Abstract
Anti-adrenergic therapy has been widely accepted as an important therapeutic intervention in patients with chronic heart failure. However, there has been continuing controversy regarding the risks and clinical significance of metabolic effects of different anti-adrenergic drugs. This review summarizes what has been learned from clinical trial evidence regarding the benefits of anti-adrenergic drugs in diabetic patients with chronic heart failure.
Introduction
It has been increasingly recognized that diabetes mellitus is an important vascular disease in patients with heart failure and cardiomyopathy. Glucose abnormalities in patients with heart failure are common, under-diagnosed, and often associated with worsening symptomatic status and poor clinical outcomes (CitationTang and Young 2001). In the RESOLVD (Randomized Evaluation of Strategies of Left Ventricular Dysfunction) substudy, up to 43% of patients with left ventricular systolic dysfunction had documented glucose abnormalities that were often previously undiagnosed (CitationSuskin et al 2000). This is in part related to the impaired glucose metabolism associated with enhanced sympathetic drive and worsening insulin resistance that is part of the pathophysiology of heart failure (CitationOpie 2004).
Diabetes can cause myocyte hypertrophy, interstitial fibrosis, impaired myocardial blood flow, and increased turnover of free fatty acids, all leading to the development of cardiomyopathy and heart failure (CitationTang and Young 2001). Clinical studies have now confirmed that patients with heart failure have up to a 4 times higher risk of developing diabetes mellitus (CitationKannel and McGee 1979), and the incidence of diabetes in patients with established heart failure has increased over previous decades (CitationFrom et al 2006). Furthermore, the risk of hospitalizations for heart failure is greatly amplified in the patients with diabetes mellitus (CitationDeedwania et al 2005).
The mechanism and consequence of insulin resistance in dysfunctional myocardium is currently unknown, but an increasing body of evidence has emerged concerning the relationship between heightened sympathetic activation and the development of myocardial and peripheral insulin resistance (CitationParsonage et al 2002). In animal studies of advanced heart failure, myocardial insulin resistance is evident in the setting of increased sympathetic nervous system activation and oxidative stress, directly leading to lipolysis, subsequent alteration in the insulin-signaling cascade, and myocyte dysfunction (CitationNikolaidis et al 2004). In small single-center studies, insulin resistance has been linked to systolic heart failure in patients without underlying diabetes mellitus. In particular, patients with heart failure have greater impairment in insulin sensitivity compared with matched controls (CitationSwan et al 1997; CitationWitteles et al 2004).
Effects of anti-adrenergic therapy on glucose metabolism
Glycemic control has been the primary therapeutic target for treating patients with diabetes mellitus, even in the setting of heart failure (CitationTang 2006). In the Kaiser registry, increasing glycosylated hemoglobin levels portends a higher incidence of subsequent heart failure (CitationIribarren et al 2001), an observation that affirms data from the United Kingdom Prospective Diabetes Study (UKPDS) (CitationStratton et al 2000). Even in the population without diabetes mellitus, elevated fasting plasma glucose or glycosylated hemoglobin levels have been associated with poorer long-term outcomes in both acute and chronic heart failure settings (CitationBhatia et al 2004; CitationGerstein et al 2005).
Treatment with anti-adrenergic drugs may increase peripheral vascular resistance, impairing peripheral blood flow leading to impaired glucose disposal to skeletal muscles. These effects are likely to be amplified in the setting of cardiac insufficiency, where vascular changes and neurohormonal upregulation occur as a compensatory response. Furthermore, blocking the sympathetic beta-stimulation of hepatic glucose production and blunting the symptoms of hypoglycemia (such as tachycardia) worsening metabolic control may have theoretical adverse consequences.
