Abstract
The degree of arterial stiffness is correlated with the risk of cardiovascular diseases and it is a powerful predictor for morbidity and mortality. Studies have shown that arterial stiffness reduction is associated with an improvement in survival. Reduction of arterial stiffness by pharmacological drugs varies according to the drugs and doses used and duration of treatment. This effect on the arteries differs among the various classes of drugs and among individual drugs in the same class. Quantification of the stiffness and other properties of the arterial wall can be used to monitor the responses to therapy in individuals with hypertension and other cardiovascular diseases. These measures can then be used as surrogate markers for the risk of clinical events. Inhibition of the renin-angiotensin system (RAS) is associated with an important decrease in cardiovascular risk. Findings from clinical trials support the hypothesis that the protective effects of RAS inhibition are partly independent from blood pressure reduction and related to several mechanisms including vascular protective effects. The aim of the TRanscend Arterial stiffNess Substudy (TRANS) is to assess the effect of an angiotensin II receptor blocker (ARB), telmisartan, on the arterial stiffness in a subgroup of patients from the Telmisartan Randomized Assessment Study in aCE iNtolerant subjects with cardiovascular Disease (TRANSCEND) trial. The TRANSCEND trial is an international, multicenter, randomized double blind placebo controlled trial of telmisartan that enrolled patients at high risk for cardiovascular events. Some clinical baseline data of the TRANS substudy are reported. When completed, the results of the TRANS substudy will show whether the beneficial effects of treatment with telmisartan on cardiovascular outcome may be related to an improvement in arterial stiffness.
Introduction
The degree of arterial stiffness, obtained in various populations, has been found to be a powerful independent marker of vascular target organ damage and an independent prognostic predictor for cardiovascular morbidity, as well as cardiovascular and all-cause mortality (CitationBlacher et al 1999; CitationLaurent et al 2001, Citation2003; CitationMeaume et al 2001; CitationBoutouyrie et al 2002; CitationCruickshank et al 2002; CitationDernellis et al 2005; CitationShokawa et al 2005; CitationSutton-Tyrrell et al 2005; CitationMattace-Raso et al 2006; CitationWillum-Hansen et al 2006). Measuring pulse wave velocity (PWV) to assess arterial stiffness is a simple and reproducible method. The underlying principles and technique of this method have been described in detail previously (CitationAsmar 1999). Several experimental studies have shown that PWV is related to the arterial wall structure, function, geometry and endothelium functions (CitationAsmar 1999). Validation studies have shown that automatic measurements of PWV are simple, non-invasive, accurate, and reproducible (CitationAsmar et al 1995; CitationVan Bortel et al 2002; CitationLaurent et al 2006), making this technique a convenient, sensitive and useful tool in physiological and pharmacological studies.
Basic pharmacological concepts of arterial stiffness
Several important points serve to better understand the effects of pharmacological intervention on arterial stiffness.
The arterial site
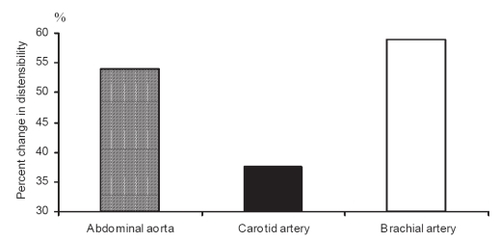
Atherosclerosis, arterial abnormalities, and their progression vary in different arterial sites. Arteries are heterogenous in structure and the arterial site has to be considered in assessment of the pharmacological treatment (CitationAsmar 1999). The impact of a given pharmacological agent may differ on the various components of the arterial wall (elastin, collagen, muscle) according to its pharmacodynamic properties. It is logical to assume that the arterial effects of a given drug administered at a given dose and period of time may differ according to the arterial site, which may be more elastic (aorta, carotid) or more muscular (radial) arteries (CitationTopouchian et al 1999). shows an example of the different effects on the arterial sites produced by the same antihypertensive drug in the same patients (CitationAsmar 1999; CitationTopouchian et al 1999).
