Abstract
In order to prevent cardiovascular events, it is essential to effectively manage overall risk of cardiovascular disease. However, despite guideline recommendations to this effect, current management of the major, modifiable cardiovascular risk factors such as hypertension and dyslipidemia is disconnected and patient adherence to therapy is poor. This is particularly important for patients with multiple cardiovascular risk factors, who are often prescribed multiple medications. The JEWEL study program investigated the use of single-pill amlodipine/atorvastatin as a strategy to improve management of these patients. The JEWEL program consisted of two 16-week, international, open-label, multicenter, titration-to-goal studies in patients with hypertension and dyslipidemia. The two studies differed based on country of enrollment and certain tertiary endpoints, but the overall designs were very similar. Patients were enrolled from 255 centers across Canada and 13 European countries. The study was designed to assess the efficacy, safety, and utility of amlodipine/atorvastatin single-pill therapy in a real-world setting. Patients were initiated at a dose of amlodipine 5 mg/atorvastatin 10 mg, unless previously treated, and were uptitrated as necessary. The primary efficacy parameter was the percentage of patients, at different levels of cardiovascular risk, achieving country-specific guideline-recommended target levels for blood pressure and lipids. A secondary analysis of efficacy measured attainment of the same single goal for blood pressure across all study participants (JEWEL I and II) and the same single goal for LDL-C across all study participants (JEWEL I and II). The program utilized a newly developed questionnaire to gain better understanding of participants’ beliefs and behaviors towards medical treatment of their multiple risk factors. Approximately 2850 patients were enrolled in the program, which was completed in August 2005. The JEWEL program assessed the effectiveness of a single pill (amlodipine/atorvastatin) in targeting the two principal risk factors for cardiovascular disease simultaneously to achieve nationally applicable treatment targets in a routine clinical practice setting.
Background
Cardiovascular disease (CVD) is the world’s most important cause of death, driven by the high rates of coronary heart disease and stroke in five out of the six World Health Organization regions (CitationWHO 2002). Since CVD also drives high healthcare costs in those surviving events such as stroke and heart failure, better strategies to facilitate the prevention and treatment of cardiovascular disease have become priorities for most healthcare systems.
Cardiovascular risk factors
The major modifiable risk factors for cardiovascular disease are well documented, with the principal three being: abnormal plasma lipid levels (high levels of low-density lipoprotein cholesterol [LDL-C] and triglycerides, and low levels of high-density lipoprotein cholesterol [HDL-C]) (CitationAnderson et al 1987; CitationVerschuren et al 1995); hypertension (CitationMacMahon et al 1990); and smoking (CitationMultiple Risk Factor Intervention Trial Group 1982; CitationGreenland et al 2003). Several large epidemiological surveys have shown that 80%–90% of patients with coronary heart disease have at least one of these three risk factors (CitationO’Meara et al 2004), and that each risk factor has a continuous, severity-dependent impact on risk for coronary heart disease (CitationNeaton et al 1992; CitationNCEP 2001; CitationThomas et al 2002). Additional cardiovascular risk factors include age (≥55 years), male gender, presence of other vascular disease (history of stroke or peripheral arterial disease), diabetes mellitus (CitationHaffner et al 1998), proteinuria, left ventricular hypertrophy, and family history of premature coronary heart disease.
Cardiovascular risk factors rarely occur in isolation and many subjects present with a combination of conditions that contribute to their total risk of cardiovascular disease (CitationKhot et al 2003; CitationJackson et al 2005). According to an analysis based on Framingham data, 78% of hypertensive men and 82% of hypertensive women have at least one other cardiovascular risk factor (CitationKannel 2000). The principal European CVD risk study, the WHO MONItoring of trends and determinants in CArdiovascular disease (MONICA) project, indicated that approximately 35% of Western Europeans have both conditions (CitationTunstall-Pedoe et al 2004). Similar studies of CVD risk factors in France showed that 84% of hypertensive men and 77% of hypertensive women have at least one other cardiovascular risk factor (CitationAsmar et al 2001), and that 20% of adults in the United Kingdom (UK) have concomitant hypertension and dyslipidemia (representing 9.2 million people) (CitationWilliams et al 2004). These findings are of major clinical significance, since even borderline elevations in both blood pressure (BP) and serum cholesterol can dramatically increase the prevalence of cardiovascular disease and the risk of coronary heart disease.
