Abstract
Over the past decade, 17 large placebo-controlled trials have established that statin therapy lowers LDL cholesterol and prevents cardiovascular events and death in patients with coronary disease or at high risk for atherosclerotic events. Nine trials of higher dose vs. lower dose statins (reporting data from 29,853 patients with coronary artery disease and 486 patients with other indications for statin therapy) have established that higher dose statin therapy is more efficacious than lower dose therapy in reducing myocardial infarctions/coronary death (by 16%) and stroke (by 18%) in patients with coronary disease but only reduces all-cause mortality in patients at high risk for coronary death (such as patients immediately after acute coronary syndrome). Higher dose statins are associated with statistically significantly increased risks of myopathy and elevated transaminases compared to lower dose statins; while relative risks for these outcomes are 1.2 and 4.0, the absolute increases are small (0.5% and 1%). Secondary analyses of these trials using individual patient data and multivariate adjustment will be needed to appropriately examine the incremental benefits of different LDL targets, and trials are needed to determine whether combinations of low dose statins plus other lipid lowering agents may achieve better clinical outcomes than higher dose statin therapy alone.
Over the past decade, an impressive number of randomized trials have confirmed that treatment with statins prevents cardiovascular events and improves survival in patients with a history of cardiovascular events as well as in patients who have not yet had an event but are at increased risk due to elevated cholesterol levels, diabetes mellitus, or hypertension (CitationLaw et al 2003; CitationCholesterol Treatment Trialists 2005). As a result of this randomized trial evidence, the indications for statin drugs have expanded rapidly and current guidelines recommend statin use in patients with, or at high risk for, atherosclerosis (CitationGrundy et al 2004; Joint British Societies 2005; CitationKhan et al 2006). Although meta-regression analyses of these randomized trials have confirmed that statins appear to exert their beneficial effects primarily via reduction of LDL cholesterol levels (CitationRobinson et al 2005), the target LDL for patients with coronary artery disease (CAD) has remained a point of debate as this evidence base has accumulated.
Although large observational studies consistently demonstrate a strong log-linear relationship between blood cholesterol levels and coronary mortality (CitationVerschuren et al 1995; CitationPadwal et al 2001), these studies have not identified a threshold level for LDL cholesterol which separates those who will suffer a coronary event from those who will not (CitationChen et al 1991; CitationO’Keefe 2004). While the cardiovascular relative risk reductions achieved with statin therapy appear to be similar regardless of baseline cholesterol levels in the large placebo-controlled randomized trials published thus far (), all of these trials enrolled patients with LDL cholesterols above 3.0 mmol/L. Further, while subgroup analyses demonstrated similar benefits across baseline LDL cholesterol levels, it should be recognized that even the lowest tertile in these analyses incorporated patients with baseline LDL cholesterols as high as 3.5 mmol/L. Thus, the question of “how low should we go in lowering LDL cholesterol” remained unanswered even after these 17 placebo-controlled statin trials were completed.
Table 1 Placebo-controlled statin trials which randomized greater than 250 participants
In an attempt to address the issue of optimal LDL cholesterol target levels, recent statin trials have compared higher dose statin therapy to the lower dose statin therapy employed in the 17 placebo-controlled trials which established the efficacy of statins for preventing cardiovascular events (outlined in ). It deserves emphasis that since these trials compared different fixed doses of statins with each other rather than titrating drug doses to achieve different target LDL levels, they cannot provide an unconfounded answer to the target LDL question. While this may seem like an argument over semantics, it is not. As pointed out by others, “compared with empirically treating patients…with statin doses similar to those used in clinical trials, titrating lipid therapy to recommended LDL cholesterol goals entails considerably greater clinical complexity, frequent use of multidrug therapy, and greater…costs” (CitationHayward et al 2006, page 521). Given the paucity of published evidence exploring the benefits of different LDL cholesterol targets in unconfounded analyses, our review will instead explore the question that can be answered from the existing trial literature – what statin dose should we use in patients with coronary heart disease? In order to answer this question, in this review we will examine the evidence from randomized trials comparing higher dose statin therapy versus lower dose statin therapy.
The randomized trials of higher dose statins versus lower dose statins
Trials reporting surrogate endpoints
The ASAP trial
The Effect of Aggressive versus conventional lipid lowering in Atherosclerosis Progression in familial hypercholesterolemia trial randomized 325 patients with familial hypercholesterolemia to atorvastatin 80 mg daily or simvastatin 40 mg daily (CitationSmilde et al 2001). The primary endpoint was change in atheroma volume as assessed by quantitative B-mode ultrasound of carotid intima media thickness (IMT). LDL cholesterol lowering was significantly greater (p = 0.0001) with atorvastatin (from 8.00 mmol/L to 3.88 mmol/L) than simvastatin (from 8.33 mmol/L to 4.81 mmol/L). After 2 years, IMT decreased in the patients randomized to atorvastatin therapy (−0.031 mm, 95% CI −0.007 to −0.055) but increased in the simvastatin-treated patients (+0.036 mm, 95% CI + 0.014 to + 0.058) – this between-group difference was highly statistically significant (p = 0.0001). Both treatment regimens were equally well tolerated.
