Abstract
Background
Ambulatory blood pressure (BP) is more sensitive than office BP and is highly correlated with the left ventricular mass (LVM) of hypertensive patients with left ventricular hypertrophy (LVH).
Methods
In this prospectively designed ancillary study of the PICXEL trial, the effects of first-line combination perindopril/indapamide on ambulatory BP were compared with those of monotherapy with enalapril in 127 patients. Hypertensive patients with LVH received once daily either perindopril 2 mg/indapamide 0.625 mg (n = 65) or enalapril 10 mg (n = 62) for 52 weeks. Dose adjustments were allowed for uncontrolled BP. Twenty-four-hour ambulatory BP and echocardiographic parameters were measured at baseline, week 24, and week 52.
Results
At study end, both treatments significantly improved ambulatory BP compared with baseline (p ≤ 0.01). Perindopril/indapamide treatment reduced 24-hour and daytime systolic BP (SBP) and pulse pressure (PP) significantly more than enalapril treatment (p < 0.01). No significant between-group differences were noted for diastolic BP (DBP) or for night-time measurements. Trough/peak ratios were higher with perindopril/indapamide than with enalapril (88.5 vs 65.8 for SBP and 86.7 vs 63.9 for DBP, respectively). The global smoothness index was higher with perindopril/indapamide than with enalapril (6.6 vs 5.2 for SBP and 5.6 vs 4.9 for DBP, respectively). With perindopril/indapamide treatment, LVM index was significantly reduced (−9.1 g/m2 from baseline; p vs baseline <0.001). More patients required dose increases with enalapril (87%) than with perindopril/indapamide (71%). No unusual safety elements were noted.
Conclusions
First-line perindopril/indapamide combination decreased ambulatory SBP and PP, and LVM more effectively than enalapril.
Introduction
Left ventricular hypertrophy (LVH) and organ damage resulting from hypertension can place patients at risk for cardiovascular events, such as myocardial infarction, heart failure, and stroke (CitationCasale et al 1986; CitationKoren et al 1991; CitationCiardullo et al 2004). Daily blood pressure variations, calculated from ambulatory blood pressure recordings, are increasingly being considered as significant contributors to such end organ damage and cardiovascular risk (CitationFrattola et al 1993; CitationKikuya et al 2000; CitationParati 2005).
In a study of 1542 subjects, a significant linear relation was noted between cardiovascular mortality risk and daytime systolic blood pressure (SBP) variations (CitationKikuya et al 2000). In addition, in several studies, 24-hour ambulatory BP measurements were more sensitive predictors of cardiovascular morbidity and mortality than conventional office BP measurements (CitationOhkubo et al 1997; CitationVerdecchia et al 1998; CitationStaessen et al 1999). Thus, regression of LVH, which has been associated with improved cardiovascular prognosis (CitationAnon. 2004), may be more closely correlated with reductions in ambulatory BP than with reductions in office blood pressure (CitationOmboni et al 1998).
The renin-angiotensin-aldosterone system (RAAS) is implicated in the development of hypertension and LVH (CitationMatsumura et al 2006). Consistent with this understanding, meta-analyses have found that RAAS inhibitors are more effective than other types of treatment in reducing left ventricular mass (LVM) ((CitationDahlöf et al 1992; CitationSchmieder et al 1996; CitationKlingbeil et al 2003). As diuretics have been shown to reduce ventricular diameter and angiotensin-converting enzyme (ACE) inhibitors to reduce wall thickness, an ACE inhibitor combined with a diuretic may be more effective than an ACE inhibitor alone (CitationDahlöf et al 1992). Indeed, first-line combination perindopril/indapamide has been shown in the 52-week PICXEL study to be more effective in reducing LVM and office BP in patients with LVH than treatment with ACE inhibitor monotherapy enalapril (CitationDahlöf et al 2005).
Perindopril/indapamide treatment has also been shown to reduce 24-hour ambulatory BP and smooth BP profiles in patients with uncomplicated essential hypertension (CitationMallion et al 2004). Consequently, to investigate further the effect of perindopril/indapamide on 24-hour BP variations in patients with LVH and to assess further the differences between first-line combination therapy perindopril/indapamide and monotherapy enalapril, ambulatory BP was monitored in a subset of patients enrolled in the PICXEL study.
