Abstract
Removal of blood-based additives from recombinant clotting factor concentrates continues to be advocated by the hemophilia community due to the history of infectious disease transmission with previous blood-derived clotting factor concentrates. In 2003, octocog-alpha, antihemophilic factor (recombinant), plasma/albumin-free method (ADVATE®) was introduced, providing the first third-generation recombinant factor VIII (rFVIII) concentrate. Completed clinical trials have demonstrated ADVATE® to be safe and effective in adult and pediatric subjects utilizing both prophylactic and on-demand treatment regimens, and for perioperative hemostatic coverage. In the five completed studies involving more than 200 previously treated patients (PTPs), a single incidence of low-titer, non-persistent inhibitor was reported. Active post authorization safety surveillance (PASS) is being performed to expand the efficacy and safety profile of ADVATE® in routine clinical practice. Laboratory studies have documented the storage and post-reconstitution stability of ADVATE®, conferring the desired versatility for home treatment. The evolving real-world experience and ongoing studies will provide further insight into ADVATE® pharmacokinetics, alternative prophylactic dosing regimens, methods for perioperative hemostatic management, and utility in immune tolerance induction. Experience with ADVATE®, and its place in today’s treatment paradigm, is the focus of this article.
Introduction to the management of hemophilia
Hemophilia A is an X-linked bleeding disorder that results from insufficient levels of factor VIII (FVIII) coagulant activity and is characterized by a prolonged clotting time, most often measured by the activated partial thromboplastin time. Patients with severe disease (FVIII levels <1%) may present with spontaneous bleeding into joints, muscles, internal organs, or subsequent to trauma or surgery. Such bleeding episodes, if left untreated, result in serious complications including permanent, disabling joint, muscle, and nerve damage, loss of musculoskeletal function, or even death (CitationArun and Kessler 2000; CitationHilgartner 2002). Over the last century, hemostatic therapy for hemophilia A has evolved from using whole blood transfusion to that of highly purified plasma-derived FVIII (pdFVIII) concentrates, and in the last 15 years, recombinant FVIII (rFVIII) concentrates. With each advancement in therapy, the quality of life for affected individuals has improved.
FVIII concentrates are commonly used on-demand (to treat bleeding episodes), prophylactically (planned regular infusions for prevention or suppression of bleeding episodes or utilized to interrupt a bleeding pattern), and to provide hemostatic coverage during and after surgical procedures. With each generation of FVIII concentrates, new features have been introduced to improve pathogen safety and convenience. Advancements in therapy have made patients less dependent on treatment centers and provided them increased control of their disorder (CitationDunn and Abshire 2006).
A major complication in hemophilia management today is the development of inhibitors, antibodies that neutralize the infused FVIII. Inhibitors most often emerge within the first 50 exposure days (ED) of replacement therapy in patients with severe hemophilia A (CitationKey 2004). Although inhibitor incidence greatly decreases after this initial high-risk period, the risk does not reach zero and persists to some small degree throughout life (CitationKempton et al 2006). The immunogenicity of new clotting factor concentrates is evaluated in studies with previously treated patients (PTPs) that have demonstrated tolerance to FVIII through an extensive history of prior exposure to licensed products (ie, 100–150 previous ED) without inhibitor development (CitationWhite et al 1999).
In contrast, the rate of inhibitor development in previously untreated patients (PUPs) or minimally transfused patients is approximately 25%–30% (CitationLusher 2000). Studies and registries of various sizes and rigor in PTPs, who have demonstrated tolerance to FVIII therapies, have reported an inhibitor incidence of 1%–3% (CitationKey 2004).
Inhibitor kinetics and persistence vary; they are measured and quantified by the Bethesda assay (CitationKasper et al 1975) in the form of Bethesda units (BU). An estimated 20%–55% of all inhibitors disappear spontaneously without specific intervention; these inhibitors tend to be low titer and are defined as transient. Inhibitors that persist are divided into two main categories based upon the highest BU level achieved; low titer inhibitors are those that have titers <5BU despite repeated exposure to FVIII concentrate whereas high responding inhibitors are those whose historical titer is ≥5BU and often rise after repeat exposure, a phenomenon called anamnesis. Patients with low titer inhibitors are often continued with FVIII concentrates in doses sufficient to achieve a hemostatic level and control bleeding (CitationDiMichele 2002). Patients with higher titer inhibitors may become tolerant to FVIII following treatment on immune tolerance programs often utilizing prolonged periods of intensive FVIII therapy (CitationWight et al 2003). In some cases, however, immune tolerance is either not utilized or fails, and the inhibitor persists indefinitely; these patients are most often treated with bypassing therapies, agents that achieve hemostasis through alternative mechanisms to obtain bleed control. Bypassing agents, although highly effective in treating bleeding episodes, are not uniformly effective, leaving this population at risk of increased morbidity and mortality (CitationAstermark et al 2007).
History of pathogen transmission and the need for blood-free recombinant FVIII technology
The pathogen transmission safety record of factor concentrates used to manage hemophilia continues to be a major concern both for those receiving and prescribing these therapies. In the 1980s, many people with hemophilia were infected with HIV and/or hepatitis C (HCV) through the use of contaminated plasma derived clotting factor concentrates. As a result, the life-span for those with hemophilia who contracted these blood borne pathogens was negatively impacted, increasing their morbidity and mortality (CitationArnold 2006). Today, an estimated 30% of people with hemophilia between the ages of 21–60 years are infected with HIV (CitationCDC 2005). As a consequence, a number of safety measures including enhanced donor screening tests and procedures, and the introduction of viral inactivation and removal technologies, have been implemented to address blood-borne disease in plasma based therapies. The desire for therapies with the highest degree of safety has remained a driving force in therapeutic research and development. As a result, a variety of rFVIII products have been developed as safer alternatives to prevent transmission of potential emerging pathogens.
