Abstract
Colesevelam HCl is a bile acid sequestrant (BAS) which has been specifically designed with a unique structure for the purpose of improving tolerability and reducing potential drug interactions compared to older BAS, such as cholestyramine and colestipol. As a class, BAS are known to reduce cholesterol and glucose levels, and to reduce atherosclerotic coronary heart disease (CHD) risk as monotherapy, and in combination with other lipid-altering drug therapies. Colesevelam HCl has specifically been shown to reduce total and low-density lipoprotein (LDL) cholesterol levels, and has been approved as a cholesterol-lowering drug since year 2000. It has also been shown to reduce glucose levels. This discussion reviews mechanisms by which BAS lower cholesterol, and potential mechanisms by which BAS lower glucose levels in patients with type 2 diabetes mellitus. Finally this paper specifically reviews colesevelam HCl’s pharmacology, lipid and glucose efficacy, safety/tolerability, and clinical use.
Introduction
Colesevelam hydrochloride (HCl) is an orally administered, polymer, bile acid sequestrant (BAS) with a high capacity for binding bile acids. As a class, BAS have been shown to reduce low-density lipoprotein cholesterol (LDL-C) levels, variably increase high-density lipoprotein cholesterol (HDL-C) and triglyceride (TG) levels, reduce glucose levels, and reduce the risk of CHD (CitationNCEP 2002). Colesevelam HCl has specifically been shown to improve hypercholesterolemia and hyperglycemia, which are major atherosclerotic coronary heart disease (CHD) risk factors. Reducing these CHD risk factors may account for BAS’s proven efficacy in reducing CHD events.
Bile acid sequestrants: lipid and CHD efficacy
One of the earliest CHD outcomes studies evaluating the efficacy of lipid-altering drugs was the Lipid Research Clinics Coronary Primary Prevention Trial (LRC-CPPT), which was a study of 3806 men without CHD wherein the BAS cholestyramine was to be taken (by protocol) at a dose of 24 g/day; the mean duration of treatment was 7.4 years. Overall, compared with baseline, LDL-C was reduced by 20.3% (12.6% compared with placebo) and HDL-C was raised by 1.6%, which was associated with a 19% reduction in fatal and nonfatal myocardial infarction in the BAS group (CitationLipid Research Clinics 1984a). Due to poor tolerance, and thus poor compliance to cholestyramine, the actual amount of drug taken by study participants varied considerably (the average daily dose was 14 grams), allowing for an analysis which revealed that the dosage of cholestyramine was directly related to the degree of LDL-C lowering, which in turn, was directly related to the degree of CHD risk reduction (CitationLipid Research Clinics 1984b).
Subsequent quantitative coronary angiography studies demonstrated that BAS, either as monotherapy or in a combination regimen, decreased progression and increased regression of atherosclerotic coronary artery lesions. A National Heart and Lung Blood Institute study of 116 men and women with CHD treated with cholestyramine 24 g/day for 5 years showed a 26% decrease in LDL-C and an 8% increase in HDL-C, with reduced atherosclerotic progression in coronary artery lesions with stenosis greater than 50% (CitationBrensike et al 1984). Both the Cholesterol Lowering Atherosclerosis Study I (CLAS) of 162 men with prior coronary artery bypass grafting treated with colestipol 30 g/day and niacin 4.3 g/day for 2 years (CitationBlankenhorn et al 1987) and the CLAS II 4-year follow-up of a 103 men subgroup of CLAS I study participants (CitationCashin-Hemphill et al 1990) revealed a LDL-C lowering of 40%–43% and HDL-C raising of 37%, with significant decreased progression and increased regression in atherosclerotic coronary lesions. The Familial Atherosclerosis Treatment Study (FATS) evaluated 38 men with coronary artery atherosclerosis and a family history of cardiovascular disease treated with colestipol 30 g/day and lovastatin 40 mg/day for 2.5 years, resulting in a 46% decrease in LDL-C and 15% increase in HDL-C levels, which was associated with significant decreased progression and increased regression of atherosclerotic coronary lesions compared with conventional therapy (CitationBrown et al 1990). Another arm of the FATS study evaluated colestipol 30 g/day and niacin 4 g/day in 36 men with the same entry criteria, also followed for 2.5 years, and found a 32% decrease in LDL-C and a 43% increase in HDL-C levels. As with the FATS data regarding colestipol and lovastatin, colestipol and niacin was also associated with significant decreased progression, and increased regression compared to conventional therapy. The University of California, San Francisco Specialized Center of Research (UCSF-SCOR) performed a study evaluating 72 men and women with heterozygous familial hypercholesterolemia treated with colestipol, niacin, and lovastatin for 2 years. LDL-C was decreased by 39%, HDL-C increased by 26%, and the treatment group had mean regression of atherosclerotic coronary artery lesions, while the control group had mean progression (CitationKane et al 1990). Finally, the St. Thomas Arteriosclerosis Regression Study (STARS) of 90 men with CHD treated with cholestyramine 16 g/day for 3 years revealed a decrease in LDL-C by 35.7%, and increase in HDL-C by 4%, associated with decreased progression and increased regression of atherosclerotic coronary artery lesions compared to “usual care” therapy (CitationWatts et al 1992).
