased risk of macrovascular disease compared to those without. Significant reductions in the risk of major cardiovascular events can be achieved with appropriate drug therapy, although patients with type 2 diabetes remain at increased risk compared with non-diabetics. The thiazolidinedione, pioglitazone, is known to offer multiple, potentially antiatherogenic, effects that may have a beneficial impact on macrovascular outcomes, including long-term improvements in insulin resistance (associated with an increased rate of atherosclerosis) and improvement in the atherogenic lipid triad (low HDL-cholesterol, raised triglycerides, and a preponderance of small, dense LDL particles) that is observed in patients with type 2 diabetes. The recent PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) study showed that pioglitazone can reduce the risk of secondary macrovascular events in a high-risk patient population with type 2 diabetes and established macrovascular disease. Here, we summarize the key results from the PROactive study and place them in context with other recent outcome trials in type 2 diabetes.
Introduction
People with type 2 diabetes have a higher risk of macrovascular disease than those without diabetes (CitationHaffner et al 1998; NCEP 2002; CitationJuutilainen et al 2005; CitationIdris et al 2006). Furthermore, prognosis after a first myocardial infarction (MI) or stroke is particularly poor in patients with type 2 diabetes (CitationMiettinen et al 1998; CitationMukamal et al 2001; CitationIdris et al 2006). As such, type 2 diabetes is associated with excess mortality in all age groups, which is largely attributable to cardiovascular disease (CVD) (CitationRoper et al 2001).
Outcome studies in patients with type 2 diabetes using antihypertensive agents, lipid-modifying drugs, and antiplatelet therapy show that significant reductions in the risk of major CV events are possible with appropriate management. Furthermore, outcome studies with glucose-lowering agents show a trend towards reduced risk of macrovascular events (CitationUKPDS33 1998). Nevertheless, these studies also show that excess risk remains in patients with type 2 diabetes relative to those without diabetes, despite contemporary interventions. This highlights the need for novel therapies for this high-risk group, especially those who also have established macrovascular disease.
Pioglitazone is an agonist for the peroxisome proliferator-activated receptor γ (PPARγ), which regulates multiple genes controlling carbohydrate and lipid metabolism and is licensed as a glucose-lowering agent for the treatment of type 2 diabetes (CitationYki-Järvinen 2004). Pioglitazone has multiple, potentially antiatherogenic properties, including effects on insulin sensitivity, lipid profiles, inflammatory markers, blood pressure, and components of the coagulation cascade that suggest it might have a beneficial impact on macrovascular outcomes. This has been investigated in the PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) study and final results have been published recently. This study, in more than 5000 high-risk patients with type 2 diabetes and a history of macrovascular disease, investigated whether pioglitazone added to standard contemporary multifactorial treatment could improve mortality and morbidity associated with major CVD progression (CitationDormandy et al 2005). This review summarizes the key results from PROactive and puts them in context with other outcome trials in type 2 diabetes.
Why should pioglitazone improve macrovascular outcomes?
Several lines of evidence suggest that pioglitazone has antiatherogenic properties and the potential to improve macrovascular outcomes in patients with type 2 diabetes. Firstly, pioglitazone produces long-term improvements in insulin sensitivity (CitationPavo et al 2003; CitationTan et al 2004a; CitationRoden et al 2005) and insulin resistance is associated with an increased rate of atherosclerosis (CitationNigro et al 2006). Furthermore, pioglitazone improves multiple established modifiable risk factors for CVD, eg, atherogenic diabetic dyslipidemia (low HDL-cholesterol, raised triglycerides, and a preponderance of small, dense LDL particles) and blood pressure (CitationTaskinen 2003; CitationBuse et al 2004; CitationLawrence et al 2004; CitationPerez et al 2004; CitationGoldberg et al 2005; CitationDerosa et al 2005a).
In line with its current role as a glucose-lowering therapy, pioglitazone produces long-term improvements in glycemic control, as evident in the sustained improvements in HbA1c and fasting plasma glucose seen in patients with type 2 diabetes in clinical trials up to 2 years in duration (CitationHanefeld et al 2004; CitationSchernthaner et al 2004; CitationTan et al 2004c; CitationCharbonnel et al 2005a, Citationb). However, it should be noted that it has not been firmly established that improving glycemia per se can significantly improve macrovascular outcomes in type 2 diabetes, although data from the United Kingdom Prospective Diabetes Study (UKPDS) suggest a trend (CitationUKPDS33 1998; CitationStratton et al 2000). In type 1 diabetes, on the other hand, intensive glucose-lowering therapy is associated with reductions in markers of inflammation, atherosclerosis and CVD, as well as a significant reduction in macrovascular events (CitationNathan et al 2003, Citation2005; CitationSchaumberg et al 2005; CitationThe DCCT Research Group 1993).
Pioglitazone also improves multiple non-traditional risk markers and emerging risk factors for CVD. Notably, pioglitazone significantly reduces C-reactive protein (CRP) levels and improves the levels of a host of other cytokines, inflammatory mediators, and markers of endothelial dysfunction with a potential role in atherosclerosis (for example adiponectin, the matrix metalloproteinase, MMP-9, and plasminogen activator inhibitor-1 [PAI-1]) (CitationSatoh et al 2003; CitationDerosa et al 2005b; CitationMiyazaki et al 2004; CitationPfutzner et al 2005). Pioglitazone has also been shown to reduce carotid intima media thickness, a surrogate marker for atherosclerosis, in a glucose-independent manner (CitationLangenfeld et al 2005; CitationPfutzner et al 2005). Pioglitazone and other thiazolidinediones also reduce restenosis and neointimal tissue proliferation after coronary stent implantation in patients with or without type 2 diabetes (CitationTakagi et al 2000, Citation2003; CitationChoi et al 2004; CitationMarx et al 2005). Studies in the isolated rat heart model suggest another potential benefit that may be relevant to improving outcomes – pioglitazone mimics ischemic preconditioning, which may protect the myocardium against subsequent prolonged episodes of lethal ischemia (CitationWynne et al 2005).