Duration of treatment
Since several mechanisms may be involved in producing reductions in arterial stiffness with a given treatment, assessment of arterial stiffness has to distinguish between the effects of acute, short-term, or long-term chronic treatments. For example, after acute administration of an antihypertensive drug, improvement of arterial stiffness is principally related to functional or mechanical mechanisms such as reduction of distension pressure, reduction of smooth muscle tone, enhancement of endothelial functions, whereas after long-term chronic treatment, additional mechanisms can be involved, eg, changes in the arterial geometry and structure, reduction in degree of fibrosis, increase in elastin/collagen ratio, remodeling of the arterial wall (CitationAsmar 1999; CitationLaurent et al 2002). Experts agree that assessment of arterial stiffness after a long-term treatment period should be preferred because of the underlying pathophysiological mechanisms involved and because acute effects may not predict long-term efficacy.
Drug doses
In assessing the efficacy of a drug, one needs to consider the dose/effect relationship. This relationship may differ from other properties of the drug such as the dose/effect of its antihypertensive properties (CitationAsmar 1999; CitationLaurent et al 2002). A clear dissociation between the antihypertensive effect and the reduction of arterial stiffness by angiotensin converting enzyme (ACE) inhibitors has been reported. There is evidence that the effect on arterial wall properties can be seen at higher doses of ACE inhibitors than with the doses required for effective blood pressure (BP) reduction in hypertensive patients (CitationAsmar et al 1992a, Citationb). These findings, independent of the drug effects on BP, have been recently confirmed by the results of large clinical studies on patients at high cardiovascular risk. In these studies, positive results on arterial stiffness have been observed with high doses of ACE inhibitors (CitationYusuf et al 2000; CitationPROGRESS Collaborative Group 2001; CitationFox et al 2003).
Effect of antihypertensive agents on arterial stiffness
Several pharmacological studies have evaluated the effects of antihypertensive drugs on arterial stiffness. shows the effects on PWV of different antihypertensive drug classes, administered double blind, either short-term (<28 days) or long-term (≥28 days) (CitationAsmar 1999; CitationAsmar et al 2002; CitationRajzer et al 2003; CitationWhite et al 2003; Handbook of Hypertension 2006). During long-term treatment, improvements in central and peripheral arterial stiffness have been found with ACE inhibitors. Less marked improvement has been reported with angiotensin II receptor blockers. Beta-blockers have exhibited variable results according to the particular drugs used. With calcium channel blockers, the results are more complex. At the aortic level, all calcium channel blockers have shown a significant reduction in arterial stiffness, in parallel with BP reduction, but at the peripheral level, arterial stiffness reduction was less evident. Diuretics have shown no significant effects. Therefore, antihypertensive treatment is associated with variable effects on arterial stiffness, due not only to BP reduction but also duration of treatment. More specific long-term studies are needed.
Table 1 Effects of antihypertensive drugs on pulse wave velocity
Arterial stiffness or distensibility, evaluated by PWV or other measurements, depends on BP level. Therefore, any blood pressure decrease (decrease of the distension pressure) is theoretically associated with a decrease of PWV. For this reason, analysis of PWV changes according to BP change is important. Different situations have been described in the literature (CitationAsmar 1999), the most frequent of which are:
- decrease of PWV and BP
- decrease of PWV and small changes of BP (this may reflect an independent arterial effect of the drug)
- decrease of BP with unchanged PWV. This may reflect a relatively “harmful” impact on the arterial wall by other mechanisms such as increasing sympathetic tone (hydralazine) or heart rate, or other mechanisms related to the impact of the drug concerned.
Taking into consideration these aspects, analysis of PWV changes should be discussed according to other hemodynamic changes such as BP, heart rate, and peripheral resistance.