Bile acid sequestrants: glucose efficacy
In addition to improving LDL-C levels, BAS are also known to reduce glucose levels in patients with type 2 diabetes mellitus (T2DM). In a 6-week (for each period) crossover study of 20 men and one woman with T2DM, cholestyramine 16 g/day decreased LDL-C by 28%, had no significant change in HDL-C, and increased triglyceride (TG) levels 13.5%. Fasting glucose was decreased by 13% and glycated hemoglobin was lowered by 0.5% (CitationGarg and Grundy 1994). In a study of 70 men and women with T2DM (baseline HbA1c of 7.7%) treated with colestimide, 6 g/day (another BAS) or pravastatin 10 mg/day for 3 months, colestimide lowered LDL-C 23%, did not significantly change HDL-C, and increased TG by 14% (although not statistically significant). Fasting glucose levels were decreased by 8% and HbA1c was lowered by 0.9% (CitationYamakawa et al 2007). Finally, in a pilot study of colesevelam HCl 3.75 g/day administered to 65 men and women with T2DM on oral anti-diabetes drugs (OAD), LDL-C was decreased by 11.7%, HDL-C was not significantly changed, and TG were increased by 7.8% (which was not statistically significant). The baseline HbA1c of 7.9% was reduced 0.5% by colesevelam HCl. Fasting glucose was reduced by 14 mg/dL (though not statistically significant), while postprandial glucose levels were significantly decreased by 31.5% (p = 0.026) (CitationZieve et al 2007).
BAS cholesterol-lowering mechanism of action
Bile is a green digestive and excretory fluid and its green color is largely derived from hemoglobin metabolites. Oxygenated arterial hemoglobin pigment is normally bright red while deoxygenated blood in veins is blue to blue-black. In bruising, the pigmented hemoglobin in red blood cells is extravated into the dermal layers and becomes deoxygenated, which accounts for the initial red, blue, and purple bruise colors (commonly seen within 48 hours). Afterwards, hemoglobin is ingested by macrophages, and converted to bilirubin, which accounts for the green, yellow, and brown colors (often seen in bruises older than 7 days, although these times vary considerably) (CitationBariciak et al 2003). Thus, when deoxygenated and chemically altered, the hemoglobin pigment may turn green. Chemical alternation of hemoglobin is thought to account for the case report finding of “dark green blood” found in a surgical patient’s arterial line, which was thought to be due to sulfhemoglobinemia. (It was concluded that the patient may have taken excessive amounts of the migraine medication, sumatriptan, which contains a sulfonamide group [CitationFlexman et al 2007].) Because bile contains bilirubin and biliverdin, which are deoxygenated final products of heme metabolism, bile has a green/yellow pigment.
From a digestive standpoint, bile contains bile acids, which are amphipathic (hydrophilic on one side; hydrophobic on the other) digestive surfactants that promote intestinal lipid absorption. From an excretory standpoint, bile contains substances that cannot be eliminated efficiently in urine because they are water insoluble or protein bound. In addition to the excretion of heme metabolic products through biliary bilirubin, bile also contains heavy metals such as iron and copper, lipophilic steroids and drug metabolites (CitationHofmann 1999). Finally, bile also contains cholesterol, which is actively excreted by hepatocytes through the adenosine triphosphate binding cassette (ABC) transporter B 11, and accounts for approximately 2/3 of the cholesterol delivered to the intestine (with the other 1/3 being dietary cholesterol) (CitationBays 2002).
Bile is the predominant mechanism by which excessive hepatic cholesterol is excreted. Additionally, hepatocytes may utilize cholesterol to synthesize bile acids, which are then excreted into the intestine through the bile. Because both the biliary excretion of cholesterol and the generation of bile acids from hepatic cholesterol may affect hepatic LDL receptor activity, these metabolic processes are integral for maintaining cholesterol homeostasis.
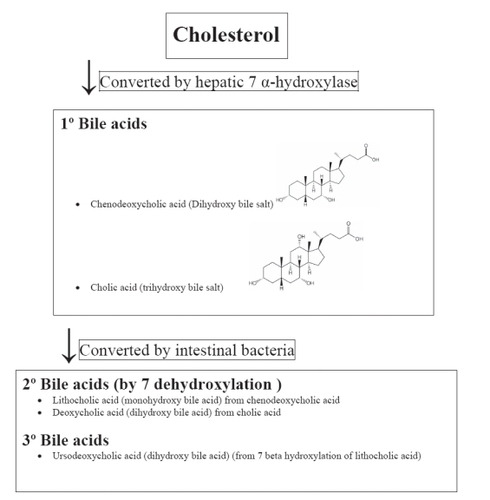
The hepatic conversion of cholesterol to bile acids occurs through the rate-limiting enzyme, cholesterol cytochrome P450 7 alpha hydroxylase (CYP7A1) (). Once conjugated with glycine or taurine, primary bile acids are then stored in the gall bladder and/or secreted into the intestine through the bile. Once in the intestine, bacterial 7-dehydroxylation results in the formation of secondary bile acids such as lithocholic and deoxycholic acid. The monohydroxy lithocholic acid can undergo 7 beta-hydroxylation, resulting in the formation of the tertiary, dihydroxy bile acid ursodeoxycholic acid. Ursodeoxycholic acid can undergo 7-dehydroxylation to form lithocholic acid. Although found in small amounts in humans, ursodeoxycholic acid is the principal bile acid produced in bears. It may decrease the intestinal absorption of cholesterol. And because an imbalance in cholesterol versus bile acids may contribute to gallstone formation, ursodeoxycholic acid is therapeutically used to prevent and treat cholesterol gallstone formation by blocking hepatic cholesterol production, decreasing biliary cholesterol, and promoting the dissolution of gallstones.