The PROactive study was designed to examine whether the incidence of macrovascular events is reduced in patients given pioglitazone or placebo in combination with their usual medication for diabetes and CVD. The primary composite endpoint of the surrogate vascular endpoints of all-cause mortality, MI, acute coronary syndrome (ACS), coronary intervention, major leg amputation, bypass surgery, or leg revascularization was chosen so as to capture all CV events.
PROactive study design
The PROactive study design is well documented elsewhere (CitationCharbonnel et al 2004; CitationDormandy et al 2005; http://www.proactive-results.com). Briefly, it was a prospective, multicenter, European (19 countries), randomized, double-blind outcome study in patients with type 2 diabetes (35–75 years). All patients had a history of pre-existing extensive macrovascular disease (MI, stroke, percutaneous coronary intervention or coronary artery bypass surgery ≥6 months before study entry, ACS ≥3 months before study entry, or objective evidence of coronary artery disease or obstructive arterial disease in the leg) making the patient population at very high-risk for macrovascular disease.
Patients were randomized to receive pioglitazone (titrated from 15 mg to 45 mg; n = 2605) or matched placebo (n = 2633) in addition to existing therapies. These included blood glucose-lowering drugs (with or without insulin), and lipid-lowering, antihypertensive, and antiplatelet therapies ( and ) – many patients were already receiving contemporary multifactorial therapy. The primary endpoint was the time from randomization to the first incidence of all-cause mortality, non-fatal MI (including silent MI), stroke, major leg amputation, ACS, cardiac intervention (bypass graft or percutaneous coronary intervention), or leg revascularization. This very challenging composite endpoint therefore included procedural endpoints and was designed to demonstrate benefit in multiple vascular beds – cardiac, cerebral, and peripheral. The main secondary endpoint was time to first incidence of all-cause mortality, non-fatal MI, or stroke – this represented the most clinically important and objectively confirmed events and is identical or similar to primary composite endpoints used in many other major CV outcome studies. Although not included in the original study design (CitationCharbonnel et al 2004), this main secondary endpoint was prespecified in the statistical analysis plan prior to unblinding (CitationDormandy et al 2006; http://www.proactive-results.com).
Table 1 Concomitant glucose-lowering medication use during the study
Table 2 Concomitant cardiovascular medication use during the study
Throughout the study, investigators were encouraged to strive for a target HbA1c <6.5% and to increase lipid-altering, antiplatelet, and antihypertensive therapy to an optimum in line with IDF Europe guidelines (CitationEuropean Diabetes Policy Group 1999). The duration of the study was event-driven – however, due to faster than anticipated enrolment and a higher event rate than predicted, the study was amended to include a minimum duration of follow-up of 30 months.
Main outcome results in PROactive
After a mean follow-up of 34.5 months, patients in the pioglitazone group experienced 803 events, of which 514 were first events, whereas those on placebo had 900 events, of which 572 were first events. For the primary composite endpoint, pioglitazone reduced the relative risk for an event by 10% compared with placebo, but this difference did not achieve statistical significance (). Nevertheless, there was a consistent reduction in most of the individual components of the primary endpoint – the risk of mortality, non-fatal MI, silent MI, stroke, major leg amputation, and ACS was lower with pioglitazone relative to placebo (). For the main secondary endpoint, pioglitazone was associated with a 16% relative risk reduction (). Adding pioglitazone to the medication of 1000 patients would, therefore, avoid 21 first MIs, strokes, or deaths over 3 years. In other words, the number needed to treat (NNT) would be 48 patients for 3 years to avoid one first major CV event.
Figure 1 Main outcome results in PROactive. A. Primary composite endpoint (all-cause mortality, non-fatal MI (including silent MI), stroke, major leg amputation, acute coronary syndrome, cardiac intervention, or leg revascularization); B. Main secondary composite endpoint (all-cause mortality, non-fatal MI, or stroke). Both figures reproduced with permission from Dormandy JA, Charbonnel B, CitationEckland DJ, et al. 2005. PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet, 366: 1279–89. Copyright © 2005 Elsevier.
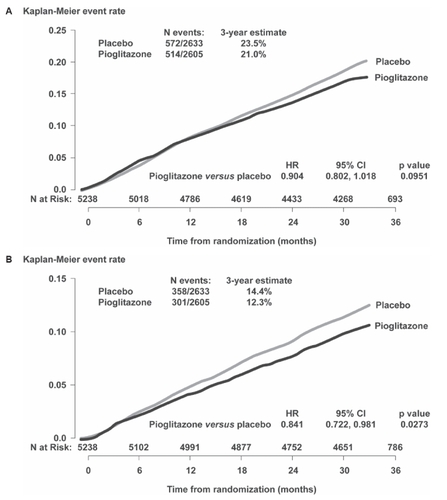
Table 3 Effects of pioglitazone and placebo on individual components of the composite endpoints and total events during the study
The difference between the two main composite endpoints was explained mainly by an unexpected increase in the number of peripheral vascular procedures performed in the pioglitazone group. There was also no decrease in the number of coronary revascularization procedures included in the primary endpoint; however, there was a decrease in the number of coronary revascularization procedures in the pioglitazone group overall during the study (including those that were not the first event) ().
Twenty-five baseline variables were prespecified for subgroup analysis. Interaction tests within these subgroups did not reveal evidence of heterogeneity. Multivariate analysis of the association of entry characteristics to the main secondary endpoint showed associations with several factors, such as statin use, insulin use, smoking, and previous MI. Nevertheless pioglitazone was associated with a 16% reduction in relative risk even after adjustment for these factors.
