Abstract
The risk for venous thromboembolism (VTE) in medical patients is high, but risk assessment is rarely performed because there is not yet a good method to identify candidates for prophylaxis.
Purpose
To perform a systematic review about VTE risk factors (RFs) in hospitalized medical patients and generate recommendations (RECs) for prophylaxis that can be implemented into practice.
Data sources
A multidisciplinary group of experts from 12 Brazilian Medical Societies searched MEDLINE, Cochrane, and LILACS.
Study selection
Two experts independently classified the evidence for each RF by its scientific quality in a standardized manner. A risk-assessment algorithm was created based on the results of the review.
Data synthesis
Several VTE RFs have enough evidence to support RECs for prophylaxis in hospitalized medical patients (eg, increasing age, heart failure, and stroke). Other factors are considered adjuncts of risk (eg, varices, obesity, and infections). According to the algorithm, hospitalized medical patients ≥40 years-old with decreased mobility, and ≥1 RFs should receive chemoprophylaxis with heparin, provided they don’t have contraindications. High prophylactic doses of unfractionated heparin or low-molecular-weight-heparin must be administered and maintained for 6–14 days.
Conclusions
A multidisciplinary group generated evidence-based RECs and an easy-to-use algorithm to facilitate VTE prophylaxis in medical patients.
Introduction
Venous thromboembolism (VTE) represents a spectrum of diseases that include deep vein thrombosis, central venous catheters associated thrombosis (CVC-thrombosis), and pulmonary embolism (PE). Both clinically symptomatic and asymptomatic episodes of VTE are common in hospitalized patients (CitationGoldhaber and Tapson 2004), and are associated with high mortality (CitationMaffei et al 1980; CitationAnderson et al 1991; CitationLindblad et al 1991; CitationGolin et al 2002). Autopsy studies have shown that approximately 10% of all inpatients deaths are due to PE, but only a small proportion of PE are suspected before death (CitationPineda et al 2001; CitationYoo and Mendes 2004). Until the mid 90s, most studies focused on surgical patients, given their high incidence of VTE. As a consequence, the notion about the need for VTE prophylaxis in surgical populations gained acceptance. More recently, randomized controlled trials have highlighted the fact that the risk of VTE in patients with medical conditions is similar to that of some surgical patients (CitationBergmann and Neuhart 1996; CitationHarenberg et al 1996; CitationLechler et al 1996; CitationSamama et al 1999; CitationKleber et al 2003; CitationLeizorovicz et al 2004). Additionally, some epidemiological studies have demonstrated that more than half of patients who develop symptomatic VTE have medical, not surgical conditions (CitationGoldhaber et al 2000). In the registry RIETE (CitationMonreal et al 2004), only 28% of acutely ill medical patients with decreased mobility had received prophylaxis, while 67% of surgical patients did. During the follow-up of these patients, both PE and fatal bleedings were more common in medical patients, underlying the need for adequate prophylactic regimens in this particular subset of patients. Therefore, the analysis of the importance of risk factors in hospitalized medical patients is crucial to define the risk-benefit of VTE prophylaxis utilization. A systematic review of risk factors for VTE was performed, evaluating the current evidence about the factors that could justify the use of VTE prophylaxis in this population. The evidence about the effectiveness of VTE prophylaxis in these specific groups was also reviewed, and evidence-based recommendations were incorporated into an easy-to-use risk-assessment algorithm for VTE.
Methods
Literature search
A computer-based literature search was performed independently by two investigators to identify studies evaluating the following conditions as risk factors for thrombosis in acutely ill medical patients: active rheumatologic diseases (ARD) and inflammatory bowel disease (IBD), acute myocardial infarction (AMI), admission to ICU, age, cancer, chemotherapy and hormonotherapy, central venous catheters (CVC), cerebral vascular accident (CVA), congestive heart failure (CHF), diabetes, hormonal contraception (HC) and hormonal replacement therapy (HRT), hypertension, infections, nephrotic syndrome, obesity, paresis and/or paralysis of the lower extremities, peripheral vascular disease, pregnancy and puerperium, previous VTE, reduced mobility, respiratory diseases (eg, chronic obstructive pulmonary disease [COPD], respiratory insufficiency, and respiratory infections), tobacco use, and thrombophilias. We also evaluated the efficacy of methods of VTE prophylaxis in acutely ill medical patients, including low dose unfractionated heparin (LDUH), low molecular weight heparins (LMWH), and mechanical methods of prophylaxis. We searched the English language and non-English language literature by using MEDLINE, LILACS databases, and the Cochrane Central Register of Controlled Trials from the earliest searchable dates through August 2004. Annals of important meetings were also searched for abstracts from 1998 onwards. The reference lists of published reviews were also evaluated.
Data collection
Inclusion criteria for the systematic review were established before the literature search. We included randomized-controlled trials, cohorts, and case-control studies with at least 10 subjects evaluating risk factors or efficacy of prophylactic methods for VTE. Two authors read all retrieved studies and made the final decision on which studies met the inclusion criteria. All data were abstracted independently and in duplicate by two of the authors using a standardized data collection form. Discrepancies in the data abstracted were resolved by consensus among the authors and the working committee.
Levels of evidence
The level of evidence of each study was classified based on the American Heart Association, American College of Cardiology, and European Society of Cardiology (AHA/ACC/ESC) guidelines for the management of patients with supraventricular arrhythmias (CitationBlomstrom-Lundqvist et al 2003). These criteria were adapted to allow evaluation of studies about efficacy of methods of VTE prophylaxis and risk factors for VTE. show the levels of evidence and shows the strength of recommendations.
Table 1 Classification of levels of evidence for the studies
Table 2 Classifi cation of recommendations
Results
Risk factors
Each risk factor was evaluated separately as much as possible. The risk factors for VTE in clinical patients, according to the level of evidence, are listed in . shows the frequency of VTE in hospitalized patients with various medical conditions.
Table 3 Risk factors for VTE in medical patients according to the level of evidence
Table 4 Frequency of DVT in hospitalized patients according to their medical condition
Active rheumatologic diseases and inflammatory bowel disease\
Although rheumatologic diseases represent a heterogeneous group of patients, they have usually been considered at-risk for thrombosis because incidences of VTE of 10% to 30% have been demonstrated in hospitalized patients with active rheumatologic diseases (CitationCohen and Quinlan 2000; CitationAlikhan et al 2003; CitationRahim et al 2003). CitationCogo and colleagues (1994) in an analysis of 540 patients with suspected DVT, detected systemic lupus erythematosus (SLE) as a risk factor for VTE (OR 4.3; 95% CI 3.1–5.5). However, Orger and colleagues (1997) in a very similar study with 277 patients, found different results (OR 0.7; p = NS), that were confirmed in a case-control study with 1,272 SLE patients (OR 1.6; p = NS) (CitationSamama 2000). In the secondary analyses of the MEDENOX study about the importance of risk factors, the association of VTE with rheumatologic diseases was not statistically significant (RR 1.6; 95% CI 0.96–2.69, p = 0.11). Based on these data, active rheumatologic diseases as a group, and SLE in particular, are not clearly related to increased VTE risk. However, lupus anticoagulant (LA) and anticardiolipin (ACL) antibodies are frequently found in rheumatologic patients and are definitely related to thrombotic phenomena. CitationFijnheer and colleagues (1996), studying 173 SLE patients, found that LA was associated with VTE with an OR of 6.4 (95% CI 2.7–15.4).
Arterial and venous thromboses are common clinical findings in patients with Behcet’ disease (BD) (CitationKoc et al 1992). ACL has been found in as many as 30% of these patients (CitationZouboulis et al 1993), but other studies demonstrate vascular thrombosis even when an ACL antibody is not found (CitationKiraz et al 1999; CitationMader et al 1999). A HLA-B51 positive is associated with a higher risk of VTE (OR 4.2; 95% CI 1.1–16.3) and a HLA-B35 has a protective effect (OR 0.2; 95% CI 0.04–0.92) (CitationKaya et al 2002).
IBD was evaluated by CitationBernstein and colleagues (2001) in a retrospective cohort including 2,857 patients with Crohn’s disease (CD) and 2,672 with ulcerative colitis (UC). Compared with controls both CD and UC patients had increased risk for DVT (RR 4.7; 95% CI 3.5–6.3 in CD and RR 2.8; 95% CI 2.1–3.7 in UC) and for PE (RR 2.9; 95% CI 1.8–4.7 in CD and RR 3.6; 95% CI 2.5–5.2 in UC) (CitationKaya et al 2002). CitationMiehsler and colleagues (2004) studied 618 patients with IBD, 243 patients with rheumatoid arthritis and equal number of gender and aged-matched control. The authors found that IBD was significantly associated with a higher chance of developing VTE (OR 3.6; 95% CI 1.7–7.8; p < 0.001), but that rheumatoid arthritis was not (OR 0.7; 95% CI 0.2–2.9; p = NS).
In summary, IBD, BD, and positive LA and ACL antibodies in SLE patients should be considered as risk factors for VTE (EVIDENCE A). Active rheumatologic diseases as a group receive a level of evidence B, while SLE without LA or ACL and rheumatoid arthritis should not be considered as risk factors for VTE.
Acute myocardial infarction
The risk for VTE in acute coronary syndromes’ patients is high, but the specific evaluation of VTE risk in this setting has become difficult because the use of heparin and other medications that interfere directly with the coagulation system is now routine. Most data about VTE risk in acute coronary syndromes come from old studies, with small number of patients, comparing placebo with some kind of prophylaxis. One study showed an incidence of DVT as high as 62.5%, when the treatment for AMI was basically absolute bedrest for 5 days (CitationZawilska et al 1989). In a study comparing LDUH with placebo, PE was detected in 12.2% (5/41) in the placebo group, compared with 0/37 in the heparin group (CitationEmerson and Marks 1977). AMI should therefore, be considered a risk factor for VTE (EVIDENCE A).
Admission to ICU
Several studies that employed screening for DVT in critically ill patients showed that admission to an ICU is a risk factor for VTE (RR 1.8 to 2.9) (CitationCade 1982; CitationIbarra-Perez et al 1988; CitationHirsch et al 1995; CitationGoldberg et al 1996; CitationMarik et al 1997; CitationKapoor et al 1999; CitationFraisse et al 2000; CitationGoldhaber et al 2000). The incidence of DVT in clinical ICUs is very high, particularly in patients receiving no prophylaxis (25% to 31%), compared with those that receive some form of prophylaxis (11 to 16%) (EVIDENCE A) (CitationCade 1982; CitationKapoor et al 1999; CitationFraisse et al 2000; CitationGoldhaber et al 2000). Besides, PE is found in up to 27% of ICU patients that undergo autopsy. Although PE contributes to death in about 12% of ICU patients, it is suspected before death in only 30% of the cases. ICU patients have in general 2 to 4 additional risk factors for VTE, not including reduced mobility (CitationRyskamp and Trottier 1998; CitationGeerts and Selby 2003). Nevertheless, according to several reports, the utilization of prophylaxis in ICU patients had been quite irregular (CitationIbrahimbacha and Alnajjar 1998; CitationLevi et al 1998; CitationRyskamp and Trottier 1998; CitationRocha and Tapson 2002; CitationGeerts and Selby 2003; CitationLacherade et al 2003; CitationRocha et al 2003).