Clinical studies suggest that various anti-adrenergic drugs may carry differential effects on insulin sensitivity (). One of the earliest investigations compared carvedilol (beta-1, beta-2, and alpha-1 selective) with metoprolol tartrate (beta-1 selective) in diabetic hypertensive subjects. Patients with non-insulin-dependent diabetes mellitus received either carvedilol (25 mg twice daily) or metoprolol tartrate (50 mg twice daily) for a period of 4 weeks and up-titrated as needed. After 4 weeks of carvedilol treatment, 23 of 25 patients (92%) showed a good response to therapy (reduction of diastolic blood pressure below 90 mmHg). Doubling of dosage in the carvedilol group did not further increase the response rate after another one month of treatment. In contrast, the response rate after 4 and 8 weeks of metoprolol treatment was 79% and 83%, respectively (CitationEhmer et al 1988). In both treatment groups, blood glucose concentrations and glycosylated hemoglobin were maintained within narrow limits. Subsequently, the differential effects were demonstrated in a 12-week isoglycemic hyperinsulinemic glucose clamp experiment (the gold standard for assessing insulin sensitivity) in patients with essential hypertension, whereby insulin sensitivity decreased significantly by approximately 14% after metoprolol tartrate but increased after carvedilol (CitationJacob et al 1996). Furthermore, a decrease in high-density lipoprotein cholesterol and an increase in triglycerides levels were observed in patients in the metoprolol-treated group, whereas these parameters remained unchanged in patients in the carvedilol-treated group. The explanation of this difference was the compensatory effects of alpha-1 adrenergic blockade in carvedilol that lead to vasodilatation, improved oxygen delivery, and reduced insulin release. There is also blockade of beta-2 adrenergic stimulation, leading to suppressed substrate preference for non-esterified fatty acid and enhanced insulin signaling. Similar effects are also seen in the post-infarction setting (CitationBasat et al 2006), suggesting that cardioselectivity of anti-adrenergic therapy may play a role in influencing the metabolic profile.
Figure 1 Potential beneficial effects of anti-adrenergic blockers in diabetic patients with heart failure.
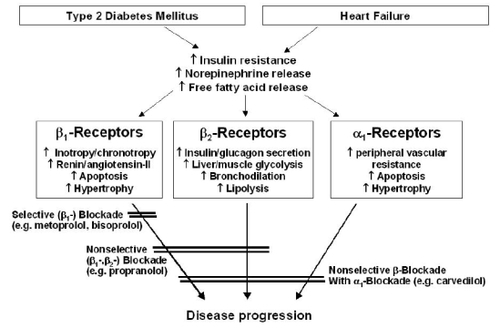
Specifically in the setting of heart failure in patients without overt diabetes, treatment with carvedilol can lead to a significant decrease in fasting insulinemia as well as inflammation (CitationHara et al 2003), and subsequent improvement or unaltered insulin sensitivity (CitationRefsgaard et al 2002; CitationFerrua et al 2005). In animal models, both carvedilol and metoprolol succinate had comparable heart rate effects, but carvedilol-treated dogs with pace-induced heart failure showed significantly greater increases in stroke volume and cardiac output and decreases in left ventricular end-diastolic pressure and systemic vascular resistance (CitationNikolaidis et al 2006). Furthermore, carvedilol may increase myocardial glucose uptake, and may blunt actions of norepinephrine and glucagon. These effects clearly illustrate the metabolic benefits of a non-selective adrenergic blockade by carvedilol.
The GEMINI (Glycemic Effects in Diabetes Mellitus: Carvedilol-Metoprolol Comparison in Hypertensives) trial constituted a milestone in the long-standing debate regarding the differential metabolic effects of different anti-adrenergic drugs. The GEMINI study randomized 1235 hypertensive patients with non-insulin-dependent type 2 diabetes mellitus in a 2-to-1 fashion to receive either metoprolol tartrate or carvedilol in addition to baseline angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) and stable anti-diabetic regimens. After 5 months of follow-up, the blood pressure effects between the two anti-adrenergic drugs were comparable. However, an increase in glycosylated hemoglobin of 0.15% was observed in the metoprolol tartrate arm, whereas an increase in glycosylated hemoglobin of 0.02% was observed in the carvedilol arm (CitationBakris et al 2004). The separation occurred as early as Month 2 of therapy and continued to diverge. Insulin resistance (estimated by homeostatic model assessment or HOMA-IR) and microalbuminuria (estimated by urinary albumin/creatinine ratio) were also significantly reduced in the carvedilol arm compared with the metoprolol tartrate arm. Interestingly, weight gain and bradycardia occurred more frequently, and there were more apparent increases in triglyceride levels and development of microalbuminuria in the metoprolol tartrate arm. Changes in low-density as well as high-density lipoprotein cholesterol levels were similar between the two groups, although only a small percentage of patients were treated with statin therapy. These data confirmed different metabolic effects among different anti-adrenergic drugs, although the results still do not specify the underlying mechanisms. Furthermore, there were no long-term outcome data to illustrate the clinical consequences of these differences in metabolic effects, and the findings can only be applied to the heart failure population by extrapolation.