Prevention guidelines from the US (CitationNCEP 2001; CitationChobanian et al 2003), Canada (CitationKhan et al 2004), and Europe (CitationGuidelines Committee 2003; CitationWilliams et al 2004) highlight the need for an overall approach towards assessment of a patient’s risk of morbidity and mortality from cardiovascular disease. These guidelines recommend assessment of total cardiovascular risk and encourage individually tailored therapy to address all risk factors.
Managing cardiovascular risk and current practice
Encouraging lifestyle modifications should be the cornerstone of all treatment programs (CitationNCEP 2001; CitationWilliams et al 2004); however lifestyle modifications are not always successful or sufficient on their own (CitationEUROASPIRE II Study Group 2001). Large clinical outcome trials have shown that cardiovascular morbidity and mortality can be reduced significantly by the use of antihypertensive agents (CitationALLHAT investigators 2002; CitationTurnbull 2003; CitationJulius et al 2004) and lipid-lowering drugs, particularly statins (CitationHeart Protection Study Collaborative Group 2002; CitationSever et al 2003) in both secondary (CitationScandinavian Simvastin Survival Group 1994) and primary prevention (CitationShepherd et al 1995).
Further evidence has accumulated to guide how intensively patients at risk should be treated. Targets for BP are based on meta-analyses from the major trials (CitationBlood Pressure Lowering Treatment Trialists’ Collaboration 2003; CitationTurnbull et al 2005) and especially the results of large outcomes trials (CitationHansson et al 1998). Targets for LDL-C were set initially on the basis of older landmark statin studies (CitationScandinavian Simvastin Survival Group 1994; CitationSever et al 2003); however there have been calls to further reduce these goals (CitationGrundy et al 2004) on the basis of more recent trials, which indicate that risk of cardiovascular disease can be further lowered by treating to more aggressive targets (CitationCannon et al 2004; CitationNissen et al 2004). Furthermore, mortality improvements have been observed in patients with multiple risk factors, but only normal to mildly elevated cholesterol at baseline (mean total cholesterol ≤6.5 mmol/L in ASCOT) (CitationSever et al 2003). For lipid lowering, it is known from MRFIT that every 0.5 mmol/L increase in total cholesterol corresponds to an increase in CHD mortality risk of 12% and an increase in mortality risk of 17% when adjusted for regression dilution bias. Furthermore, the major lipid reduction trials have shown that every 1 mmol/L reduction in TC was associated with a 23% reduction in CHD and 21% reduction in total vascular risk (CitationBaigent et al 2005). There is also strong evidence from intervention trials that benefit of this magnitude is achieved whichever method is used to reduce cholesterol levels.
Guidelines for management of cardiovascular risk factors rely on physicians and patients recognizing the need to reduce cardiovascular risk and accepting the huge evidence-base supporting the recommended interventions. However, the observed suboptimal treatment patterns and failure to achieve guideline-recommended treatment goals () are compounded by patient non-concordance with prescribed therapies – up to 50% of patients choose to stop their medication of their own volition for a variety of reasons (CitationInsull 1997), and often within a few months of starting treatment. Clearly, adherence to treatment will influence whether benefits are translated into reductions in morbidity and mortality (CitationShepherd et al 1995; CitationFlack et al 1996). Factors reported to influence adherence include patient education, cost, patients’ attitudes towards treatment, differences in dosing regimens, history of cardiovascular disease, numbers of concomitant medications and side effects (CitationSchroeder et al 2004; CitationChapman et al 2005). There are reasons in addition to non-adherence, which may help to explain why treated patients do not attain target levels, for example, lack of response to a specific therapy.
Physician performance in delivering guideline recommendations for CVD risk
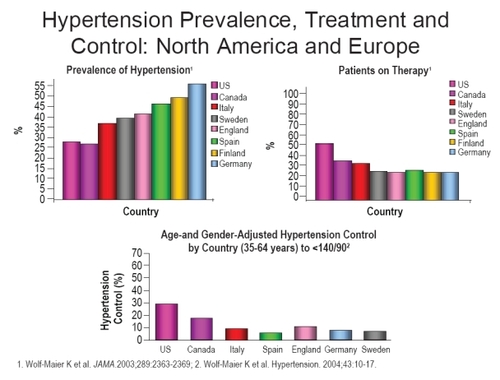
Despite the widely acknowledged severe burden of cardiovascular disease, previous studies conducted in a variety of locations and settings have demonstrated that many patients at risk remain unidentified and or untreated. One survey conducted in Europe and Canada has indicated that up to 60% of treatment-eligible patients are not receiving treatment for their hypertension, ranging from 38% in Canada, to 59% in Germany (CitationWolf-Maier et al 2004). A similar pattern has been recorded for patients with coronary heart disease and elevated lipid levels in Europe (EUROASPIRE I and II Group).