Future perspectives
Activation of the renin-angiotensin system (RAS) has been implicated in the pathogenesis of a wide variety of diseases affecting the cardiovascular system. Clinical trials have shown that inhibition of the RAS, particularly by ACE inhibitors, is associated with important decreases in cardiovascular risk in a broad range of patients (CitationYusuf et al 2000; CitationPROGRESS Collaborative Group 2001; CitationFox et al 2003; CitationHOPE/HOPE-TOO Study Investigators 2005). More recently, angiotensin II receptor blockers (ARBs) have been reported to reduce cardiovascular events in different populations, such as patients with diabetes, hypertension, or heart failure (CitationLacourcière et al 1998; CitationNeutel et al 1998; CitationDalhöf et al 2002; CitationYoung et al 2004). Both pathophysiological studies and clinical trials support the hypothesis that the protective effects of RAS inhibition observed with ACE inhibitors and ARBs are, at least partly, independent from their blood-pressure-lowering effects. Consistent improvement in arterial stiffness seems to be observed with some classes of drugs that target the RAS. Whether this effect on arterial stiffness may be observed with other drugs, such as ARBs, in different populations needs clarification.
There are several observations regarding ACE inhibitors (eg, adverse effects and lack of full RAS blockade during chronic treatment) and ARBs (powerful blocking effect on angiotensin II receptors) that can be discussed. While the relationship between reduction in arterial stiffness and decreased cardiovascular risk has been shown with ACE inhibitors, this relationship is not clear with ARBs. Thus, it is important to assess the arterial effect of ARBs after long-term chronic treatment in high risk patients.
Telmisartan is an ARB with a specific pharmacokinetic profile (CitationLacourcière et al 1998; CitationNeutel et al 1998). Several studies have investigated the effects of telmisartan on end-organ damage and demonstrated beneficial results. Results of a pilot crossover, placebo-controlled study showed that telmisartan reduced arterial stiffness in hypertensive patients with type 2 diabetes (CitationAsmar et al 2002). The TRANSCEND study (Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease) seeks to determine whether the ARB, telmisartan, is superior to placebo in high risk patients who are intolerant to ACE inhibitors, in reducing the risk of cardiovascular death, myocardial infarction, stroke, and hospitalization for heart failure. Details of the TRANSCEND trial have been published previously elsewhere (CitationLacourcière et al 1998; CitationNeutel et al 1998; CitationTeo et al 2004; CitationAsmar 2006). The TRanscend Arterial stiffNess Substudy (TRANS) is designed to determine the effect of telmisartan on arterial stiffness. This will assess whether the expected results of the TRANSCEND trial might be explained by the vascular protective effects of telmisartan and estimate whether the efficacy of telmisartan on cardiovascular outcomes may be related to an improvement in arterial stiffness.
Methods
Study design
The TRANSCEND study was an international, multicenter, randomized, double-blind, placebo-controlled clinical trial (CitationTeo et al 2004). The primary objective of the TRANSCEND study was to determine if telmisartan 80 mg daily was superior to placebo in reducing the composite endpoint of cardiovascular death, myocardial infarction, stroke, or hospitalization for heart failure. In this substudy (TRANS), assessment of arterial stiffness was performed initially during the run-in period before randomization, after 6 months of randomized treatment and yearly for 3 years. The primary objective of the TRANS study was to investigate whether the lower incidence of cardiovascular events with telmisartan was correlated with an improvement in arterial stiffness. Secondary objectives were to compare early changes in arterial stiffness between the treatment groups, to estimate the prognostic factors relating to changes in arterial stiffness, and to determine if an early change in arterial stiffness might be a prognostic factor for cardiovascular events.
Patients
Patient eligibility, inclusion, and exclusion criteria of the TRANSCEND study have been described elsewhere (CitationTeo et al 2004). Approximately 6000 high risk patients were enrolled.