The ARBITER trial
The Arterial Biology for the Investigation of the Treatment Effects of Reducing cholesterol trial randomized 161 patients meeting National Cholesterol Education Program II criteria for lipid-lowering therapy (46% of whom had known cardiovascular disease) to atorvastatin 80 mg daily or pravastatin 40 mg daily (CitationTaylor et al 2002). The primary endpoint was change in carotid IMT. LDL cholesterol lowering was significantly greater (p < 0.001) with atorvastatin (from 3.80 mmol/L to 1.95 mmol/L) than pravastatin (from 3.98 mmol/L to 2.82 mmol/L) at 12 months. After 12 months, IMT decreased in the patients randomized to atorvastatin therapy (−0.034 mm ± 0.021 mm) but did not appreciably change in the simvastatin-treated patients (+0.025 mm ± 0.017 mm) – this between-group difference was statistically significant (p = 0.03). No patient in either treatment arm suffered a drug-related side effect.
Trials reporting clinical endpoints
The Post-CABG trial
The Post Coronary Artery Bypass Graft Trial randomized 1351 patients who had undergone bypass surgery in the preceding decade, still had at least one patent vein graft on angiography, and who had an LDL cholesterol level between 3.34 mmol/L and 4.49 mmol/L to aggressive or moderate intensity treatment to lower LDL cholesterol levels (with lovastatin and, if needed, cholestyramine) and, using a two-by-two factorial design, to treatment with warfarin or placebo (CitationThe Post Coronary Artery Bypass Graft Trial Investigators 1997). The primary endpoint was the per-patient percentage of initially patent major grafts that had substantial progression of atherosclerosis (a decrease of 0.6 mm or more in lumen diameter) at the site of greatest change at follow-up. During follow-up, patients assigned to the aggressive lipid treatment group were taking a mean of 76 mg lovastatin daily (30% were also taking 8 g of cholestyramine daily) and patients assigned to the moderate lipid treatment group were taking a mean of 4 mg of lovastatin daily (and only 5% were also taking 8 g of cholestyramine daily). LDL cholesterol was reduced to 2.4 mmol/L in the aggressive treatment group and 3.5 mmol/L in the moderate treatment arm. After a mean follow-up of 4.3 years, the number of grafts demonstrating substantial progression of disease was statistically significantly reduced in the aggressive-treatment group (27% vs. 39%, p < 0.001). No significant differences in angiographic outcomes were observed between the warfarin and placebo groups and there was no interaction between warfarin and aggressive lipid lowering. Clinical events and adverse effects were uncommon and did not differ significantly between groups.
The REVERSAL trial
The REVERSal of atherosclerosis with Aggressive Lipid lowering Trial randomized 654 patients with angiographically proven CAD to atorvastatin 80 mg daily or pravastatin 40 mg daily (CitationNissen et al 2004). The primary endpoint was change in atheroma volume assessed by intravascular ultrasound. LDL cholesterol lowering was significantly greater (p < 0.001) with atorvastatin (from 3.86 mmol/L to 2.03 mmol/L) than pravastatin (from 3.86 mmol/L to 2.84 mmol/L) at the end of the 18 month follow-up in this trial. After 18 months, atheroma volume progressed in the pravastatin arm (+2.7%, 95% CI 0.24% to 4.67%) but was stable in the patients randomized to atorvastatin therapy (−0.4%, 95% CI −2.35% to +1.49%) – this between-group difference was statistically significant (p = 0.02). The beneficial effects of atorvastatin on atheroma progression were seen in all 23 subgroups examined. Both treatment regimens were equally well tolerated, although there were too few cardiovascular events (15) or drug-related discontinuations/adverse effects (43) for useful comparisons between treatment arms.
The PROVE IT- TIMI 22 trial
The Pravastatin or Atorvastatin Evaluation and Infection Therapy – Thrombolysis in Myocardial Infarction 22 Trial randomized 4,162 patients with acute coronary syndrome to either atorvastatin 80 mg daily or pravastatin 40 mg daily (CitationCannon et al 2004). The primary endpoint was a composite of death from any cause, myocardial infarction, unstable angina requiring hospitalization, revascularization (performed at least 30 days after randomization), or stroke. LDL cholesterol lowering was significantly greater (p < 0.001) with atorvastatin (from 2.74 mmol/L to 1.60 mmol/L) than pravastatin (from 2.74 mmol/L to 2.46 mmol/L) at the end of the trial (24 months). At the end of 24 months, the rates of the primary endpoint were 22.4% in the atorvastatin group and 26.3% in the pravastatin group – this between-group difference was statistically significant (p = 0.005). The beneficial effects of atorvastatin on the primary endpoint were consistent across all 17 subgroups examined, with the benefits appearing to be greater in those patients with baseline LDL cholesterol levels of at least 3.2 mmol/L (p = 0.02 for interaction). The intensive treatment arm had a statistically significant increase in rates of alanine aminotransferase elevations when compared to the moderate lipid lowering treatment arm (p < 0.001), but there were no significant differences between groups in myopathy or drug discontinuation rates.