The role of anti-oxidant properties of anti-adrenergic drugs is less clear. Oxidative stress has been implicated in the pathophysiology of heart failure, although the mechanisms are complex. In both heart failure and type 2 diabetes mellitus, reactive oxygen species are increased and endothelial nitric oxide synthase is diminished. By reducing nitric oxide availability, nitric-oxide derived vasodilatation is reduced, which can lead to endothelial dysfunction and poor long-term mortality in heart failure (CitationKatz et al 2005). There has been evidence to suggest that the transcardiac gradient of plasma oxidized low-density lipoprotein is significantly lower in patients who receive carvedilol compared with those who do not (CitationTsutamoto et al 2001). Therefore, the possibility of carvedilol as a scavenger of oxidative free radicals, a chelator of metal ions, and its ability to improve insulin sensitivity through reduction of oxidative stress in patients with diabetes mellitus is encouraging, although such relationships have yet to be confirmed in the human heart failure population (CitationGiugliano et al 1997).
Anti-adrenergic therapy in patients with heart failure and diabetes mellitus
Patients with diabetes mellitus have been included in the majority of large-scale clinical trials in chronic heart failure; therefore data have emerged regarding the benefits versus risks of anti-adrenergic therapy in this population through post-hoc analyses. There have been several limitations, however, to this work. First, subgroup analyses (even when pre-specified) are often underpowered and potentially biased, in part because diabetes mellitus status has not been used as a variable for stratified randomization. Furthermore, diabetes mellitus is often documented as a dichotomous variable. Therefore, the mere presence of a diagnosis of diabetes mellitus cannot provide insight into the degrees of glycemic control and vascular abnormalities of the patient.
One of the first published papers in this topic was the subgroup analysis from the Second Cardiac Insufficiency Bisoprolol Study (CIBIS-II) with the use of bisoprolol. The relative risk of bisoprolol versus placebo for mortality was 0.81 (19% reduction, 95% confidence interval [CI] 0.51–1.28) in patients with diabetes mellitus, compared with 0.66 (95% CI of 0.54–0.81) in patients without diabetes mellitus (CitationErdmann et al 2001). These results may have been affected by the relatively small sample size of subjects with diabetes mellitus in CIBIS-II (only 12% of the study population). Meanwhile in the MERIT-HF trial, a larger proportion of subjects (n = 985, or 25%) presented with a diagnosis of diabetes mellitus, among whom 199 had severe heart failure (NYHA III-IV, LVEF <25%) (CitationDeedwania et al 2005). A pre-specified subgroup analysis in the cohort of patients with diabetes mellitus demonstrated a mortality reduction following treatment with metoprolol succinate when compared with placebo (10.1% vs 12.7%, or an 18% reduction). This compares with a 31% mortality reduction in the non-diabetes subgroup (CitationDeedwania et al 2005). Nevertheless, the risk of hospitalization for heart failure was reduced by 37% with metoprolol succinate in the MERIT-HF trial. One very reassuring point from this report was the lack of significant adverse effects of metoprolol succinate compared with placebo in patients with diabetes mellitus, even in those with severe heart failure.
The largest experience of anti-adrenergic therapy use in patients with chronic heart failure and diabetes mellitus has been with the use of carvedilol. Beneficial effects of carvedilol have been seen across the spectrum of heart failure trials, irrespective of the presence or absence of diabetes mellitus. A recent meta-analysis summarized this clinical trial experience with several of the landmark carvedilol heart failure studies looking at patients with diabetes mellitus. Among a total of 1411 subjects with diabetes mellitus enrolled in 7 studies (25% of all enrollees), carvedilol demonstrated a statistically significant survival benefit (34% risk reduction, ) (CitationBell et al 2006), even after adjustment for age, left ventricular ejection fraction, ischemic etiology, diabetes history, and weight. The authors claimed that the number of patients needed to treat with carvedilol for 1 year to prevent 1 death was 23 for the overall population, and 25 for patients with diabetes mellitus. These estimates are still more favorable than that from SOLVD (estimated number needed to treat with enalapril being 78 for 1 year to prevent 1 death) (CitationDries et al 2001).