Over 95% of bile acids are transported into enterocytes in the terminal ileum and returned to the liver via the enterohepatic circulation. Binding to bile acids with BAS such as colesevelam HCl impedes the delivery or flux of bile acids back to the liver. As the hepatic bile acid pool becomes diminished, CYP7A1 becomes up-regulated, increasing the conversion of cholesterol to bile acids, and increasing the activity of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase (the rate-limiting step of cholesterol synthesis). The number of hepatic LDL receptors is also increased, which increases the clearance of LDL-C from the circulation, thus decreases LDL-C blood levels (CitationBays and Dujovne 2003).
BAS glucose-lowering mechanism of action
The mechanism of action by which BAS decrease glucose levels is largely unknown. Proposed mechanisms include alterations in luminal bile acid composition, increases in the incretin cholecystokinin, and effects related to hepatocyte nuclear factor 4 alpha (CitationBays and Cohen 2007).
The mechanism of action that might represent the most logical explanation would be the effects of BAS upon the nuclear receptors of farnesoid X receptors (FXR) and liver X receptors (LXR). Bile acids are a natural ligand for FXR, and activation of FXR results in repression of CYP7A1, thereby reducing bile acid synthesis. It is thought that FXR serves to protect the hepatocyte from bile acid excess, which can be toxic. Because BAS bind bile acids as their targeted mechanism of action, a decrease in enterohepatic bile acid availability would essentially “deactivate” or repress FXR, and impair hepatic FXR’s promotion of gluconeogenic genes such as phosphoenolpyruvate (PEPCK), a key enzyme in gluconeogenesis. Thus, repressed FXR activity from BAS may reduce PEPCK activity, reduce gluconeogensis, and thus lower glucose levels (CitationStayrook et al 2005).
But other data has not been consistent with these direct effects of repressed FXR activity. Another study suggests that FXR activation actually impairs hepatic gluconeogenic genes such as PEPCK (CitationMa et al 2006), and increases hepatic glycogen synthesis, all potentially mediated through enhanced insulin sensitivity. Further more, activation of FXR has been shown to lower glucose levels in mice with diabetes mellitus (CitationZhang et al 2006). Thus, in these models, repression of FXR activity through BAS therapy would be expected to increase, not decrease, glucose levels (CitationZhang et al 2006).
Similarly, FXR positively regulates expression of fibroblast growth factor 19 (FGF19). FGF19 normally suppresses CYP7A1 (CitationHolt et al 2003), possibly as a feedback response to increased cellular exposure to bile acids. Additionally, FGF19 has been suggested to increase metabolic rate, decrease body weight, and improve glucose homeostasis (CitationFu et al 2004; CitationStrack and Myers 2004). BAS decreases FGF19 activity, likely the result of repressed FXR activity (CitationLundasen et al 2006). Therefore, the administration of BAS would be expected to decrease the FXR-regulated expression of FGF19, with potential increases in glucose levels. This is another example suggesting that the direct effects of FXR repression from BAS therapy are not consistent with a potential to lower glucose levels, but instead are more consistent with a potential to raise glucose levels.
But FXR activation not only has direct effects, but also has important indirect effects that may affect glucose metabolism. As noted before, activation of FXR lowers glucose levels in mice with diabetes mellitus (CitationZhang et al 2006). This glucose lowering may be the net result of the direct and indirect effects of FXR activation. One of the important indirect effects of FXR activation relates to LXR. LXR has been described as a “glucose sensor” (CitationMitro et al 2007). Among the glucose-related effects of LXR activity are an increase in insulin secretion from the pancreas (CitationEfanov et al 2004), and increased adipogenesis (CitationSeo et al 2004). An increase in the number of functional adipocytes, particularly in patients with T2DM, may improve adiposopathy, which is defined as pathogenic adipose tissue promoted by positive caloric balance and sedentary lifestyle in genetically and environmentally susceptible patients. Adiposopathy is often characterized by hypertrophic visceral adipose tissue accumulation, and physiologically results in adverse metabolic and immune consequences resulting in clinical metabolic disease (CitationBays et al 2005, Citation2006c; CitationBays and Ballantyne 2006; CitationBays and Dujovne 2006; CitationBays 2006a). Specifically, increased LXR activity increases adipogenesis through increased expression of Adipocyte Determination and Differentiation-dependent Factor 1/Sterol Regulatory Element Binding Protein 1c, FAS genes, peroxisome proliferators-activated receptor gamma, and adipose protein 2 (CitationSeo et al 2004).
FXR activation normally induces expression of small heterodimer partner (SHP), which in turn, inhibits LXR activity, as well as CYP7A1 (CitationBrendel et al 2002). Repressed FXR activity, through the binding of bile acids with BAS, would be expected to decrease SHP, and release the inhibition of LXR. Thus, this indirect effect of FXR repression would be a relative increase in hepatic LXR activity, which downregulates enzymes that may contribute to hepatic insulin resistance and glucose intolerance. Increased LXR activity may also suppress hepatic gluconeogenesis, improve hepatic glucose utilization, and increase hepatic glucose uptake. Specifically, increased LXR activity downregulates 11 beta-hydroxysteroid dehydrogenase type 1 (CitationStulnig et al 2002), downregules PPAR gamma, coactivator-1 alpha, and gluconeogenic enzymes such as PEPCK and glucose-6-phosphatase (CitationCao et al 2003; CitationLaffitte et al 2003) and increases expression of glucokinase and GLUT-4 (CitationLaffitte et al 2003).