In 2006, the FDA released a statement that the risk of acquiring variant Creutzfeld-Jakob Disease (vCJD) is low, but could not be deemed to be zero, in people using US-licensed pdFVIII products (CitationFDA 2006). Based on these conclusions, the Great Lakes Hemophilia Foundation (GLHF) recommended that physicians and patients consider this risk when choosing an appropriate therapy (CitationMedical Advisory #406 2006). To this end, the National Hemophilia Foundation Medical and Scientific Advisory Council (MASAC) also recommended recombinant FVIII products as the treatment of choice for patients with hemophilia A (CitationMASAC #177 2006). The UK Department of Health independently created a “Recombinant for All” policy to ensure that hemophilia patients have access to the safest treatments (CitationUKHCDO 2003). Similar policies are now in effect in a number of countries, including Austria, Germany, Ireland, the Netherlands, Australia, and Canada (CitationAHCDC 2006; CitationEHC 2006; CitationNH 2006).
To date, there have not been any documented viral- or prion-based disease transmission reports from rFVIII products (CitationDunn and Abshire 2006). Despite the excellent safety record of rFVIII therapeutics, the risk for potential transmission of blood-borne infectious agents through the use of human- or animal-derived plasma proteins in rFVIII concentrates remained a concern. No current viral inactivation/elimination method, such as solvent/detergent methods or heat treatment, is infallible (CitationBrown 2001; CitationHoots 2001; CitationLlewelyn et al 2004). For example, three viruses are known to be a potential threat to the supply of blood and blood products; hepatitis A (HAV) is resistant to the solvent/detergent viral inactivation step, parvovirus B is both heat and solvent/detergent resistant, and transfusion transmitted virus (TTV) is resistant to heat, detergent, and disinfectants (CitationValentino and Oza 2006). Despite the present use of effective pathogen inactivation methods, international guidelines recommend that manufacturers make all efforts to remove human and animal proteins, including human albumin and other plasma-derived proteins, from the manufacturing process of recombinant products, and that such differences be taken into consideration when selecting an appropriate therapy (CitationMASAC #177 2006; CitationUKHCDO 2003; CitationAHCDC 2006).
Nearly all rFVIII concentrates use human- or animal-derived plasma proteins at some point in the processing. Recombinant FVIII concentrates now are classified into first, second, and third generation based on the use of human- or animal-derived materials at various stages of processing, as shown in .
Table 1 Commercial recombinant FVIII therapies
The first-generation product relies on plasma proteins in the production, purification, and final formulation of the product (CitationJing et al 2002; Package Insert, Recombinate®, Baxter BioScience). Second-generation products use plasma-derived proteins in the production and/or purification phase (Package Insert Kogenate® FS, Bayer Healthcare; Package Insert ReFacto®, Wyeth Pharmaceuticals, Inc), and trace levels of such proteins have been detected in the final container of selected products (CitationEriksson et al 2001; EPAR Kogenate Bayer; EPAR Helixate NexGen). In 2003, ADVATE® (Antihemophilic Factor [Recombinant], Plasma/Albumin-Free Method [rAHF-PFM]) was licensed as the first rFVIII that does not include animal or human plasma protein additives during the cell culture, purification, or formulation processes. The clinical experience of this full-length rFVIII is the focus of this article.
Development of the plasma/albumin-free platform
To develop ADVATE®, the Chinese hamster ovary (CHO) cell line used to produce the first-generation FVIII concentrate, Recombinate®, was adapted to grow in a proprietary culture medium, free of human and animal derived additives. This cell line, which co-expresses full-length FVIII and von Willebrand Factor (VWF), did not undergo any genetic manipulations, and the cDNA coding sequence of the recombinant proteins remained identical to those used for Recombinate®. To attain consistent and stable production of rFVIII, a continuous (chemostat) perfusion bioreactor culture is used to harvest the clotting factor with minimal environmental changes to the cell line.
The purification techniques utilized for ADVATE® are based on the methods used for its first generation predecessor. First, the rFVIII-containing cell culture medium is filtered to remove the CHO cells, followed by a sterile filtration step. The rFVIII protein is then purified in a stepwise fashion, using multiple chromatography columns, including immunoaffinity (to eliminate non-FVIII proteins) and cation exchange (using electrical charge differences to eliminate other impurities). This is followed by a solvent/detergent (S/D) step, a viral inactivation process which preserves the integrity of the structure and function of the FVIII molecule yet inactivates lipid enveloped infectious agents (CitationHorowitz et al 1993). Following the S/D step, the eluate is further purified using anion exchange chromatography. Finally, ADVATE® is pooled, frozen, and stored until ready for final formulation, filling, and lyophilization.
The absence of human- or animal-plasma protein additives eliminates the risk of pathogen transmission. Formulation components including the sugars trehalose and mannitol mimic the stabilizing function of albumin and have been substituted to replace plasma protein stabilizers (CitationProduct Monograph 2004).
Preclinical evaluation
Biochemical and preclinical analyses assessed toxicity, tolerability, and hemostatic activity of ADVATE®. The new formulation was compared with its well-established predecessor, Recombinate® (CitationBray et al 1994; CitationWhite et al 1997), demonstrating that ADVATE® and Recombinate® have structural and functional equivalence (CitationEwenstein 2004).
The approved route of administration for ADVATE® is via intravenous bolus infusion; however, in specific clinical circumstances such as surgery or severe or life-threatening bleeding events, FVIII products are administered via continuous infusion (CitationBatorova and Martinowitz 2002). Therefore, the stability and feasibility of ADVATE® delivery by continuous infusion were assessed in in vitro experiments that simulated this clinical application (CitationFernandez et al 2006). In the presence and absence of heparin (2 U/mL), ADVATE® at mid- (522 IU/vial) and high-potency (1210 IU/vial) doses, were run through a CADD-1 infusion pump at a rate of 1.5 mL/minute, at 30 °C, for 48 hours. Reconstituted control vials of the same lot were maintained at the same temperature, over the same duration. Stability and recovery of at least 80% of baseline potency was observed for up to 48 hours after reconstitution and running through the CADD-1 infusion pump at room temperature (30 °C) both in the presence and absence of added heparin (CitationFernandez et al 2006). Additional stability tests regarding the storage conditions of unreconstituted ADVATE® were also performed and will be discussed in the “Patient perspectives” section.