Age
Several epidemiologic studies have shown that the incidence of VTE increases exponentially with aging. It is not clear if the reasons for this are changes in clotting mechanisms or the presence of thrombogenic comorbidities (CitationAnderson et al 1991; CitationSilverstein et al 1998). In a study conducted in Oslo, the incidence of VTE increased from 1:10,000 at age 20 to 1:1,000 at age 50 (CitationStrekerud et al 1998). Other studies showed different age cutoffs for significant increase in risk of VTE: RR 1.75 for age >65 years, 1.51 for age >75 years and 2.0 for age >85 years (CitationKniffin et al 1994; CitationAlikhan et al 2004; CitationOger 2000). In the study EPI-GETBO, the authors screened 234 medical patients on the day of admission with Doppler ultrasound, and found that the prevalence of asymptomatic DVT, among patients older than 80 years, reached 17.8% (95% CI 8.5–32.6) and the incidence 6.0 per 1,000 patients-day (95% CI, 0–12.7) (CitationOger et al 2002a). In the study performed in the community of Worcester, MA, the risk of VTE almost doubled at each decade from the 5th decade on (RR 1.9) and the incidence of VTE was 62:100,000, starting at age 50 (CitationAnderson et al 1991). For the same age, others studies showed an incidence of VTE reaching 100:100,000 (CitationSilverstein et al 1998; CitationHansson et al 1999). Therefore, we conclude that there is a progressive increase in VTE risk with advancing age, and that age over the 5th decade should be considered as an additional risk factor for VTE in medical patients (EVIDENCE A).
Cancer, chemotherapy, and hormonotherapy
Association between cancer and thromboembolism is known since 1865, when Trousseau described migratory thrombophlebitis as a sign for underlying pancreatic cancer (EVIDENCE A). Since then, many others neoplasias have been associated with VTE () (CitationPinzon et al 1986; CitationThodiyil and Kakkar 2002). This high incidence of VTE may be explained not only by the hypercoagulability but also by the action of some antineoplastic agents and the frequent venous catheterization. In some studies the higher incidences of DVT were observed in patients with pancreas, ovarium, liver, and brain cancer, while others indicate breast, lung, genital, urinary, stomach and colon cancers as the most frequently related to VTE (CitationCoon 1976; CitationBussani and Cosatti 1990; CitationSorensen et al 1998). In a series of 21.530 autopsies, 29% of the patients had cancer and the most common were ovarium, extrahepatic biliary tree, and stomach (34.6%, 31.7%, and 15.2%, respectively), while esophagus, larynges, leukemia, and lymphoma had the lower prevalences (CitationCoon 1976; CitationBussani and Cosatti 1990; CitationSorensen et al 1998).
Table 5 Relative risk for VTE in different neoplasias
Chemotherapy and hormonotherapy may also be thrombogenic (EVIDENCE A). However, it’s difficult to separate the effect of the treatment from the effect of the cancer itself. Several studies have shown that VTE is more common during chemotherapy in breast cancer patients, compared with the period without treatment (6.8% vs 17.6%) (CitationGoodnough et al 1984; CitationLevine et al 1988; CitationSaphner et al 1991; CitationPritchard et al 1996). CitationSaphner et al 1991 observed a higher incidence of VTE when tamoxifen was used. In a study with mieloma patients, CitationZangari and colleagues (2001) observed that addition of thalidomide to chemotherapy was associated with an important increase in DVT incidence (28% vs 4%, p = 0.002).
Central venous catheters
Several variables are implicated in the increased thrombogenicity associated with central venous catheters (CVC-thrombosis) and DVT in patients using catheters (). Given the high variability of catheter-related factors and underlying diseases, studies evaluating CVC-thrombosis are quite heterogeneous. Besides, the diagnostic methods for thrombosis and the primary objectives of these studies are extremely variable, which make it difficult to group then in order to make specific recommendations. For these reasons, we discuss thrombosis prophylaxis according to the purpose of the catheter: for chemotherapy in cancer patients, for parenteral nutrition (PN) and for general use in ICU patients. We briefly discuss also the evidence for pulmonary artery catheters and hemodialysis catheters as risk factors for thrombosis.
Table 6 Factors associated with venous thrombosis in patients with catheters
Several studies show that the incidence of thrombosis is higher in the catheterized veins than in contralateral veins (CitationRaad et al 1994; CitationDurbec et al 1997b; CitationMian et al 1997; CitationMartin et al 1999; CitationJoynt et al 2000). Besides, in RCTs of prophylaxis, the incidence of CVC-thrombosis is 5%–18% in the groups that receive prophylaxis and 4%–62% in the groups that do not receive prophylaxis. Therefore, central venous catheters constitute additional risk factors for VTE in clinical patients (CitationHeit et al 2000) in general and in oncologic patients (CitationBern et al 1990; CitationBalestreri et al 1995; CitationDe et al 1997) in particular (EVIDENCE A).
Pulmonary artery catheters (Swan-Ganz)
The rate of clot formation in pulmonary artery catheters is time dependent and ranges from 66% to almost 100% in noncoated catheters with heparin (CitationHoar et al 1981; CitationChastre et al 1982; CitationMangano 1982; CitationMollenholt et al 1987). Nevertheless, the frequency of thrombotic complications reported varies considerably, depending on the method of detection of the thrombus (CitationElliott et al 1979). Similarly to other CVC, pulmonary artery catheters lead to a 4.5 higher relative risk of thrombosis of the catheterized vein as compared with the contralateral veins (CitationMeredith et al 1993), and constitute an additional risk factor for VTE (CitationRocha et al 2003) (EVIDENCE A).
Hemodialysis catheters
Although moderate to severe renal insufficiency is associated with higher risk of bleeding (CitationLohr and Schwab 1991), thromboembolic events are also quite common in patients with renal failure. Chronic hemodialysis patients present high incidence of thrombophilias, frequently utilize recombinant erithropoetin, which may have a prothrombotic effect and present also VTE risk factors that are less commonly recognized, such as hyperhomocysteinemia, endothelial dysfunction and markers of systemic inflammation (CitationCasserly and Dember 2003). In a study of the US Renal Data System with 76,718 renal failure patients on chronic dialysis in 1996, the incidence of PE was 149.9/100,000, while the incidence of PE in the general US population was 24.6/100,000 (CitationTveit et al 2002). Furthermore, thrombosis of the venous access is another well established condition in these patients, affecting arterial-venous fistulas, grafts and double-lumen catheters for hemodialysis (CitationFan and Schwab 1992). These catheters are most commonly used as temporary vascular accesses but in some instances, allow hemodialysis for longer periods (CitationShusterman et al 1989). Catheter malfunction is quite common and the incidence of CVC-thrombosis in these patients is up to 46% (CitationFan and Schwab 1992; CitationBeenen et al 1994), however, the need for catheter removal is rare. Also, the cannulation of subclavian veins lead more frequently to thrombosis and/or stenosis than the cannulation of internal jugular veins (CitationVanherweghem et al 1986; CitationBeenen et al 1994; CitationAgraharkar et al 1995), particularly on the left side (CitationClark et al 1990). Thus, there is evidence that hemodialysis catheters, similarly to other CVC constitute an additional risk factor for VTE (EVIDENCE A).
Cerebral vascular accident
Hospitalized patients with CVA present one of the highest rates of VTE among general medical patients, ranging from 28% to 75% and affecting particularly the paralyzed limb (CitationMcCarthy et al 1977). In a retrospective cohort were analyzed 1,953 patients with hemorrhagic CVA and 15,599 patients with ischemic CVA (CitationGregory and Kuhlemeier 2003). The authors found a prevalence of VTE four-fold higher for patients with hemorrhagic CVA and even after controlling for severity of disease and duration of hospitalization, hemorrhagic CVA was an independent risk factor for VTE with an OR of 2.6 (95% CI 1.5–4.6). In another study, PE was found to be the immediate cause of death in 5% of stroke patients (CitationPambianco et al 1995). For these reasons both ischemic and hemorrhagic CVA must be considered important risk factors for VTE (EVIDENCE A).
Congestive heart failure
Many studies have shown that CHF is related to VTE, especially in patients with reduced mobility. In a case-control study with 790 patients, CHF increased significantly the risk for VTE (OR 2.6; 95% CI 1.4–4.7) (CitationHowell et al 2001). Besides, the lower the ejection fraction the higher the risk of VTE: OR 38.3 for EF <20%; 2.8 for EF from 20% to 40%, and 1.7 for EF >45%. In a cohort study with 1,250 patients, CitationHeit and colleagues (2000) detected CHF as an independent risk factor for VTE in patients presenting with fatal PE at autopsy (OR 2.8; 95% CI 1.8–4.2). Samama, in another case-control study, also identified CHF as a risk factor for VTE in ambulatorial patients (OR 2.9; 95% CI 1.5–5.6) (CitationSamama 2000). Even in patients treated with anticoagulants due to a previous VTE episode, CHF was considered an independent risk factor for new VTE episodes (OR 2.3; 95% CI 1.1–5.0) (CitationDouketis et al 2000). Although an analysis of the MEDENOX study failed to consider CHF as an independent risk factor for VTE, the risk associated with CHF was observed to be higher among patients with more severe functional compromise (RR 0.87; 95% CI 0.6–1.3 for New York Heart Association [NYHA] class III and RR 1.3; 95% CI 0.74–2.34 for NYHA class IV). CHF must be considered an important risk factor for VTE, particularly among patients with NYHA classes III and IV (EVIDENCE A).
Hormonal contraception and hormonal replacement therapy
HRT and HC increase the risk for VTE by 2 to 6 times (EVIDENCE A). Recently, two well designed RCTs confirmed the association between VTE and HRT (CitationHulley et al 1998; CitationRossouw et al 2002). In the HERS Study (Heart and Estrogen/progestin Replacement Study), 2,763 women with coronary disease were followed prospectively and an increase in the risk for VTE was noted among those women treated with estrogen and progesterone (RR 2.7; 95% CI 1.4–5.0 for VTE and RR 2.8; 95% CI 0.9–8.7 for PE). A few years later, in the Women’s Health Initiative (WHI), the group receiving HRT had a RR of 2.1 (CI 95% 1.6–2.8) for the development of VTE when compared to the placebo group. The risk is higher in the first year of HRT use (CitationPerez et al 1997; CitationHoibraaten et al 1999; CitationMiller et al 2002), especially in the presence of previous history of VTE (CitationHoibraaten et al 2000). CitationScarabin and colleagues (2003) showed that the risk of VTE was higher with oral, compared with transdermic, administration of HRT (RR 4.0; 95% CI 1.9–8.3).