As discussed, some of the data about FXR’s direct effects suggest that BAS-induced repression of FXR would increase, not decrease glucose levels. It may actually be that LXR is the dominant regulator of the glucose effect (CitationGupta et al 2002). Therefore, if direct effects of FXR repression from BAS do indeed promote hyperglycemia, this effect may be overcome by the indirect increase in LXR activity, resulting in a net decrease in glucose levels.
Explaining some of the BAS metabolic effects through increased LXR activity has appeal because LXR agonists are known to lower glucose, lower LDL-C, and raise HDL-C levels (CitationLaffitte et al 2003; CitationTontonoz and Mangelsdorf 2003). It may be especially pertinent to note that LXR agonists have been shown to raise TG levels (CitationBays and Stein 2003). Thus, if increased LXR activity is an important mechanism of action accounting for BAS metabolic effects, then not only would this explain the glucose-lowering effects of BAS, but would also explain the variable increase in both HDL-C and TG levels observed with BAS (CitationBays and Cohen 2007).
Several questions remain, however. For example, activation of CYP7A1 is one of the most fundamental enzyme effects of BAS treatment. Studies have shown that the activation of this enzyme may be only partially mediated by FXR (CitationKerr et al 2002) while other studies have suggested that the activation of CYP7A1 is independent of either LXR or FXR activity (CitationShibata et al 2007). This suggests that important metabolic effects associated with BAS therapy may not be significantly influenced by either of these nuclear receptors. Nevertheless, while the glucose-lowering effect of BAS is not entirely clear, increased LXR activity from BAS may represent a unifying explanation as to why BAS may decrease glucose, increase HDL-C, and increase TG levels (CitationBays and Cohen 2007).
Colesevelam HCl pharmacology
Colesevelam HCl is hydrophilic, insoluble in water, and is administered orally as a solid off white tablet containing 625 mg colesevelam HCl, which is not hydrolyzed by digestive enzymes and does not undergo intestinal absorption. It is excreted exclusively in the feces. Colesevelam HCl may be administered as 6 tablets once a day, or 3 tablets twice a day with meals.
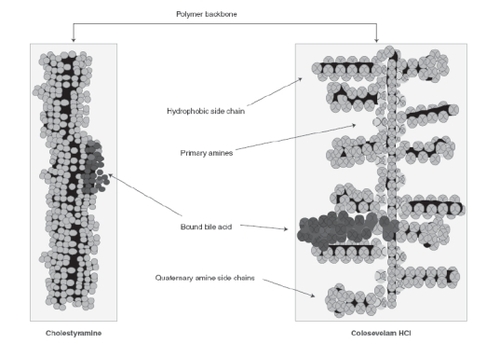
In a study of 16 healthy volunteers administered colesevelam HCl 1.9 g twice a day for 28 days, an average of only 0.05% of a single 14C-labeled colesevelam HCl dose was excreted in the urine (CitationWelchol® Product Information 2007) supporting its limited systemic uptake. Its structure is unique compared to other BAS, being a polyallylamine crosslinked with epichlorohydrin and alkylated with 1-bromodecane plus 6-bromohexyltrimethylammonium bromide (). This results in a more gelatinous consistency (and thus improved tolerability) compared with the more sandy-textured cholestyramine and colestipol. The positioning of the colesevelam HCl side chains maximizes interactions with bile salts, allowing for high capacity, specificity, and affinity for bile salts, and accounts for the more limited potential for drug interactions (CitationBays and Dujovne 2003).
Figure 2 Comparative structure of bile acid sequestrants. Rreproduced with permission from CitationBays H, Dujovne C. 2003. Colesevelam HCl: a non-systemic lipid-altering drug. Expert Opin Pharmacother,4: 779-90. © 2003 Informa Healthcare.
In monotherapy, the recommended starting dose is 3 tablets twice per day with meals (including liquids) or 6 tablets once per day with a meal (including liquids). If needed, colesevelam HCl may be increased to 7 625-mg tablets per day, although this was rarely studied in the clinical trials. In combination therapy, colesevelam HCl has been shown to be safe and effective when used with statins, at doses of 4–6 tablets per day.
Colesevelam HCl cholesterol efficacy studies
Early monotherapy trials demonstrated that compared to placebo, six 625 mg tablets colesevelam HCl per day decreased LDL-C by 15%–21%, increased HDL-C by 3%–9%, and increased TG levels by 2%–16% compared with placebo (CitationBays and Dujovne 2003). Early statin combination trials demonstrated that compared with statin alone, six 625-mg colesevelam HCl tablets per day in combination with statins, further decreased LDL-C by 10%–16%, increased HDL-C by 3%–7%, and increased TG levels by 5%–23%.