Composite endpoints of cardiovascular events, referred to as major adverse cardiovascular events (MACE), are standard measures for comparing treatments in large cardiovascular outcome studies. In addition to the main secondary endpoint, analyses of other prespecified and post-hoc MACE endpoints have been presented recently for the overall patient population (CitationWilcox and Kupfer 2006). Pioglitazone treatment resulted in significant relative risk reductions in the prespecified endpoint of risk of fatal/non-fatal MI (excluding silent MI) (HR = 0.77, 95% CI [0.60,1.00]; p = 0.046), and prespecified endpoint of the composite of CV death, non-fatal MI (excluding silent MI), or non-fatal stroke (HR = 0.82, 95% CI [0.70, 0.97]; p = 0.02). A post-hoc analysis also showed a significant relative risk reduction for the composite of all-cause mortality, non-fatal MI (excluding silent MI), non-fatal stroke, or ACS (HR = 0.83, 95% CI [0.72, 0.96]; p = 0.010).
Metabolic and laboratory results
Laboratory data showed significant improvements in HbA1c (−0.5%), triglycerides (−13.2%), HDL-cholesterol (+8.9%) and LDL-/HDL-cholesterol ratio (−5.3%) with pioglitazone relative to placebo (). The improvement in glycemic control occurred despite a 50% reduction in rate of progression to permanent insulin use (defined as daily insulin use for a period of ≥90 days, or ongoing use at death/final visit) in the pioglitazone group compared with placebo – 183 patients on pioglitazone progressed to permanent insulin use during the study compared with 362 on placebo (CitationMassi-Benedetti et al 2006). The decreased need for insulin in the pioglitazone group was irrespective of baseline treatment. Furthermore, among patients receiving insulin at baseline, insulin doses progressively decreased in the pioglitazone group during the study, but progressively increased with placebo and were significantly different by study end (CitationScheen and Charbonnel 2006). There were no differences in changes in the use of other medications during the study, apart from a slight decrease in metformin use in the pioglitazone group compared with placebo (). Significant improvements in HbA1c of similar magnitude to the overall pioglitazone group were seen in patients who were on insulin at baseline (CitationScheen and Charbonnel 2006) or on dual oral agent therapy with metformin plus sulfonylureas at baseline (when pioglitazone was added as a third agent) (CitationCharbonnel and Scheen 2006).
Table 4 Change in metabolic parameters from baseline to final visit
Systolic blood pressure was significantly improved in the pioglitazone group compared with placebo (between-group difference of −3 mmHg), with only a slight increase in the use of antihypertensive agents. Analyses of laboratory assessments for liver function (alanine aminotransferase [ALT], aspartate aminotransferase [AST], and alkaline phosphatase [AP]) have also been presented recently (CitationHeine et al 2006). The results showed a general shift toward normalization of ALT and AST values in the pioglitazone group from baseline to final visit compared with no change or an increase in the placebo group (p < 0.001 for difference between groups at final visit). Changes in ALT with pioglitazone may reflect a reduction in liver fat due to improvement in hepatic insulin sensitivity.
Subgroup analyses
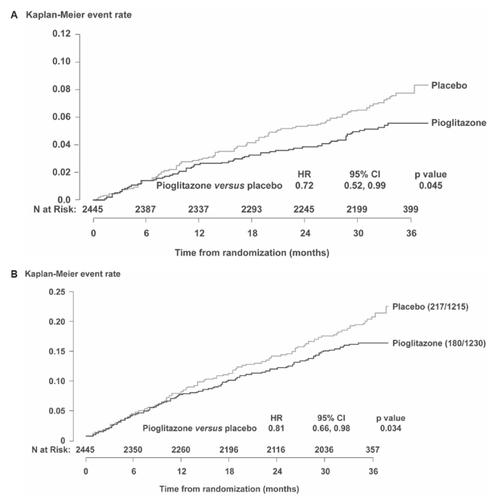
The first prespecified subgroup analysis involved the cohort of 2445 patients with a previous MI ≥ 6 months prior to randomization (CitationErdmann et al 2007). Prespecified endpoints were time to fatal or non-fatal MI (excluding silent MI), time to CV death or non-fatal MI, and time to CV death, non-fatal MI, or stroke. Time to fatal or non-fatal MI was significantly reduced by 28% in the pioglitazone group relative to placebo (). Risk of CV death or non-fatal MI, and risk of CV death, non-fatal MI, or stroke were both reduced by 15% with pioglitazone relative to placebo, but neither reached statistical significance. In addition, several post-hoc exploratory endpoints were analyzed for this subgroup. Risk of experiencing an event in a cardiac composite endpoint of cardiac death, non-fatal MI (excluding silent MI), ACS, and coronary revascularization was significantly reduced by 19% in the pioglitazone group relative to the placebo group (). There was no significant reduction in relative risk when silent MI was included in this composite endpoint or in any of its individual components, except for risk of an ACS, which was significantly reduced by 37% with pioglitazone relative to placebo.
Figure 2 Outcomes in the subgroup of patients with a previous myocardial infarction (MI). A. Prespecified endpoint of fatal/non-fatal MI (excluding silent MI); B. Exploratory composite cardiac endpoint (cardiac death, non-fatal MI, coronary revascularization, or acute coronary syndrome). Figure 2A reproduced with permission from CitationErdmann E, Dormandy JA, Charbonnel B, et al; on behalf of the PROactive investigators 2007. The effect of pioglitazone on recurrent myocardial infarction in 2445 patients with type 2 diabetes and previous myocardial infarction – Results from PROactive (PROactive 05). J Am Coll Cardiol, 49: 1772–80. Copyright © 2007 Elsevier.
Another prespecified subgroup analysis compared outcomes in patients with (n = 984) or without (n = 4254) prior stroke (CitationWilcox et al 2007). Risk of a recurrent stroke was significantly reduced by 47% with pioglitazone relative to placebo in patients with prior stroke (HR = 0.53; 95% CI [0.34, 0.85]; p = 0.0085), whereas no significant difference in the relative risk of stroke was seen in patients without a prior stroke. For the composite of CV death, MI, or stroke there was also a significant relative risk reduction with pioglitazone in patients with prior stroke (HR = 0.72; 95% CI [0.52, 1.00]; p = 0.0467).