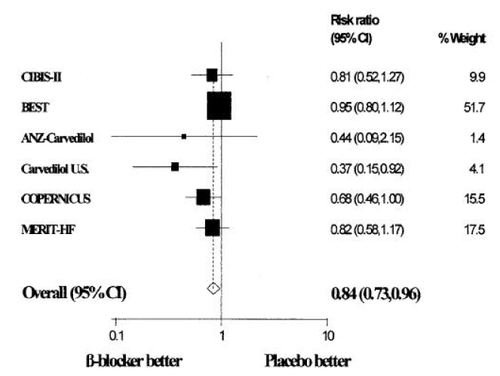
Figure 2 Mantel-Haenszel relative risk (fixed effects) plot of anti-adrenergic drugs versus placebo in patients with diabetes mellitus and chronic heart failure for all-cause mortality Reproduced with permission from CitationHaas SJ, Vos T, Gilbert RE, et al 2003. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trials. Am Heart J, 146:848–53. Copyright © 2003 Elsevier.
The latest data came from BEST (Beta-blocker Evaluation of Survival Trial), a database of 2708 patients with advanced heart failure (36% with diabetes mellitus) randomized to bucindolol versus placebo (CitationDomanski et al 2003). While in the overall trial bucindolol did not demonstrate a significant survival benefit, the use of bucindolol was associated with a significant benefit compared with placebo in subjects with diabetes mellitus when death plus heart failure hospitalizations were combined as endpoints.
Combining the experience of the landmark anti-adrenergic therapy clinical trials, several meta-analyses have been conducted to determine whether there are differences in mortality and morbidity benefits with different anti-adrenergic drugs. All analyses found that patients with chronic heart failure and diabetes mellitus had higher event rates compared to those without diabetes (CitationHaas et al 2003; CitationShekelle et al 2003; CitationDeedwania et al 2005). In the pooled analysis by Shekelle and colleagues, anti-adrenergic therapy use was associated with a 23% mortality reduction in patients with diabetes mellitus versus a 35% mortality reduction in those without diabetes mellitus (CitationShekelle et al 2003). This finding was consistent with a larger meta-analysis, which concluded that anti-adrenergic therapy for heart failure was beneficial in patients with diabetes mellitus (relative risk of 0.84; 95% CI 0.73–0.96; p = 0.01, see ) (CitationHaas et al 2003).
Unanswered questions
It is important to emphasize that while carvedilol appears to have favorable metabolic effects over metoprolol or bisoprolol, all three approved anti-adrenergic drugs can be used effectively in the setting of diabetes mellitus. Indeed, there is only inferred evidence but no prospective data to support the switch from stable regimens of metoprolol succinate or bisoprolol to carvedilol in patients with heart failure and diabetes mellitus at present. The incremental long-term clinical benefit of carvedilol in this population over other currently approved anti-adrenergic drugs is still debated. In particular, several unanswered questions remain despite the wide range of studies that have emerged over the years as well as the extensive use of carvedilol, bisoprolol, and metoprolol succinate in the clinical setting.
First, what is the impact of the differential metabolic effects of anti-adrenergic drugs on long-term outcomes in the clinical heart failure setting? If the underlying pathophysiology of heightened risk of developing diabetes in the heart failure population is the presence of overactive sympathetic drive (which is counteracted by any anti-adrenergic drug), the differences among anti-adrenergic drugs on long-term outcomes should be minimal. The carvedilol investigators have argued that a consistent 35% reduction has been observed in both diabetic and non-diabetic subgroups of patients with heart failure, particularly in the COPERNICUS (Carvedilol Prospective Randomized Cumulative Survival) study (CitationMohacsi et al 2001; CitationBell et al 2006). They further use the results of COMET (Carvedilol or Metoprolol Evaluation Trial) to justify their argument, stating that the head-to-head comparison in COMET showed a 17% mortality reduction, a 25% relative reduction in vascular events (stroke or myocardial infarction), and a 12% relative reduction in the development of new-onset diabetes mellitus with long-term treatment using carvedilol over metoprolol tartrate in patients with chronic heart failure (CitationPoole-Wilson et al 2003; CitationTorp-Pedersen et al 2007; CitationRemme et al 2007). However, the MERIT-HF investigators responded by demonstrating a non-significant statistical test for treatment interaction in their own post-hoc meta-analysis between CIBIS-II, MERIT-HF, and COPERNICUS (CitationDeedwania et al 2005).