The way physicians increment therapy also contributes to under-treatment: typically, physicians treat patients with additional cardiovascular risk factors by managing each risk factor separately, often sequentially rather than in parallel (CitationPearson et al 2000; CitationRosal et al 2004). This approach adds to pill burden and requires multiple physician visits to achieve appropriate drug titration for each risk factor. Even among those patients receiving adequate treatment for both conditions, few patients are at goal. This treatment gap could widen as target levels are reduced (CitationGrundy et al 2004). There is also suboptimal management and little improvement over time of other cardiovascular disease risk factors, such as smoking and obesity (CitationRosal et al 2004; CitationAgarwal et al 2006).
Patient factors that influence patient concordance with therapy
In a retrospective cohort study of patients in a US managed-care plan, it was found that adherence to concomitant antihypertensive and lipid-lowering therapy was poor, with only one in three patients adherent to both medications at 6-months (CitationWolf-Maier et al 2004). Pill count is an important issue, since patients are less likely to refill their antihypertensive and lipid-lowering prescriptions as their total number of prescriptions increases (CitationWolf-Maier et al 2004). Synchronization of antihypertensive and lipid-lowering therapy initiation (ie, initiating both therapies simultaneously) also improves adherence in comparison with patients starting one therapy more than 30-days prior to the other (CitationAgarwal et al 2006), and adherence decreases as the time between initiation of antihypertensive and lipid-lowering therapies is prolonged (CitationWolf-Maier et al 2004). Increased cost of multiple prescriptions has been shown to have a large impact on adherence. For example, 25% of senior (>65 years) Medicare beneficiaries in a 2003 survey reported forgoing prescription medications in the past year because of cost (CitationSafran et al 2005).
Physicians may therefore be able to significantly improve medication adherence by initiating antihypertensive and lipid-lowering therapy concomitantly and by reducing pill burden and costs to the patient – goals that may be aided by single-pill therapy combining an antihypertensive with a lipid lowering agent. Patient adherence to medication has been shown to be significantly greater with a single-pill regimen compared with a two-pill regimen for antihypertensive therapy alone (CitationJulius et al 2004), and also when antihypertensive and lipid-lowering therapies are initiated together versus sequentially (CitationWolf-Maier et al 2004; CitationDahlof et al 2005).
Rationale for amlodipine besylate/atorvastatin calcium combination
With evidence showing that drug combinations improve patient compliance and persistence with therapy, this provides the rationale for the amlodipine besylate/atorvastatin calcium combination – a single-pill medication that treats both hypertension and dyslipidemia.
Amlodipine component
Amlodipine besylate is approved for the treatment of hypertension and both vasospastic and chronic stable angina, alone or in combination with other agents. Amlodipine has been demonstrated to be well tolerated and effective at lowering BP in a wide variety of patients at risk of cardiovascular disease (CitationALLHAT Investigators 2002; CitationBisognano et al 2004; CitationJulius et al 2004; CitationDahlof et al 2005). It has been shown to reduce cardiovascular events in both hypertensive patients (CitationDahlof et al 2005; CitationSafran et al 2005; CitationAgarawal et al 2006) and normotensive patients with coronary artery disease (CitationSafran et al 2005).
The primary action of calcium channel blockers is to inhibit calcium (Ca2+) entry through voltage-gated transmembrane L-type channels, thus decreasing intracellular Ca2+ concentration and inducing smooth muscle relaxation. Amlodipine has been tested extensively in clinical trials and has been used for the treatment of hypertension since 1990 in Europe and 1992 in the US.
Atorvastatin component
Atorvastatin calcium is a synthetic lipid-lowering agent, available in dosage strengths of 10, 20, 40, or 80 mg. Atorvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, which catalyzes the conversion of HMG-CoA to mevalonate. This action occurs at an early and rate-limiting step in cholesterol biosynthesis and, consequently, is an important target for the development of cholesterol-lowering agents (CitationJukema et al 2004). Inhibition of HMG-CoA reductase leads to upregulation of LDL-receptors in the liver, mediated by activation of sterol regulatory element-binding proteins and enhanced clearance of LDL from the circulation. Because lipoproteins that infiltrate the vessel wall initiate the development of atherosclerotic lesions, and oxidized LDL particles may trigger or enhance atherogenicity, HMG-CoA reductase inhibitors (statins) have an important role in therapy to prevent atherosclerosis.