Were there any potential safety or tolerability issues in PROactive?
The overall frequency of serious adverse events was slightly lower in the pioglitazone group, even after events contributing to the primary composite endpoint were excluded. The incidence of adverse events reported as “heart failure” has received considerable attention in the critical appraisal of the results from PROactive (CitationYki-Järvinen 2005). Regarding serious heart failure as reported by the investigators, 5.7% of pioglitazone-treated patients were reported with heart failure leading to hospitalization compared with 4.1% of placebo-treated patients; however, rates of mortality due to heart failure were similar between the two groups (0.96% [n = 25 out of 2605] vs 0.84% [n = 22 out of 2633] for pioglitazone and placebo, respectively). Despite more reports of heart failure in the pioglitazone group, overall CV outcomes were improved and the number of CV endpoints after serious heart failure was also similar between treatment groups (CitationErdmann et al 2006). Furthermore, most investigator-reported cases of serious heart failure resolved and were not treatment-limiting. Only 34 of the 113 patients in the pioglitazone group and 17 out of 89 patients in the placebo group who were on therapy at the time of diagnosis of heart failure discontinued study drug. Therefore, only a little more than 1% of patients in the pioglitazone group discontinued study drug for this reason. Edema in the absence of heart failure was reported in 21.6% of patients treated with pioglitazone compared with 13.0% on placebo. An independent review of the heart failure cases was conducted by assessing the strength of evidence of the patients having a history of heart failure before entering the study and also for each reported case of serious heart failure. This confirmed the accuracy of the original investigator diagnoses of greater rates of non-serious and serious heart failure in the pioglitazone arm, but comparable rates of mortality due to heart failure (CitationRydén et al 2007).
It should be emphasized that there is no evidence available in the literature to suggest that pioglitazone or other thiazolidinediones have any adverse effect on cardiac function. In clinical practice, initiation of pioglitazone therapy (alone or as an addition to pre-existing therapies, such as other oral agents and/or insulin) does not appear to be associated with increased hospitalization due to heart failure over a 10-month follow-up compared with initiation of sulfonylureas as a standard reference diabetes therapy (CitationKarter et al 2005). Initiation of insulin, on the other hand, is associated with a significant increase. Furthermore, 52 weeks of therapy with rosiglitazone was shown to have no adverse effect on cardiac structure or function (left ventricular [LV] mass index, ejection fraction, and left ventricular end-diastolic volume) (CitationSt John Sutton et al 2002). In fact, pioglitazone has been shown actually to improve LV diastolic function without LV mass regression in hypertensive patients in proportion to the amelioration of insulin resistance (CitationHorio et al 2005). Animal models support these observations. Pioglitazone improved LV remodeling and function in mice with post-MI heart failure, an effect that was associated with an attenuated LV expression of inflammatory cytokines (CitationShiomi et al 2002). Such studies have led to the suggestion that thiazolidinediones may, in fact, have therapeutic potential in patients with advanced heart failure, rather than being a cause for concern (CitationNikolaidis and Levine, 2004). However, it should be noted that edema is more frequent in patients treated with pioglitazone plus insulin – fluid retention is a dose-dependent side-effect of both drugs.
In PROactive, average weight gain from baseline with pioglitazone was 3.6 kg (compared with −0.6 kg for placebo). However, weight gain led to permanent discontinuation in only 0.8% of patients compared with 0.2% on placebo. Weight gain with thiazolidinediones is a consistent finding and may reflect a combination of both fat increase and fluid retention (CitationHollenberg 2003). Any possible detrimental effect of increased body fat is offset by potentially beneficial qualitative effects – thiazolidinediones shift fat distribution away from the more metabolically active visceral depots to less active subcutaneous depots (an effect associated with improvements in hepatic and peripheral insulin sensitivity) (CitationMiyazaki et al 2002). The overall incidence of cancer in PROactive was equivalent in the pioglitazone and placebo groups (3.7% vs 3.8%, respectively). Although there was a slight increase in the number of bladder tumors (n = 14 vs 6) and a slight decrease in breast tumors (n = 3 vs 11) reported in the pioglitazone group, an external independent review of blinded data concluded that any causal link was unlikely.
How does PROactive compare with other outcome studies in type 2 diabetes?
Several intervention trials using antihypertensive agents, lipid-modifying drugs (principally statins), glucose-lowering agents, or multifactorial intervention strategies have looked at macrovascular outcomes in patients with type 2 diabetes. However, much of the data pertaining to patients with diabetes are post-hoc or, in some instances, predefined subgroup analyses from larger cohorts (). Comparison between the results of PROactive and previous outcome studies is complicated by differences in predefined endpoints, study population, study duration, concomitant medication use, and a range of other methodological issues.
Table 5 Placebo-controlled outcome trials
To date, no prospective studies have been able to establish conclusively whether improving hyperglycemia per se in patients with type 2 diabetes can improve macrovascular outcomes. However, the UKPDS showed a clear trend towards a reduction in primary macrovascular events (based on a secondary endpoint of fatal/non-fatal MI) in patients whose hyperglycemia was more intensively managed with pharmacologic therapy (using sulfonylureas or insulin) compared with conventional management using lifestyle interventions (CitationUKPDS33 1998). Subsequent observational analyses showed that each 1% reduction in HbA1c was associated with a 14% relative reduction in risk for MI (CitationStratton et al 2000). However, in a substudy of overweight patients, tight glycemic control with metformin significantly reduced the two secondary endpoints of MI and any macrovascular disease (CitationUKPDS34 1998), suggesting an effect independent of glucose control in this patient group. Secondary endpoint measures from a prospective placebo-controlled diabetes prevention study suggested that acarbose (an α-glucosidase inhibitor that reduces postprandial hyperglycemia) may reduce the development of major CV events in people with impaired glucose tolerance, although the total number of events was low (CitationChiasson et al 2003). In type 1 diabetes, the benefits of reducing hyperglycemia appears to be less ambiguous, and the recent 17-year follow-up of the Diabetes Control and Complications Trial (DCCT) showed that intensive treatment caused a significant 57% reduction in macrovascular events among intensively-treated patients that was explained principally by a sustained decrease in HbA1c (HbA1c decreases at Year 11 were the same in both the intensive and conventional treatment groups; CitationNathan et al 2005).