Several observational studies and some RCTs have documented a RR 3 to 6 times higher in HC users, compared with nonusers (EVIDENCE A) (CitationJick et al 1995; CitationLewis et al 1996; CitationDouketis et al 1997; CitationVandenbroucke et al 2001). In a multicentric, international case-control study including 1,143 cases of VTE and 2.998 controls, HC was associated with an OR of 4.1 (95% CI 3.1–5.6) in European women and to an OR of 3.2 (95% CI 2.6–4.1) in women from developing countries (WHO 1995). As for HRT, the risk is higher during the first year of use (CitationSuissa et al 1997; CitationLidegaard et al 1998), and it seems also higher for the 3rd generation hormones (desogestrel and gestoden) (CitationKemmeren et al 2001).
Infections
Several cohorts and prospective randomized trials have indicated the association between infections and VTE. However, most patients included in these studies have lung infections and are described in the specific section about respiratory diseases. The SIRIUS study (CitationSamama 2000) included 1,272 ambulatory patients and demonstrated that infection is a risk factor for VTE (OR 1.95; 95% CI 1.31–2.92). However, in this study the site of the infections are not reported. CitationKierkegaard and colleagues (1987) studied 101 acutely ill patients with labeled fibrinogen and found that those with pneumonia or cardiac diseases had an incidence of DVT of 20% while the patients with other diagnoses had an incidence of DVT of only 4%. In this study, 4 of the 22 patients with pneumonia had DVT, while none of the patients with urinary tract infection, bronchitis, acute enterocolitis or sepsis developed DVT. In the prospective registry ‘DVT FREE’, CitationGoldhaber and Tapson (2004) evaluated 5,451 patients with acute DVT confirmed by Doppler ultrasound, demonstrating that 22% of the patients had an infection as one of the comorbidities (pneumonia in 7%, sepsis in 5%, and other infections in 10%). They also showed that thoracic infections were more common among those with PE and DVT, than among those with DVT alone (10% vs 8% p = 0.04). CitationAlikhan and colleagues (2004) analyzed the data of 866 patients of the MEDENOX study, demonstrating that the acute infections were one of the main risk factors for VTE in the univariate (RR 1.47; 95% CI 1.47–2.14) and multivariate analyses (OR 1.74; 95% 1.12–2.75). These data suggest that infections are an additional and frequent risk factor for VTE, especially in hospitalized patients (EVIDENCE A for pulmonary infection and B for the other infections).
Nephrotic syndrome
The association between nephrotic syndrome (NS) and thromboembolic events is recognized since 1837 (CitationTrew et al 1978). Until the 70s, some authors suggested that renal vein thrombosis was possibly the cause of the NS (CitationKendall et al 1971; CitationBennett 1975). Nevertheless, several studies confirmed that the thrombotic episodes are a consequence of the factors leading to the hypercoagulability found on the NS (CitationLlach et al 1975, Citation1980; CitationNoel et al 1979; CitationLlach 1985; CitationBellomo et al 1993) The global incidence of thrombosis in NS is 43% (CitationBellomo and Atkins 1993), but PE and DVT affect about 11% of the patients (CitationNickolas et al 2003). The frequency of renal vein thrombosis in the membranous NS, in adults, varies among studies from 5% to 60% (CitationOrth and Ritz 1998; CitationNickolas et al 2003). These episodes are symptomatic in only 10% of the cases (CitationOrth and Ritz 1998), and all episodes can be accurately detected by computed tomography (CitationGatewood et al 1986). CitationRostoker and colleagues (1995) reviewed 13 prospective studies, including 682 patients with NS, showing that the incidence of renal vein thrombosis was 21.4% (95% CI 18%–25%). These authors reviewed also three other studies with 148 patients and found that incidence of PE was 14% (95% CI 9%–21%). Thromboembolic phenomena, particularly renal vein thrombosis, are more common with membranous lesions (CitationWagoner et al 1983; CitationLlach 1985; CitationNickolas et al 2003), and possibly when serum albumin is <2g/dL (CitationKauffmann et al 1978; CitationKuhlmann et al 1981; CitationRobert et al 1987; CitationNickolas et al 2003). For hospitalized patients with reduced mobility, NS is a risk factor for VTE (EVIDENCE A).
Obesity
There are many studies that evaluate obesity direct or indirectly as a risk factor for VTE leading to some debate about the importance of it as a risk factor. CitationAlikhan and colleagues (2004), analyzing the data of the MEDENOX study, did not find a significant correlation between obesity and VTE (RR 1.04; 95% CI 0.69–1.60). CitationGrady and colleagues (2000), evaluating women with body mass index (BMI) above 27, also failed to find such a correlation. It is important to mention that in both studies the identification of obesity as a risk factor was a secondary objective and the analysis was done post hoc. Besides, the definition of obesity currently accepted as the BMI ≥30 Kg/m2, is not used in all studies. For example, in a study of consecutive out-patients with clinical suspicion of VTE, CitationCogo and colleagues (1994) defined obesity as 30% excess above ideal body weight and did not find that obesity was a risk factor for VTE. CitationHeit and colleagues (2000), in a population based case-control study reached the same conclusion.
On the other hand, several studies do implicate obesity as a risk factor for VTE. CitationBlaszyk and colleagues (1999) found in an autopsy study (n = 7,227) a higher incidence of VTE in obese (67%) than in nonobese (14%), RR 2.97 (95% CI 1.78–4.93). Four prospective cohort studies support theses findings, with RR ranging from 2.0 to 3.92 (CitationCogo et al 1994; CitationGoldhaber et al 1997, Citation1983; CitationHansson et al 1999). Although there is some debate, the evidence from prospective trials evaluating risk factors support the correlation between obesity and VTE. Nevertheless, the RR for obesity is relatively low (between 2 and 3) but increases significantly when there are additional risk factors for VTE. Indeed, the RR rises from 2 to 10 with the use of HC in obese patients (CitationAbdollahi et al 2003). In summary, we conclude that obesity is an important coadjuvant for the development of VTE (EVIDENCE B).
Paresis/paralysis of the lower extremities
There is supporting evidence that paresis or paralysis of the lower extremities is associated with VTE, even when not secondary to CVA (EVIDENCE A). In a study with 143 patients that developed acute hemiplegia, the incidence of VTE was 26% and the risk was higher during the first 4 weeks (CitationRentsch 1987). CitationPottier and colleagues (2002) studied the presence of risk factors among 450 hospitalized medical patients and found that paralysis was associated with an increased chance of VTE (calculated OR 12.5; 95% CI 1.5–104.5). The same result was seem in a case-control study with 620 patients older than 65 years (OR 2.1; 95% CI 1.0–4.1) (CitationWeill-Engerer et al 2004). Patients with paresis or paralysis of the legs must be considered as at-risk for VTE, particularly during the acute setting (EVIDENCE A).
Peripheral vascular diseases
The impact of varices of the lower extremities as an additional risk factor for VTE in medical patients is controversial. There are few studies evaluating the theme and there is no evidence that surgical treatment of the varicose veins decreases the potential risk of VTE. CitationKakkar and colleagues (1970) showed that the incidence of VTE by labeled fibrinogen in surgical patients with mild to severe varices was 56.5%, leading to a RR of 2.3. The reason for such a high incidence of VTE in patients with varices is not known but one of the possibilities is that the varices may be a consequence of previous and undiagnosed DVT. Recently, CitationHeit and colleagues (2000) demonstrated in a population-based, case-control study that varices are associated with risk of VTE in medical patients, but the risk decreases with age: OR 4.2 at age 45, 1.9 at age 60 and 0.9 at age 75. In another analysis of the same data (CitationHeit et al 2002), the attributable risk of VTE to varices was 6%; but after adjustments for other confounders, such as hospitalization, trauma, cancer and chemotherapy, CHF and CVA, CVC or pacemaker, the risk associated with varices became zero. Some cohort studies also failed to demonstrate an independent association between varices and VTE (CitationGoldhaber et al 1983; CitationKierkegaard et al 1987). On the other hand, prospective studies with good methodology (CitationAlikhan et al 2003; CitationOger et al 1997) and case control studies (CitationSamama 2000; CitationPottier et al 2002) showed that varices (OR ≥ 2.5 and RR 4.2) and venous insufficiency (OR ≥ 1.7) were significantly associated with VTE in medical patients. Furthermore, one case-control study in ambulatory patients with clinical suspicion of DVT or PE showed that peripheral arteriopathy was related to an higher chance of VTE (OR 1.9) (CitationCogo et al 1994). Therefore, peripheral venous and arterial diseases may be considered as cofactors for the development of thrombosis (EVIDENCE B).
Pregnancy and puerperium
Pregnancy is a well known condition related to increased risk of thrombosis (CitationSamama 2000), that persists even a few months after delivery (EVIDENCE A). It is estimated that the risk for VTE during pregnancy increases 3 to 4 times, probably because of the increase in procoagulant factors, such as factor VIII and in the resistance to activated protein C. CitationSamuelsson and Hagg (2004), in a population-based study with more than 24,000 women, reported an incidence of VTE during pregnancy and postpartum of 103:100,000 (95% CI 55–177). Besides, fatal PE remains one of the most important complications during pregnancy and puerperium, especially in those women older than 40 years (CitationFranks et al 1990).
Previous VTE
Previous VTE has been consistently described as a risk factor for the development of VTE in several scenarios: hospitalized and ambulatorial patients and in the general population (EVIDENCE A). The case-control study SIRIUS (CitationSamama 2000) revealed a strong tendency to new thrombotic events in ambulatorial patients with previous history of VTE (OR 15.6). In a prospective study, CitationOger and colleagues (1997) showed that medical patients with suspected DVT and history of VTE had increased chance of confirming the diagnosis by venography (OR 1.7). In 2003, Tosseto and colleagues (2003) also demonstrated increased risk of VTE in individuals with previous history of this condition (OR 6.8). A case-control study showed that previous history was an important risk factor for VTE also in hospitalized medical patients (OR 4.7) (CitationBonifacj et al 1997). Another case-control study showed that in hospitalized medical patients older than 64 years-old, previous VTE was independently associated with the development of VTE during hospitalization (OR 3.4) (CitationWeill-Engerer et al 2004). In the analysis of VTE risk factors based on the MEDENOX study (CitationAlikhan et al 2004), previous history was associated with the development of VTE, in univariate (RR 1.8; p = 0.02) and multivariate logistic regression (RR 2.1; p = 0.02). Thus, previous history of thrombosis should be considered as an additional risk factor for VTE in medical patients (EVIDENCE A).