Methods
This study is an ancillary study of the previously published, randomized, double-blind, parallel-group, PICXEL study performed from June 1999 to March 2002 (CitationGosse et al 2002; CitationDahlöf et al 2005). This analysis will focus on materials and methods pertinent to this substudy, which was prospectively designed to measure ambulatory BP.
Patient population
Patients enrolled in the PICXEL study (n = 679) were given the option to participate concomitantly in this ancillary study. No additional or specific entry criteria were specified. The main inclusion criteria for PICXEL were essential systolic and diastolic or isolated systolic hypertension (140 mmHg ≤ sitting SBP <210 mmHG) and LVM index ([LVMI] >120 g/m2 for men and >100 g/m2 for women). The main exclusion criteria were severe hypertension (diastolic BP [DBP] ≥115 mmHg and/or SBP ≥210 mmHg), and secondary or complicated hypertension (with the exception of LVH).
Study design
As in PICXEL, after a 4-week placebo run-in period, patients received either combination perindopril 2 mg/indapamide 0.625 mg or enalapril 10 mg once a day for 52 weeks (CitationGosse et al 2002; CitationDahlöf et al 2005). Dose adjustments to combination perindopril 4 mg/indapamide 1.25 mg or enalapril 20 mg and then to combination perindopril 8 mg/indapamide 2.5 mg or enalapril 40 mg were requested if BP was not controlled. Adjustments were based on office BP measurements and specific predefined criteria (CitationGosse et al 2002; CitationDahlöf et al 2005). Adjustments took place at office visits at weeks 6, 12, 24, and 36. Treatment compliance was assessed during the double-blind treatment period by counting returned unused capsules.
Ambulatory BP
Patients were fitted between 8 AM and 11 AM with a non-invasive automated blood pressure recorder at baseline (week 0), week 24, and study end (week 52). Use of a recorder that met the validation requirements of the British Hypertension Society and/or American Association for the Advancement of Medical Instrumentation was recommended. Drug intake took place within 30 minutes after fitting. Recordings were performed every 15 minutes during the daytime (7 AM to 10 PM), every 30 min during the night-time (10 PM to 7 AM), and for at least 25 hours.
To ensure data quality, all ambulatory BP recordings were blindly reviewed by a central ambulatory BP monitoring (ABPM) committee. The prospectively established validation criteria were: ambulatory BP needed to have been recorded for ≥25 hours after dosing; ≥75% of each recording was eligible for analysis; no two consecutive hourly average blood pressure values were missing; both 2nd to 8th and 23rd to 24th averages were present; ≥2 valid measurements per hour over the daytime period and 1 per hour in the night-time period were available; a baseline recording and at least 1 post-baseline recording was available. The following artifacts were systematically excluded: any DBP > SBP; SBP <60 mmHg; SBP >250 mmHg; DBP <40 mmHg; DBP >150 mmHg; a blood pressure differential (SBP – DBP) <10 mmHg with a SBP >110 mmHg. Non-valid recordings could be repeated within a week.
Daytime, night-time, and 24-hour means were calculated for ambulatory SBP, DBP, and PP. Pulse pressure was defined as SBP – DBP. Two-hourly means, global trough/peak ratio, and global smoothness index were also calculated. The trough (minimum treatment effect/highest BP) was defined as the 12th 2-hourly mean (just before the next dose). The peak (maximum treatment effect/lowest BP) was defined as the lowest reading from the 2nd to the 5th 2-hourly mean. The smoothness index was defined as the mean of the hourly mean changes from baseline to study end divided by its standard deviation.
Other variables
Echocardiography was performed at week −4, week 0, week 24, and week 52 (Wend). Patients were examined in the left lateral supine position after 15 minutes of rest. Left ventricular mass, end-diastolic left ventricular internal diameter (LVIDd), end-diastolic left ventricular posterior wall thickness (PWTd), and end-diastolic interventricular septal wall thickness (IVSTd) were measured. Echocardiograms were read by the Central Echocardiography Committee using a Iô 3.2 unit (IôDP, Paris, France). Data presented are the results from the final central blinded reading.