Clinical study program
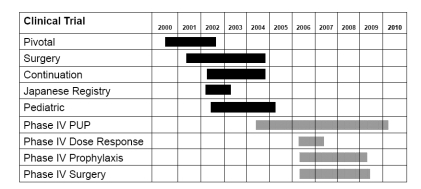
Five completed clinical trials have investigated the use of ADVATE® in the prevention and treatment of bleeding episodes in PTPs. illustrates the studies, patients, and endpoints measured in these completed phase II/III clinical studies.
Table 2 Global clinical program of ADVATE®
Pivotal efficacy and safety study
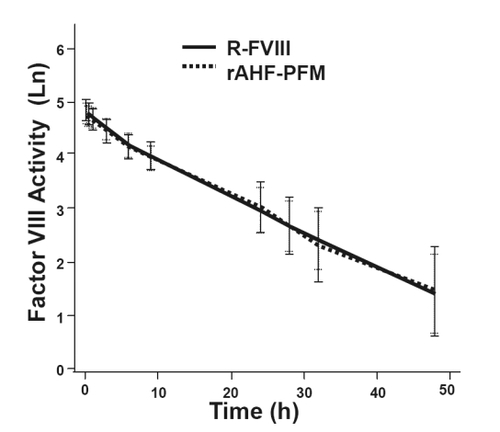
Results from a three-part study investigating the use of ADVATE® in 10- to 65-year-old PTPs without previous or detectable inhibitors have previously been reported (CitationTarantino et al 2004). Double-blinded, randomized, crossover pharmacokinetic (PK) evaluations compared pilot-scale ADVATE® (produced in Orth, Austria) with both commercial-scale ADVATE® (produced in Neuchatel, Switzerland) and with Recombinate®. Fifty-six subjects were randomized to Part 1 of the study comparing PK parameters of ADVATE® with Recombinate®. Subjects with severe or moderately severe hemophilia A (baseline FVIII level ≤2%) received an initial infusion (50 ± 5 IU/kg) of either ADVATE® or Recombinate® with analysis post-infusion per ISTH guidelines out to 48 hours. Following a wash-out period, subjects received a second infusion of the opposite product at a dose identical to the first infusion administered. Mean post-infusion plasma FVIII activity profiles were almost identical across all time points (), and the authors concluded bioequivalence in terms of area under the curve (AUC) and adjusted recovery.
Figure 1 PK comparisons of Recombinate® and ADVATE®. Post-infusion factor VIII levels (logarithmically adjusted for illustrative purposes) over time were similar with R-FVIII and ADVATE®. Data shown represent the natural logarithm FVIII activity (Ln) as a function of time in hours (h). Reprinted with permission from CitationTarantino MD, Collins PW, Hay CR, et al 2004. Clinical evaluation of an advanced category antihaemophilic factor prepared using a plasma/albumin-free method: pharmacokinetics, efficacy, and safety in previously treated patients with haemophilia A. Haemophilia, 10:428–37. Copyright © 2004 Blackwell Publishing.
Part 2 of the study was an open-label assessment of the efficacy, safety, and immunogenicity of ADVATE®. One hundred and eight subjects (baseline FVIII level ≤2%) followed a standard fixed prophylactic regimen over a period of at least 75 ED. The study product was infused while the subject was in a steady (non-bleeding) state, 3–4 infusions/week at a dose of 25–40 IU/kg, with the specific dose prescribed by the investigator and rounded up to the nearest whole vial. While on this treatment regimen, 30% of subjects (n = 32) did not experience a breakthrough bleeding episode, while 54% of the 510 bleeding episodes (n = 274) occurred in 70% of subjects (n = 75). In a post-hoc analysis, the overall bleed rate was correlated inversely with the degree of compliance with the prescribed prophylactic regimen. Subjects who were infused with ≤25 IU/kg per dose of ADVATE® for more than 20% of prophylactic infusions, and/or those who administered less than three infusions per week for more than 20% of the study (n = 37), experienced a 2.3-fold higher rate of bleeding when compared to subjects who complied with the prescribed prophylactic regimen, infusing at least 80% of the time with the appropriate prescribed dose (n = 70; p< 0.03). Of the 510 bleeding episodes that occurred during the study, 93% were resolved with 1–2 infusions of ADVATE®, and 86% were rated as Excellent/Good hemostatic response.
Part 3 was a double-blind, randomized, cross-over comparison of the pharmacokinetics of pilot-scale prepared ADVATE® with commercial-scale prepared ADVATE® to confirm bioequivalence of the product produced via two scales, pilot versus commericial. On completion of Part 2, 55 subjects were randomly assigned to receive an initial study infusion of either pilot-scale or commercial-scale ADVATE® at a dose of 50 ± 5 IU/kg. Following a wash-out period, subjects received a second infusion of the product produced from the other facility. The authors concluded that the products from the two facilities were bioequivalent (CitationTarantino et al 2004).
Of the 108 subjects who received ADVATE® during this study, one subject developed a low-level titer (2.0 BU), non-persistent inhibitor that was not detectable at an 8-week follow up visit. In addition, of the 867 adverse experiences (AE) categorized as non-serious, only 19, reported in 7 subjects, were judged to be possibly (n = 12) or probably (n = 7) related to the administration of ADVATE®. The 19 non-serious, related AEs consisted of taste perversion (n = 3), headache (n = 2), fever (n = 1), diarrhea (n = 1), dizziness (n = 3), hot flashes (n = 2), abdominal pain (n = 1), lower chest pain (n = 1), shortness of breath (n = 1), sweating (n = 1), nausea (n = 1), rigors (n = 1), and itching at the infusion site (n = 1). No subject withdrew due to an AE related to the study product. None of the serious AEs were regarded as possibly or probably related to study medications (CitationTarantino et al 2004).