Colesevelam HCl was the first BAS reported to reduce C-reactive protein (CRP) when added to statins (CitationBays et al 2006b). A smaller study of 48 mildly hypercholesterolemic patients has reported that colesevelam HCl monotherapy lowers CRP compared with placebo, with no correlation to LDL cholesterol lowering (CitationDevaraj et al 2006). It is possible that these findings are not unique to colesevelam HCl, given that CRP was not routinely measured during the development of older BAS such cholestyramine and colestipol. Therefore, the lack of prior reported CRP effects with other BAS was likely because the data had never been assessed.
Colesevelam HCl has also been evaluated in combination with non-statin lipid-altering drugs. Colesevelam HCl added to ezetimibe significantly reduced LDL-C by 32%, with non-significant increases in HDL-C and TG levels (CitationBays et al 2006a), which may have clinical relevance for statin intolerant patients. When combined with fenofibrate, colesevelam HCl decreased LDL-C by 12.7% compared with fenofibrate monotherapy, with no statistically significant effect on TG levels (CitationMcKenney et al 2005), which may have clinical relevance for hypertriglyceridemic patients with persistent hypercholesterolemia after fibrate therapy.
Colesevelam HCl glucose efficacy studies
After the previously mentioned pilot study demonstrating that colesevelam HCl reduced glucose, HbA1C, and cholesterol levels in T2DM patients, a phase III program was initiated, evaluating the effects of colesevelam HCl in a larger, and more broad population of T2DM patients. In a 26-week study of 316 men and women with T2DM and treated with metformin monotherapy, or metformin combined with other OAD, colesevelam HCl six 625-mg tablets per day decreased LDL-C by 15.9% and increased HDL-C 0.9%, with a non-statistically significant increase in TG levels of 4.7%. The mean baseline HbA1c was 8.2%, and colesevelam HCl therapy lowered HbA1c by 0.54% and fasting glucose levels by 13.9 mg/dL (CitationBays et al 2007). In another 26-week study of 461 men and women with T2DM treated with sulfonylurea monotherapy, or combined with other OAD, colesevelam HCl six 625-mg tablets per day decreased LDL-C by 16.7%, non-statistically increased HDL-C by 0.1%, and increased TG levels by 17.7%. The mean baseline HbA1c was 8.2%, and colesevelam HCl lowered HbA1c by 0.54% and decreased fasting glucose levels 13.5 mg/dL (CitationFonseca et al 2007). Finally, in a 16-week study of 287 men and women with T2DM treated with insulin monotherapy or insulin in combination with OAD, colesevelam HCl six 625-mg tablets per day decreased LDL-C by 12.8%, non-significantly decreased HDL-C by 0.9%, and significantly increased TG by 21.5%. The mean baseline HbA1c was 8.3%, and colesevelam HCl therapy lowered HbA1c by 0.5% and fasting glucose levels by 14.6 mg/dL (CitationGoldberg and Truitt 2006). Study medication compliance was over 90% in both the colesevelam HCl and placebo groups of all three studies.
Head-to-head comparative trials of the glucose-lowering effects of colesevelam HCl compared with other anti-diabetes drug therapies have not been reported. It is perilous to compare clinical trial data from different clinical trial programs, conducted at different time periods (sometimes decades apart), in patients at different stages of their disease, managed at different research sites, under different protocol designs, and with varying degrees of dietary/lifestyle intervention. Nonetheless, it may be of some relevance that the current prescribing information of sitagliptin (approved as an oral anti-diabetes drug therapy in 2006), reports that in T2DM patients with a mean baseline HbA1c of 8.0%, sitagliptin 100 mg reduced HbA1c by 0.5%–0.7% compared with placebo (CitationJanuvia 2007). Thus, the HbA1c lowering of colesevelam HCl is in the same general range as reported with sitagliptin, a recently approved anti-diabetes drug. A factor that contributes to reports of colesevelam HCl’s moderate HbA1c lowering is the protocol design, which as is more typical of recent anti-diabetes drug trial protocols. The entry criteria for HbA1c was restricted to a range of 7.5%–9.5%, with the top HbA1c level being lower than often found in other diabetes mellitus drug trials. Capping the top HbA1c entry criteria to lower levels blunts the mean HbA1c percent reduction because the higher the baseline HbA1c, the greater percent reduction in HbA1c with anti-diabetes drug therapies.
Drug interactions
Human drug interaction studies with colesevelam HCl have found no significant effect on the bioavailability of digoxin, fenofibrate, lovastatin, metoprolol, quinidine, valproic acid, warfarin, or statins (CitationWelchol® Product Information 2007). This is important because one of the major concerns and challenges with older BAS (cholestyramine and colestipol) was the high potential to impair the absorption of many common drugs, particularly anionic, acidic materials. Examples of concomitantly administered drugs in which cholestyramine has been described to potentially impair intestinal absorption include digitalis, diuretics, estrogens, hydrocortisone, penicillin G, phenobarbital, phenylbutazone, phosphate supplements, progesterones, propranolol, tetracycline, thiazides, thyroid and thyroxine preparations, and warfarin (CitationBays and Dujovne 2003). Examples of drugs in which colestipol has been described to potentially impair intestinal absorption include digoxin (possibly), furosemide, gemfibrozil, hydrocortisone, oral phosphate supplements, penicillin G, propranolol, tetracycline, and thiazide diuretics (CitationBays and Dujovne 2003). BAS may also theoretically interfere with fat absorption, potentially impairing absorption of fat soluble vitamins such as A, D, E, and K. Because of these important drug interactions, and because not all drugs have been evaluated for pharmacokinetic interactions, it is generally recommended that any concurrent drugs be administered at least 1 hour before, or 4 hours after cholestyramine or colesitpol. Colesevelam HCl has no such dosing requirement, with the possible exception of administering drugs with a narrow therapeutic index or margin of safety that has not been evaluated in a formal drug interaction study. In this case, the concomitant drug should be administered at least one hour before or four hours after colesevelam HCl, and if available, drug blood levels should be monitored. A practical example is the use of colesevelam HCl with thyroid hormone, in that post marketing reports have suggested increases in thyroid stimulating hormone (TSH) levels in patients who have received colesevelam HCl co-administered with thyroid hormone replacement therapy (CitationWelchol® Product Information 2007).