Office SBP and DBP were measured by mercury sphygmomanometer at each visit according to the European Society of Hypertension guidelines.
Safety
The safety assessment was based on the incidence of adverse events among all the patients participating in the PICXEL study.
Statistics
This ancillary study was exploratory; no sample size estimate was performed. The efficacy population included all randomized patients having taken at least one dose of study treatment, with a valid ambulatory reading at baseline and at least one valid ambulatory reading post-baseline.
Descriptive statistics were provided for all criteria. Effects of treatment with perindopril/indapamide vs enalapril on ambulatory parameters and sphygmomanometer BPs were compared using an analysis of covariance (α = 2.5% with a 95% confidence interval [CI]). Analyses were adjusted for the baseline value and used the last observation post-baseline. The significance of the changes from baseline in ambulatory parameters and sphygmomanometer BPs was tested in each group, without adjustment, using the last observation post-baseline and a one tailed Student’s t-test for dependent samples (α = 2.5% with a 95% CI).
The between-group differences perindopril/indapamide vs enalapril on LVMI were compared after adjustment for baseline value and gender using an analysis of covariance (α = 5% with a 95% CI). The within-group changes from baseline in LVMI were tested, without adjustment, using the last observation post-baseline and a one-tailed Student’s t-test for dependent samples (α = 2.5% with a 95% CI). Complementary analyses were performed to assess between-group differences in LVMI at baseline (using a two-sided Student’s t-test; α = 5%) and to compare dose increases between groups (using a Chi-square test).
The multiple linear regression was performed using a stepwise selection method.
The statistical analysis was performed using SAS version 8.2 and Statgraphics version 5.0.
Ethics
The study was conducted in accordance with the ethical principles stated in the 1964 Declaration of Helsinki and revised in Somerset West in 1996 and in Edinburgh in 2000. The protocol was approved by the independent Ethics Committees of each country. All the patients gave written informed consent.
Results
One hundred and forty-six patients from a total of 28 centers in 7 countries participated in this ancillary study. The efficacy population of 127 subjects was defined as all randomized patients who took as least one dose of studied treatment with a valid ABPM at M0 performed before M0 visit and at least one valid ABPM post-baseline. The efficacy population was made up of 65 perindopril/indapamide-treated patients and 62 enalapril-treated patients. Study groups were well balanced in terms of demographics and baseline characteristics (). Patients were on average 55 ± 9 years of age; 11% presented with a complication other than LVH such as retinopathy or hypertensive encephalopathy, 68% had a family history of hypertension; and most patients (88.2%) had received at least one previous treatment for hypertension. No clinically relevant differences were observed between groups for baseline office and ambulatory BP measurements and for baseline LVMI (). There were significant relationships between baseline LVMI and ambulatory SBP, DBP, and PP (correlation coefficients from 0.20 to 0.30, p < 0.05). Relationships between baseline LVMI and office SBP and PP were non-significant (correlation coefficients less than 0.12, NS) and significant only for office DBP (correlation coefficient 0.20, p < 0.05). Multiple linear regression shows that factors with an impact on baseline LVMI are sex (p < 0.0001), nocturnal mean SBP (p = 0.0018) and BMI (p = 0.1190) (R2 = 0.23, p < 0.0001) ().
Table 1 Demographics, baseline blood pressure measurementsTable Footnotea and echocardiographic parametersTable Footnoteb
Table 2 Influencing factors of left ventricular mass index at baseline
Patients were exposed to treatment for a mean of 365 ± 19 days and 360 ± 44 days in the perindopril/indapamide and enalapril groups, respectively. Overall compliance was 99.8 ± 3.4% and 100.5 ± 3.3% in the perindopril/indapamide and enalapril groups, respectively.
Dose increases were required significantly more frequently in the enalapril group than in the perindopril/indapamide group at week 24 (80.6% vs 61.6%, respectively; p = 0.03). At week 52, the same tendency was observed but the difference was not statistically significant (87.1% vs 70.8%, respectively; p = 0.08).