Long-term efficacy and safety
The long-term efficacy and safety of ADVATE® in PTPs with severe or moderately severe hemophilia A (baseline FVIII level ≤2%) and no previous or detectable FVIII inhibitors were evaluated in an open-label, two-part continuation study of subjects who completed the pivotal phase III ADVATE® study (CitationTarantino et al 2004). The pharmacokinetics and safety profile of a single infusion of ADVATE® (50 ± 5 IU/kg) in subjects was compared before and after ≥75 ED to ADVATE®. Safety, immunogenicity, and hemostatic efficacy of ADVATE® of three different therapeutic regimens were evaluated: standard prophylaxis of 25–40 IU/kg, 3–4 times a week; modified prophylaxis as determined by the investigator; and on-demand therapy (CitationGruppo et al 2006). No clinically relevant differences were detected between pharmacokinetic parameters measured at the initiation of trial product and after ≥75 EDs in either the per-protocol (n = 22) or the intent-to-treat populations (n = 34).
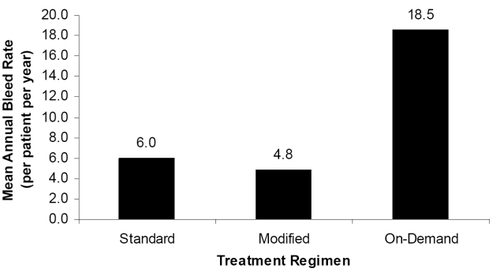
Eleven of 81 subjects reported no bleeding episodes, and all 11 were on a prophylactic treatment regimen for ≥90% of Part 2. Seventy of 81 subjects experienced 822 bleeding episodes, of which 820 were treated; 88.5% of the treated bleeding episodes were managed with one or two infusions. As shown in , annual bleed rates for patients on standard prophylaxis (n = 54), modified prophylaxis (n = 53), or on-demand treatment (n = 9) were 6.0, 4.8, and 18.5, respectively. Protocol-compliant subjects on standard prophylaxis experienced lower annual bleed rates (n = 30, 4.5 bleeds/year) compared with non-compliant subjects (n = 24, 7.9 bleeds/year). Hemostatic efficacy outcomes were sustained throughout a median participation interval of 617.5 days (CitationGruppo et al 2006).
Figure 2 Mean annual total bleed episode rate per regimen. Subjects on prophylactic regimens experienced fewer bleeding episodes than those using on-demand treatment.
No subjects developed a detectable inhibitor during the continuation study. After a combined total of 20,832 infusions, 79 of 82 subjects reported a total of 506 AEs. Four AEs, none of which were serious, were classified to be at least possibly related to the product, for an overall rate of 0.02% related AEs (4 AEs/20,832 infusions). Three of these were considered mild (headache, dysgeusia, and elevated liver function tests), and one moderate (moderately severe migraine). Of the 11 serious AEs reported by seven subjects, none was considered related to ADVATE® treatment. The authors concluded that pharmacokinetic parameters and the safety profile of ADVATE® were stable with long-term use (CitationGruppo et al 2006).
Efficacy during surgical, dental, or other invasive procedures
Medical management during surgery is complicated for patients with hemophilia due to the need to attend to hemostatic control. Surgery is commonly used to address complications resulting from repeated bleeding into joints and muscles. Surgery may also be required to address general medical issues as experienced in the non-hemophilic population. Management of hemostasis during surgical procedures requires the establishment and maintenance of appropriate target FVIII levels to prevent bleeding both during the operative, post-operative, and rehabilitative phases. Treatment regimens typically involve pre-, intra-, and post-operative administration of FVIII during hospitalization and continued replacement therapy following discharge dependent upon the length of hospitalization and the procedure performed.
The safety and hemostatic efficacy of ADVATE® in PTPs (≥150 ED) undergoing surgical, dental, or other invasive procedures was assessed in a multicenter, prospective, open-label, uncontrolled surgery study. Severe or moderately severe hemophilia A subjects (baseline FVIII level ≤2%) greater than 5 years of age without history or presence of an inhibitor were eligible to participate and were allowed to enroll repeatedly for distinct surgical procedures. Each procedure was counted individually (CitationNegrier et al 2006).
A total of 58 subjects underwent 65 procedures. Procedures were classified as either major (moderate to critical risk and/or estimated blood loss ≥500 mL), minor (mild risk and/or estimated blood loss <500 mL), or dental. ADVATE® was administered by continuous infusion in 18 procedures (4 major, 14 minor) and by bolus infusion in 47 procedures (18 major, 21 minor, and 8 dental). Blood loss was less than or within the expected range compared to a normal individual undergoing the same procedure in 55 of 58 assessed procedures, as determined by the surgeon. Intra- and postoperative hemostatic efficacy was rated “Excellent” or “Good” in 100% (n = 61 and 62, respectively) of assessed procedures (CitationNegrier et al 2006).
No serious AEs related to ADVATE® were reported during this study. Eight non-serious AEs were considered at least possibly related to ADVATE®: 2 mild AEs (lymphangitis and pruritus), 4 moderate AEs (one decreased hematocrit, one hemorrhage at time of drain removal, and 2 cases of edema at surgical site), and 2 severe (decreased coagulation FVIII coincident with a central venous access device-related infection and hematoma at surgical site). No patient developed an inhibitor during this study (CitationNegrier et al 2006).