Safety/tolerability
Colesevelam HCl is pregnancy category B, which suggests that while no animal studies have demonstrated evidence of harm to the fetus, no adequate and well-controlled studies have been done in pregnant women. Thus, colesevelam HCl should be used during pregnancy only if clinically required. No special considerations, such as alteration in doses, are required when colesevelam HCl is administered to older patients. As a class, BAS are approved for use in children with significant hypercholesterolemia (CitationTonstad 2000), although the safety and efficacy of colesevelam HCl has not specifically been established in pediatric patients.
According to the prescribing information for colesevelam HCl (CitationWelchol® Product Information, 2007), when compared to placebo in an integrated safety analysis, the treatment-emergent adverse experiences that occurred in greater than 2% did not increase with colesevelam HCl with regard to “Body as a Whole” or “Respiratory System”. From a musculoskeletal standpoint, myalgias were reported in 2% of colesevelam HCl administered study participants, compared with 0% of those administered placebo. When adverse experiences relative to placebo were reported to increase with colesevelam HCl, they were predominantly in the digestive system, and included constipation (11% vs 7%) and dyspepsia (8% vs 3%). This is generally consistent with the clinical trial experience with colesevelam HCl in that the total treatment-emergent adverse experiences are usually similar to placebo, of the same severity, with a few percentage point increases in the rate of constipation and dyspepsia. It should be noted that colesevelam HCl trials most often excluded study participants with major gastrointestinal abnormalities. Given this, colesevelam HCl is contraindicated in individuals with a history of bowel obstruction, and should be used with caution in patients with dysphagia, swallowing disorders, severe gastrointestinal motility disorders or major gastrointestinal tract surgery.
Patients with TG levels greater than 300 mg/dL were also excluded from colesevelam HCl clinical trials. Given the clinical trial evidence of a variable increase in TG with BAS, caution should be exercised when using any BAS in patients with TG levels greater than 300 mg/dL, particularly in patients with TG-induced pancreatitis, or otherwise at risk for pancreatitis.
Finally, although colesevelam HCl has not demonstrated reductions in vitamins A, D, E, or K during human clinical trials of up to one year, caution should be exercised when treating patients with a susceptibility to vitamin K or fat-soluble vitamin deficiencies.
Conclusions and place in therapy
Colesevelam HCl is indicated as an adjunct to diet and exercise for the reduction of elevated LDL-C in patients with primary hypercholesterolemia, either alone or in combination with statins. Although other BAS have been shown to reduce CHD risk, colesevelam HCl has not been evaluated in a CHD outcome study, and thus does not have an approved indication to reduce CHD risk either alone, or in combination with statins. However, when combined with a statin, colesevelam HCl has been shown to not only improve lipid levels (such as LDL-C and HDL-C), but also improve inflammatory markers associated with increased CHD risk, such as CRP. The clinical use of colesevelam HCl is not unlike that of other lipid-altering drugs in that therapy should be considered as one component of an overall strategy of concomitant CHD risk-factor intervention. Before initiating therapy with colesevelam HCl, secondary causes of hypercholesterolemia should be evaluated and managed, such as poorly controlled hyperglycemia, untreated hypothyroidism, nephrotic syndrome, obstructive liver disease, and drug-induced dyslipidemias (eg, protease inhibitors, some immunosupressants).
Currently, 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors (“statins”) are the lipid-altering drugs of first choice for many patients towards the goal of decreasing LDL-C, and reducing CHD risk, because of the substantial clinical outcomes trial evidence and their demonstrated safety (CitationJones et al 1998; CitationBaigent et al 2005; CitationBays 2006b, Citationc). Recent guidelines have reduced the LDL-C treatment goals for patients at highest CHD risk (CitationGrundy et al 2004) and have thus increased the number of high-risk patients who do not achieve their target levels. In other words, patients at highest CHD risk (CitationGrundy et al 2004) including those with T2DM and CHD, are often the same patients who have the lowest achievement of LDL-C treatment goals (CitationDavidson et al 2005; CitationDavidson 2006). As such, statin monotherapy is frequently insufficient to achieve LDL-C less than 70 mg/dL goals in very high CHD risk patients, especially when higher statin doses cannot be employed due to statin intolerance, safety considerations, or fear of potential adverse experiences (rational or otherwise) at the highest statin doses (CitationBays 2006c; CitationJones 2006). This has prompted a call to use other lipid-altering drugs in combination with statins, or as an alternative to statins in patients intolerant to statins. (CitationGagne et al 2002; CitationBays et al 2003; CitationBays 2004; CitationBays et al 2004; CitationVasudevan and Jones 2006).