Atorvastatin, a second-generation statin, was introduced in 1996 and gives LDL-C reductions of 41%–61% in hypercholesterolemic patients. Individualization of drug dosage should be based on therapeutic response. Atorvastatin has been extensively tested in clinical trials and has shown impressive reductions in cardiovascular events (CitationGrundy et al 2004). The efficacy of atorvastatin monotherapy also has been demonstrated in diverse patient types analyzed in major clinical endpoint trials (CitationAthyros et al 2002; CitationCannon et al 2004; CitationColhoun et al 2004).
In addition to the treatment of hypercholesterolemia, atorvastatin is indicated for reducing the risk of cardiovascular disease events in diabetic patients without clinically evident coronary heart disease, but with at least one additional cardiovascular risk factor. Furthermore, details of the CV benefits observed in hypertensive patients with 3 or more additional risk factors in ASCOT-LLA are included in the UK label, although an indication for the prevention of CV events in this group of patients is not mentioned specifically.
Amlodipine and atorvastatin have therefore been shown in major outcome studies to be safe and effective. There are no reported safety issues with the combined use of these two medications. Each is effective in the target population of the other, and neither component negatively impacts the efficacy of the other. In terms of pharmacokinetics, both agents have long half-lives, allowing once-daily administration. Both agents can be dosed at any time of the day. Neither drug has any interaction with foods, or affects the other drug’s pharmacokinetic profile.
Simplifying drug regimens and reducing “pill burden” have been shown to enhance patient adherence. This could be particularly relevant for patients at high risk of cardiovascular disease who will often be prescribed complex drug regimens to appropriately manage their multiple cardiovascular risk factors. The logic of combining multiple risk interventions for this multifactorial disease has been widely debated following the concept of the “polypill” for cardiovascular disease prevention (CitationLaw et al 2003; CitationWald and Law 2003).
Clinical evidence for amlodipine/atorvastatin (Caduet) combination therapy
Pre-registration studies of Caduet were extensive, using Atorvastatin and Amlodipine pills separately but concurrently. Co-administration of amlodipine and atorvastatin has been demonstrated to be safe and effective for lowering both BP and LDL-C in patients with concomitant hypertension and dyslipidemia (CitationBlank et al 2005; CitationDorval et al 2005; CitationMesserli et al 2006). The half-lives of both agents facilitate once-daily dosing, and both can be administered at any time of day with or without food (CitationPreston et al 2004). Neither drug has any adverse effects on the other’s efficacy or tolerability (CitationPreston et al 2005a, Citationb; CitationHobbs et al 2006). Bioequivalence has been demonstrated for both amlodipine and atorvastatin when fixed-dose combination tablets in strengths of 5/10 or 10/80 mg amlodipine/atorvastatin were compared with commercially available amlodipine and atorvastatin tablets coadministered in matching doses (CitationFlack et al 2003).
Following successful registration, amlodipine/atorvastatin (Caduet) tablets are available for oral administration in 11 dose-strength combinations for use in the US. In some countries of the European Union two dose strengths are available (5 mg amlodipine/10 mg atorvastatin, and 10 mg amlodipine/10 mg atorvastatin). Single-pill amlodipine/atorvastatin is indicated in Europe for the prevention of cardiovascular events in hypertensive patients, with three concomitant cardiovascular risk factors, normal to mildly elevated cholesterol levels, and without clinically evident coronary heart disease where combined use of amlodipine and low dose atorvastatin is appropriate.
Since registration, several clinical trials have been conducted to assess the efficacy and safety of the combination therapy.
GEMINI (CitationWilson et al 1998)
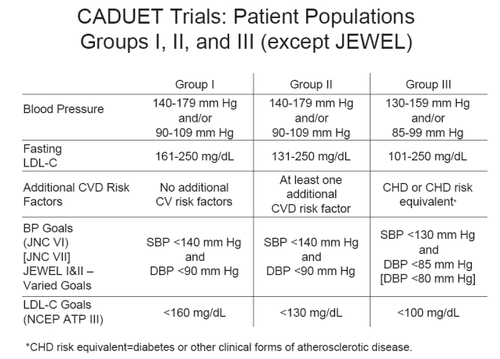
GEMINI was the first Real World trial conducted to examine the use of amlodipine/atorvastatin (Caduet) single-pill therapy for the concomitant treatment of hypertension and dyslipidemia (CitationWilson et al 1998). This 14-week, open-label, non-comparative, multicenter trial based in the US demonstrated the broad utility of the combination treatment in getting 1220 patients with uncontrolled hypertension and concurrent dyslipidemia to achieve both BP and LDL-C goals, based on JNC VI and NCEP ATP guidelines (). At study end, more than half of the patients had reached both their BP and LDL-C therapeutic targets. Over 65% of patients reached their respective Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure VI (JNC VI) goal at study end, and 82% attained their National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) goal. shows the percentages of patients reaching their BP, LDL-C or both therapeutic targets, based on baseline CVD risk category (CitationWilson et al 1998). Data were also presented regarding the performance of Caduet in patients who meet the IDF criteris for the metabolic syndrome (IDF).