Efficacy and safety in children
The pharmacokinetics, efficacy, safety, and immunogenicity of ADVATE® in PTPs less than 6 years of age with severe or moderately severe hemophilia A (baseline FVIII level ≤2%) were evaluated in a pediatric PTP study. This open-label, multi-center, prospective, uncontrolled, two-part study evaluated the use of ADVATE® in 53 children (mean age 3.1 ± 1.5 years) with previous FVIII history of at least 50 EDs, and without a history of an inhibitor. Part 1 consisted of a modified pharmacokinetic evaluation following a single 50 IU/kg infusion of ADVATE®. Part 2 evaluated the efficacy, immunogenicity, and safety of ADVATE® in pediatric PTPs while on standard prophylaxis (25–50 IU/kg, 3–4 times/week), investigator-modified prophylaxis, or on-demand therapy, as prescribed by the investigating physician. Mean plasma terminal phase half-life (9.88 ± 1.89 h) and adjusted in vivo recovery (1.90 ± 0.43 IU/dL per IU/kg) were lower in these young children compared to the adult population in the pivotal trial. These differences may have been influenced by methodology, including a delayed first time point in the pediatric patients (1 hour post-infusion), which is beyond the maximum peak level derived in many adult subjects in the pivotal trial. However, these differences may also have been impacted by physiological differences between young children and adults, as these differences were positively correlated with age and body mass index in the pediatric patients (CitationBlanchette et al 2006; CitationCollins et al 2006).
Bleeding episodes were infrequent in children on prophylaxis, possibly due to less underlying joint disease compared to their adult counterparts enrolled in the previously described trials. The majority (90.1%) of the 354 bleeding episodes were managed with one or two infusions of ADVATE®, and treatment efficacy was rated as “Excellent” or “Good” for 93.8% of the bleeding episodes (CitationBlanchette et al 2006).
Participants received a combined total of 7980 infusions of ADVATE® over a median of 156 exposure days (range: 14–384 days). During the study, six AEs in two subjects were considered to be related to ADVATE®, all of which were non-serious. No inhibitors were detected (CitationBlanchette et al 2006).
Perioperative management in children
The complexity of hemostatic management during surgery in children is compounded by challenges related to limited venous access and differing pharmacokinetic patterns such as decreased half-life and lower AUC, Cmax, and recovery compared with adults (CitationBlanchette et al 2006).
Efficacy and safety data of ADVATE® in pediatric (<16 years of age) PTPs undergoing surgical procedures were collected (CitationShapiro et al 2006) from the surgery and pediatric studies described herein. The subjects had no inhibitors and underwent elective or emergency surgical, dental, or an otherwise invasive procedure. Some individual subjects enrolled multiple times for separate surgical interventions; each procedure was counted individually. The study product was administered per each study site’s investigator-determined standard of care for surgical management of hemophilia patients. Patients could be treated via bolus injection or continuous infusion, and continuous infusion could be supplemented with bolus injections as needed. Procedures were classified as either major or minor as defined previously.
A total of 18 patients underwent 19 procedures: 2 major, 1 moderate, 13 minor, and 3 dental procedures. Sixteen procedures used one or more bolus injections, and 3 used continuous infusion with supplemental bolus injections. Intra-operative efficacy was rated as “Excellent” or “Good” in 100% (n = 14) of the assessed procedures. Postoperative efficacy was rated as “Excellent” or “Good” in 100% (n = 15) of the assessed procedures (CitationShapiro et al 2006). As shown in , 13 of 14 evaluable procedures had less than, or were within the predicted blood loss range for an unaffected individual undergoing the procedure. Although one patient was reported to experience greater than predicted blood loss in one procedure, no supplemental blood or blood products were required to maintain hemostasis (CitationShapiro et al 2006).
Table 3 Predicted and actual blood loss associated with surgical procedures
This case series highlights some key surgical scenarios encountered in the pediatric management of hemophilia. Known differences in pharmacokinetic parameters between children and adults have been documented (CitationBlanchette et al 2006; CitationTarantino et al 2004); however, the hemostatic assessment results of this study closely reflect the surgical experience reported in PTPs between 7 and 65 years of age most likely due to attention to these differences through monitoring of levels achieved (CitationNegrier et al 2006).
In this evaluation of pediatric patients, a total of 349 ADVATE® infusions were administered to PTPs <16 years old. Six serious AEs were reported, none of which were related to the product. Ninety-seven non-serious AEs were reported, of which one was judged as related to the product (mild pruritus). No inhibitors were detected, and no subject withdrew due to an AE (CitationShapiro et al 2006).
Summary of clinical data
Based on data from the completed clinical studies, ADVATE® has been shown to be efficacious, well-tolerated, and non-immunogenic in adult and pediatric PTPs with hemophilia A. When used prophylactically, on-demand, as well as perioperatively, ADVATE® was effective in preventing and/or controlling bleeding events (CitationTarantino et al 2004; CitationBlanchette et al 2006; CitationGruppo et al 2006; CitationNegrier et al 2006; CitationShapiro et al 2006). As expected, annual bleed rates were lower in subjects using ADVATE® prophylactically, especially in those adherent to prescribed regimens, compared to an on-demand treatment regimen (CitationTarantino et al 2004; CitationGruppo et al 2006).
The comprehensive clinical program for ADVATE® is illustrated in . This current report focuses on the completed studies in large cohorts of patients. Future work will focus on the ongoing Phase IV studies investigating dose responses, prophylactic outcomes, and comparative surgical outcomes in PTPs, as well as use in PUPs.
Safety and tolerability
The risk of inhibitor development with ADVATE® has been evaluated in a broad range of therapeutic settings and in patient groups that represent somewhat different inhibitor risk profiles. Data compiled from 5 completed clinical trials and registries include 208 unique PTPs, 198 of which had ≥10 ED and/or 6 months of observation during the study. Of these, one low-titer, non-persistent inhibitor was detected in one subject, for an overall incidence rate of 0.51% (95% confidence interval 0.03%–2.91%) (Unpublished data, Baxter BioScience). An on-going prospective post-licensure safety surveillance study designed to evaluate the first full year experience of new patients on ADVATE® will continue to accumulate data regarding the immunogenicity in a larger hemophilia population, including patients with mild or moderate hemophilia A, as well as patients with a history of inhibitor development. Thus, this study may provide a more accurate estimate of the incidence of inhibitors in real world practice.