Efficacy
At study end, treatment of both groups with perindopril/indapamide and enalapril significantly reduced office () and ambulatory SBP, DBP, and PP measured over 24 hours, during the daytime, and during the night-time (p vs baseline ≤0.01; ).
Figure 1 Changes in ambulatory blood pressure after 52 weeks of treatment with perindopril/indapamide (n = 65) or enalapril (n = 62). A. Over 24 hours; B. Daytime; C. Night-time. Mean changes from baseline and standard deviations in parentheses are presented. *p vs baseline ≤0.01; **p vs baseline ≤0.001, †p vs enalapril <0.01.
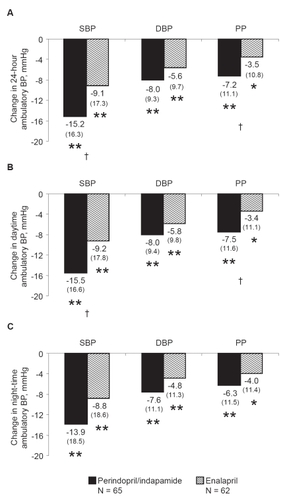
Table 3 Change in office blood pressure between baseline and study end
Decreases in 24-hour and daytime ambulatory SBP and PP were significantly greater in the perindopril/indapamide group than in the enalapril group. Between-group differences of 6.1 ± 2.4 mmHg and 4.1 ± 1.6 mmHg were noted for 24-hour SBP and PP, respectively (p vs enalapril <0.01; ). Between-group differences of 6.0 ± 2.5 mmHg and 4.3 ± 1.6 mmHg were noted for daytime SBP and PP, respectively (p vs enalapril <0.01; ). Decreases in night-time ambulatory SBP and PP were greater in the perindopril/indapamide group than in the enalapril group, but differences were not statistically significant (). Similar results were recorded for 24-hour SBP measurements at week 24 (data not shown).
There was a tendency towards greater decreases in ambulatory DBP in the perindopril/indapamide group than in the enalapril group. These differences, however, were not significant (between-group differences of 2.1 ± 1.5 mmHg, 1.8 ± 1.5 mmHg, and 2.2 ± 1.7 for 24-hour, daytime, and night-time measurements, respectively; ). Similar results were recorded for 24-hour measurements at week 24 (data not shown).
At baseline, circadian variations of SBP, DBP, and PP, averaged every 2 hours over the 24-hour period, were similar for both groups. At study end, the circadian variation chronograms of SBP, DBP, and PP were lower in the perindopril/indapamide group than in the enalapril group throughout the 24-hour period ().
Figure 2 Variations in blood pressure over 24 hours in perindopril/indapamide (n = 65) and enalapril (n = 62). A. SBP; B. DBP; C. PP. Mean baseline and end-of-study ambulatory blood pressure calculated every 2 hours are plotted.
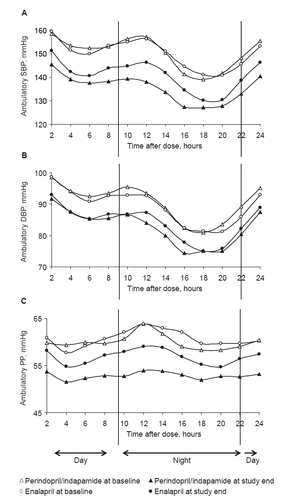
For SBP and DBP, the global trough/peak ratio tended to be higher after perindopril/indapamide treatment than after enalapril treatment (88.5 vs 65.8 for SBP and 86.7 vs 63.9 for DBP). The absolute value of the global smoothness index tended to be higher after perindopril/indapamide treatment than after enalapril treatment (6.6 vs 5.2 for SBP and 5.6 vs 4.9 for DBP respectively; ).