Figure 2 Blood pressure and lipid targets for the GEMINI and CAPABLE trials, based on JNC VI blood pressure and NCEP ATP lipid recommendations.
The open-label GEMINI study also demonstrated that the amlodipine/atorvastatin single pill has a safety profile in a real-world office setting consistent with its components, as previously documented in randomized, controlled clinical trials, including RESPOND and AVALON (CitationFlack et al 2003; CitationHobbs et al 2006). In GEMINI, the most frequently reported adverse events (regardless of cause) were respiratory tract infections (11.9%), peripheral edema (8.8%), headache (5.4%) and myalgia (4.2%).These data demonstrated that co-administered amlodipine plus atorvastatin is well tolerated in patients with hypertension and additional risk factors, and that the adverse events observed are similar in nature, severity and frequency to those seen with amlodipine or atorvastatin administered alone.
The JEWEL Program (CitationHobbs et al 2006)
This program consisted of two 16-week, international, open-label, multicenter, titration-to-goal studies (JEWEL I and II) in 2219 patients with hypertension and dyslipidemia. The two studies differed based on country of enrollment (I with 1135 patients was based in the UK and Canada (CitationNichol et al 2006), and II with 1034 patients was based in 8 other major European countries) and primary endpoints in terms of treatment targets – these were determined by what the prevailing targets were by country (Canada, UK [audit targets], and European Society of Cardiology [CitationConroy et al 2003] guidelines) as seen in . Patients were initiated at a dose of amlodipine besylate 5 mg/atorvastatin calcium 10 mg, and the dose was uptitrated as necessary.
Figure 3 Percentage of GEMINI patients at blood pressure, lipid, or combined targets by cardiovascular disease risk category (NCEP ATP I to III risk categories) at baseline.
CV Risk Group I – Hypertension and dyslipidemia
CV Risk Group II – Hypertension and dyslipidemia and presence of at least one other CVD risk factor (ie, men ≥ 45 years, women ≥ 55 years, premature CHD in first-degree relative, current smoker, HDL-C <40 mg/dL)
CV Risk Group III – Hypertension and dyslipidemia with CHD or CHD equivalents (ie, diabetes mellitus, any atherosclerotic disease)
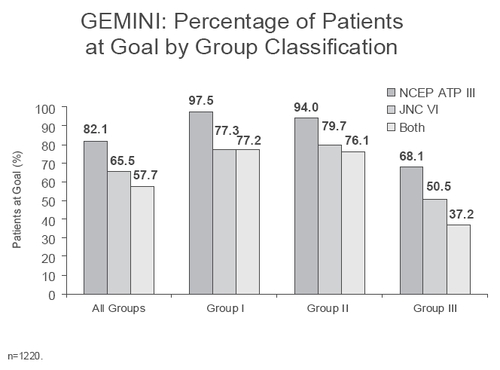
Figure 4 Blood pressure and lipid targets for the JEWEL I and II studies, based on country specific recommendations.
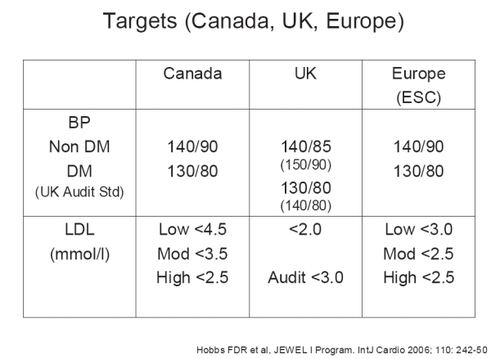
Figure 5 Proportions of patients meeting either blood pressure or lipid targets and both in JEWEL I and II studies after 16 weeks of amlodipine/atorvastatin (Caduet) therapy in 2219 patients.