Inhibitor management
Immune tolerance induction (ITI) in patients with inhibitors has been reported to be successful in a number of cases using ADVATE®. In one study, 3 PUPs (13–16 months of age) who developed inhibitors while on ADVATE® (1 low titer inhibitor patient and 2 high titer inhibitor patients) were successfully tolerized using ADVATE® (CitationRecht and Shapiro 2005). A follow-up chart review of 9 children (≤23 months old) who developed inhibitors on various recombinant products documented the use of ADVATE® for ITI (CitationValentino et al 2006). This report included 5 patients with low titer inhibitors (≤5 BU), 3 patients with high titer inhibitors (>5 BU) and 1 patient with a very high titer inhibitor (peak titer 1823 BU). The inhibitors were successfully eradicated in 8 of the 9 patients. Despite aggressive ITI, the inhibitor persisted in the patient with very high titer. No AEs were reported during this treatment period (CitationValentino et al 2006).
Patient perspectives
Hemophilia care has shifted from the paradigm of hospital or clinic based treatment of bleeding events to home based therapy utilizing diverse regimens ranging from on-demand to primary prophylaxis to immune tolerance induction (CitationRosendaal et al 1990). Prophylaxis as a long-term treatment regimen requires FVIII infusions 2–4 times per week and has been documented to be the best therapy for prevention of joint disease associated with musculoskeletal hemorrhage (CitationManco-Johnson 2003). Such a treatment regimen requires not only a commitment by the patient and/or caregiver, but requires safe and readily available infusion products; many of these previous barriers to prophylaxis have been addressed by the introduction of recombinant products. In addition to potential improvements in musculoskeletal outcomes, prophylaxis may also improve the psychosocial development of children by eliminating the anticipatory concern associated with potential future bleeding events (CitationBlanchette et al 2004).
FVIII concentrates that eliminate the risk of pathogen transmission not only increase patient confidence in the safety of the product but may also have long-term economical benefits as well. Pharmacoeconomic models of medical costs associated with co-morbidities suggest that hemophilia patients with HIV and HCV have higher medical costs compared to non-infected hemophilia patients due to increased hospitalization, more frequent office visits to physicians and specialists, and use of long-term health resources (CitationChan et al 2005; CitationLi-McLeod et al 2006). Although these scenarios are specific to HIV and HCV, it can be hypothesized that similar pharmacoeconomic findings might be applicable with possible emerging pathogens causing a chronically infected state. Thus, the use of therapies least likely to cause infectious co-morbidity may lead to avoidance cost-savings.
Patient convenience
To make home treatment practical coagulation products have been improved to make them more convenient, easier to use, and stable for longer periods of time under a variety of storage conditions. In addition, needle-less reconstitution devices have been developed for all rFVIII products. Such devices decrease the risk of patient or caregiver injury.
Because patients today are less reliant on hemophilia treatment centers, manufacturers have improved the stability and portability of rFVIII products to accommodate patients’ lifestyles. The US prescribing information for ADVATE® states that the lyophilized product should be stored under refrigeration (2–8 °C [36–46 °F]), but it may be stored at room temperature (up to 30 °C C[86 °F]) for a period of up to six months, not exceeding the expiration date (Package Insert, ADVATE®). To test real-world treatment situations, additional in vitro analyses demonstrated that ADVATE® remains stable beyond the recommended duration of storage (CitationParti et al 2005). Samples were stored for 2 weeks at 40 °C prior to being stored at 5, 25, or 30 °C for up to 24 months, and the activity was measured at 88.9%, 85.7%, and 83.5% of initial activity, respectively (CitationParti et al 2005). Likewise, when cycled three times through room temperature and refrigerated storage, the stability of ADVATE® was maintained (CitationParti et al 2006). Although not recommended by the manufacturer, these studies support the stability profile of ADVATE®.
Discussion
In response to the needs of the hemophilia community, ADVATE® was developed as the first third-generation hemophilia product with a safety and efficacy profile comparable with Recombinate® (R-FVIII, Baxter BioScience). The removal of blood-based additives from the entire manufacturing process eliminates the risk of blood borne disease transmission associated with such additives. All clinical trials completed to date demonstrate that ADVATE® is effective in adult and pediatric PTPs for the management of hemophilia A. As part of the continuing clinical program, four additional Phase IV studies are ongoing: 1) a PUP study, evaluating the pharmacokinetic parameters, efficacy, and safety profile of the product in previously untreated patients less than 6 years of age; 2) a dose response study, investigating the effect of 3 doses of ADVATE® on major pharmacokinetic parameters in PTPs with severe hemophilia A; 3) a prophylaxis study, comparing the efficacy, safety and immunogenicity of ADVATE® in PTPs with moderately severe to severe hemophilia A during standard prophylaxis, alternate modified prophylaxis, and on-demand regimens; and 4) a randomized surgery study comparing the efficacy and safety of ADVATE® when administered by continuous or bolus infusion during the intra- and post-operative setting of PTPs undergoing total knee replacement.
The availability of safe, effective, and convenient genetically engineered FVIII therapies has the potential to improve patient compliance and therefore enhance the quality of life for people living with hemophilia A.