Phase Z of the A to Z trial
Phase Z of the A to Z Trial randomized 4,497 patients with acute coronary syndrome (ACS) to receive 40 mg of simvastatin daily for 1 month followed by 80 mg daily thereafter or placebo for 4 months followed by 20 mg of simvastatin daily (Citationde Lemos et al 2004). The primary endpoint was a composite of cardiovascular death, nonfatal myocardial infarction, readmission for ACS, or stroke. LDL cholesterol lowering was significantly greater (p < 0.001) with the higher dose simvastatin group (from 2.90 mmol/L to 1.71 mmol/L) than the lower dose simvastatin group (from 2.87 mmol/L to 2.10 mmol/L) at the end of the trial (24 months). After 24 months, the primary endpoint occurred in 14.4% of the higher dose simvastatin group and 16.7% in the lower dose group – this between-group difference was not statistically significant (p = 0.14). There were no significant treatment interactions in any of the 21 subgroups examined. The higher dose treatment arm demonstrated statistically significant increases in liver enzyme elevations and myopathy when compared to the lower dose treatment arm.
The Vascular Basis for the Treatment of Myocardial Ischemia Study
The Vascular Basis for the Treatment of Myocardial Ischemia Study randomized 300 patients with stable CAD and a positive exercise stress test to one of 3 groups: atorvastatin 80 mg daily, atorvastatin 80 mg daily plus vitamins C and E, or the control group of low dose (median 5 mg) lovastatin (CitationStone et al 2005). The primary endpoint was number of ischemic episodes on ambulatory ECG monitoring. LDL cholesterol lowering was significantly greater (p < 0.0001) in both of the atorvastatin groups (from 3.9 mmol/L to 2.2 mmol/L) than in the lovastatin (from 3.9 mmol/L to 3.2 mmol/L) at the end of the trial (12 months). After 12 months, patients in all 3 treatment arms experienced significant declines in the frequency and duration of myocardial ischemia episodes – between 31% and 61%, but with no statistically significant difference between groups (p = 0.15). There were too few clinical events (12 deaths, MI, unstable angina, or stroke) to draw conclusions between treatment arms and no adverse events were reported.
The TNT trial
The Treating to New Targets Trial randomized 10,001 patients with stable CAD to either atorvastatin 80 mg daily or atorvastatin 10 mg daily after completion of an open-label run-in period (CitationLaRosa et al 2005). The primary endpoint was the occurrence of a first major cardiovascular event, defined as death from coronary heart disease, nonfatal non-procedure-related myocardial infarction, resuscitation after cardiac arrest, or fatal/nonfatal stroke. LDL cholesterol lowering was significantly greater (p < 0.001) with high dose atorvastatin (from 2.6 mmol/L to 2.0 mmol/L) than low dose atorvastatin (remained unchanged from post run-in period value of 2.6 mmol/L) at the end of the trial (4.9 years). After 4.9 years, 8.7% of patients in the higher dose group had a primary event vs 10.9% in the lower dose group – this between-group difference was statistically significant (p < 0.001). When compared to patients receiving 10 mg of atorvastatin, those receiving 80 mg of atorvastatin demonstrated statistically significant increases in liver enzyme elevations as well as increased rates of drug discontinuation due to adverse events (p < 0.001 for both).
The IDEAL trial
The Incremental Decrease in End points through Aggressive Lipid lowering Trial randomized 8,888 with stable CAD to atorvastatin 80 mg daily or simvastatin 20 mg daily (CitationPedersen et al 2005). The primary endpoint was time to first occurrence of a major coronary event, defined as coronary death, hospitalization for nonfatal acute myocardial infarction, or cardiac arrest with resuscitation. LDL cholesterol lowering was significantly greater (p < 0.001) with atorvastatin (from 3.15 mmol/L to 2.07 mmol/L) than simvastatin (from 3.14 mmol/L to 2.58 mmol/L). After 4.8 years, 9.3% of the atorvastatin group had suffered a primary outcome vs 10.4% of the simvastatin group – this between-group difference was not statistically significant (p = 0.07). Patients in the high dose treatment arm had a statistically significant increases in liver enzyme elevations and adverse events leading to drug discontinuation when compared to those in the lower dose treatment arm (both p < 0.001).
Summary of the higher dose vs lower dose statin trials
These 9 trials report data from 29,853 patients with coronary artery disease and 486 patients with other indications for statin therapy randomized to higher dose vs. lower dose statin therapy. Study participants in the 7 trials of secondary prevention were demographically similar, although baseline LDL cholesterols ranged between 2.74 mmol/L in PROVE IT to 3.98 mmol/L in Post-CABG (). These trials were methodologically robust (all scored greater than 3 on the 5-point Jadad scale for randomized trials) but some potential threats to the generalizability of study results are worth pointing out. For example, the TNT trial excluded almost half of those initially screened and included a run-in phase before randomization (both design features can bias the results towards an underestimation of adverse effects and an overestimation of benefits since patients who are identified to be at increased risk for adverse effects due to comorbidities at screening, or indeed suffer adverse effects during the run-in phase, are excluded). Further, the proportion of patients using statins prior to randomization varied widely amongst the trials, with only A-to-Z excluding anyone previously using statins (inclusion of patients previously exposed to a medication can also bias results towards an underestimation of adverse effects and an overestimation of benefits).