Statin use in PROactive was not completely optimal for such a high-risk patient group (approximately 43% at baseline increasing to 55% at study end in both groups). Nevertheless, it reflects or possibly even exceeds the levels seen in contemporary practice among patients with diabetes with or without established coronary heart disease (CHD) and other high-risk patients (CitationBrown et al 2004; CitationKo et al 2004; CitationEmberson et al 2005; CitationBhatt et al 2006).
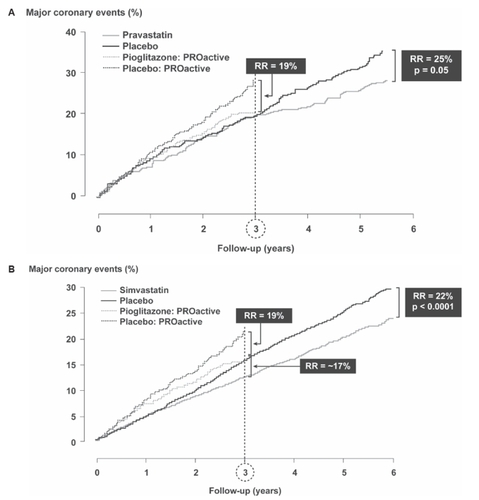
The 5-year Cholesterol And Recurrent Events (CARE) trial looked at secondary prevention in patients with a previous MI and had major coronary events (CHD death, non-fatal MI, or coronary revascularization) as a primary endpoint (CitationGoldberg et al 1998). This provides similar criteria for comparison with the previous MI subgroup in PROactive using the post-hoc exploratory composite endpoint of cardiac death, non-fatal MI, ACS, or coronary revascularization (). Patients in PROactive had a higher event rate than patients in CARE. At the 3-year point, pravastatin therapy in CARE produced no benefit in these patients, whereas in PROactive there was a significant 19% relative risk reduction with pioglitazone. An analysis of the 5963 patients with diabetes in the Heart Protection Study (HPS), around 50% of whom had a previous history of CVD, showed that simvastatin reduced the relative risk of major vascular events by approximately 17% after 3 years (). In the pravastatin in elderly individuals at risk of vascular disease (PROSPER) study (CitationShepherd et al 2002), which included individuals with a history of (or risk of) vascular disease, the length of follow-up (3.2 years) was very similar to that in PROactive. Among the 623 patients with diabetes, those treated with pravastatin appeared to have a non-significant 27% increase in the risk of an event (CV death, stroke, or MI), although there was a significant risk reduction in the population overall, and the authors considered the number of patients with diabetes too small to permit accurate interpretation of any treatment effect. The recent FIELD study was the largest intervention study to date in patients with type 2 diabetes (CitationKeech et al 2005). The rather heterogeneous study population included patients with varying levels of dyslipidemia, only 22% of whom had a prior history of CVD. After a median 5 years of follow-up, the primary outcome (non-fatal MI or CHD death), was reduced by 11% with fenofibrate compared with placebo, but this did not achieve statistical significance. A significant 24% decrease in non-fatal MI was offset by a non-significant 19% increase in CHD mortality. However, patients were statin-naïve at baseline and significantly more patients in the placebo group commenced statin therapy during the study so this may have been a confounding factor. Interestingly, fenofibrate seemed to reduce events only in those patients with no previous CVD (19% decrease). In patients with prior CVD, there was a slight (2%) increase in relative risk. For the main secondary outcome (composite of MI, stroke, CVD death, and coronary and carotid revascularization), there was a statistically significant 11% reduction in risk overall.
Figure 3 A. Major cardiac outcomes in the PROactive subgroup with previous myocardial infarction (MI) compared with outcomes in the CARE study. All patients in CARE had diabetes and a previous MI (from data of CitationGoldberg 1998); B. Major vascular outcomes (major coronary events, stroke, and revascularization in patients with diabetes) in the HPS study (from data of CitationHeart Protection Study Collaborative Group 2003). Figure 3A reproduced with permission from CitationGoldberg RB, Mellies MJ, Sacks FM, et al. 1998 for the Care Investigators. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels. Subgroup analyses in the cholesterol and recurrent events (CARE) trial. Circulation, 98: 2513–9. Copyright © 1998 Lippincott Williams and Wilkins. Figure 3B reproduced with permission from CitationHeart Protection Study Collaborative Group. 2003. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet, 361:2005–16. Copyright © 2003 Elsevier.
The vast majority of patients in PROactive were receiving antihypertensive and antiplatelet therapy with comparable proportions in the two treatment groups, and all patients were treated to an HbA1c goal of <6.5% (96% were using glucose-lowering agents) ( and ). The significant impact of antihypertensive therapy on macrovascular outcomes in type 2 diabetes has been demonstrated in several large-scale trials, including the UKPDS (primary prevention), the Heart Outcomes Prevention Evaluation (HOPE) study (mixed secondary/primary prevention) and GISSI-3 (secondary prevention) (CitationZuanetti et al 1997; CitationUKPDS38 1998; CitationHOPE 2000). An analysis from the HOPE study, which included 3577 patients with diabetes (approximately two-thirds with a history of CVD and one-third with ≥1 CV risk factor), compared treatment with the ACE inhibitor ramipril with placebo. By 3 years, a relative risk reduction of approximately 25% was seen in the ramipril group for the primary composite endpoint of CV death, MI, or stroke (CitationHOPE 2000).