Since improvements in lipid levels reduce macrovascular CHD risk, and since improvements in glycemia reduce microvascular, and possibly macrovascular disease, patients with T2DM often receive both lipid and anti-diabetes drugs (CitationBays and Cohen 2007). Colesevelam HCl is approved as a cholesterol-lowering drug. In December of 2006, the manufacturer filed with the US Food and Drug Administration for approval of colesevelam HCl as a diabetes mellitus drug treatment. While clinical trial data is lacking as to whether the glucose-lowering effects of colesevelam HCl improves microvascular disease in patients with T2DM, if approved, colesevelam HCl will be the first LDL-C lowering medication also indicated for the glycemic treatment of T2DM, which may significantly help T2DM patients achieve both their LDL-C and HbA1C goals.
References
- BaigentCKeechAKearneyPMEfficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statinsLancet200536612677816214597
- BariciakEDPlintACGabouryIDating of bruises in children: an assessment of physician accuracyPediatrics2003112804714523170
- BaysHEzetimibeExpert Opin Investig Drugs20021115871604
- BaysHEExtended-release niacin/lovastatin: the first combination product for dyslipidemiaExpert Rev Cardiovasc Ther2004248550115225109
- BaysHAdiposopathy – defining, diagnosing, and establishing indications to treat “sick fat”: what are the regulatory considerations?US Endocrine Disease2006a1214
- BaysHWhat are the long-term effects of statin therapy?Nat Clin Pract Cardiovasc Med2006b31289
- BaysHStatin safety: an overview and assessment of the data – 2005Am J Cardiol2006c976C26C
- BaysHAbateNChandaliaMAdiposopathy: Sick fat causes high blood sugar, high blood pressure, and dyslipidemiaFuture Cardiology20051395919804060
- BaysHBallantyneCAdiposopathy: why do adiposity and obesity cause metabolic disease?Future Lipidology20061389420
- BaysHBlondeLRosensonRAdiposopathy: How do diet, exercise, weight loss and drug therapies improve metabolic disease?Expert Rev Cardiovasc Ther2006c48719517173503
- BaysHECohenDERationale and design of a prospective clinical trial program to evaluate colesevelam’s glucose-lowering effects in patients with type 2 diabetes mellitusCurr Med Res Opin20072316738417588297
- BaysHEDavidsonMJonesMREffects of colesevelam hydrochloride on low-density lipoprotein cholesterol and high-sensitivity C-reactive protein when added to statins in patients with hypercholesterolemiaAm J Cardiol2006b971198120516616026
- BaysHDujovneCColesevelam HCl: a non-systemic lipid-altering drugExpert Opin Pharmacother200347799012740000
- BaysHEDujovneCAMcGovernMEComparison of once-daily, niacin extended-release/lovastatin with standard doses of atorvastatin and simvastatin. the ADvicor Versus Other Cholesterol-Modulating Agents Trial Evaluation [ADVOCATE]Am J Cardiol2003916677212633795
- BaysHDujovneCAAdiposopathy is a more rational treatment target for metabolic disease than obesity aloneCurr Atheroscler Rep200681445616510049
- BaysHEGoldbergRBTruittKAddition of colesevelam HCl to patients with type 2 diabetes mellitus inadequately controlled on a metformin-based therapy improves glycemic control2007Abstract poster Presentation American Association of Clinical Endocrinologists 16th Annual Meeting and Clinical CongressApril 14, 2007Seattle Washington USA
- BaysHEOseLFraserNA multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemiaClin Ther20042617587315639688
- BaysHRhyneJAbbySLipid-lowering effects of colesevelam HCl in combination with ezetimibeCurr Med Res Opin2006a22219120017076980
- BaysHSteinEAPharmacotherapy for dyslipidaemia – current therapies and future agentsExpert Opin Pharmacother2003419013814596646
- BlankenhornDHNessimSAJohnsonRLBeneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass graftsJAMA19872573233403295315
- BrendelCSchoonjansKBotrugnoOAThe small heterodimer partner interacts with the liver X receptor alpha and represses its transcriptional activityMol Endocrinol20021620657612198243
- BrensikeJFLevyRIKelseySFEffects of therapy with cholestyramine on progression of coronary arteriosclerosis: results of the NHLBI Type II Coronary Intervention StudyCirculation198469313246360414
- BrownGAlbersJJFisherLDRegression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein BN Engl J Med19903231289982215615
- CaoGLiangYBroderickCLAntidiabetic action of a liver X receptor agonist mediated by inhibition of hepatic gluconeogenesisJ Biol Chem20032781131612414791
- Cashin-HemphillLMackWJPogodaJMBeneficial effects of colestipol-niacin on coronary atherosclerosis. A 4-year follow-upJAMA19902643013172243429
- DavidsonMHDifferences between clinical trial efficacy and real-world effectivenessAm J Manag Care200612S405S1117112328
- DavidsonMHMakiKCPearsonTAResults of the National Cholesterol Education. NCEP. Program Evaluation ProjecT Utilizing Novel E-Technology. NEPTUNE. II survey and implications for treatment under the recent NCEP Writing Group recommendationsAm J Cardiol2005965566316098311
- DevarajSAutretBJialalIEffects of colesevelam hydrochloride. WelChol. on biomarkers of inflammation in patients with mild hypercholesterolemiaAm J Cardiol200698641316923452