Table 2 Randomized trials comparing higher dose statin therapy with lower dose statin therapy in patients with coronary artery disease
Pooled results from the seven higher dose vs lower dose statin trials in patients with coronary disease
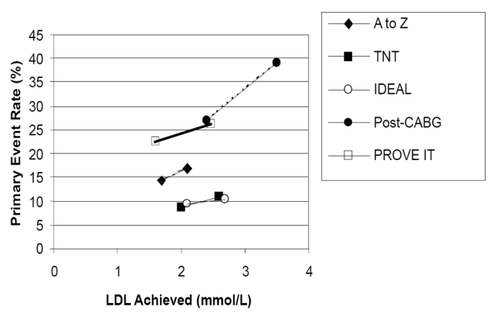
Changes in LDL cholesterol and reductions in primary endpoints ()
Patients treated with higher dose statins in all trials achieved lower LDL cholesterols than those treated with lower dose statins () and higher dose statins were associated with relative reductions in each study’s primary endpoint compared to lower intensity statin treatment (from 11% in IDEAL to 20% in TNT). The differences in achieved LDL levels between the higher and lower dose statin arms ranged from a low of 0.39 mmol/L in the A-to-Z Trial to 1.0 mmol/L in the Vascular Basis Trial.
Figure 1 Association between achieved LDL cholesterol levels and primary outcome in both arms of the higher dose versus lower dose statin trials. The results for the less intensive arm of each trial are expressed on the right of each trial’s line; the results for the more intensive arm of each trial are expressed on the left of each trial’s line.
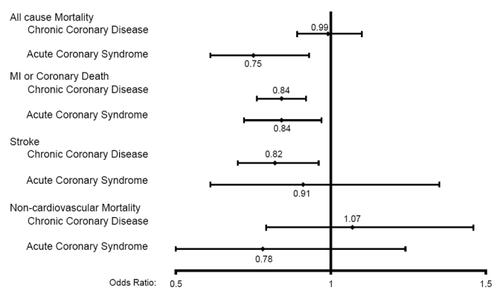
All-cause mortality ()
Higher dose statin therapy was associated with non-significant trends towards lower all-cause mortality rates in 4 of the 7 secondary prevention trials (with OR ranging from 0.71 (95% CI 0.49–1.02) in PROVE-IT TIMI 22 to 0.98 (95% CI 0.84–1.14) in IDEAL) – pooling the data across all 7 trials using a random effects model revealed a 25% reduction in mortality in patients after acute coronary syndromes (OR 0.75; 95% CI 0.61–0.93) with higher dose statin therapy, but no apparent impact on mortality in patients with chronic CAD (OR 0.99; 95% CI 0.89–1.10) (CitationJosan et al 2007).
Myocardial infarction or coronary death ()
Higher dose statins were associated with a significant reduction in this composite endpoint in the TNT trial (OR 0.79, 95% CI 0.68−0.91) and non-significant trends towards lower event rates in 5 of the other 6 trials (with OR ranging from 0.57 (95% CI 0.16–1.95) in REVERSAL to 0.88 (95% CI 0.71–1.01) in IDEAL). Pooling the data across all 7 trials using a random effects model confirmed that higher dose statins provided further reductions (over lower dose statin therapy) in myocardial infarction or coronary death in patients after acute coronary syndromes (OR 0.84; 95% CI 0.72–0.97) and in patients with chronic CAD (OR 0.84; 95% CI 0.76–0.92) (CitationJosan et al 2007).
Stroke ()
Higher dose statins significantly reduced stroke in the TNT trial (OR 0.75, 95% CI 0.59–0.96) and was associated with trends to benefit in 3 of the other 6 trials. Pooling the data across all 7 trials using a random effects model confirmed that, compared to lower dose statin therapy, higher dose statins further reduced stroke in patients with chronic CAD (OR 0.82; 95% CI 0.70–0.96); although a similar trend was observed in patients after acute coronary syndromes (OR 0.91; 95% CI 0.61–1.35) there were too few events in this smaller subgroup of patients for the data to be definitive (CitationJosan et al 2007).
Non-cardiovascular mortality ()
There was no appreciable difference between higher or lower dose statin therapy in any of the trials or either in patients with chronic CAD (OR 1.07, 95% CI 0.79–1.46) or in patients after acute coronary syndromes (OR 0.78, 95% CI 0.49–1.24) when the data were pooled across trials.