Reduced mobility
It is not known exactly what level and duration of immobility is associated with increased risk of VTE. What is recognized is that when important risk factors are present, even subtle reductions in mobility increase the overall risk of VTE. It is believed that when patients are able to ambulate to the bathroom or on the hallways, but have to come back, for any reason (eg, need for intravenous infusions or oxygen therapy, generalized weakness, pain, or dyspnea on exertion), and stay in bed or chair while hospitalized with an acute illness, they are at-risk for VTE.
Some studies tried to identify the loss of mobility as the main factor leading to VTE. CitationMotykie and colleagues (2000) evaluated 1,000 patients with Doppler ultrasound for suspected DVT and noted that there was a significant correlation between loss of mobility for more than 3 days and development of DVT. In a large case-control study with 1,272 ambulatory patients, CitationSamama (2000) showed that standing for more than 6 hours and resting in bed or chair were associated with an increased odds of VTE (OR 1.9; 95% CI 1.1–3.1 and OR 5.6; 95% CI 2.3–13.7, respectively). Similarly, CitationHeit and colleagues (2000) showed that hospitalization or admission to a long-term care facility increased the risk of VTE (OR 8.0; 95% CI 4.5–14.2). Other studies identify the reduction of mobility as a risk factor for VTE, but the definition of decreased mobility is either not clearly stated or it is quite variable, including complete bed rest for ≥ 5 days (CitationAnderson et al 1992), partial or complete bedrest for at least 10 days (CitationHarenberg et al 1996), reduced mobility for more than half of the day, at least during 7 days (CitationLechler et al 1996). Other authors noted that more serious loss of mobility, such as the incapacity to walk independently for more than 10 meters were frequently associated with the development of VTE (CitationAlikhan et al 2003). In a recent case-control study of hospitalized patients older than 65 years, reduced mobility was an independent risk factor for VTE (OR 1.73 to 5.64), depending on the degree of immobility () (CitationWeill-Engerer et al 2004). The risk was higher in patients with more severe limitation of mobility (bedrest vs wheel-chair) and when the loss of mobility was recent (<15 days vs ≥30 days).
Table 7 Reduced mobility as a risk factor for VTE
Based on presented data, we believe that the patient who stays in bed or chair for more than half of the day (excluding sleep time) must be considered as having reduced mobility and is at-risk for VTE (EVIDENCE B). The immobility that is more recent and severe is more strongly associated with the development of VTE.
Respiratory diseases
Respiratory diseases such as COPD and pneumonia are frequently cited as VTE risk factors, but studies evaluating specifically the impact of these conditions in the incidence of VTE are rare. Besides, the diagnosis of VTE in COPD patients is usually a challenge because PE may present simply as worsening of dyspnea in a patient with chronic respiratory failure. On the study performed in the community of Worcester, MA, the diagnosis of COPD was present in 18% of those with DVT and in 34% of patients with PE (CitationAnderson et al 1991). In autopsy studies, DVT has been diagnosed in 18% to 51% of COPD patients, giving an OR of 1.6 for emphysema as compared with controls with median age >81 years-old (CitationMitchell et al 1968; CitationJanssens et al 2001). In prospective cohorts of patients with exacerbation of COPD, DVT was detected in 9% of patients by venography and/or labeled fibrinogen (CitationPrescott et al 1981), in 11% by Doppler ultrasound (CitationSchonhofer and Kohler 1998; CitationErelel et al 2002), and in 29%, when combining ventilation-perfusion scanning and Doppler ultrasound (CitationMispelaere et al 2002). Patients treated for VTE with oral anticoagulation in a RCT were analyzed, and the authors demonstrated that chronic respiratory diseases increased the risk for recurrence of VTE (OR 1.91; 95% CI 0.85–4.26) (CitationDouketis et al 2000). On the other hand, the analyses of patients with risk factors for VTE in the MEDENOX study surprisingly revealed that chronic respiratory disease was not significantly associated with an increased risk (OR 0.6; 95% CI 0.38–0.92)(CitationAlikhan et al 2004). However, it is worthy noticing that these analyses were performed post hoc and based on secondary objectives of the MEDENOX study.
Prospective controlled studies evaluating the efficacy of VTE prophylaxis are helpful in identifying pulmonary conditions as risk factors for VTE because the rates of thrombosis can be compared between patients on control and treatment groups. CitationBelch and colleagues (1981), in a small study with patients with thoracic infection or CHF, showed a significant difference in the incidence of DVT diagnosed by labeled fibrinogen, favoring LDUH (5,000 IU 8–8h) versus placebo (4% vs 26%, respectively, p < 0.01). In another RCT, patients with exacerbation of COPD, requiring ventilatory support (the majority with thoracic infection), were randomized to LMWH (nadroparin 3,800 IU AXa or 5,700 IU AXa) or placebo (CitationFraisse et al 2000). Those receiving LMWH had significantly lower incidence of VTE, when compared with placebo (28.2% vs 15.5% respectively, p = 0.045). In the study THE-PRINCE (CitationKleber et al 2003), patients with severe respiratory diseases (SRD) or CHF were randomized to enoxaparin 40 mg/daily or LDUH 5,000 IU three times daily. The authors demonstrated that the incidence of VTE by venography was high in all patients, without significant differences among all patients receiving enoxaparin and LDUH (8.4% vs 10.4%), or among patients with SRD (7.1% vs 5.9%). The definition of SRD in this study was the presence of abnormalities in the pulmonary function tests, arterial blood gas analyses or both, and at least one of the following conditions: acute exacerbation of COPD, severe secondary pulmonary hypertension, pneumonia, interstitial lung disease, lung cancer and/or metastasis and life expectancy of less than 2 months. This description is broad enough to include the main pulmonary diagnoses that are associated with an increase risk of VTE in hospitalized medical patients, and was therefore, incorporated to our algorithm instead of each pulmonary disease separately. In summary, there is some controversy about the role of specific respiratory diseases as risk factors for VTE in hospitalized medical patients. However, in general, patients presenting diagnostic criteria for SRD have increased risk for VTE (EVIDENCE A).
Thrombophilias
Hereditary thrombophilias, particularly antithrombin III (ATIII), protein C (PC) and protein S (PS) deficiencies, and factor V Leiden (FVL) are well known risk factors for VTE. Several case-control studies and some prospective registries show level A evidence for these thrombophilias as risk factors for thrombosis (). In a recent prospective registry of thrombophilic families, the incidence of VTE was 16% among relatives with thrombophilia, against 1% among those without thrombophilia (RR 15.7; 95% CI 9.6–28.0) (CitationVossen et al 2004). In a multicenter study with 233 Italian families, CitationBucciarelli and colleagues (1999) showed that ATIII deficiency is associated with a higher risk for VTE than other genetic conditions (RR 4.4 for ATIII vs FVL, 2.6 for ATIII vs PS, and 1.9 for ATIII vs PC). CitationSimioni and colleagues (1999) reported that conditions such as surgery, trauma, immobilization, pregnancy, puerperium, and hormonal contraception increase the risk for thrombosis in patients with ATIII, PS or PC deficiencies. Mutation of the prothrombin gene has also been associated with increased risk for VTE in different populations (OR 2.0 to 11.5) (CitationPoort et al 1996; CitationBrown et al 1997; CitationCumming et al 1997; CitationHillarp et al 1997; CitationKapur et al 1997; CitationLeroyer et al 1998; CitationSouto et al 1998; CitationAznar et al 2000). It is appropriate to conclude that hereditary thrombophilias confer additional risk for VTE in hospitalized patients (EVIDENCE A).
Table 8 Annual incidence and VTE risk in hereditary thrombophilias
Hyperhomocysteinemia (HHC) has been considered a risk factor not only for arterial disease, but also for venous thrombosis. In 1998, den Heijer and colleagues (1998) reviewed 8 case-control studies in a meta-analysis, and showed correlations between fasting HHC and VTE (OR 2.5; 95% CI 1.8–3.5), and methionine-induced HHC and VTE (OR 2.6; 95% CI 1.6–4.4). Furthermore, the risk seems to be even higher in patients older than 60 years (OR 4.4; 95% CI 1.9–9.8) (Ray 1998). Thus, HHC should be considered a risk factor for VTE (EVIDENCE A).
Other risk factors
Although some conditions, such as, systemic arterial hypertension, diabetes mellitus and tobacco smoking are cited occasionally as potential risk factors for VTE, we did not find enough evidence to justify their inclusion on the list of factors that predispose hospitalized medical patients to the development of venous thrombosis.
VTE prophylaxis
Compared with surgical patients, there are few studies evaluating VTE prophylaxis in medical patients. Besides, the great range of clinical conditions and variations in individual characteristics make it difficult to create a single recommendation suitable for all patients or even define if there is superiority of one type or one particular regimen of heparin over the others. shows the evidence-based recommendations for prophylaxis, as they are found in the literature, for specific conditions and not for medical patients as a group. also shows that, in most studies, the regimens of heparin involve high prophylactic doses: LDUH 5.000 IU every 8 hours, enoxaparin 40 mg daily, dalteparin 5.000 IU daily or nadroparin also in high doses (3.800 IU for patients with less than 70 kg and 5.700 IU for those weighing 70 kg or more). All these studies have proved the efficacy of these regimens in decreasing the incidence of VTE. This leads to the initial conclusion that medical patients benefit from high prophylactic doses of heparin. Therefore, these high prophylactic doses are the ones recommended (GRADE I) for most hospitalized medical patients on the algorithm (). Only a few studies, usually with very few patients and some methodological flaws (CitationHarenberg et al 1996; CitationLechler et al 1996; CitationSamama et al 1999; CitationLeizorovicz et al 2004) have shown that low prophylactic doses of heparin have efficacy. Besides, in the MEDENOX study (CitationSamama et al 1999) that compared the high (40 mg) and low (20 mg) prophylactic doses of enoxaparin with placebo, only the higher dose reduced significantly the incidence of VTE. In the groups receiving 20 mg of enoxaparin, the incidence of DVT detected by phlebography was similar to that of the placebo group (14.5% vs 15.0%). Finally, a large randomized placebo-controlled trial, published after this review had been concluded (CitationCohen et al 2006) showed that fondaparinux, a synthetic, selective inhibitor of factor Xa, at a dose of 2.5 mg/day subcutaneously for 14 days was also effective for the prevention of VTE in older acutely ill medical patients.