Second, are there specific subgroups of patients with diabetes mellitus that may limit the effectiveness and potentiate adverse effects of anti-adrenergic drugs? In other words, are there any noticeable differences between insulin-dependent versus non-insulin-dependent diabetes mellitus regarding the benefits and risks of anti-adrenergic drugs in the heart failure setting? What is clear from the clinical trial experiences is that irrespective of the type of anti-adrenergic drugs used, patients with diabetes compared with those without diabetes had significantly greater weight gain, hyperglycemia, hypoglycemia, elevated creatinine levels, syncope, myocardial infarction, and cerebrovascular accidents when treated with anti-adrenergic therapy. The results from GEMINI and many other small mechanistic studies have confirmed the potential differences in metabolic consequences between anti-adrenergic drugs. Hence, there may be theoretical advantages in using carvedilol in patients with brittle diabetes or with evidence of vascular compromise (such as microalbuminuria).
The United States Food and Drug Administration (FDA) has recently added warnings to the label of anti-adrenergic drugs (such as carvedilol) regarding their use in patients with heart failure and diabetes mellitus. The FDA stated that patients subject to spontaneous hypoglycemia and patients with diabetes mellitus receiving insulin/oral hypoglycemic drugs should be warned of the possibility that anti-adrenergic therapy may mask manifestations of hypoglycemia (particularly tachycardia), potentiate insulin-induced hypoglycemia, and possibly delay recovery of serum glucose levels. Therefore, close monitoring of blood glucose levels is advised on initiation, adjustment, or discontinuation of any anti-adrenergic therapy in patients with diabetes mellitus.
Conclusion
Patients with chronic heart failure and diabetes mellitus should be treated with anti-adrenergic therapy because of its consistent mortality benefits observed in pivotal trials. The differential metabolic effects of carvedilol over other anti-adrenergic therapies have largely been implicated by logical deduction from experimental heart failure models as well as data from two head-to-head clinical trials. Regardless of which anti-adrenergic drug is being used to treat chronic heart failure, careful monitoring of glycemic parameters and adverse effects remains important, particularly in the presence of diabetes mellitus.
Disclosures
Dr. Tang has served as a consultant for GlaxoSmithKline Pharmaceuticals and AstraZeneca Pharmaceuticals. He has grant support from the American Heart Association. Dr. Tang is supported by the American Heart Association Ohio Valley Associates (0465266B) and the Cleveland Clinic General Clinical Research Center (MO1 RR018390).
References
- BakrisGLFonsecaVKatholiRMetabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trialJAMA200429222273615536109
- BasatOUcakSSeberSAfter myocardial infarction carvedilol improves insulin resistance compared to metoprololClin Res Cardiol2006959910416598518
- BellDSLukasMHoldbrookFKThe effect of carvedilol on mortality risk in heart failure patients with diabetes: results of a meta-analysisCurr Med Res Opin2006222879616466600
- BhatiaVWildingGEDhindsaGAssociation of poor glycemic control with prolonged hospital stay in patients with diabetes admitted with exacerbation of congestive heart failureEndocr Pract2004104677116033717
- DeedwaniaPCGilesTDKlibanerMEfficacy safety and tolerability of metoprolol CR/XL in patients with diabetes and chronic heart failure: experiences from MERIT-HFAm Heart J20051491596715660048
- DomanskiMKrause-SteinraufHDeedwaniaPThe effect of diabetes on outcomes of patients with advanced heart failure in the BEST trialJ Am Coll Cardiol2003429142212957443
- DriesDLSweitzerNKDraznerMHPrognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunctionJ Am Coll Cardiol200138421811499733
- EhmerBvan der DoesRRudorfJInfluence of carvedilol on blood glucose and glycohaemoglobin A1 in non-insulin-dependent diabeticsDrugs198836Suppl 6136402908300
- ErdmannELechatPVerkennePResults from post-hoc analyses of the CIBIS II trial: effect of bisoprolol in high-risk patient groups with chronic heart failureEur J Heart Fail200134697911511434