Adverse events
Discontinuation of study drug due to adverse events was higher in the higher dose statin arms of these trials (pooled estimate 7.7% versus 5.1% for the lower dose statin arms, p < 0.0001) (CitationJosan et al 2007). In contrast, the discontinuation rate attributed to study drug in the placebo-controlled trials in was 7.7% in 25,723 placebo-treated patients and 7.8% in 25,742 statin-treated patients (p = 0.98). The frequency of elevated transaminases (AST or ALT greater than 3 times the upper limit of normal) was significantly greater with higher dose statin therapy vs. lower dose therapy (1.4% vs. 0.4%, p < 0.0001) (CitationJosan et al 2007). In contrast, the frequency of elevated transaminases in the statin placebo-controlled trials was 1.2% in 33,465 placebo-treated patients and 1.6% in 33,494 statin-treated patients (p = 0.0001). Myopathic adverse events were inconsistently reported in these trials and in the placebo-controlled statin trials. While the A-to-Z trial reported a small but statistically significant increase in cases of rhabdomyolysis among the patients receiving higher dose statin therapy (0.4% vs 0.04%, p = 0.02), neither the IDEAL (0.05% vs 0.07%, p = 0.99) nor TNT (0.04% vs 0.06%, p = 0.99) trials found any significant difference in rhabdomyolysis risk. The pooled frequency of statin-associated myopathy (as per the American College of Cardiology/American Heart Association definition, myopathy covers any muscle complaints, with or without elevated CK levels) (CitationPasternak et al 2002) was 3.3% in patients randomized to higher dose statins and 2.8% in patients randomized to lower dose statins (p = 0.008); in comparison, the rates were 0.9% in 34,830 placebo-treated patients and 0.9% in 34,848 statin-treated patients in the trials (p = 0.89).
Discussion
The expected relative risk reductions associated with lower dose statin regimens over placebo are 23% for myocardial infarction, 17% for stroke, and 13% for all-cause mortality (CitationCholesterol Treatment Trialists 2005). Compared to lower dose statin therapy, higher dose statin therapy reduces myocardial infarctions by a further 16% and strokes by 18% in patients with CAD – both of these values are relative risk reductions and the absolute benefits depend on the baseline risk of the patients in which these drugs are used. Although there is no appreciable effect on survival in patients with chronic CAD, in those patients at higher risk for death due to recent acute coronary syndromes higher dose statin therapy does confer an additional 25% relative reduction in all-cause mortality over and above the reductions expected with low dose statin therapy. Higher dose statin regimens are also associated with statistically significantly increased risks of myopathy, elevated transaminases, and drug discontinuation compared to lower dose statins. However, although relative risks for these outcomes range from 1.2 to 4.0, the absolute increases are small (particularly when balanced against the absolute reductions of 1.6% in myocardial infarction and 0.5% in stroke seen in the trials reviewed above).
However, there are two caveats to our findings. First, although the current literature supports the use of higher dose statin regimens in patients with established CAD, it provides limited insight into whether high or low dose statins should be employed in patients without CAD but with elevated cholesterols and/or multiple atherosclerotic risk factors. Although the ASAP and ARBITER Trials have shown that higher dose statin therapy does reduce the surrogate outcome of progression in carotid intima media thickness in patients with familial hypercholesterolemia (CitationSmilde et al 2001) or a wide variety of conditions which placed them at risk for atherosclerosis (CitationTaylor et al 2002), the number of clinically apparent events (ie, MI, stroke, or death) was far too few in these trials to make definitive conclusions at this time. We do not believe that the secondary prevention trials that we reviewed can be generalized to patients without coronary disease and this is an area that should be a research priority (particularly given a recent secondary analysis of TNT suggesting that patients with metabolic syndrome may derive even greater benefits from higher dose statin therapy than other patients (CitationDeedwania et al 2006).
Second, although our analysis provides information on the benefits and safety of higher dose versus lower dose statin therapy, none of these trials provide data which can directly answer the question of optimal LDL targets as none provide a breakdown of event rates by LDL achieved. Although patients randomized to lower dose statins in the dose comparison trials we reviewed had higher event rates, it is possible that only a subset of the patients in the randomized arms contributed to the difference. That is, the worse outcomes in the lower dose statin group may be the result of events occurring in those patients with persisting LDL elevations rather than a result of the mean LDL achieved not being low enough (CitationMann 2006). As previously mentioned, multidrug therapy is frequently required to achieve lower LDL targets thus resulting in increased risk of adverse effects and/or patient non-adherence to prescribed therapy. Although short-term trials with LDL cholesterol endpoints have demonstrated that non-statin agents can further lower LDL cholesterol when added to statin therapy (CitationBrown et al 2001; CitationStein et al 2004; CitationBissonette et al 2006), there is currently a paucity of long-term trials proving that these agents provide further reductions in clinical outcomes (such as myocardial infarction, stroke, or death) above those achieved with statin therapy alone.