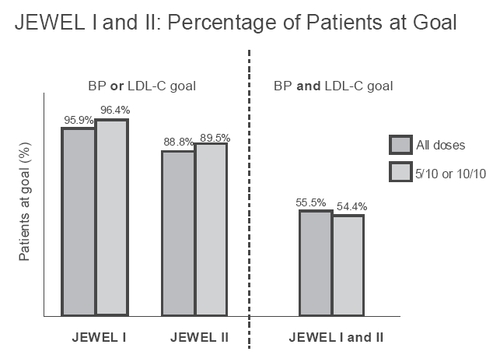
Different combinations of doses of amlodipine besylate/atorvastatin calcium were used in the study, and the majority of patients achieved their BP and LDL-C targets (varied between UK, Canada and Europe) with the 5/10 and 10/10 combination doses. illustrates the percentages of patients attaining the BP and/or LDL-C goals.
In terms of safety profile, peripheral edema and myalgia occurred in a small proportion of patients, with myalgia occurring at a lower than expected rate.
GEMINI AALA
With a study design similar to that of GEMINI conducted in the United States, this trial included 1649 patients with diverse ethnic backgrounds across 27 countries in Asia, Africa, the Middle East and Latin America. Fifty-two percent of the patients in GEMINI AALA were of Asian and 15% of Hispanic ethnicity. Preliminary results show that an overall of 55.2% of patients achieved both JNC VI BP and NCEP ATP III LDL-C therapeutic goals after 14 weeks of treatment. A post-hoc analysis conducted among a subgroup of patients in Eastern Asian countries (excluding Australia, India, and Pakistan) further shows that the mean Framingham 10-year CHD risk score was reduced by 51.6% with Caduet single-pill treatment.
The treatment was generally well tolerated by patients. The majority of peripheral edema cases was mild to moderate in severity, and resulted in only 4 discontinuations. The incidence of myalgia was lower than expected, and none resulted in discontinuations.
CAPABLE
The CAPABLE study conducted in the US, investigated the efficacy, safety and clinical utility of single-pill amlodipine/atorvastatin therapy among 499 African-American patients) with concomitant hypertension and dyslipidemia (under-researched group (CitationFlack et al 2006a). Research to study therapies for the reduction of cardiovascular risk in the African-American population is particularly important as hypertension is more common among African-Americans than other US ethnic groups (CitationFlack et al 2003). The design of the CAPABLE study was similar to that of the GEMINI study, but enrolled 500 African-American men and women and analyzed BP and LDL-C goal attainment over 20 weeks rather than all ethnic groups with 14 weeks of follow up. Overall, at the end of CAPABLE 48.3% of patients reached both their blood pressure and LDL-C goals (compared with 0.8% at baseline) indicating the utility of amlodipine/atorvastatin to lower BP and lipid levels in this ethnic minority population, which is at a high risk of CVD events. A substudy of CAPABLE also demonstrated that amlodipine/atorvastatin improves 24 hour ambulatory BP (CitationFlack et al 2006b).
Real-life prescribing data
The Caduet Concordance Research Program and Education (CARPE)-PBM Concordance Study (CitationNichol et al 2006) utilized claims data from a large US database, to assess differences in concordance between single-pill amlodipine/atorvastatin therapy in comparison with separate antihypertensive and lipid-lowering therapies. The study showed that the probability of achieving concordance with single-pill amlodipine/atorvastatin was almost twice that for amlodipine and atorvastatin taken separately. Compared with patients taking another antihypertensive and another statin separately, patients receiving amlodipine/atorvastatin were almost three times as likely to be adherent to both classes of medication (CitationNichol et al 2006).
Conclusion
CVD is the most important cause of death and disability in the world. The most important treatable risk factors are hypertension and dyslipidemia. Despite effective therapies, patient concordance with multiple therapies is problematic. The amlodipine/atorvastatin single pill (Caduet) is the first combination therapy treating two risk factors simultaneously, targeting patients with co-existing hypertension and dyslipidemia. It has been shown to improve rates of goal attainment and to be as well tolerated as the parent compounds given individually.