Table 9 Evidence-based recommendations for VTE prophylaxis in patients with specific medical conditions Table FootnoteΨ
Four important RCTs evaluated VTE prophylaxis in medical patients as a group (CitationHarenberg et al 1996; CitationLechler et al 1996; CitationSamama et al 1999; CitationLeizorovicz et al 2004). Together they included 7,735 patients, with average ages of 68 to 74 years-old. Diseases and conditions listed as risk factors are presented on . The most common reasons for admission were CHF, respiratory insufficiency and infection. The most common risk factors were obesity, varices, cancer, active rheumatologic disorders and previous VTE. Enoxaparin (CitationLechler et al 1996; CitationSamama et al 1999), dalteparin (CitationLeizorovicz et al 2004), nadroparin (CitationHarenberg et al 1996), and LDUH (CitationHarenberg et al 1996; CitationLechler et al 1996) were used in these studies. CitationHarenberg and colleagues (1996) compared nadroparin 3.600 IU with LDUH 5.000 IU, every 8 hours in 1,968 medical patients. Although there were no differences on the efficacy of prophylaxis or on the rate of bleeding, more patients in the nadroparin group died (2.8% vs 1.2% in LDUH group, p = 0.02). However, CitationFraisse and colleagues (2000) evaluated nadroparin as VTE prophylaxis in 223 patients with acute exacerbation of COPD requiring mechanical ventilation and demonstrated the efficacy of this LMWH against placebo, without increased bleeding or death rates.
Table 10 The most common diseases and risk factors for VTE found in four large RCTs about efficacy of prophylaxis in hospitalized medical patientsTable Footnote1–Table Footnote4
Another important issue is how long the prophylaxis should be maintained. It is common belief among physicians that as soon as the patient is able to ambulate, the risk is over and prophylaxis could be discontinued. However, there is no support in the literature for this, and in all studies that included hospitalized medical patients with risk factors to VTE, prophylaxis was maintained for at least 6 to 14 days (CitationHarenberg et al 1996; CitationLechler et al 1996; CitationSamama et al 1999; CitationKleber et al 2003; CitationLeizorovicz et al 2004). In the PREVENT (CitationLeizorovicz et al 2004) study, authors are specific about the point that all patients received the medication (dalteparin or placebo) for 14 days, even if they were discharged earlier. Besides, in the MEDENOX (CitationHarenberg et al 1996; CitationLechler et al 1996; CitationSamama et al 1999; CitationLeizorovicz et al 2004) and in the PREVENT (CitationLeizorovicz et al 2004) studies, patients were reevaluated several weeks after prophylaxis was finished, and symptomatic VTE episodes were detected. We have not found any studies testing prophylaxis for less than 6 days. Until new data is available, it’s recommended that VTE prophylaxis be maintained for at least 6 to 14 days. There is only one study that we are aware of—the EXCLAIM study (Extended Prophylaxis for Venous Thromboembolism in Acutely Ill Medical Patients with Prolonged Immobilization)—that has evaluated the extension of prophylaxis beyond 14 days in medical patients. The preliminary results of this study were recently presented at the XXIth Congress of the International Society on Thrombosis and Haemostatis and suggest the extension of VTE prophylaxis with enoxaparin 40 mg/day for an additional 28 days, is beneficial for acutely ill medical patients that remain with severely impaired mobility and for those with moderate impairment of mobility plus ≥75 years-old, cancer, or history of VTE. The final manuscript has not yet been published.
Some conditions represent contraindications to heparin use and must have their risk weighed against the potential benefit of the prophylaxis. Active bleeding, allergies, and previous type II heparin induced thrombocytopenia are absolute contraindications; thrombocytopenia for other reasons, coagulopathies, recent surgeries, specially cranial and ocular, lumbar puncture, uncontrolled hypertension, active pepticulcer without bleeding, and renal failure with clearance lower than 30 mL/min are also considered contraindications (CitationLeizorovicz et al 2004; CitationSamama et al 1999). In patients with mild to moderate renal failure, LDUH is preferred over LMWH for VTE prophylaxis in medical patients, based on level C of evidence (Class IIa). If LMWH are chosen for patients with renal insufficiency, the measurement of anti-Xa activity is recommended to adjust LMWH doses (CitationHulot et al 2004).
Conclusion
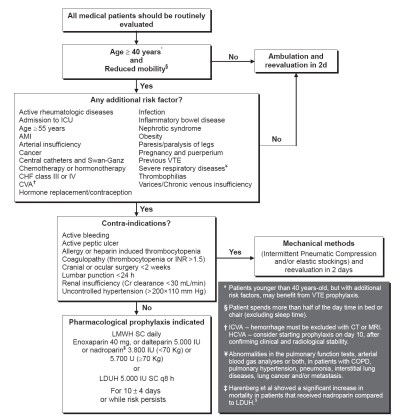
In summary, VTE prophylaxis is recommended for acutely ill, hospitalized medical patients, age 40 years or older, with reduced mobility and at least one additional risk factor for VTE, as suggested in the algorithm below (). Patients younger than 40 years of age, but presenting with important risk factors, may benefit from prophylaxis. When the algorithm for risk assessment indicates that VTE prophylaxis is recommended, LMWH once a day (enoxaparin 40 mg, dalteparin 5.000 IU, nadroparin 3.800 IU if ≤70 Kg or 5.700 IU if >70 Kg) or LDUH 5.000 UI SC every 8 h should be used and maintained for 6 to 14 days, even if the patient resumes ambulation or has early discharge. For patients older than 60 years, fondaparinux 2.5 mg once a day is also an option. If there is contraindication for pharmacological prophylaxis, mechanical methods of prophylaxis may be considered. However, all patients must be frequently reevaluated for the appearance of new indications or contraindications for prophylaxis during the hospitalization.
Key points
Hospitalized medical patients have increased risk of thromboembolic complications.
Our multidisciplinary group created an easy-to-use algorithm to facilitate the implementation of evidence-based recommendations for prophylaxis of hospitalized medical patients into practice.
In the absence of contraindications, hospitalized medical patients that are older than 40 years of age, have reduced mobility and at least one additional risk factor for VTE should be given high prophylactic doses of LDUH or LMWH for 6 to 14 days.
For the working committee
From the Academia Brasileira de Neurologia, Charles André, Marcia Maiumi Fukujima and Gabriel R de Freitas; from the Associação de Medicina Intensiva Brasileira, Silvia Lage; from the Federação Brasileira das Associações de Ginecologia e Obstetrícia, Cláudio Bonduki; do GETH, Carlos Carvalho, Eduardo Ramacciotti and Vera Lúcia Teixeira; from the Sociedade Brasileira de Angiologia e Cirurgia Vascular, Paulo R Mattos da Silveira; from the Sociedade Brasileira de Cancerologia, Clarissa Mathias; da Sociedade Brasileira de Cardiologia, José C Nicolau; from the Sociedade Brasileira de Clínica Médica, Renato D Lopes; da Sociedade Brasileira de Geriatria e Gerontologia, Salo Buksman and Verônica Hagemeyer; from the Sociedade Brasileira de Hematologia e Hemoterapia, élbio D’Amico; from the Sociedade Brasileira de Pneumologia e Tisiologia, Mário Terra Filho and from the Sociedade Brasileira de Reumatologia, Roger A Levy and Alexandre Wagner Silva de Souza.
References
- AbdollahiMCushmanMRosendaalFRObesity: risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive useThromb Haemost200389349349812624633
- AgraharkarMIsaacsonSMendelssohnDPercutaneously inserted silastic jugular hemodialysis catheters seldom cause jugular vein thrombosisASAIO J19954121691727640421
- AlikhanRCohenATCombeSRisk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX StudyArch Intern Med2004164996396815136304
- AlikhanRCohenATCombeSPrevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX studyBlood Coagul Fibrinolysis200314434134612945875
- AndersonFAJrWheelerHBGoldbergRJThe prevalence of risk factors for venous thromboembolism among hospital patientsArch Intern Med19921528166016641497399
- AndersonFAJrWheelerHBGoldbergRJA population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT StudyArch Intern Med199115159339382025141
- AznarJVayaAEstellesARisk of venous thrombosis in carriers of the prothrombin G20210A variant and factor V Leiden and their interaction with oral contraceptivesHaematologica200085121271127611114134
- BalestreriLDeCMMatovicMCentral venous catheter-related thrombosis in clinically asymptomatic oncologic patients: a phlebographic studyEur J Radiol19952021081117588863
- BathPMIddendenRBathFJLow-molecular-weight heparins and heparinoids in acute ischemic stroke : a meta-analysis of randomized controlled trialsStroke20003171770177810884486
- BeenenLvanLRDeenikBThe incidence of subclavian vein stenosis using silicone catheters for hemodialysisArtif Organs19941842892928024477
- BelchJJLoweGDWardAGPrevention of deep vein thrombosis in medical patients by low-dose heparinScott Med J19812621151177291971
- BellomoRAtkinsRCMembranous nephropathy and thromboembolism: is prophylactic anticoagulation warranted?Nephron19936332492548446259
- BellomoRWoodCWagnerIIdiopathic membranous nephropathy in an Australian population: the incidence of thromboembolism and its impact on the natural historyNephron19936322402418450923
- BennettWMLetter: Renal vein thrombosis in nephrotic syndromeAnn Intern Med19758345775781166998
- BergmannJFNeuhartEA multicenter randomized double-blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in-patients bedridden for an acute medical illness. The Enoxaparin in Medicine Study GroupThromb Haemost19967645295348902991
- BernsteinCNBlanchardJFHoustonDSThe incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatary bowel disease: a population-based cohort studyThromb Haemost20018543043411307809
- BernMMLokichJJWallachSRVery low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trialAnn Intern Med199011264234282178534
- BlaszykHWollanPCWitkiewiczAKDeath from pulmonary thromboembolism in severe obesity: lack of association with established genetic and clinical risk factorsVirchows Arch1999434652953210394888
- Blomstrom-LundqvistCScheinmanMMAliotEMACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias – executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias)Circulation2003108151871190914557344
- BoeerAVothEHenzeTEarly heparin therapy in patients with spontaneous intracerebral haemorrhageJ Neurol Neurosurg Psychiatry19915454664671865215
- BonifacjCQuereIDupuyCCase control studies of the risk factors for deep-vein thrombosis in an adult population hospitalized in internal medicineRev Epidemiol Sante Publique19974564654739496577
- BozzettiFScarpaDTernoGSubclavian venous thrombosis due to indwelling catheters: a prospective study on 52 patientsJPEN J Parenter Enteral Nutr1983765605626418913