An analogy has been made between PROactive and the Steno-2 study, which had an almost identical primary endpoint (CitationGaede et al 2006). Steno-2 showed a 20% absolute risk reduction and a 53% relative risk reduction after intensified multifactorial intervention compared with standard multifactorial intervention (CitationGaede et al 2003, Citation2006). However, this was a comparatively small study (n = 180), with an 8-year follow-up in patients with microalbuminuria, the majority of whom had no pre-existing major CVD. Furthermore, significantly fewer patients in the control group received statins (n = 57 vs 14), antihypertensives, or antiplatelet therapy, whereas in PROactive, patients in both groups were receiving comparable multifactorial interventions at baseline and at study end (statins, fibrates, antihypertensives, and antiplatelet therapy). Any effect of pioglitazone should therefore be considered as additional to these interventions. Use of antihypertensives and antiplatelet therapy was similar between PROactive and the intensive group in Steno-2 (although statin use was lower in PROactive (43% increasing to 55% at study end)).
Conclusion
What are the implications of PROactive?
PROactive has shown that pioglitazone can significantly reduce the risk of secondary macrovascular events in a very high-risk patient population with established macrovascular disease, the majority of whom were receiving optimal treatment of established CV risk factors. As this patient population carries an excess risk, even with attention to established risk factors, pioglitazone may therefore provide an additional option to reduce residual events further as part of a multifactorial intervention strategy.
What questions remain unanswered after PROactive?
Several outcomes studies with thiazolidinediones are currently underway including IRIS (Insulin Resistance Intervention After Stroke) (http://iristrial.org) and RECORD (Rosiglitazone Evaluated of Cardiac Outcomes and Regulation of Glycemia in Diabetes) that aim to evaluate the long-term impact of these effects on CV outcomes and on long-term glycemic control in people with type 2 diabetes (CitationHome et al 2005) and BARI 2D (Bypass Angioplasty Revascularization Investigation in Type 2 Diabetics; CitationSobel et al 2003). The results of the Diabetes Reduction Approaches with ramipril and rosiglitazone Medications (DREAM) study were presented at the 2006 European Association for the Study of Diabetes (EASD) meeting and have been published in The Lancet (CitationDREAM Trial Investigators 2006). In the enrolled population of 5,269 people with impaired glucose tolerance and/or impaired fasting glucose, rosiglitazone did not reduce all-cause mortality within 3 years of treatment (30 patients [1.1%] in the rosiglitazone group and 33 [1.3%] in the placebo group died). There was a trend for a higher number of events from the CV composite in the rosiglitazone group (75 [2.9%] events vs 55 [2.1%] in the placebo group; HR 1.37; 95% CI 0.97, 1.94; p = 0.08) that was driven by a higher rate of heart failure in the rosiglitazone group (0.5%; n = 14) than in the placebo group (0.1%, n = 2; HR 7.03; 95% CI 1.60, 30.9; p = 0.01). There were no cases of fatal heart failure during the study. It should be noted, however, that DREAM was a trial designed for primary prevention of manifest type 2 diabetes in patients with the metabolic syndrome and as such targeted towards glycemia outcomes and not towards prevention of CV risk.
PROactive has presented a range of questions that will hopefully be clarified with further analyses and through the results of these ongoing outcomes studies. For instance, it is unclear which effects of pioglitazone underlie its antiatherogenic effects – glucose-lowering, lipid regulation (effects on HDL-cholesterol, triglycerides, or LDL particle size), other pleiotropic factors (effects on inflammatory mediators, such as CRP), or a combination of factors. It is also unclear whether these results can be extrapolated to thiazolidinediones in general, considering some of their mechanistic distinctions, such as differing effects on lipid profiles (CitationGoldberg et al 2005). Furthermore, it is not known whether a longer duration study would have resulted in a significant impact on the primary endpoint, or how effective pioglitazone would be in these patients if attention to antiplatelet therapy, blood pressure therapy, and, in particular, lipid-modifying therapy had been fully optimized. PROactive was a secondary prevention study, and we do not know if these results can be extrapolated to the wider population of patients with type 2 diabetes (such as in primary prevention), or indeed to those with prediabetes or high-risk patients without diabetes. Finally, the real impact (if any) of pioglitazone on heart failure is unclear at present.