References
- AgarwalSMcLaughlinTJoyceASynchronizing antihypertensive and lipid-lowering therapies increases patient adherenceJ Clin Hypertens20068Suppl A 5A214P519
- ALLHAT investigatorsMajor outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)JAMA200228829819712479763
- AndersonKMCastelliWPLevyDCholesterol and mortality. 30 years of follow-up from the Framingham studyJAMA19872572176803560398
- AsmarRVolSPannierBHigh blood pressure and associated cardiovascular risk factors in FranceJ Hypertens20011917273211593091
- AthyrosVGPapageorgiouAAMercourisBRTreatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) studyCurr Med Res Opin200218220812201623
- BaigentCKeechAKearneyPMEfficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statinsLancet200536612677816214597
- BisognanoJMcLaughlinTRobertsCSIncremental effectiveness of amlodipine besylate in the treatment of hypertension with single and multiple medication regimensAm J Hypertens2004176768315323063
- BlankRLaSalleJReevesRSingle-pill therapy in the treatment of concomitant hypertension and dyslipidemia (the Amlodipine/Atorvastatin Gemini Study)J Clin Hypertens2005726473
- Blood Pressure Lowering Treatment Trialists’ CollaborationEffects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trialsLancet200336215273514615107
- CannonCPBraunwaldEMcCabeCHIntensive versus moderate lipid lowering with statins after acute coronary syndromesN Engl J Med2004350149550415007110
- ChapmanRHBennerJSPetrillaAAPredictors of adherence with antihypertensive and lipid-lowering therapyArch Intern Med200516511475215911728
- ChobanianAVBakrisGLBlackHRThe Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 ReportJAMA200328925607212748199
- ColhounHMBetteridgeDJDurringtonPNPrimary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trialLancet20043646859615325833
- ConroyRMPyoralaKFitzgeraldAPEstimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE projectEur Heart J200324987100312788299
- DahlofBSeverPSPoulterNRPrevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trialLancet200536689590616154016
- DorvalJFAndersonTBuithieuJReaching recommended lipid and blood pressure targets with amlodipine/atorvastatin combination in patients with coronary heart diseaseAm J Cardiol2005952495315642561
- EUROASPIRE I and II GroupClinical reality of coronary prevention guidelines: a comparison of EUROASPIRE I and II in nine countries. European Action on Secondary Prevention by Intervention to Reduce EventsLancet2001357995100111293642
- EUROASPIRE II Study GroupLifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries; principal results from EUROASPIRE II Euro Heart Survey ProgrammeEur Heart J2001225547211259143
- FlackJMFerdinandKCNasserSAEpidemiology of hypertension and cardiovascular disease in African AmericansJ Clin Hypertens20035511
- FlackJMNovikovSVFerrarioCMBenefits of adherence to anti-hypertensive drug therapyEur Heart J199617Suppl A16208737196
- FlackJMVictorRWatsonKAmlodipine/atorvastatin single-pill dual therapy improves goal attainment in the treatment of concomitant hypertension and dyslipidemia in African-Americans: the CAPABLE trialJ Clin Hypertens2006a8456
- FlackJMVictorRWatsonKAmlodipine/Atorvastatin Single-Pill Dual Therapy Improves 24 Hour Ambulatory Blood Pressure in African-Americans: The CAPABLE TrialJ Clin Hypertens2006b8Suppl A 5A47P-96
- GreenlandPKnollMDStamlerJMajor risk factors as antecedents of fatal and nonfatal coronary heart disease eventsJAMA2003290891712928465
- GrundySMCleemanJIMerzCNImplications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelinesCirculation20041102273915249516
- Guidelines CommitteeEuropean Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertensionJ Hypertens20032110115312777938
- HaffnerSMLehtoSRonnemaaTMortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarctionN Engl J Med1998339229349673301
- HanssonLZanchettiACarruthersSGEffects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study GroupLancet19983511755629635947
- Heart Protection Study Collaborative GroupMRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trialLancet200236072212114036
- HobbsFDRGensiniGManciniGBJCan combining different risk interventions into a single formulation contribute to improved cardiovascular disease risk reduction? Rationale and design for an international open-label program to assess the effectiveness of a single pill (amlodipine/atorvastatin) to attain recommended target levels for blood pressure and lipids (The JEWEL Program)Int J Cardiol20061102425016338012
- InsullWThe problem of compliance to cholesterol altering therapyJ Intern Med1997241317259159603
- [IDF] International Diabetes FederationA new worldwide definition of the metabolic syndrome [online]2005 URL:http://http://www.idf.org/home/index.cfm?node=1401
- JacksonRLawesCMBennettDATreatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular riskLancet20053654344115680460
- JukemaJWvan der HoornJWAmlodipine and atorvastatin in atherosclerosis: a review of the potential of combination therapyExpert Opin Pharmacother200454596814996641
- JuliusSKjeldsenSEWeberMOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet200436320223115207952