Table 4 Global trough/peak ratio and smoothness index at study end
At study end, mean LVMI significantly decreased compared to baseline after perindopril/indapamide treatment (−9.1 ± 21.6 g/m2; p vs baseline <0.001), but not after enalapril treatment (+1.8 ± 24.8 g/m2; p vs baseline = 0.7). The adjusted difference between groups was significant (p vs enalapril = 0.004). Slight reductions in LVIDd, PWTd, and IVSTd were also observed in the perindopril/indapamide group, whereas slight increases were noted in the enalapril group (data not shown). The correlations between changes (end – baseline) in ambulatory blood pressure and in LVMI (over 24 hours, daytime, night-time) were significant in the enalapril group (correlation coefficients from 0.30 to 0.40, p < 0.05) and non significant in the perindopril/indapamide group (correlation coefficients less than 0.07, NS; and ). Multiple linear regression shows that the factors with an impact on LVMI change during the follow up of this study are baseline LVMI (p < 0.0001) and treatment group (p = 0.0046), (R2 = 0.17; p < 0.0001) ().
Figure 3 Relationship between mean systolic blood pressure over 24 hours (mmHg) change (Wend − W0) and left ventricular mass index change in enalapril group (n = 62). ρ: Pearson correlation coefficient.
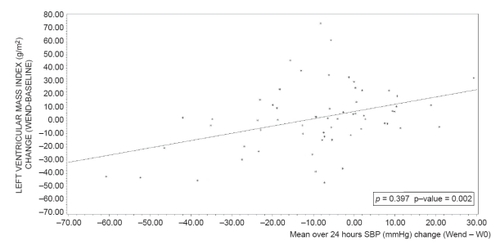
Figure 4 Relationship between mean SBP over 24 hours (mmHg) change (Wend − W0) and left ventricular mass index change (Wend − baseline) in perindopril/indapamide group (n = 65). ρ: Pearson correlation coefficient.
Regression equation: LVMI change (WEND-Baseline) (ENA) = 6.8649 + 0.551855 *SBPMEAN24_CHANGE AT WEND
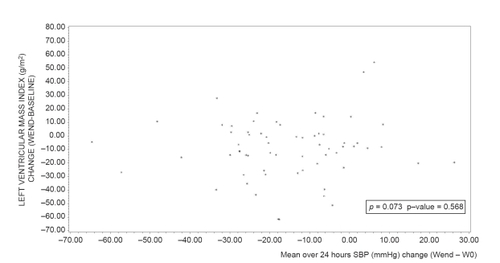
Table 5 Influencing factors of left ventricular mass index (LVMI) change
Safety
Safety in this substudy was similar to that of the main PICXEL study (CitationDahlöf et al 2005). In the main PICXEL study (679 patients) adverse events related to treatment occurred in 17.3% of the perindopril/indapamide group and in 15.7% of the enalapril group.
Discussion
In this 1-year study of hypertensive patients with LVH, treatment with the first-line combination perindopril/indapamide was significantly more effective in reducing ambulatory SBP and PP over 24 hours and during the daytime than treatment with enalapril. These data, which were, overall, consistent with those recorded in the ambulatory BP REASON substudy (CitationMallion et al 2004), suggest that perindopril/indapamide has a sustained, antihypertensive effect that attenuates blood pressure over a full 24 hours. In addition, the data suggesting a decrease in LVH, as measured by LVMI, are consistent with those recorded in the main PICXEL and in the REASON echocardiography study (CitationDe Luca et al 2004; CitationDahlöf et al 2005).
Twenty-four-hour monitoring provides a snapshot of the effect of treatment on BP variations over the course of a day. Several studies have suggested that these fluctuations contribute significantly to cardiovascular morbidity and mortality (CitationFrattola et al 1993; CitationKikuya et al 2000). A treatment that reduces the difference between BP troughs and peaks is likely to improve cardiovascular outcomes (CitationParati 2005). A high smoothness index (≥1), for example, has been correlated with positive changes in carotid artery wall thickness (CitationRizzoni et al 2001). Results of this study showed that perindopril/indapamide treatment reduced BP peaks and troughs and smoothed the BP curve (smoothness index ≥5) compared with baseline. Although this study was not designed to assess cardiovascular outcomes, the decrease in LVMI suggests an improvement in cardiovascular health.