References
- BhattDLStegPGOhmanEMREACH Registry InvestigatorsInternational prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosisJAMA2006295180916403930
- BrownLCJohnsonJAMajumdarSREvidence of suboptimal management of cardiovascular risk in patients with type 2 diabetes mellitus and symptomatic atherosclerosisCMAJ200417111899215534311
- BuseJBTanMHPrinceMJThe effects of oral anti-hyperglycaemic medications on serum lipid profiles in patients with type 2 diabetesDiabetes Obes Metab200461335614746579
- CharbonnelBDormandyJErdmannEPROactive Study GroupThe prospective pioglitazone clinical trial in macrovascular events (PROactive): can pioglitazone reduce cardiovascular events in diabetes? Study design and baseline characteristics of 5238 patientsDiabetes Care20042716475315220241
- CharbonnelBScheenAPioglitazone in triple oral therapy: long-term glycaemic results from PROactive [abstract]Diabetes200655Suppl 1A106
- CharbonnelBHMatthewsDRSchernthanerGQUARTET Study GroupA long-term comparison of pioglitazone and gliclazide in patients with Type 2 diabetes mellitus: a randomized, double-blind, parallel-group comparison trialDiabet Med2005a2239940515787663
- CharbonnelBSchernthanerGBrunettiPLong-term efficacy and tolerability of add-on pioglitazone therapy to failing monotherapy compared with addition of gliclazide or metformin in patients with type 2 diabetesDiabetologia2005b48109310415889234
- ChiassonJLJosseRGGomisRSTOP-NIDDM Trial Research GroupAcarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trialJAMA20032904869412876091
- ChoiDKimSKChoiSHPreventative effects of rosiglitazone on restenosis after coronary stent implantation in patients with type 2 diabetesDiabetes Care20042726546015505001
- ColhounHMBetteridgeDJDurringtonPNCARDS investigatorsPrimary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomized placebo-controlled trialLancet20043646859615325833
- The Diabetes Control and Complications Trial Research GroupThe effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitusN Engl J Med1993329977868366922
- DerosaGCiceroAFDangeloAThiazolidinedione effects on blood pressure in diabetic patients with metabolic syndrome treated with glimepirideHypertens Res2005a289172416555581
- DerosaGCiceroAFGaddiAA comparison of the effects of pioglitazone and rosiglitazone combined with glimepiride on prothrombotic state in type 2 diabetic patients with the metabolic syndromeDiabetes Res Clin Pract2005b6951315955382
- DormandyJon behalf of the PROactive Writing CommitteePROactive study [letter]Lancet2006367267
- DormandyJACharbonnelBEcklandDJPROactive investigatorsSecondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trialLancet200536612798916214598
- The DREAM (Diabetes Reduction Assessment with ramipril and rosiglitazone Medication) Trial InvestigatorsEffect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomized controlled trialLancet2006368109610516997664
- EmbersonJRWhincupPHLawlorDACoronary heart disease prevention in clinical practice: are patients with diabetes special? Evidence from two studies of older men and womenHeart200591451515772196
- ErdmannEDormandyJACharbonnelBon behalf of the PROactive investigatorsThe effect of pioglitazone on recurrent myocardial infarction in 2445 patients with type 2 diabetes and previous myocardial infarction – Results from PROactive (PROactive 05)J Am Coll Cardiol20074917728017466227
- ErdmannEDormandyJAKupferSMorbidity after reports of serious heart failure in type 2 diabetes patients with underlying cardiovascular disease: results from PROactive [abstract]Circulation2006114Suppl 11848
- European Diabetes Policy Group. 1998–1999A Desktop Guide to Type 2 Diabetes Mellitus Accessed 17 September 2006. URL: http://www.staff.ncl.ac.uk/philip.home/t2dgw97r.doc
- GaedePParvingHHPedersenOPROactive studyLancet200636723416399142
- GaedePVedelPLarsenNMultifactorial intervention and cardiovascular disease in patients with type 2 diabetesN Engl J Med20033483839312556541
- GoldbergRBKendallDMDeegMAGLAI Study InvestigatorsA comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemiaDiabetes Care20052815475415983299
- GoldbergRBMelliesMJSacksFMfor the Care InvestigatorsCardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels. Subgroup analyses in the cholesterol and recurrent events (CARE) trialCirculation199898251399843456
- HaffnerSMLehtoSRönnemaaTMortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarctionN Engl J Med1998339229349673301
- HanefeldMBrunettiPSchernthanerGHQUARTET Study GroupOne-year glycemic control with a sulfonylurea plus pioglitazone versus a sulfonylurea plus metformin in patients with type 2 diabetesDiabetes Care200427141714693980
- HanssonLZanchettiACarruthersSGfor the HOT Study GroupEffects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study GroupLancet19983511755629635947
- Heart Outcomes Prevention Evaluation (HOPE) Study InvestigatorsEffects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudyLancet2000355253910675071
- Heart Protection Study Collaborative GroupMRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trialLancet200336120051612814710
- HeineRSchindhelmRDiamantMLong-term pioglitazone treatment improves markers of liver function: results from PROactive [Abstract]Diabetes200655Suppl 1A115
- HollenbergNKConsiderations for management of fluid dynamic issues associated with thiazolidinedionesAm J Med2003115Suppl 8A111S5S14678876
- HomePDPocockSJBeck-NielsenHRosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD): study design and protocolDiabetologia20054817263516025252
- HorioTSuzukiMSuzukiKPioglitazone improves left ventricular diastolic function in patients with essential hypertensionAm J Hypertens2005189495716053992
- IdrisIThomsonGASharmaJCDiabetes mellitus and strokeInt J Clin Pract200660485616409428
- JuutilainenALehtoSRönnemaaTType 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjectsDiabetes Care2005282901716306552
- KarterAJAhmedATLiuJPioglitazone initiation and subsequent hospitalization for congestive heart failureDiabet Med2005229869316026362
- KeechASimesRJBarterPFIELD study investigatorsEffects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trialLancet200536618496116310551
- KoDTMamdaniMAlterDALipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradoxJAMA200429118647015100205
- LangenfeldMRForstTHohbergCPioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus: results from a controlled randomized studyCirculation200511125253115883215
- LawrenceJMReidJTaylorGJFavorable effects of pioglitazone and metformin compared with gliclazide on lipoprotein subfractions in overweight patients with early type 2 diabetesDiabetes Care20042741614693964
- The Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study GroupPrevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levelsN Engl J Med19983391349579841303
- MarxNWohrleJNusserTPioglitazone reduces neointima volume after coronary stent implantation: a randomized, placebo-controlled, double-blind trial in nondiabetic patientsCirculation20051122792816246947