- FerruaSBobbioMCatalanoEDoes carvedilol impair insulin sensitivity in heart failure patients without diabetesJ Card Fail20051159059416230261
- FromAMLeibsonCLBursiFDiabetes in heart failure: prevalence and impact on outcome in the populationAm J Med2006119591916828631
- GersteinHCPogueJMannJFThe relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysisDiabetologia20054817495516059716
- GiuglianoDAcamporaRMarfellaRMetabolic and cardiovascular effects of carvedilol and atenolol in non-insulin-dependent diabetes mellitus and hypertension.A randomized, controlled trialAnn Intern Med199712695599182472
- HaasSJVosTGilbertREAre beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trialsAm Heart J20031468485314597934
- HaraYHamadaMShigematsuYEffect of beta-blockers on insulin resistance in patients with dilated cardiomyopathyCirc J200367701412890914
- IribarrenCKarterAJGoASGlycemic control and heart failure among adult patients with diabetesCirculation200110326687311390335
- JacobSRettKWicklmayrMDifferential effect of chronic treatment with two beta-blocking agents on insulin sensitivity: the carvedilol-metoprolol studyJ Hypertens199614489948761899
- KannelWBMcGeeDLDiabetes and cardiovascular disease. The Framingham studyJAMA197924120358430798
- KatzSDHryniewiczKHriljacIVascular endothelial dysfunction and mortality risk in patients with chronic heart failureCirculation20051113101415655134
- MohacsiPFowlerMBKrumHShould physicians avoid the use of beta-blockers in patients with heart failure who have diabetes? Results of the COPERNICUS study [Abstract 3551]Circulation2001104II754
- NikolaidisLAPoornimaIParikhPThe effects of combined versus selective adrenergic blockade on left ventricular and systemic hemodynamics, myocardial substrate preference, and regional perfusion in conscious dogs with dilated cardiomyopathyJ Am Coll Cardiol20064718718116682315
- NikolaidisLASturzuAStolarskiCThe development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathyCardiovasc Res20046129730614736546
- OpieLHThe metabolic vicious cycle in heart failureLancet20043641733415541431
- ParsonageWHetmanskiDCowleyADifferentiation of the metabolic and vascular effects of insulin in insulin resistance in patients with chronic heart failureAm J Cardiol20028969670311897212
- Poole-WilsonPASwedbergKClelandJGComparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trialLancet200336271312853193
- RefsgaardJThomsenCAndreasenFCarvedilol does not alter the insulin sensitivity in patients with congestive heart failureEur J Heart Fail200244455312167382
- RemmeWJTorp-PedersenCClelandJGCarvedilol protects better against vascular events than metoprolol in heart failure: results from COMETJ Am Coll Cardiol200769637117336720
- ShekellePGRichMWMortonSCEfficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trialsJ Am Coll Cardiol20034115293812742294
- StrattonIMAdlerAINeilHAAssociation of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational studyBMJ20003214051210938048
- SuskinNMcKelvieRSBurnsRJGlucose and insulin abnormalities relate to functional capacity in patients with congestive heart failureEur Heart J20002113687510952826
- SwanJWAnkerSDWaltonCInsulin resistance in chronic heart failure: relation to severity and etiology of heart failureJ Am Coll Cardiol199730527329247528
- TangWHGlycemic control and treatment patterns in patients with heart failureHeart Fail Monit20065101416547530
- TangWHYoungJBCardiomyopathy and heart failure in diabetesEndocrinol Metab Clin North Am20013010314611727399
- Torp-PedersenCMetraMCharlesworthAEffects of metoprolol and carvedilol on pre-existing and new onset diabetes in patients with chronic heart failure:data from the Carvedilol Or Metoprolol European Trial (COMET)Heart2007939687317237130
- TsutamotoTWadaAMatsumotoTRelationship between tumor necrosis factor-alpha production and oxidative stress in the failing hearts of patients with dilated cardiomyopathyJ Am Coll Cardiol20013720869211419892
- WittelesRMTangWHJamaliAHInsulin resistance in idiopathic dilated cardiomyopathy: a possible etiologic linkJ Am Coll Cardiol200444788115234411