Increasing evidence suggests that office and ambulatory PP and SBP are independent predictors of cardiovascular disease (CitationVerdecchia et al 1998; CitationFranklin et al 1999; CitationMillar et al 1999; CitationStaessen et al 1999). This understanding of hypertension together with the data presented here suggest that perindopril/indapamide, by its greater control of SBP and PP (ambulatory and office), may reduce cardiovascular risk more significantly than enalapril.
In this study, not only was first-line combination therapy with perindopril/indapamide more effective than monotherapy with enalapril, but fewer perindopril/indapamide-treated patients required dose increases. These data are consistent with the fact that monotherapies are often less effective than combination therapies in achieving BP control (CitationDickerson et al 1999; CitationHansson et al 1998). Enalapril, as monotherapy, does not target as many pathways and feedback mechanisms as ACE inhibitor/diuretic combination therapy with perindopril/indapamide. In comparative clinical trials, the combination perindopril/indapamide was more effective than atenolol, losartan, amlodipine, or enalapril monotherapy (CitationChanudet and de Champvallins 2001, CitationMogensen et al 2003; CitationDe Luca et al 2004; CitationMallion et al 2004; CitationMourad et al 2004; CitationDahlöf et al 2005).
In this ancillary study, patients were comparably distributed among treatment groups and baseline characteristics were similar. The small variations noted between groups were neither statistically significant nor considered clinically relevant. The difference between the ambulatory and office BP measurements can be explained by the subjective nature and the observed bias of office measurements which tend to be higher (CitationBobrie et al 2005). Lastly, with the exception of LVMI, which was lower in this ancillary study, baseline characteristics were similar to those in the overall PICXEL study (CitationDahlöf et al 2005).
Because the correlations between changes in ambulatory BP and in LVMI were significant in the enalapril group and non-significant in the perindopril/indapamide group, the effect of perindopril/indapamide on LVMI seems to be not only pressure dependent. Different mechanisms can be postulated to explain the changes relatively independent from BP reduction in the perindopril/indapamide group. These mechanisms include the LV functional and structural improvement with perindopril (CitationGrandi et al 1995). Another mechanism involving indapamide has been discussed in the main PICXEL study (CitationDahlöf et al 2005).
Conclusions
These data suggest that perindopril/indapamide has a sustained antihypertensive effect that decreases 24-hour and daytime SBP and PP as well as decreasing LVMI more effectively than enalapril. The results from this trial contribute to the growing pool of evidence that treatment with the combination perindopril/indapamide has significant beneficial effects on the cardiovascular health of a wide range of hypertensive patients including elderly and diabetic patients and patients with LVH (CitationChalmers et al 2000; CitationMogensen et al 2003; CitationDe Luca et al 2004; CitationDahlöf et al 2005).
Acknowledgements
The authors would like to thank all the investigators who participated in this ancillary study. The study was funded by I.R.I.S. (Courbevoie, France).