Specific non-inclusion criteria for the TRANS study were: known significant peripheral vascular disease with proximal artery stenosis or limb amputation, history of vascular surgery at the level of the carotid artery, femoral artery, or on the aorta, body mass index >40 kg/m2, and atrial fibrillation. The purpose of these additional exclusion criteria was to avoid conditions that may impair the quality and reliability of PWV measurements.
Assessment of arterial stiffness
The Complior® device (Artech Medical, Paris, France) was used to record PWV in this study because of its reproducibility (CitationAsmar 1999) and simplicity of use. These properties were important in this large international multicenter study, with many sites obtaining recordings and a centralized assessment in a core-laboratory (CitationAsmar et al 2001). This device also allowed a valid measurement of the velocity of the same pulse wave recorded simultaneously in different sites of the arterial tree. Basic principles of PWV measurements have been described elsewhere (CitationAsmar 1999). The pressure pulse generated by ventricular ejection is propagated along the arterial tree at a speed determined by the geometric and elastic properties of the arterial wall. PWV is calculated from measurements of pulse transit time and the distance traveled by the pulse between two recording sites, according to the following formula: PWV (m/s) = distance (m)/transit time (s). All measurements are recorded on the right side of the body with the patient lying at ease in the supine position.
Blood pressure measurement
Blood pressure (BP) was measured before PWV recording and according to standard published guidelines (CitationO’Brien et al 2005). In order to minimize its variability the following recommendations were followed: patients in supine position, their arms bared, and supported at heart level. Patients refrained from smoking or ingesting caffeine during the 30 minutes preceding the measurement. Measurements began after at least 5 minutes of rest. The appropriate cuff size was used to ensure accurate measurement.
Logistics and organization
A total of 34 centers (from 12 countries) were selected; all were provided with Complior devices. All investigators participated in specific training sessions on PWV assessment organized by the study core laboratory. In order to limit inter-center variability of the PWV measurements, and to ensure data homogeneity, a pre-study assessment was carried out as part of the investigators’ certification to participate in the study.
Data analysis
Demographic and baseline characteristics
Demographic and baseline characteristics, risk factors, and relevant clinical variables (eg, cardiovascular history and medication), current status of diabetes, current medical conditions, and concurrent medications were summarized by treatment group and overall inclusion.
Efficacy analysis
Primary efficacy criterion
The primary efficacy parameter was the change from baseline, after 3 years of treatment, in the aortic stiffness as assessed by carotid-femoral PWV. The change from baseline was defined as the mean value at the end of the treatment period minus the mean value at baseline. The change from baseline was compared between the treatment groups using a t-test. If the data did not conform to the assumptions of normality, then a non-parametric test was used. Multiple regression analysis was used to explore BP changes and baseline characteristics. The same analysis was performed with the change from baseline after 6 months of treatment. In a second step, repeated measures of analysis of variance with PWV at 6 months, 1 year, 2 years and 3 years of treatment and between treatment groups was performed.
Secondary efficacy criteria
Regression analyses were performed to estimate factors related to carotid-femoral PWV at baseline. Only known factors or factors significantly related to PWV in simple regression analysis were included in a stepwise selection multiple regression analysis. If the treatment effect on PWV was not constant throughout the trial, regression analyses were performed for changes after 6 months of treatment and for changes between 2 and 3 years of treatment.
Results
Logistics and organization
Among the centers participating in the TRANSCEND trial, 34 centers from 12 countries participated in the TRANS study. All of these centers were provided with Complior devices. Investigators from all centers participated in specific training sessions and received their certification by the end of 2003. Twenty-six of the 34 centers participated actively by including patients in the TRANS study. shows the numbers of centers and patients enrolled per center.
Table 2 TRANS investigating centers distribution and number of patients enrolled
Study population
Recruitment for the TRANS study took place from November 2003 to June 2004. During this period 296 patients from 26 centers were enrolled. shows their geographic distribution. At randomization, 17 patients were excluded because their PWV recordings were judged (n = 17) to be of insufficient quality by the PWV Core laboratory. Therefore a total of 279 patients were included in the TRANS study. Their main baseline clinical characteristics are shown in .