Acknowledgements
The following investigators comprised the ADVATE Clinical Study Group:
Thomas Abshire, MD, USA; Carmen Altisent, MD, Spain, Erik Berntorp, MD, PhD, Sweden; Victor Blanchette, MD, Canada; Peter Collins, MD, UK; Donna Di Michele, MD, USA; Jorge Di Paola, MD, USA; Paul Giangrande, MD, UK; Joan Gill, MD, USA; Alessandro Gringeri, MD, Italy; Ralph Gruppo, MD, USA; Hideji Hanabusa, MD, Japan; Yoshiro Hatae, MD, Japan; Charles Hay, MD, UK; Hans-Hermann Brackmann, MD, Germany; Fernando Hernández, MD, Spain; Keith Hoots, MD, USA; Anne Marie Hurlet-Jensen, MD, USA; Katsuyuki Fukutake, MD, Japan; Jorgen Ingerslev, MD, Denmark; Eizou Kakishita, MD, Japan; Wolfhart Kreuz, MD, Germany; Roshni Kulkarni, MD, USA; Thierry Lambert, MD, France; Christine Lee, MD, UK; Raina Liesner, MD, UK; Christoph Male, MD, Austria; Marilyn Manco-Johnson, MD, USA; Catherine Manno, MD, USA; Pier Mannuccio Mannucci, MD, Italy; Pier Mannuccio Mannucci, MD, Italy; Peter Marks, MD, PhD, USA; Junichi Mimaya, MD, Japan; Claude Négrier, MD, France; Rachelle Nuss, MD, USA; Idith Ortiz, MD, Puerto Rico; Ingrid Pabinger, MD, Austria; Pia Petrini, MD, Sweden; Claire Philipp, MD, USA; Steven Pipe, MD, USA; Hartmut Pollmann, MD, Germany; Margaret Ragni, MD, USA; Bruce Ritchie, MD, Canada; Chantal Rothschild, MD, France; Hideaki Sakai, MD, Japan; Inge Scharrer, MD, Germany; Wolfgang Schramm, MD, Germany; Amy Shapiro, MD, USA; Midori Shima, MD, Japan; Akira Shirahata, MD, Japan; Martti Siimes, MD, Finland; Junki Takamatsu, MD, Japan; Masashi Taki, MD, Japan; Michael Tarantino, MD, USA; Alexis Thompson, MD, USA; Arthur Thompson, MD, USA; Marijke van den Berg, MD, The Netherlands; Jozef Vermylen, MD, Belgium; Mario Von Depka, MD, Germany; Irwin Walker, MD, Canada; Indira Warrier, MD, USA; Wing-Yen Wong, MD, USA.
The author gratefully acknowledges Kathleen Casey PhD, Gerald Spotts PhD, and Bruce M. Ewenstein MD, PhD for their thoughtful review and input on the manuscript.
References
- AHCDC, Association of Hemophilia Clinic Directors of Canada website [online] Accessed 11/20/2006. URL: http://www.ahcdc.ca/vWDManagement.html
- ArnoldDMJulianJAWalkerIRAssociation of hemophilia Clinic Director of CanadaMortality rates and causes of death among all HIV-positive individuals with hemophilia in Canada over 21 years of follow-upBlood2006108460416551974
- ArunBKesslerCMColmanRWClowesAWHirshJClinical manifestations and therapy of the hemophiliasHemostasis and thrombosis: basic principles and clinical practice20004Philadelphia, PALippincott, Williams Wilkins
- AstermarkJDonfieldSMDiMicheleDMA randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) studyBlood20071095465116990605
- BatorovaAMartinowitzUContinuous infusion of coagulation factorsHaemophilia200281707712010406
- BlanchetteVSManco-JohnsonMSantagostinoEOptimizing factor prophylaxis for the haemophilia population: where do we stand?Haemophilia200410Suppl 49710415479380
- BlanchetteVShapiroALiesnerRADVATE Antihemophilic Factor Plasma/Albumin Free Method (rAHF-PFM): Pharmacokinetics, safety and efficacy in previously treated patients less than 6 years old [poster]The Hemophilia 2006 World Congress2006Vancouver, Canada
- BrayGLGompertsEDCourterSA multicenter study of recombinant factor VIII (recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. The Recombinate Study GroupBlood1994832428358167332
- BrownPBovine spongiform encephalopathy and variant Creutzfeldt-Jakob diseaseBMJ2001322841411290640
- CDCCenters for Disease Control and Prevention. Report on the Universal Data Collection Program2005719
- ChanWWFriedmanHLi-McLeodJImpact of HIV and HCV co-infection on hospitalization and resource use among hemophilia enrollees in a managed care population200545th Annual Interscience Conference on Antimicrobial Agents and ChemotherapyWashington, D.C
- CollinsPBlanchetteVFischerKClinical implications of pharmacokinetic variables in the management of patients with severe hemophilia A [poster]Blood2006Orlando, FloridaAmerican Society of Hematology 2006
- DiMicheleDInhibitors: resolving diagnostic and therapeutic dilemmasHaemophilia20028280712010424
- DunnALAbshireTCCurrent issues in prophylactic therapy for persons with hemophiliaActa Haematol20061151627116549891
- EHC, European Haemophilia Consortium website [online]. Accessed 20 Nove 2006. URL: http://www.wfh.org/2/docs/Resources/EHC/EHC_Fact-sheet_2006.pdf
- EPAR Helixate NexGenScientific Discussion [online] Assesed 16 Feb 2007. URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/Helixatenexgen/301100en6.pdf
- EPAR Kogenate BayerScientific Discussion. [online]. Accessed 16 Feb 2007. URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/Kogenatebayer/101600en6.pdf
- ErikssonRKFengeCLindner-OlssonEThe manufacturing process for B-domain deleted recombinant factor VIIISemin Hematol200138243111449332
- EwensteinBMCollinsPTarantinoMDHemophilia therapy innovation: development of an advanced category recombinant factor VIII by a plasma/albumine-free methodSemin Hematol2004411, Suppl 211815071785
- [FDA] Food and Drug Administration; Center for Biologics Evaluation and Research2006Transmissible Spongiform encephalopathies advisory committee meetingDecember 15, 2006 Issue Summary
- FernandezMYuTBjornsonEStability of ADVATE, Antihemophilic Factor (Recombinant) Plasma/Albumin-Free Method, during simulated continuous infusionBlood Coagul Fibrinolysis2006171657116575253