- BrismarBHardstedtCJacobsonSDiagnosis of thrombosis by catheter phlebography after prolonged central venous catheterizationAnn Surg198119467797837305496
- BrownKLuddingtonRWilliamsonDRisk of venous thromboembolism associated with a G to A transition at position 20210 in the 3′-untranslated region of the prothrombin geneBr J Haematol19979849079099326187
- BucciarelliPRosendaalFRTripodiARisk of venous thromboembolism and clinical manifestations in carriers of antithrombin, protein C, protein S deficiency, or activated protein C resistance: a multicenter collaborative family studyArterioscler Thromb Vasc Biol19991941026103310195932
- BussaniRCosattiCPulmonary embolism: epidemiologic analysis of 27,410 autopsies during a 10-year periodMedicina (Firenze)199010140432381281
- CadeJFHigh risk of the critically ill for venous thromboembolismCrit Care Med19821074484507044682
- CasserlyLFDemberLMThrombosis in end-stage renal diseaseSemin Dial200316324525612753687
- ChastreJCornudFBouchamaAThrombosis as a complication of pulmonary-artery catheterization via the internal jugular vein: prospective evaluation by phlebographyN Engl J Med198230652782817054699
- ClarkDDAlbinaJEChazanJASubclavian vein stenosis and thrombosis: a potential serious complication in chronic hemodialysis patientsAm J Kidney Dis19901532652682305766
- CogoABernardiEPrandoniPAcquired risk factors for deep-vein thrombosis in symptomatic outpatientsArch Intern Med199415421641688285811
- CohenAQuinlanDPEP trial. Pulmonary Embolism PreventionLancet20003569225247110963215
- CohenATDavidsonBLGallusASEfficacy and safety of fondaparinux for the prevention of verous thromboembolism in older acute medical patients’ randomized placebo controlled trailBMJ200633232532916439370
- CookDAttiaJWeaverBVenous thromboembolic disease: an observational study in medical-surgical intensive care unit patientsJ Crit Care200015412713211138871
- CoonWWRisk factors in pulmonary embolismSurg Gynecol Obstet19761433385390959958
- CoubanSGoodyearMBurnellMA Randomized double-blind placebo-controlled study of low dose warfarin for the prevention of symptomatic central venous catheter-associated thrombosis in patients with cancer [abstract]Blood100suppl703a Blood 100(Suppl.), 703a. 2002. Ref Type: Abstract
- CounsellCSandercockPLow-molecular-weight heparins or heparinoids versus standard unfractionated heparin for acute ischaemic strokeCochrane Database Syst Rev2001no. 4CD00011911687069
- CummingAMKeeneySSaldenAThe prothrombin gene G20210A variant: prevalence in a U.K. anticoagulant clinic populationBr J Haemato1997982353355
- DeCMMatovicMBalestreriLAntithrombin III deficiency as a risk factor for catheter-related central vein thrombosis in cancer patientsThromb Res19957821271377482430
- DeCMMatovicMBalestreriLCentral venous thrombosis: an early and frequent complication in cancer patients bearing long-term silastic catheter. A prospective studyThromb Res19978621011139175232
- denHMRosendaalFRBlomHJHyperhomocysteinemia and venous thrombosis: a meta-analysisThromb Haemost19988068748779869152
- DickmannUVothESchichaHHeparin therapy, deep-vein thrombosis and pulmonary embolism after intracerebral hemorrhageKlin Wochenschr19886623118211833062268
- DouketisJDFosterGACrowtherMAClinical risk factors and timing of recurrent venous thromboembolism during the initial 3 months of anticoagulant therapyArch Intern Med2000160223431343611112236
- DouketisJDGinsbergJSHolbrookAA reevaluation of the risk for venous thromboembolism with the use of oral contraceptives and hormone replacement therapyArch Intern Med199715714152215309236553
- DurbecOViviandXPotieFA prospective evaluation of the use of femoral venous catheters in critically ill adultsCrit Care Med1997a2512198619899403747
- DurbecOViviandXPotieFLower extremity deep vein thrombosis: a prospective, randomized, controlled trial in comatose or sedated patients undergoing femoral vein catheterizationCrit Care Med1997b2512198219859403746
- ElliottCGZimmermanGAClemmerTPComplications of pulmonary artery catheterization in the care of critically ill patients. A prospective studyChest1979766647652510002
- EmersonPAMarksPPreventing thromboembolism after myocardial infarction: effect of low-dose heparin or smokingBr Med J1977160521820831965
- ErelelMCuhadarogluCEceTThe frequency of deep venous thrombosis and pulmonary embolus in acute exacerbation of chronic obstructive pulmonary diseaseRespir Med200296751551812194636
- FaioniEMValsecchiCPallaAFree protein S deficiency is a risk factor for venous thrombosisThromb Haemost1997785134313469408016
- FanPYSchwabSJVascular access: concepts for the 1990sJ Am Soc Nephrol1992311111391700
- FijnheerRHorbachDADondersRCFactor V Leiden, antiphospholipid antibodies and thrombosis in systemic lupus erythematosusThromb Haemost19967645145178902988
- FinazzisGBarbuiTDifferent incidence of venous thrombosis in patients with inherited deficiencies of antithrombin III, protein C and protein SThromb Haemost199471115188165635
- FolsomARAleksicNWangLProtein C, antithrombin, and venous thromboembolism incidence: a prospective population-based studyArterioscler Thromb Vasc Biol2002a2261018102212067914
- FolsomARCushmanMTsaiMYA prospective study of venous thromboembolism in relation to factor V Leiden and related factorsBlood2002b9982720272511929758
- FraisseFHolzapfelLCoulandJMNadroparin in the prevention of deep vein thrombosis in acute decompensated COPD. The Association of Non-University Affiliated Intensive Care Specialist Physicians of FranceAm J Respir Crit Care Med20001614 Pt 11109111410764298
- FranksALAtrashHKLawsonHWObstetrical pulmonary embolism mortality, United States, 1970–85Am J Public Health19908067207222343959
- GardlundBRandomised, controlled trial of low-dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study GroupLancet19963479012135713618637340
- GatewoodOMFishmanEKBurrowCRRenal vein thrombosis in patients with nephrotic syndrome: CT diagnosisRadiology198615911171223952296
- GeertsWSelbyRPrevention of venous thromboembolism in the ICUChest20031246 Suppl357S363S14668418
- GoldbergSKLippmanMLWalkersteinMDThe prevalence of DVT among patients in respiratory failure: the role of DVT prophylaxis [abstract]Am J Respir Crit Care Med1996153SupplA94
- GoldhaberSZDunnKMacDougallRCNew onset of venous thromboembolism among hospitalized patients at Brigham and Women’s Hospital is caused more often by prophylaxis failure than by withholding treatmentChest200011861680168411115458
- GoldhaberSZGrodsteinFStampferMJA prospective study of risk factors for pulmonary embolism in womenJAMA199727786426459039882
- GoldhaberSZKettDHCusumanoCJLow molecular weight heparin versus minidose unfractionated heparin for prophylaxis against venous thromboembolism in medical intensive care unit patients: a randomized controlled trial [abstract]J A Coll Cardiol200035suppl325A
- GoldhaberSZSavageDDGarrisonRJRisk factors for pulmonary embolism. The Framingham StudyAm J Med1983746102310286859053
- GoldhaberSZTapsonVFA prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosisAm J Cardiol200493225926214715365
- GolinVSprovieriSRBedrikowRPulmonary thromboembolism: retrospective study of necropsies performed over 24 years in a university hospital in BrazilSao Paulo Med J2002120410510812436156
- GoodnoughLTSaitoHManniAIncreased incidence of thromboembolism in stage IV breast cancer patients treated with a five-drug chemotherapy regimen. A study of 159 patientsCancer1984547126412686547874
- GouldJRCarlossHWSkinnerWLGroshong catheter-associated subclavian venous thrombosisAm J Med19939544194238213875
- GradyDWengerNKHerringtonDPostmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement StudyAnn Intern Med2000132968969610787361
- GregoryPCKuhlemeierKVPrevalence of venous thromboembolism in acute hemorrhagic and thromboembolic strokeAm J Phys Med Rehabil200382536436912704275
- HanssonPOErikssonHWelinLSmoking and abdominal obesity: risk factors for venous thromboembolism among middle-aged men: the study of men born in 1913Arch Intern Med1999159161886189010493318
- HarenbergJRoebruckPHeeneDLSubcutaneous low-molecular-weight heparin versus standard heparin and the prevention of thromboembolism in medical inpatients. The Heparin Study in Internal Medicine GroupHaemostasis19962631271398738587
- HarenbergJSchomakerUFloshbachCWEnoxaparin is superior to unfractionated heparin in the prevention of thromboembolic events in medical patients at increased thromboembolic risk [abstract]Blood199994suppl.1399a
- HeatonDCHanDYInderAMinidose (1 mg) warfarin as prophylaxis for central vein catheter thrombosisIntern Med J2002323848811885848
- HeitJAO’FallonWMPettersonTMRelative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based studyArch Intern Med2002162111245124812038942
- HeitJASilversteinMDMohrDNRisk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control studyArch Intern Med2000160680981510737280
- HillarpAZollerBSvenssonPJThe 20210 A allele of the prothrombin gene is a common risk factor among Swedish outpatients with verified deep venous thrombosisThromb Haemost19977839909929308741
- HillbomMErilaTSotaniemiKComparison of the efficacy and safety of the low-molecular-weight heparin enoxaparin with unfractionated heparin in the prevention of deep vein thrombosis in patients with acute ischemic stroke [abstract 183a]Blood19999410 Suppl 1183a
- HirschDRIngenitoEPGoldhaberSZPrevalence of deep venous thrombosis among patients in medical intensive careJAMA199527443353377609264
- HoarPFWilsonRMManganoDTHeparin bonding reduces thrombogenicity of pulmonary-artery cathetersN Engl J Med1981305179939957024812
- HoibraatenEAbdelnoorMSandsetPMHormone replace-ment therapy with estradiol and risk of venous thromboembo-lism – a population-based case-control studyThromb Haemost19998241218122110544901
- HoibraatenEQvigstadEArnesenHIncreased risk of recurrent venous thromboembolism during hormone replacement therapy – results of the randomized, double-blind, placebo-controlled estrogen in venous thromboembolism trial (EVTET)Thromb Haemost200084696196711154141
- HowellMDGeraciJMKnowltonAACongestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case-control studyJ Clin Epidemiol200154881081611470390
- HulleySGradyDBushTRandomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research GroupJAMA199828076056139718051
- HulotJSVantelonCUrienSEffect of renal function on the pharmacokinetics of enoxaparin and consequences on dose adjustmentTher Drug Monit200426330531015167633
- Ibarra-PerezCLau-CortesEColmenero-ZubiateSPrevalence and prevention of deep venous thrombosis of the lower extremities in high-risk pulmonary patientsAngiology19883965055133377270