Table 3 Baseline clinical characteristics of the TRANS study population
The mean values of arterial hemodynamic parameters of the study population remained within the upper limit of normal values both for blood pressure (139/80 mmHg) and pulse wave velocity (carotid-femoral = 10.0 ± 2.6 m/s; carotid-radial = 9.1 ± 1.4 m/s). These relatively “normal” values may be related to the baseline treatments received by this population at high cardiovascular risk.
and show that patients included in the TRANS sub-study are at high cardiovascular risk, as intended for the TRANSCEND study. Results show that about 68% of patients had previous coronary artery disease, more than 30% had experienced previous stroke, 13% had high-risk diabetes with evidence of end organ damage and about 4% experienced recent Transient Ischemic Attacks (TIA). Details of biochemical results are shown in .
Table 4 Diagnosis and previous diseases of the TRANS study population
Table 5 Biochemical results of the TRANS study population
Conclusion
The TRANS arterial stiffness sub-study of the TRANSCEND trial assessed the effect of telmisartan on arterial stiffness in patients at high cardiovascular risk. The results of this sub-study should be helpful in the interpretation of the results of both the TRANSCEND and the parallel ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET) trials. In fact, among the several mechanisms supporting the cardioprotective effects of RAS inhibition, improvement of arterial stiffness is of major importance. Moreover, the TRANS substudy will provide information about direct assessment of the arterial wall which constitutes the major target and the site of treatment of cardiovascular disease.
References
- AsmarRArterial stiffness and pulse wave velocity – Clinical applications1999ParisElsevier943
- AsmarRSafarMEPulse wave velocity. Principles and measurementsArterial stiffness and pulse wave velocity – clinical applications1999ParisElsevier2553 Chap. III
- AsmarRSafarMEPulse wave velocity and therapyArterial stiffness and pulse wave velocity – clinical applications1999ParisElsevier14357 Chap. VII
- AsmarRBenetosATopouchianJAssessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studiesHypertension199526485907649586
- AsmarRBenetosADarneBConverting enzyme inhibition: dissociation between antihypertensive and arterial effectsJ Hum Hypertens1992a638151464895
- AsmarRIannascoliFBenetosADose optimisation study of arterial changes associated with angiotensin converting enzyme inhibition in hypertensionJ Hypertens1992b10Suppl 51319
- AsmarRGossePTopouchianJEffects of telmisartan on arterial stiffness in Type 2 diabetes patients with essential hypertensionJ Renin Angiotensin Aldosterone Syst200231768012563568
- AsmarRTopouchianJPannierBon behalf of the Scientific, Quality control, Coordination and Investigation Committees of the Complior StudyPulse Wave velocity as endpoint in large-scale intervention trial. Complior® studyJ Hypertens2001198131811330885
- AsmarRTargetting effective blood pressure control with angiotensin receptor blockersInt J Clin Pract2006603152016494647
- BarnettAHBainSCBouterPAngiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathyN Engl J Med200435119526115516696
- BlacherJGuerinAPPannierBImpact of aortic stiffness on survival in end-stage renal diseaseCirculation1999992434910318666
- BoutouyriePTropeanoAIAsmarRAortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal studyHypertension200239101511799071
- BrennerBMCooperMEde ZeeuwDEffects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathyN Engl J Med2001345861911565518
- CruickshankKRisteLAndersonSGAortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function?Circulation200210620859012379578
- DalhöfBDevereuxRBKjeldsenSECardiovascular Morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (life): A randomised trial against atenololLancet2002359995100311937178
- DernellisJPanaretouMAortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjectsHypertension2005454263115710784
- FoxKEuropean Trial On reduction of cardiac events with Perindopril in Stable Coronary Artery Disease InvestigatorsEfficacy of Perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study)Lancet2003362782813678872