Without individual patient data we cannot calculate risk reductions per mmol/L reduction in LDL cholesterol but extrapolating from the expected benefits based on the Cholesterol Treatment Trialists’ Collaboration suggests that the benefits seen in these trials are generally consistent with what would have been expected given the mean LDL reduction observed in these trials (0.61 mmol/L) and their relatively long duration. This latter point is relevant since the placebo-controlled statin trials demonstrated that the benefits of statins are approximately half as large in the first year of use as in subsequent years (CitationCholesterol Treatment Trialists’ Collaboration 2005). Although the general consistency of the clinical event reductions for the observed LDL reductions lends support to those asserting that the lower the LDL the better (CitationO’Keefe et al 2004), it does not provide any information about whether there is a lower threshold below which further reduction in LDL cholesterol is not helpful (or is even harmful). Indeed, it could be argued that although the data from the 7 intensive therapy trials is generally consistent with that from the placebo-controlled trials, the clinical event reduction seen in these trials is slightly lower than would be expected for trials of such long duration. Thus, although it is commonly asserted that for every 1% reduction in LDL levels, the relative risk for major coronary events is reduced by approximately 1% (CitationGrundy 2004), all 4 of the intensive therapy trials that reported data on major coronary events failed to show such a relationship. For example, although LDL levels were reduced by 35% in PROVE IT, this was associated with only a 16% relative risk reduction in major coronary events and although LDL levels were reduced by 22% in IDEAL the major coronary event rate was only reduced by 11%. While the current literature is inadequate to assess whether there may be an LDL threshold below which further reductions in LDL do not improve clinical outcomes, such a study could be done with the trial data at hand if a cohort analysis to explore the association between LDL levels and clinical outcomes was conducted with multivariate adjustment for prescribed therapies (statins and concomitant anti-atherosclerotic therapies), adherence, and changes in other risk factors as well as baseline imbalances in prognostic risk factors (CitationHayward et al 2006).
Although the Cholesterol Treatment Trialists suggested that there was an approximately linear relationship between the LDL cholesterol achieved and the reductions in clinical outcomes, the mean pre-treatment LDL cholesterol of 3.79 mmol/L in their meta-analysis is considerably higher than that of patients in the higher vs. lower dose statin trials reviewed in this manuscript. As a result, there were undoubtedly fewer patients that had baseline LDLs lower than 2.59 mmol/L in the placebo-controlled trials. Indeed, while several of the placebo-controlled trials did examine whether patients with lower baseline LDL levels derived the same benefits from LDL reduction as those starting from higher LDL levels, the results were mixed. For example, while the CARE trial (CitationSacks et al 1996) demonstrated no benefit with statin therapy in patients with baseline LDL level <3.2 mmol/L (22% rate of major coronary events vs 21% in placebo-treated patients) and the LIPID trial (CitationLIPID study group 1998) reported less benefit with statins in patients with baseline LDL < 3.5 mmol/L (mortality relative risk reduction 16% vs 30% benefit in those patients with higher baseline LDL levels), the Heart Protection Study (CitationMRC/BHF 2002), PROSPER (CitationShepherd et al 2002), CARDS (CitationColhoun et al 2004), and MIRACL (CitationSchwartz et al 2001) trials all demonstrated similar benefits across subgroups regardless of baseline LDL level.
Although they are most effective at decreasing LDL levels, statins are also known to exert varying effects on triglyceride and HDL levels (CitationJones 2003) and statins do differ in their non-cholesterol pleiotropic effects (CitationDavignon 2004). However, as the trials comparing more intensive vs less intensive statin therapy do not consistently report post treatment triglyceride and HDL levels or non-cholesterol risk factors (such as C-reactive protein) one cannot determine the contribution of these factors. As a result, we believe there is currently no evidence to support assertions that a higher dose of one statin is more efficacious than higher doses of any other statin (assuming equipotent dosing and equal reductions in LDL cholesterol) for clinical outcomes.
In considering the benefits of lipid lowering with higher dose statin therapy, one must also weigh the potential harms to the patient. After all, cholesterol is a cell membrane component and plays a key role in vitamin synthesis. While ecological and cohort data did raise concerns that very low cholesterol levels may be associated with increases in non-cardiovascular mortality, intracranial hemorrhage, cancer, or suicide, these concerns have been proven unfounded by the randomized trial literature (and likely reflected the fact that systemic illnesses which predisposed to those outcomes also lowered cholesterol levels). Indeed, a recent meta-analysis of 35 randomized placebo-controlled statin trials (with data on over 74,000 patients followed for a mean of 17 months) documented that there was no appreciable increase in the risk of myalgias, CK elevations, rhabdomyolysis, or drug discontinuation with non-cerivastatin statins over placebo-treated patients, and although the risk of transaminase elevations was higher with statin treatment, the absolute risk was only 4 cases per 1000 patients (CitationKashani 2006). In our analysis of trials comparing different doses of statins (none of which were included in the aforementioned meta-analysis), we found that although adverse event rates (particularly myopathy and hepatotoxicity) were higher with higher dose statin therapy than with lower dose therapy, the absolute rates of adverse events were low in these trials (and in the placebo-controlled statin trials). Further, post-hoc analyses suggest that adverse events are not related to achieved LDL cholesterol levels (CitationWiviott 2005).