- IbrahimEHIreguiMPrenticeDDeep vein thrombosis during prolonged mechanical ventilation despite prophylaxisCrit Care Med200230477177411940743
- IbrahimbachaAAlnajjarNImprovement in the utilization of venous thromboembolism (VTE) prophylaxis in the medical intensive care unit (MICU) [abstract]Chest1998114suppl392S
- JanssensJPHerrmannFMacGeeWCause of death in older patients with anatomo-pathological evidence of chronic bronchitis or emphysema: a case-control study based on autopsy findingsJ Am Geriatr Soc200149557157611380749
- JickHJickSSGurewichVRisk of idiopathic cardiovascular death and nonfatal venous thromboembolism in women using oral contraceptives with differing progestagen componentsLancet19953468990158915937500750
- JimenezRKupferYTesslerSPneumatic stockings do not decresase the incidence of deep venous trhombophlebitis in the critically ill [abstract]Crit Care Med200129suppl12A98 poster 317
- JoyntGMKewJGomersallCDDeep venous thrombosis caused by femoral venous catheters in critically ill adult patientsChest2000117117818310631217
- KakkarVVHoweCTNicolaidesANDeep vein thrombosis of the leg. Is there a high risk group?Am J Surg197012045275304097038
- KamranSIDowneyDRuffRLPneumatic sequential compression reduces the risk of deep vein thrombosis in stroke patientsNeurology1998506168316889633711
- KapoorMTesslerSKupferYSubcutaneous heparin prophylaxis significantly reduces the incidence of venous thromboembolic events in the critically ill [abstract]Crit Care Med19992712(Suppl.)A69
- KapurRKMillsLASpitzerSGA prothrombin gene mutation is significantly associated with venous thrombosisArterioscler Thromb Vasc Biol19971711287528799409269
- KauffmannRHVeltkampJJVan TilburgNHAcquired antithrombin III deficiency and thrombosis in the nephrotic syndromeAm J Med1978654607613707521
- KayaTIDurHTursenUAssociation of class I HLA antigens with the clinical manifestations of Turkish patients with Behcet’s diseaseClin Exp Dermatol200227649850112372094
- KemmerenJMAlgraAGrobbeeDEThird generation oral contraceptives and risk of venous thrombosis: meta-analysisBMJ2001323730513113411463678
- KendallAGLohmannRCDossetorJBNephrotic syndrome. A hypercoagulable stateArch Intern Med19711276102110275578557
- KerrTMLutterKSMoellerDMUpper extremity venous thrombosis diagnosed by duplex scanningAm J Surg199016022022062382774
- KierkegaardANorgrenLGraduated compression stockings in the prevention of deep vein thrombosis in patients with acute myocardial infarctionEur Heart J19931410136513688262083
- KierkegaardANorgrenLOlssonCGIncidence of deep vein thrombosis in bedridden non-surgical patientsActa Med Scand198722254094143425393
- KirazSErtenliIBenekliMClinical significance of hemostatic markers and thrombomodulin in systemic lupus erythematosus: evidence for a prothrombotic stateLupus19998973774110602446
- KleberFXWittCVogelGRandomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory diseaseAm Heart J2003145461462112679756
- KlerkCPSmorenburgSMBullerHRThrombosis prophylaxis in patient populations with a central venous catheter: a systematic reviewArch Intern Med2003163161913192112963564
- KniffinWDJrBaronJABarrettJThe epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderlyArch Intern Med199415488618668154949
- KocYGulluIAkpekGVascular involvement in Behcet’s diseaseJ Rheumatol19921934024101578454
- KoksoyCKuzuAErdenIThe risk factors in central venous catheter-related thrombosisAust N Z J Surg199565117967987487729
- KosterTRosendaalFRBrietEProtein C deficiency in a controlled series of unselected outpatients: an infrequent but clear risk factor for venous thrombosis (Leiden Thrombophilia Study)Blood19958510275627617742536
- KuhlmannUSteurerJRhynerKPlatelet aggregation and beta-thromboglobulin levels in nephrotic patients with and without thrombosisClin Nephrol19811552292356166421
- KuriakosePColon-OteroGPaz-FumagalliRRisk of deep venous thrombosis associated with chest versus arm central venous subcutaneous port catheters: a 5-year single-institution retrospective studyJ Vasc Interv Radiol200213217918411830624
- LacheradeJCCookDHeylandDPrevention of venous thromboembolism in critically ill medical patients: a Franco-Canadian cross-sectional studyJ Crit Care200318422823714691896
- LechlerESchrammWFlosbachCWThe venous thrombotic risk in non-surgical patients: epidemiological data and efficacy/safety profile of a low-molecular-weight heparin (enoxaparin). The Prime Study GroupHaemostasis199626Suppl (2)49568707167
- LeizoroviczACohenATTurpieAGRandomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patientsCirculation2004110787487915289368
- LensenRRosendaalFVandenbrouckeJFactor V Leiden: the venous thrombotic risk in thrombophilic familiesBr J Haematol2000110493994511054086
- LeroyerCMercierBOgerEPrevalence of 20210 A allele of the prothrombin gene in venous thromboembolism patientsThromb Haemost199880149519684784
- LeviDKupfterYCereviratnCComputerized order entry sets and intensive education improve the rate of prophylaxis for deep vein thrombophlebitis [abstract]Chest1998114supp280S
- LevineMNGentMHirshJThe thrombogenic effect of anticancer drug therapy in women with stage II breast cancerN Engl J Med198831874044073340118
- LewisMAHeinemannLAMacRaeKDThe increased risk of venous thromboembolism and the use of third generation progestagens: role of bias in observational research. The Transnational Research Group on Oral Contraceptives and the Health of Young WomenContraception19965415138804801
- LidegaardOEdstromBKreinerSOral contraceptives and venous thromboembolism. A case-control studyContraception19985752913019673836
- LindbladBSternbyNHBergqvistDIncidence of venous thromboembolism verified by necropsy over 30 yearsBMJ199130267787097112021744
- LlachFHypercoagulability, renal vein thrombosis, and other thrombotic complications of nephrotic syndromeKidney Int19852834294393906225
- LlachFArieffAIMassrySGRenal vein thrombosis and nephrotic syndrome. A prospective study of 36 adult patientsAnn Intern Med19758318141096703
- LlachFPapperSMassrySGThe clinical spectrum of renal vein thrombosis: acute and chronicAm J Med19806968198277446547
- LohrJWSchwabSJMinimizing hemorrhagic complications in dialysis patientsJ Am Soc Nephrol1991259619751760540
- LokichJJBeckerBSubclavian vein thrombosis in patients treated with infusion chemotherapy for advanced malignancyCancer1983529158615896616416
- MaderRZivMAdawiMThrombophilic factors and their relation to thromboembolic and other clinical manifestations in Behcet’s diseaseJ Rheumatol199926112404240810555901
- MaffeiFHFalleirosATVenezianCAIncidence and pathological anatomy of pulmonary thromboembolism in autopsies (author’s transl)AMB Rev Assoc Med Bras19802617106968934
- ManganoDTHeparin bonding and long-term protection against thrombogenesisN Engl J Med1982307148948957110267
- MarikPEAndrewsLMainiBThe incidence of deep venous thrombosis in ICU patientsChest199711136616649118705
- MartinCViviandXSauxPUpper-extremity deep vein thrombosis after central venous catheterization via the axillary veinCrit Care Med199927122626262910628601
- MartinelliIMannucciPMDeSVDifferent risks of thrombosis in four coagulation defects associated with inherited thrombophilia: a study of 150 familiesBlood1998927235323589746774
- MateoJOliverABorrellMIncreased risk of venous thrombosis in carriers of natural anticoagulant deficiencies. Results of the family studies of the Spanish Multicenter Study on Thrombophilia (EMET study)Blood Coagul Fibrinolysis19989171789607121
- MazzoneCChiodoGFSandercockPPhysical methods for preventing deep vein thrombosis in strokeCochrane Database Syst Rev2004no. 4CD00192215495020
- McCarthySTTurnerJLow-dose subcutaneous heparin in the prevention of deep-vein thrombosis and pulmonary emboli following acute strokeAge Ageing198615284883962762
- McCarthySTTurnerJJRobertsonDLow-dose heparin as a prophylaxis against deep-vein thrombosis after acute strokeLancet19772804280080171605
- MeredithJWYoungJSO’NeilEAFemoral catheters and deep venous thrombosis: a prospective evaluation with venous duplex sonographyJ Trauma19933521871908355295
- MerrerJDeJBGolliotFComplications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trialJAMA2001286670070711495620
- MianNZBaylyRSchreckDMIncidence of deep venous thrombosis associated with femoral venous catheterizationAcad Emerg Med1997412111811219408426
- MiddeldorpSHenkensCMKoopmanMMThe incidence of venous thromboembolism in family members of patients with factor V Leiden mutation and venous thrombosisAnn Intern Med1998128115209424976
- MiddeldorpSMeinardiJRKoopmanMMA prospective study of asymptomatic carriers of the factor V Leiden mutation to determine the incidence of venous thromboembolismAnn Intern Med2001135532232711529695
- MiehslerWReinischWValicEIs inflammatory bowel disease an independent and disease specific risk factor for thromboembolism?Gut200453454254815016749
- MillerJChanBKNelsonHDPostmenopausal estrogen replacement and risk for venous thromboembolism: a systematic review and meta-analysis for the U.S. Preventive Services Task ForceAnn Intern Med2002136968069011992304
- MillerRRLiesJECarrettaRFPrevention of lower extremity venous thrombosis by early mobilization. Confirmation in patients with acute myocardial infarction by 125I-fibrinogen uptake and venographyAnn Intern Med1976846700703937882
- MispelaereDGlerantJCAudebertMPulmonary embolism and sibilant types of chronic obstructive pulmonary disease decompensationsRev Mal Respir200219441542312417857
- MitchellRSWalkerSHSilversGWThe causes of death in chronic airway obstruction. I. The unreliability of death certificates and routine autopsiesAm Rev Respir Dis19689846016105677146
- MollenholtPErikssonIAnderssonTThrombogenicity of pulmonary-artery cathetersIntensive Care Med198713157593558936
- MonrealMAlastrueARullMUpper extremity deep venous thrombosis in cancer patients with venous access devices–prophylaxis with a low molecular weight heparin (Fragmin)Thromb Haemost19967522512538815570
- MonrealMKakkarAKCapriniJAThe outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registryJournal of Thrombosis and Haemostasis20042111892189815550017
- MonrealMRaventosALermaRPulmonary embolism in patients with upper extremity DVT associated to venous central lines–a prospective studyThromb Haemost19947245485507878630
- MotykieGDCapriniJAArcelusJIRisk factor assessment in the management of patients with suspected deep venous thrombosisInt Angiol2000191475110853685
- MuirKWWattABaxterGRandomized trial of graded compression stockings for prevention of deep-vein thrombosis after acute strokeQJM200093635936410873185