- Massi-BenedettiMScheenACharbonnelBPioglitazone delays the need for permanent insulin use: results from PROactive [abstract]Diabetes200655Suppl 1A124
- MeinertCLKnatterudGLProutTEA study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality resultsDiabetes197019Suppl7898304926376
- MiettinenHLehtoSSalomaaVImpact of diabetes on mortality after the first myocardial infarction. The FINMONICA Myocardial Infarction Register Study GroupDiabetes Care19982169759538972
- MiyazakiYMahankaliAMatsudaMEffect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patientsJ Clin Endocrinol Metab20028727849112050251
- MiyazakiYMahankaliAWajcbergEEffect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patientsJ Clin Endocrinol Metab2004894312915356026
- MukamalKJNestoRWCohenMCImpact of diabetes on long-term survival after acute myocardial infarction: comparability of risk with prior myocardial infarctionDiabetes Care2001241422711473080
- NathanDMClearyPABacklundJYDiabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research GroupIntensive diabetes treatment and cardiovascular disease in patients with type 1 diabetesN Engl J Med200535326435316371630
- NathanDMLachinJClearyPDiabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research GroupIntensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitusN Engl J Med2003348229430312788993
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III)Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final reportCirculation2002106314342112485966
- NigroJOsmanNDartAMInsulin resistance and atherosclerosisEndocr Rev2006272425916492903
- NikolaidisLALevineTBPeroxisome proliferator activator receptors (PPAR), insulin resistance, and cardiomyopathy: friends or foes for the diabetic patient with heart failure?Cardiol Rev2004121587015078585
- PavoIJermendyGVarkonyiTTEffect of pioglitazone compared with metformin on glycemic control and indicators of insulin sensitivity in recently diagnosed patients with type 2 diabetesJ Clin Endocrinol Metab20038816374512679450
- PerezAKhanMJohnsonTPioglitazone plus a sulphonylurea or metformin is associated with increased lipoprotein particle size in patients with type 2 diabetesDiab Vasc Dis Res20041445016305056
- PfutznerAMarxNLubbenGImprovement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the pioneer studyJ Am Coll Cardiol20054519253115963388
- PyöräläKPedersenTRKjekshusJfor the Scandinavian Simvastatin Survival Study (4S) GroupCholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S)Diabetes Care199720614209096989
- RodenMLaaksoMJohnsDLong-term effects of pioglitazone and metformin on insulin sensitivity in patients with Type 2 diabetes mellitusDiabet Med2005221101616026380
- RoperNABilousRWKellyWFExcess mortality in a population with diabetes and the impact of material deprivation: longitudinal population based studyBMJ200132213899311397742
- RydénLThráinsdóttirISwedbergKAdjudication of serious heart failure in patients from PROactive [letter]Lancet200736818990
- SatohNOgawaYUsuiTAntiatherogenic effect of pioglitazone in type 2 diabetic patients irrespective of the responsiveness to its antidiabetic effectDiabetes Care2003262493912941708
- SchaumbergDAGlynnRJJenkinsAJEffect of intensive glycemic control on levels of markers of inflammation in type 1 diabetes mellitus in the diabetes control and complications trialCirculation200511124465315867184
- ScheenACharbonnelBReduced insulin requirements and improved glycaemic control with pioglitazone in insulin-treated patients with type 2 diabetes: results from PROactive [abstract]Diabetes200655Suppl 1A134
- SchernthanerGMatthewsDRCharbonnelBQuartet Study GroupEfficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trialJ Clin Endocrinol Metab20048960687615579760
- ShepherdJBlauwGJMurphyMBPROSPER study groupPROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trialLancet200236016233012457784
- ShiomiTTsutsuiHHayashidaniSPioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarctionCirculation200210631263212473562
- SobelBEFryeRDetreKMBypass Angioplasty Revascularization Investigation 2 Diabetes Trial. Burgeoning dilemmas in the management of diabetes and cardiovascular disease: rationale for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) TrialCirculation20031076364212566379
- StrattonIMAdlerAINeilAWon behalf of the UK Prospective Diabetes Study GroupAssociation of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational studyBMJ20003214051210938048
- St John SuttonMRendellMDandonaPA comparison of the effects of rosiglitazone and glyburide on cardiovascular function and glycemic control in patients with type 2 diabetesDiabetes Care20022520586412401757
- TakagiTAkasakaTYamamuroATroglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with non-insulin dependent diabetes mellitus: a serial intravascular ultrasound studyJ Am Coll Cardiol20003615293511079654
- TakagiTYamamuroATamitaKPioglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with type 2 diabetes mellitus: an intravascular ultrasound scanning studyAm Heart J2003146E512891212
- TanMHGlazerNBJohnsDJPioglitazone as monotherapy or in combination with sulfonylurea or metformin enhances insulin sensitivity (HOMA-S or QUICKI) in patients with type 2 diabetesCurr Med Res Opin2004a20723815140339
- TanMHJohnsDJGonzalez GalvezGGLAD Study GroupEffects of pioglitazone and glimepiride on glycemic control and insulin sensitivity in Mexican patients with type 2 diabetes mellitus: A multicenter, randomized, double-blind, parallel-group trialClin Ther2004b266809315220012
- TanMHJohnsDJStrandJGLAC Study GroupSustained effects of pioglitazone vs. glibenclamide on insulin sensitivity, glycaemic control, and lipid profiles in patients with Type 2 diabetesDiabet Med2004c218596615270789
- TaskinenMRDiabetic dyslipidaemia: from basic research to clinical practiceDiabetologia2003467334912774165
- UK Prospective Diabetes Study (UKPDS) GroupIntensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33)Lancet1998352837539742976
- UK Prospective Diabetes Study (UKPDS) GroupEffect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34)Lancet1998352854659742977
- UK Prospective Diabetes Study GroupTight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38BMJ1998317703139732337
- WilcoxRBousserM-GPiragVPROactive 04: Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke – results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular EventsStroke2007388657317290029
- WilcoxRKupferSEffects of pioglitazone on major adverse cardiovascular events (MACE) and myocardial infarction: results from PROactive [abstract]Diabetes200655Suppl 1A74
- WynneAMMocanuMMYellonDMPioglitazone mimics preconditioning in the isolated perfused rat heart: a role for the prosurvival kinases PI3K and P42/44MAPKJ Cardiovasc Pharmacol2005468172216306807
- Yki-JärvinenHThiazolidinedionesN Engl J Med200435111061815356308
- Yki-JärvinenHThe PROactive study: some answers, many questions [commentary]Lancet20053661241216214581
- ZuanettiGLatiniRMaggioniAPEffect of the ACE inhibitor lisinopril on mortality in diabetic patients with acute myocardial infarction. Data from the GISSI-3 studyCirculation1997964239459416888