- EfanovAMSewingSBokvistKLiver X receptor activation stimulates insulin secretion via modulation of glucose and lipid metabolism in pancreatic beta-cellsDiabetes200453Suppl 3S75S815561926
- FlexmanAMDelVGSchwarzSKDark green blood in the operating theatreLancet2007369197217560450
- FonsecaVRosenstockJTruittKColesevelam HCl added to sulfonylurea-based therapy in patients with inadequately controlled type 2 diabetes mellitus improves glycemic control2007Abstract poster presentation American Association of Clinical Endocrinologists 16th Annual Meeting and Clinical CongressApril 14th, 2007Seattle Washington USA
- FuLJohnLMAdamsSHFibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetesEndocrinology2004145259460314976145
- GagneCBaysHEWeissSREfficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemiaAm J Cardiol20029010849112423708
- GargAGrundySMCholestyramine therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. A short-term, double-blind, crossover trialAnn Intern Med1994121416228053615
- GoldbergRBTruittKColesevelam HCl improves glycemic control in type 2 diabetes mellitus subjects managed with insulin therapy2006Abstract poster presentation. American Heart Association Annual MeetingNovember 12–15, 2006Chicago Illinois USA
- GrundySMCleemanJIMerzCNImplications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelinesCirculation20041102273915249516
- GuptaSPandakWMHylemonPBLXR alpha is the dominant regulator of CYP7A1 transcriptionBiochem Biophys Res Commun20022933384312054605
- HofmannAFThe continuing importance of bile acids in liver and intestinal diseaseArch Intern Med199915926475810597755
- HoltJALuoGBillinANDefinition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesisGenes Dev20031715819112815072
- JanuviaStagliptin [online]2007 URL: http://www.januvia.com.
- JonesPKafonekSLauroraIComparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia. the CURVES studyAm J Cardiol19988158279514454
- JonesPHRationale for intensive statin treatment in high-risk patientsPrev Cardiol2006991516603827
- KaneJPMalloyMJPortsTARegression of coronary atherosclerosis during treatment of familial hypercholesterolemia with combined drug regimensJAMA19902643007122243428
- KerrTASaekiSSchneiderMLoss of nuclear receptor SHP impairs but does not eliminate negative feedback regulation of bile acid synthesisDev Cell200227132012062084
- LaffitteBAChaoLCLiJActivation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissueProc Natl Acad Sci USA200310054192412697904
- Lipid Research ClinicsThe Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart diseaseJAMA1984a251351646361299
- Lipid Research ClinicsThe Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol loweringJAMA1984b251365746361300
- LundasenTGalmanCAngelinBCirculating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in manJ Intern Med2006260530617116003
- MaKSahaPKChanLFarnesoid X receptor is essential for normal glucose homeostasisJ Clin Invest20061161102916557297
- McKenneyJJonesMAbbySSafety and efficacy of colesevelam hydrochloride in combination with fenofibrate for the treatment of mixed hyperlipidemiaCurr Med Res Opin20052114031216197659
- MitroNMakPAVargasLThe nuclear receptor LXR is a glucose sensorNature20074452192317187055
- [NCEP] National Cholesterol Education ProgramThird Report of the National Cholesterol Education Program. NCEP. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Adult Treatment Panel III. final reportCirculation2002106314342112485966
- SeoJBMoonHMKimWSActivated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor gamma expressionMol Cell Biol20042434304415060163
- ShibataSHayakawaKEgashiraYRoles of nuclear receptors in the up-regulation of hepatic cholesterol 7alpha-hydroxylase by cholestyramine in ratsLife Sci2007805465317107691
- StayrookKRBramlettKSSavkurRSRegulation of carbohydrate metabolism by the farnesoid X receptorEndocrinology20051469849115564327
- StrackAMMyersRWModulation of metabolic syndrome by fibroblast growth factor 19. FGF19?Endocrinology20041452591315140837
- StulnigTMOppermannUSteffensenKRLiver X receptors downregulate 11beta-hydroxysteroid dehydrogenase type 1 expression and activityDiabetes20025124263312145154
- TonstadSRole of lipid-lowering pharmacotherapy in childrenPaediatr Drugs20002112210937455
- TontonozPMangelsdorfDJLiver X receptor signaling pathways in cardiovascular diseaseMol Endocrinol2003179859312690094
- VasudevanARJonesPHEffective use of combination lipid therapyCurr Atheroscler Rep20068768416455018
- WattsGFLewisBBruntJNEffects on coronary artery disease of lipid-lowering diet, or diet plus cholestyramine, in the St Thomas’ Atherosclerosis Regression Study. STARSLancet199233956391347091
- Welchol® Product InformationCholesevelam hydrochloride2007 Accessed July 1, 2007. URL: http://www.welchol.com/pdfs/fullPI.pdf
- YamakawaTTakanoTUtsunomiyaHEffect of colestimide therapy for glycemic control in type 2 diabetes mellitus with hypercholesterolemiaEndocr J20075453817102570
- ZhangYLeeFYBarreraGActivation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic miceProc Natl Acad Sci USA200610310061116410358
- ZieveFJKalinMFSchwartzSLResults of the glucose-lowering effect of WelChol study. GLOWS: A randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetesClin Ther200729748317379048