- NickolasTLRadhakrishnanJAppelGBHyperlipidemia and thrombotic complications in patients with membranous nephropathySemin Nephrol200323440641112923730
- NoelLHZanettiMDrozDLong-term prognosis of idiopathic membranous glomerulonephritis. Study of 116 untreated patientsAm J Med19796618290420255
- OgerEIncidence of venous thromboembolism: a community-based study in Western France. EPI-GETBP Study Group. Groupe d’Etude de la Thrombose de Bretagne OccidentaleThromb Haemost200083565766010823257
- OgerEBressolletteLNonentMHigh prevalence of asymptomatic deep vein thrombosis on admission in a medical unit among elderly patientsThromb Haemost2002a88459259712362229
- OgerELacutKLeGGVanDPIs APC resistance a risk factor for venous thromboembolism in patients over 70 years?Thromb Haemost2002b88458759112362228
- OgerELeroyerCLeMEThe value of a risk factor analysis in clinically suspected deep venous thrombosisRespiration19976453263309311047
- OrthSRRitzEThe nephrotic syndromeN Engl J Med199833817120212119554862
- PabingerIKyrlePAHeistingerMThe risk of thromboembolism in asymptomatic patients with protein C and protein S deficiency: a prospective cohort studyThromb Haemost19947144414458052960
- PambiancoGOrchardTLandauPDeep vein thrombosis: prevention in stroke patients during rehabilitationArch Phys Med Rehabil19957643243307717832
- PerezGSGarcia RodriguezLACastellsagueJHormone replacement therapy and risk of venous thromboembolism: population based case-control studyBMJ199731470837968009081000
- PinedaLAHathwarVSGrantBJClinical suspicion of fatal pulmonary embolismChest2001120379179511555511
- PinzonRDrewinkoBTrujilloJMPancreatic carcinoma and Trousseau’s syndrome: experience at a large cancer centerJ Clin Oncol1986445095143958764
- PittAAndersonSTHabersbergerPGLow dose heparin in the prevention of deep-vein thromboses in patients with acute myocardial infarctionAm Heart J19809955745787369096
- PoortSRRosendaalFRReitsmaPHA common genetic variation in the 3’-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosisBlood19968810369837038916933
- PottierPPlanchonBPistoriusMARisk factors for venous thromboembolism in hospitalized patients in internal medicine: case-control study of 150 patientsRev Med Interne2002231191091812481391
- PrasadBKBanerjeeAKHowardHIncidence of deep vein thrombosis and the effect of pneumatic compression of the calf in elderly hemiplegicsAge Ageing198211142447072559
- PrescottSMRichardsKLTikoffGVenous thromboembolism in decompensated chronic obstructive pulmonary disease. A prospective studyAm Rev Respir Dis1981123132367458085
- PrinsMHGelsemaRSingAKProphylaxis of deep venous thrombosis with a low-molecular-weight heparin (Kabi 2165/Fragmin) in stroke patientsHaemostasis19891952452502550335
- PritchardKIPatersonAHPaulNAIncreased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site GroupJ Clin Oncol19961410273127378874334
- RaadIILunaMKhalilSAThe relationship between the thrombotic and infectious complications of central venous cathetersJAMA199427113101410168139059
- RahimSAPanjuAPaiMVenous thromboembolism prophylaxis in medical inpatients: a retrospective chart reviewThromb Res200311145215219
- RandolphAGCookDJGonzalesCABenefit of heparin in central venous and pulmonary artery catheters: a meta-analysis of randomized controlled trialsChest199811311651719440585
- RayJGMeta-analysis of hyperhomocysteinemia as a risk factor for venous thromboembolic diseaseArch Intern Med199815819210121069801176
- RentschHPThromboembolism complications following acute hemiplegiasSchweiz Med Wochenschr198711747185318553423773
- RobertAOlmerMSampolJClinical correlation between hypercoagulability and thrombo-embolic phenomenaKidney Int19873138308352437353
- RochaATMuhlbaierMGovertJVenous Thromboembolism Prophylaxis With Sequential Compression Devices in Critically Ill Medical Patients: Analysis of the Project IMPACT Database [abstract]Chest2003124132Sa
- RochaATTapsonVFChoice of venous thromboembolic disease prophylaxis in medcial intensive care unit patients [abstract]Chest20021224 (suppl)212S12114328
- RodeghieroFTosettoAActivated protein C resistance and factor V Leiden mutation are independent risk factors for venous thromboembolismAnn Intern Med1999130864365010215560
- RossouwJEAndersonGLPrenticeRLRisks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trialJAMA2002288332133312117397
- RostokerGDurand-ZaleskiIPetit-PharMPrevention of thrombotic complications of the nephrotic syndrome by the low-molecular-weight heparin enoxaparinNephron199569120287891793
- RyskampRPTrottierSJUtilization of venous thromboembolism prophylaxis in a medical-surgical ICUChest199811311621649440584
- SamamaMMAn epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius studyArch Intern Med2000160223415342011112234
- SamamaMMCohenATDarmonJYA comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study GroupN Engl J Med19993411179380010477777
- SamuelssonEHaggSIncidence of venous thromboembolism in young Swedish women and possibly preventable cases among combined oral contraceptive usersActa Obstet Gynecol Scand200483767468115225194
- SandercockPAvan den BeltAGLindleyRIAntithrombotic therapy in acute ischaemic stroke: an overview of the completed randomised trialsJ Neurol Neurosurg Psychiatry199356117258429318
- SansonBJSimioniPTormeneDThe incidence of venous thromboembolism in asymptomatic carriers of a deficiency of antithrombin protein C, or protein S: a prospective cohort studyBlood199994113702370610572082
- SaphnerTTormeyDCGrayRVenous and arterial thrombosis in patients who received adjuvant therapy for breast cancerJ Clin Oncol1991922862941988575
- SarasinFPSchifferliJAProphylactic oral anticoagulation in nephrotic patients with idiopathic membranous nephropathyKidney Int19944525785858164448
- ScalaPJThiolletMMidavaineMDeep venous thrombosis and left ventricular thrombosis prophylaxis by low molecular weight heparin in acute myocardial infarctionHaemostasis19902063683691965978
- ScarabinPYOgerEPlu-BureauDifferential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism riskLancet2003362938242843212927428
- SchonhoferBKohlerDPrevalence of deep-vein thrombosis of the leg in patients with acute exacerbation of chronic obstructive pulmonary diseaseRespiration19986531731779670296
- ShustermanNHKlossKMullenJLSuccessful use of double-lumen, silicone rubber catheters for permanent hemodialysis accessKidney Int19893538878902709681
- SilversteinMDHeitJAMohrDNTrends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based studyArch Intern Med199815865855939521222
- SimioniPSansonBJPrandoniPIncidence of venous thromboembolism in families with inherited thrombophiliaThromb Haemost199981219820210063991
- SimioniPTormeneDPrandoniPIncidence of venous thromboembolism in asymptomatic family members who are carriers of factor V Leiden: a prospective cohort studyBlood20029961938194211877263
- SorensenHTMellemkjaerLSteffensenFHThe risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolismN Engl J Med199833817116911739554856
- SoutoJCCollILlobetDThe prothrombin 20210A allele is the most prevalent genetic risk factor for venous thromboembolism in the Spanish populationThromb Haemost19988033663699759610
- StrekerudFJohansenAMAbildgaardUVenous thromboembolism – incidence and risk factors in OsloTidsskr Nor Laegeforen199811825393439389830338
- SuissaSBlaisLSpitzerWOFirst-time use of newer oral contraceptives and the risk of venous thromboembolismContraception19975631411469347203
- The International Stroke Trial (IST)a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative GroupLancet19973499065156915819174558
- ThodiyilPAKakkarAKVariation in relative risk of venous thromboembolism in different cancersThromb Haemost20028761076107712083490
- TimsitJFFarkasJCBoyerJMCentral vein catheter-related thrombosis in intensive care patients: incidence, risks factors, and relationship with catheter-related sepsisChest199811412072139674471
- TosettoAFrezzatoMRodeghieroFPrevalence and risk factors of non-fatal venous thromboembolism in the active population of the VITA ProjectJ Thromb Haemost2003181724172912911584
- TrewPABiavaCGJacobsRPRenal vein thrombosis in membranous glomerulonephropathy: incidence and associationMedicine (Baltimore)19785716982618442
- TrottierSJVeremakisCO’BrienJFemoral deep vein thrombosis associated with central venous catheterization: results from a prospective, randomized trialCrit Care Med199523152598001386
- TveitDPHypoliteIOHshiehPChronic dialysis patients have high risk for pulmonary embolismAm J Kidney Dis20023951011101711979344
- van BovenHHVandenbrouckeJPBrietEGene-gene and gene-environment interactions determine risk of thrombosis in families with inherited antithrombin deficiencyBlood19999482590259410515862
- Van RoodenCJRosendaalFRMeindersAEThe contribution of factor V Leiden and prothrombin G20210A mutation to the risk of central venous catheter-related thrombosisHaematologica200489220120615003896
- VandenbrouckeJPRosingJBloemenkampKWOral contraceptives and the risk of venous thrombosisN Engl J Med2001344201527153511357157
- VanherweghemJLYassineTGoldmanMSubclavian vein thrombosis: a frequent complication of subclavian vein cannulation for hemodialysisClin Nephrol19862652352383802586
- VossenCYConardJFontcubertaJFamilial thrombophilia and lifetime risk of venous thrombosisJournal of Thrombosis and Haemostasis2004291526153215333025
- WagonerRDStansonAWHolleyKERenal vein thrombosis in idiopathic membranous glomerulopathy and nephrotic syndrome: incidence and significanceKidney Int19832323683746842962
- Weill-EngererSMeaumeSLahlouARisk factors for deep vein thrombosis in inpatients aged 65 and older: a case-control multicenter studyJ Am Geriatr Soc20045281299130415271117
- [WHO] World Health OrganisationVenous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone ContraceptionLancet19953468990157515827500748
- YooHHMendesFGAchados clínicopatológicos na tromboembolia pulmonar: estudo de 24 anos de autópsias*J Bras Pneumol2004305426432
- ZangariMAnaissieEBarlogieBIncreased risk of deep-vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapyBlood20019851614161511520815
- ZangariMBarlogieBAnaissieEDeep vein thrombosis in patients with multiple myeloma treated with thalidomide and chemotherapy: effects of prophylactic and therapeutic anticoagulationBr J Haematol2004126571572115327525
- ZawilskaKPsujaPLewandowskiKLow-dose heparin in the prevention of thrombotic complications following acute myocardial infarctionCor Vasa19893131791852670447
- ZouboulisCCButtnerPTebbeBAnticardiolipin antibodies in Adamantiades-Behcet’s diseaseBr J Dermatol199312832812848471511