References
- Meta-analysis suggests regression of left ventricular hypertrophy during antihypertensive treatment is linked to reduced risk of cardiovascular diseaseEvid Based Cardiovasc Med2004822316379885
- BobrieGDeloncaJMoulinCA home blood pressure monitoring study comparing the antihypertensive efficacy of two angiotensin II receptor antagonist fixed combinationsAm J Hypertens2005181482816280286
- CasalePNDevereuxRBMilnerMValue of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive menAnn Intern Med198610517382942070
- ChalmersJCastaigneAMorganTLong-term efficacy of a new, fixed, very-low-dose angiotensin-converting enzyme-inhibitor/diuretic combination as first-line therapy in elderly hypertensive patientsJ Hypertens2000183273710726720
- ChanudetXde ChampvallinsMAntihypertensive efficacy and tolerability of low-dose perindopril/indapamide combination compared with losartan in the treatment of essential hypertensionInt J Clin Pract200155233911406907
- CiardulloAVAzzoliniLBeviniMA diagnosis of left ventricular hypertrophy on ECG is associated with a high cardiovascular risk: findings from a 40- to 69-year-old cohort in general practiceFam Pract20042163514760047
- DahlöfBGossePGuéretPPerindopril/indapamide combination more effective than enalapril in reducing blood pressure and left ventricular mass: the PICXEL studyJ Hypertens20052320637016208150
- DahlöfBPennertKHanssonLReversal of left ventricular hypertrophy in hypertensive patients. A metaanalysis of 109 treatment studiesAm J Hypertens19925951101532319
- de LucaNMallionJMO’RourkeMFRegression of left ventricular mass in hypertensive patients treated with perindopril/indapamide as a first-line combination: the REASON echocardiography studyAm J Hypertens200417660715323062
- DickersonJEHingoraniADAshbyMJOptimisation of antihypertensive treatment by crossover rotation of four major classesLancet199935320081310376615
- FranklinSSKhanSAWongNDIs pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart studyCirculation19991003546010421594
- FrattolaAParatiGCuspidiCPrognostic value of 24-hour blood pressure variabilityJ Hypertens199311113378258679
- GossePDubourgOGuéretPEfficacy of very low dose perindopril 2 mg/indapamide 0.625 mg combination on left ventricular hypertrophy in hypertensive patients: the P.I.C.X.E.L. study rationale and designJ Hum Hypertens200216653912214263
- GrandiAMBignottiMGaudioGAmbulatory blood pressure and left ventricular changes during antihypertensive treatment: Perindopril versus IsradipineJ Cardiovasc Pharmacol199526737418637188
- HanssonLZanchettiACarruthersSGEffects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study GroupLancet19983511755629635947
- KikuyaMHozawaAOhokuboTPrognostic significance of blood pressure and heart rate variabilities: the Ohasama studyHypertension200036901611082164
- KlingbeilAUSchneiderMMartusPA meta-analysis of the effects of treatment on left ventricular mass in essential hypertensionAm J Med200311541612867233
- KorenMJDevereuxRBCasalePNRelation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertensionAnn Intern Med1991114345521825164
- MallionJMChamontinBAsmarRTwenty-four-hour ambulatory blood pressure monitoring efficacy of perindopril/indapamide first-line combination in hypertensive patients: the REASON studyAm J Hypertens2004172455115001199
- MatsumuraKFujiiKOnikiHRole of aldosterone in left ventricular hypertrophy in hypertensionAm J Hypertens200619131816461184
- MillarJALeverAFBurkeVPulse pressure as a risk factor for cardiovascular events in the MRC Mild Hypertension TrialJ Hypertens19991710657210466460
- MogensenCEVibertiGHalimiSEffect of low-dose perindopril/indapamide on albuminuria in diabetes: preterax in albuminuria regression: PREMIERHypertension20034110637112654706
- MouradJJWaeberBZannadFComparison of different therapeutic strategies in hypertension: a low-dose combination of perindopril/indapamide versus a sequential monotherapy or a stepped-care approachJ Hypertens20042223798615614033
- OhkuboTImaiYTsujiIPrediction of mortality by ambulatory blood pressure monitoring versus screening blood pressure measurements: a pilot study in OhasamaJ Hypertens199715357649211170
- OmboniSFogariRPalatiniPReproducibility and clinical value of the trough-to-peak ratio of the antihypertensive effect: evidence from the sample studyHypertension19983242499740606
- ParatiGBlood pressure variability: its measurement and significance in hypertensionJ Hypertens200523SupplS1925
- RizzoniDMuiesanMLSalvettiMThe smoothness index, but not the trough-to-peak ratio predicts changes in carotid artery wall thickness during antihypertensive treatmentJ Hypertens2001197031111330873
- SchmiederREMartusPKlingbeilAReversal of left ventricular hypertrophy in essential hypertension. A meta-analysis of randomized double-blind studiesJAMA19962751507138622227
- StaessenJAThijsLFagardRPredicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial InvestigatorsJAMA19992825394610450715
- VerdecchiaPSchillaciGBorgioniCAmbulatory pulse pressure: a potent predictor of total cardiovascular risk in hypertensionHypertension19983298389856961