- KannelWBFifty years of Framingham Study contributions to understanding hypertensionJ Hum Hypertens200014839010723112
- KhanNAMcAlisterFACampbellNRThe 2004 Canadian recommendations for the management of hypertension: Part II – TherapyCan J Cardiol200420415414968142
- KhotUNKhotMBBayzerCTPrevalence of conventional risk factors in patients with coronary heart diseaseJAMA200329089890412928466
- LawMRWaldNJRudnickaARQuantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysisBMJ2003326142312829554
- MacMahonSPetoRCutlerJBlood pressure, stroke, and coronary heart disease. Part 1 Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution biasLancet1990335765741969518
- MesserliFNeutelJHoustonMMulticenter evaluation of the efficacy and safety of atorvastatin plus amlodipine versus either agent alone in patients with concomitant hypertension and dyslipidemia: the AVALON StudyJ Clin Hypertens2006857181
- Multiple risk factor intervention trial. Risk factor changes and mortality results. Multiple Risk Factor Intervention Trial Research GroupJAMA19822481465777050440
- [NCEP] National Cholesterol Education ProgramExecutive Summary of The Third Report of The NCEP Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III)JAMA200128524869711368702
- NeatonJDWentworthDSerum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research GroupArch Intern Med199215256641728930
- NicholMBPatelBVThiebaudPA single pill combining antihypertensive and statin therapies improves patient concordance compared with multi-drug combinations: Results from the Caduet® Concordance Research Program and Education (CARPE) – PBM Concordance StudyJ Clin Hypertens20068456
- NissenSETuzcuEMSchoenhagenPEffect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trialJAMA200429110718014996776
- O’MearaJGKardiaSLArmonJJEthnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA studyArch Intern Med20041641313815226165
- PrestonRAHarveyPHerfertOThe efficacy and safety of fixed-dose combinations of amlodipine and atorvastatin in the treatment of patients with concomitant hypertension and dyslipidemia [abstract]Am J Hypertens200417185A Abstract #P-413
- PrestonRAHarveyPHerfertOReduction in Framingham cardiovascular risk with concomitant treatment of hypertension/dyslipidemia with amlodipine/atorvastatinAm J Hypertens2005a18A226
- PearsonTALauroraIChuHThe lipid treatment assessment project (L-TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goalsArch Intern Med20001604596710695686
- PrestonRASunFTarasenkoLSafety and tolerability of coadministered amlodipine and atorvastatin in patients with concomitant hypertension and dyslipidemia in the Respond studyAm J Hypertens2005b18A92A3
- RosalMCOckeneJKLuckmannRCoronary heart disease multiple risk factor reduction Providers’ perspectivesAm J Prev Med200427546015275674
- SafranDGNeumanPSchoenCPrescription drug coverage and seniors: findings from a 2003 national surveyHealth Aff (Millwood)2005Suppl Web ExclusivesW5-152W5-6615840625
- Scandinavian Simvastin Survival GroupRandomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastin Survival Study (4S)Lancet1994344138397968073
- SchroederKFaheyTEbrahimSHow can we improve adherence to blood pressure-lowering medication in ambulatory care? Systematic review of randomized controlled trialsArch Intern Med20041647223215078641
- SeverPSDahlöfBPoulterNRPrevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trialLancet200336111495812686036
- ShepherdJCobbeSMFordIPrevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study GroupN Engl J Med1995333130177566020
- ThomasFBeanKGuizeLCombined effects of systolic blood pressure and serum cholesterol on cardiovascular mortality in young (<55 years) men and womenEur Heart J2002235283511922642
- Tunstall-PedoeHChenRKramarzPPrevalence of individuals with both raised blood pressure and raised cholesterol in WHO MONICA Project population surveys 1989–97Pharmacoepidemiol Drug Saf200413Suppl 1S307S08
- TurnbullFEffects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trialsLancet200336215273514615107
- TurnbullFNealBAlgertCBlood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trialsArch Intern Med20051651410915983291
- VerschurenWMJacobsDRBloembergBPSerum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries studyJAMA199527413167596000
- WaldNJLawMRA strategy to reduce cardiovascular disease by more than 80%BMJ2003326141912829553
- WilliamsBWilsonKLaceyLThe prevalence and management of patients with co-existing hypertension and hypercholesterolaemia in the UKEur Heart J200425abstract suppl5289
- WilliamsBPoulterNRBrownMJGuidelines for management of hypertension: report of the fourth working party of the British Hypertension Society 2004-BHS IVJ Hum Hypertens2004181398514973512
- WilsonPWD’AgostinoRBLevyDPrediction of coronary heart disease using risk factor categoriesCirculation1998971837479603539
- Wolf-MaierKCooperRSKramerHHypertension treatment and control in five European countries, Canada, and the United StatesHypertension20044310714638619
- [WHO] World Health OrganizationIntegrated management of cardiovascular risk. Report of a WHO meeting2002GenevaWHO