However, it should be acknowledged that adverse events may be more common in clinical practice since trial participants tend to be healthier and more closely followed than usual patients. Indeed, these trials excluded over half of all patients screened because of various factors known to increase the risk of adverse events: advanced age, renal failure, hepatic failure, hypothyroidism, or concomitant use of fibrates, macrolide antibiotics, antifungal agents, HIV protease inhibitors, verapamil, or cyclosporine (CitationGrundy 2005). Having raised this objection, however, it should be acknowledged that adverse events with statin therapy appear to be relatively similar when used in the non-trial setting as in the randomized trials, at least based on analyses of large cohort studies, administrative databases, and FDA reports published so far (CitationThompson et al 2003; CitationBays 2005; CitationCharles 2005). However, the relatively short time frame of the randomized trials of higher dose vs. lower dose statin therapy conducted thus far should be acknowledged and emphasizes the importance of post-marketing surveillance to track complication rates over longer time periods, and in larger samples (witness the problems with cerivastatin which only became apparent when scores of thousands of patients had been prescribed the drug in North America). The ongoing SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) Trial will provide much needed safety information on high dose statin therapy (simvastatin 80 mg daily vs. 20 mg daily) over a longer timeframe (12,064 study participants, with an expected average follow-up of 7 years).
In addition to the adverse events attributed to statin use, attention should be drawn to the overall discontinuation rate with higher dose vs. lower dose statin therapy which likely reflects adverse effects such as nausea, diarrhea, and abdominal pain. These symptoms are typically dismissed as nuisance effects rather than true adverse events and thus not systematically captured in randomized trial case reports. However, if such nuisance effects lead patients to discontinue potentially life saving drugs, they are clearly important contributors to patient outcomes. Thus, the argument could be made that in those intolerant to higher dose statin therapy, it may be beneficial to derive some benefit from a low dose statin than no benefit from a discontinued high dose statin.
In closing, the current literature does prove conclusively that higher dose statin therapy (for example, 80 mg of simvastatin or atorvastatin) in patients with established CAD provides incremental benefits over and above those expected with lower dose statin therapy; however, this literature is insufficient to define optimal LDL targets in these patients. Secondary analyses of the existing randomized trial data using individual patient data and multivariate adjustment will be needed to appropriately examine the incremental benefits of different LDL targets (CitationHayward 2006), and future trials will have to determine whether lower dose statin therapy plus other lipid lowering agents may achieve better LDL levels and clinical outcomes than maximal dose statin therapy. Indeed, further research is needed to conclusively establish whether the benefits associated with statin treatment are determined by the LDL level achieved, the percent reduction in LDL, the absolute reduction in LDL, or the dose of the statin. Based on the current evidence base, the use of higher dose statin therapy should be restricted to patients with established CAD at this time.
Sources of support
No project specific funding for this study. FAM receives career salary support from the Alberta Heritage Foundation for Medical Research and the Canadian Institutes of Health Research, and is also supported by the Merck Frosst/Aventis Chair in Patient Health Management at the University of Alberta.
References
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research GroupMajor outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT-LLT)JAMA20022882998300712479764
- BaysHStatin safety: An overview and assessment of the data – 2005Am J Cardiol200697Suppl6C26C
- BissonnetteSHabibRSampalisFEfficacy and tolerability of ezetimibe 10 mg/day co administered with statins in patients with primary hypercholesterolemia who do not achieve target LDL-C while on statin monotherapy: A Canadian, multicentre, prospective study – the Ezetrol Add-On StudyCan J Cardiol20062210354417036098
- British Cardiac Society, British Hypertension SocietyDiabetesUKJBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practiceHeart200591Suppl 5v15216365341
- BrownBGZhaoXQChaitASimvastatin and niacin, antioxidant vitamins, or their combination for the prevention of coronary diseaseN Engl J Med200134515839211757504
- CannonCPBraunwaldEMcCabeCHIntensive versus moderate lipid lowering with statins after acute coronary syndromesN Engl J Med2004350149550415007110
- CharlesECOlsonKLSandhoffBGEvaluation of cases of severe statin-related transaminitis within a large health maintenance organizationAm J Med20051186182415922693
- ChenZPetoRCollinsRSerum cholesterol concentration and coronary heart disease in population with low cholesterol concentrationsBMJ1991303276821888927
- Cholesterol Treatment Trialists’ CollaboratorsEfficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statinsLancet200536612677816214597
- ColhounHMBetteridgeDJDurringtonPNPrimary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trialLancet20043646859615325833
- DavignonJBeneficial cardiovascular pleiotropic effects of statinsCirculation20041093943
- DeedwaniaPBarterPCarmenaRReduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets StudyLancet20063689192816962881
- de LemosJABlazingMAWiviottSDEarly intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trialJAMA200429213071615337732
- DownsJRClearfieldMWeisSPrimary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPSJAMA19982791615229613910
- GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico)Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge?Ital Heart J200018102011302109
- GrundySMCleemanJIMerzCNImplications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III GuidelinesCirculation20041102273915249516
- GrundySMThe issue of statin safety. Where do we stand?Circulation20051113016915911705
- HaywardRAHoferTPVijanSNarrative review: Lack of evidence for recommended low-density lipoprotein treatment targets: A solvable problemAnn Intern Med20061455203017015870
- Heart Protection Study Collaborative GroupMRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trialLancet200236072212114036
- HoldaasHFellstromBJardineAGEffect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomized, placebo-controlled trialLancet200336120243112814712
- JadadARMooreRACarrollDAssessing the quality of reports of randomized clinical trials: Is blinding necessary?Control Clin Trials1996171128721797
- JonesPHDavidsonMHSteinEASTELLAR study groupComparison and efficacy of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across dosesAm J Cardiol2003921526012860216
- JosanKMajumdarSRMcAlisterFAThe efficacy and safety of intensive statin therapy: A meta-analysis of randomized trialsCMAJ2007 under review
- KashaniAPhillipsCOFoodyJMRisks associated with statin therapy. A systematic overview of randomized clinical trialsCirculation200611427889717159064
- KhanNAMcAlisterFARabkinSWThe 2006 Canadian Hypertension Education Program (CHEP) recommendations for the management of hypertension: Part 2 – TherapyCan J Cardiol2006225839316755313
- LaRosaJCGrundySMWatersDDTreating to new targets (TNT) InvestigatorsIntensive lipid lowering with atorvastatin in patients with stable coronary diseaseN Engl J Med200535214253515755765
- LawMRWaldNJRudnickaARQuantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysisBMJ20033261423
- LiemAHvan BovenAJVeegerNJFluvastatin On Risk Diminishment after Acute myocardial infarction (FLORIDA) study groupEffect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trialEur Heart J2002231931712473255
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study GroupPrevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levelsN Engl J Med19983391349579841303
- MannSJLetter to the editorJAMA20062952477816757715
- NissenSETuzcuEMSchoenhagenPEffect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized control trialJAMA200429110718014996776
- NissenSEHigh-dose statins in acute coronary syndromes. Not just lipid levelsJAMA20062921365715337731
- O’KeefeJHCordainLHarrisWHOptimal low-density lipoprotein is 50 to 70 mg/dl. Lower is better and physiologically normalJ Am Coll Cardiol2004432142615172426
- Pablos MendezABarrRGSheaSRun-in periods in randomized trials: implications for the application of results in clinical practiceJAMA199827922259438743
- PadwalRStrausSEMcAlisterFACardiovascular risk factors and their impact on the decision to treat hypertension: an evidence-based reviewBMJ20013229778011312234
- PasternakRCSmithSCBairey-MerzCNACC/AHA/NHLBI clinical advisory on the use and safety of statinsJ Am Coll Cardiol2002405677212142128
- PedersenTRFaergemanOKasteleinJJHigh-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trialJAMA200529424374516287954
- PittBWatersDBrownWVAggressive lipid-lowering therapy compared with angioplasty in stable coronary artery diseaseN Engl J Med199934170610395630
- The Post Coronary Artery Bypass Graft Trial InvestigatorsThe effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass graftsN Engl J Med1997336153628992351
- RobinsonJGSmithBMaheshwariNPleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysisJ Am Coll Cardiol20054618556216286171
- SacksFMPfefferMAMoyeLAfor the Cholesterol and Recurrent Events Trial InvestigatorsThe effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levelsN Engl J Med1996335100198801446
- Scandinavian Simvastatin Survival Study GroupRandomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S)Lancet1994344138397968073
- SchwartzGGOlssonAGEzekowitzMDEffects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trialJAMA20012851711811277825
- SerruysPWde FeyterPMacayaCFluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trialJAMA200228732152212076217
- SeverPSDahlofBPoulterNRPrevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trialLancet200336111495812686036
- ShepherdJBlauwGJMurphyMBPravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trialLancet200236016233012457784
- ShepherdJCobbeSMFordIPrevention of coronary heart disease with pravastatin in men with hypercholesterolemiaN Engl J Med1995333130177566020
- SmildeTJvan WillsenSWollersheimHEffect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomized, double-blind trialLancet20013575778111558482
- SteinEStenderSMataPAchieving lipoprotein goals in patients at high risk with severe hypercholesterolemia: efficacy and safety of ezetimibe co-administered with atorvastatinAm Heart J20041484475515389231
- StonePHLloyd-JonesDMKinlaySEffect of intensive lipid lowering, with or without antioxidant vitamins, compared with moderate lipid lowering on myocardial ischemia in patients with stable coronary artery disease: the Vascular Basis for the treatment of myocardial ischemia studyCirculation200511117475515809368
- The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) InvestigatorsHigh-dose atorvastatin after stroke or transient ischemic attackN Engl J Med20063555495916899775
- TaylorAJKentSMFlahertyPJARBITER: Arterial biology for the investigation of the treatment effects of reducing cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thicknessCirculation200210620556012379573
- ThompsonPDClarksonPKarasRHStatin-associated myopathyJAMA200328916819012672737
- VerschurenWMJacobsDRBloembergBPSerum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five year follow-up of the seven countries studyJAMA199527413167596000
- WiviottSDCannonCPMorrowDACan low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy. A PROVE-IT TIMI 22 SubstudyJ Am Coll Cardiol2005461411616226163