- HollenbergNKSeverPSThe past, present and future of hypertension management : a potential role for AT1-receptor antagonistsJ Renin Angiotensin Aldosterone Syst2000151011967784
- HOPE/HOPE-TOO Study InvestigatorsLong-term effects of Ramipril on Cardiovascular Events and on Diabetes. Results of the HOPE Study ExtensionCirculation200511213394616129815
- LacourcièreYLenisJOrchardRA comparison of the efficacy and duration of action of the angiotensin II receptor blocker telmisartan to amlodipineBlood Press Monit1998329530210212369
- LaurentSBoutouyriePAsmarRAortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patientsHypertension20013712364111358934
- LaurentSKatsahianSFassotCAortic stiffness is an independent predictor of fatal stroke in essential hypertensionStroke2003341203612677025
- LaurentSBoutouyriePSafarMEO’RourkeMFDetermination of systemic and regional arterial stiffnessHandbook of hypertension – arterial stiffness in hypertension2006ParisElsevier5362 Chap. I–4
- LaurentSKingwellBBankAClinical applications of arterial stiffness: therapeutics and pharmacologyAm J Hypertens200215453812022248
- LewisEJHunsickerLGClarkeWRRenoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med20013458516011565517
- Mattace-RasoFUvan der CammenTJHofmanAArterial stiffness and risk of coronary heart disease and stroke: the Rotterdam StudyCirculation2006113657316461838
- MeaumeSBenetosAHenryOFAortic pulse wave velocity predicts cardiovascular mortality in subjects.70 years of ageArterioscler Thromb Vasc Biol20012120465011742883
- NeutelJMSmithDHGDose response and antihypertensive efficacy of the AT1 receptor antagonist telmisartan in patients with mild to moderate hypertensionAdv Ther19981520617
- O’BrienEAsmarRBeilinLPractice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurementJ Hypertens20052369770115775768
- ParvingHHLehnertHBrochner-MortensenJThe effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetesN Engl J Med2001345870811565519
- PROGRESS Collaborative GroupRandomised trial of a perindopril-based blood pressure lowering regimen among 6105 individuals with previous stroke or transient ischaemic attackLancet200135810334111589932
- RajzerMKlocekMKawecka-JaszczKEffect of Amlodipine, Quinapril, and Losartan on pulse wave velocity and plasma collagen markers in patients with mild-to-moderate arterial hypetensionAm J Hypertens2003164394412799091
- Therapeutic aspects of arterial stiffness and wave reflectionsSafarMEO’RourkeMFHandbook of hypertension – arterial stiffness in hypertension2006ParisElsevier459580 Chap. V
- ShokawaTImazuMYamamotoHPulse wave velocity predicts cardiovascular mortality: findings from the Hawaii-Los Angeles-Hiroshima studyCirc J2005692596415731528
- StrawnWBDeanRHFerrarioCMNovel mechanisms linking angiotensin II and early atherogenesisJ Renin Angiotensin Aldosterone Syst20001111711967786
- Sutton-TyrrellKNajjarSSBoudreauRMHealth ABC StudyElevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adultsCirculation200511133849015967850
- TeoKYusufSSleightPONTARGET/TRANSCEND InvestigatorsRationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trialsAm Heart J2004148526115215792
- TopouchianJAsmarRSayeghFChanges in arterial structure and function under trandolapril-verapamil combination in hypertensionStroke19993010566410229744
- Van BortelLMDuprezDStarmans-KoolMJApplications of arterial stiffness, Task Force III: recommendations for user proceduresAm J Hypertens2002154455212022247
- WhiteWBDuprezDSt HilaireREffects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertensionHypertension2003421021614557281
- Willum-HansenTStaessenJATorp-PedersenCPrognostic value of aortic pulse wave velocity as index of arterial stiffness in the general populationCirculation20061136647016461839
- YoungJBDunlapMEPfefferMAMortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trialsCirculation200411026182615492298
- YusufSSleightPPogueJEffects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study InvestigatorsN Engl J Med20003421455310639539