- GruppoRCollinsPShapiroALong-term clinical evaluation of safety, efficacy and immunogenicity of rFVIII Plasma/Albumin Free Method (rAHF-PFM) in previously treated hemophilia A patients – Final Report [poster]The Hemophilia 2006 World Congress2006Vancouver, Canada
- HilgartnerMWCurrent treatment of hemophilic arthropathyCurr Opin Pediatr20021446911880733
- HootsWKHistory of plasma-product safetyTransfus Med Rev20011531011441415
- HorowitzBPrinceAMHorowitzMSViral safety of solvent-detergent treated blood productsDev Biol Stand199381147618174797
- JiangRMonroeTMcRogersRManufacturing challenges in the commercial production of recombinant coagulation factor VIIIHaemophilia20028Suppl 21511966844
- KasperCKAledortLMCountsRBA more uniform measurement of factor FVIII inhibitorsThromb Diath Haemorrh197534869721209552
- KemptonCLSoucieJMAbshireTCIncidence of inhibitors in a cohort of 838 males with hemophilia A previously treated with factor VIII concentratesJourn Thromb Haemost20064257681
- KeyNInhibitors in congenital coagulation disordersBr J Haematol20041273799115521914
- KwonJStuartRAddressing the need for improved convenience in hemophilia therapy: A vial reduction analysis in the United StatesThe Hemophilia 2006 World Congress2006Vancouver, Canada
- Li-McLeodJFriedmanHTencerTEvaluation of office care for hemophilia patients with HIV and HCV co-infection in a managed care populationHemophilia 2006 World Congress2006Vancouver, Canada
- LlewelynCAHewittPEKnightRSPossible transmission of variant Creutzfeldt-Jakob disease by blood transfusionLancet20043634172114962520
- LusherJMHemophilia treatment. Factor VIII inhibitors with recombinant products: prospective clinical trialsHaematologica20008525 discussion:5–611187864
- LuuHSpottsGEwensteinBImmunogenicity profile of ADVATE antihemophilic factor (recombinant), plasma/albumin-free method (rAHF-PFM) [poster]The Hemophilia 2006 World Congress2006Vancouver, Canada
- Manco-JohnsonMJUpdate on treatment regimens: prophylaxis versus on-demand therapySemin Hematol2003443, Suppl 33914690062
- MASAC #177Recommendations for physicians treating patients with hemophilia A and B, von Willebrand Disease, and other congenital bleeding disorders2006
- Medical Advisory #406FDA confirms low risk for Creutzfeldt-Jakob disease among persons using plasma-derived factor VIII products licensed in the US2006
- NegrierCShapiroABerntopEClinical efficacy and safety evaluation of ADVATE Antihemophilic Factor (Recombinant) Plasma/Albumin-Free Method (rAHF-PFM) during surgical and invasive procedures [poster]The Hemophilia 2006 World Congress2006Vancouver, Canada
- NHNew government contracts for recombinant haemophilia products (July 2006)National Hemophilia200615414
- Package InsertADVATE® [Antihemophilic factor (recombinant), plasma/albumin-free method] Prescribing InformationBaxter BioScience
- Package InsertKogenate FS® [Antihemophilic Factor (Recombinant), Formulated with Sucrose] Package InsertBayer HealthCare
- Package InsertReFacto® [Antihemophilic Factor (Recombinant)] Prescribing InformationWyeth Pharmaceuticals, Inc.
- Package InsertRecombinate® [Antihemophilic Factor (Recombinant]) Prescribing InformationBaxter BioScience
- PartiRLeeHYangLStability of plasma/albumin free full-length human factor FVIII (ADVATE rAHF-PFM) after temperature cycling between refrigerated and room temperatureThe Hemophilia 2006 World Congress2006Vancouver, Canada
- PartiRSchoppmannALeeHStability of lyophilized and reconstituted plasma/albumin-free recombinant human factor VIII (ADVATE rAHF-PFM)Haemophilia200511492616128893
- Product MonographADVATE [Antihemophilic Factor (Recombinant), Plasma-Albumin-Free Method]Baxter BioScience2004
- RechtMShapiroAInduction of immune tolerance utilizing third generation recombinant factor VIII [ADVATE]: A report of three cases [Poster]2005Sydney, AustraliaISTH
- RosendaalFRSmitCVarekampIModern haemophilia treatment: medical improvements and quality of lifeJ Intern Med1990228633402280241
- ShapiroAAbshireTHernandezFEfficacy and safety of ADVATE rAHF-PFM for perioperative management of hemostasis in previously treated children with hemophilia A [poster]The Hemophilia 2006 World Congress2006Vancouver, Canada
- TarantinoMDCollinsPWHayCRClinical evaluation of an advanced category antihaemophilic factor prepared using a plasma/albumin-free method: pharmacokinetics, efficacy, and safety in previously treated patients with haemophilia AHaemophilia2004104283715357767
- UKHCDOUnited Kingdom Haemophilia Centre Doctors’ Organisation. Guidelines on the selection and use of therapeutic products to treat haemophilia and other hereditary bleeding disordersHaemophilia20039123
- ValentinoLAOzaVMBlood safety and the choice of anti-hemophilic factor concentratePediatr Blood Cancer2006472455416724312
- ValentinoLARechtMDiPaolaJExperience with a third-generation recombinant factor VIII concentrate (ADVATE) for the induction of immune toleranceHemophila 2006 World Congress2006Vancouver, Canada
- WhiteGC2ndCourterSBrayGLA multicenter study of recombinant factor VIII (Recombinate) in previously treated patients with hemophilia A. The Recombinate Previously Treated Patient Study GroupThromb Haemost19977766079134639
- WhiteGCDiMicheleDMertensKUtilization of previously treated patients (PTPs), noninfected patients (NIPs), and previously untreated patients (PUPs) in the evaluation of new factor VIII and factor IX concentratesThromb Haemost19998146210102478
- WightJPaisleySKnightCImmune tolerance induction in patients with haemophilia A with inhibitors: a systematic reviewHaemophilia200394366312828679