Abstract
Objectives
In the present study, patients with severely compromized left ventricular function underwent magnetic resonance imaging (MRI) before and after coronary artery bypass grafting (CABG). Although improvement of global myocardial contractile function has been shown before, we sought to evaluate whether a functional contractile improvement may be determinable on a myocardial segmental basis after CABG surgery.
Methods
Thirty-three CABG patients with left ventricular ejection fraction (LVEF) ≤30% prospectively underwent MRI to compare pre- and postoperative functional data. At follow-up, all survivors underwent clinical assessment. In 16 patients (three patients died perioperatively, 13 could were lost to MRI follow-up because of cardiac resynchronization therapy and other reasons) postoperative MRI scanning was performed.
Results
In-hospital mortality was 9%. At 20 ± 2 months after surgery, New York Heart Association class improved from 3.0 ± 0.1 to 2.2 ± 0.2 (p < 0.01). Left ventricular end-diastolic volumes decreased significantly from 229 ± 14 mL to 189 ± 19 mL (p < 0.05). LV end-systolic volumes decreased significantly from 163 ± 13 mL to 126 ± 17 mL (p < 0.05). LVEF improved from 30 ± 2% to 36 ± 3% (p < 0.05). On a segmental basis, 42 out of 875 segments (4.8%) had normal function before surgery, at follow-up, 177 segments (20.4%) had normal regional function (p < 0.05).
Conclusions
Patients who undergo CABG surgery with severely compromized left ventricular function, postoperative MRI shows improved global and segmental cardiac function at mid-term follow-up. At the same time there is considerable clinical improvement.
Introduction
A subset of patients with severe ischemic cardiomyopathy appears to benefit from revascularization. However, the selection of these patients for revascularization is often difficult. On the one hand, there are increased preoperative risks and co-morbidities in these patients, which must be weighed against the potential to improve contractile function, prognosis, and symptoms. This provides the rationale for noninvasive viability testing by using magnetic resonance imaging (MRI). Besides the quantification of myocardial scar tissue using late enhancement (CitationKim et al 1999; CitationHunold et al 2005), MRI has also been shown to reliably assess the extent of regional myocardial contraction (CitationSelvanagayam et al 2004) as well as global volume and function parameters (CitationWestenberg et al 2005).
Left ventricular (LV) function is among the most important determinants of prognosis in patients with coronary artery disease (CAD) (CitationBonow et al 2002). In patients with ischemic LV dysfunction, CABG is associated with a significant independent reduction in mortality (CitationVeenhuyzen et al 2001). In the absence of myocardial viability, however, myocardial revascularisation is not associated with any significant difference in outcomes, irrespective of treatment strategy (CitationAllman et al 2002). In CABG patients with preoperative LV dysfunction, MRI hyperenhancement has been shown to reliably indicate myocardial scar tissue (CitationKlein et al 2002). Using magnetic resonance imaging, cardiac function can be assessed globally and regionally (CitationSelvanagayam et al 2004). In the present study, we pre- and postoperatively determined global and segmental cardiac function with cardiac MRI. We were therefore interested whether, in addition to an improvement of global myocardial contractility, functional improvement could also be observed on a myocardial segmental basis after CABG surgery.
Material and methods
Patients
Thirty-three patients with a left ventricular ejection fraction ≤30% (as determined by coronary angiography) prospectively underwent MRI scanning before coronary artery bypass grafting (CABG). According to the protocol, the patients were contacted after 18 months and a second MRI scan was performed thereafter. Eventually, of the 33 patients who were scanned preoperatively, 16 underwent postoperative MRI after a mean follow-up of 20 ± 2 months. All patients gave their informed consent, and the study was approved by the institutional ethics committee.
Magnetic resonance imaging
A 1.5 T scanner (Magnetom Sonata, Siemens Medical Solutions, Erlangen, Germany) was used for all MRI examinations. The MRI protocol included a functional study of the left ventricle using an ECG-triggered breath-hold segmented steady-state free precession (SSFP; TrueFISP®) cine sequence (TR, 3.0 ms; TE, 1.5 ms, flip angle, 60°) with a slice thickness of 8 mm. After three standard long axes (horizontal and vertical, long axis left ventricular outflow tract), contiguous short axis slices were acquired to cover the entire LV without interslice gap. After injection of 0.2 mmol/kg bw Gd-DTPA (Magnevist™, Schering AG, Berlin, Germany), “late enhancement” (LE) scans were collected in three long and all short axis orientations using a breath-hold ECG-triggered 2D inversion recovery TurboFLASH sequence (TR, 8 ms; TE, 4 ms; flip angle, 25°) as described elsewhere (CitationSimonetti et al 2001). Images were acquired subsequently 8 to 15 minutes after injection. The inversion time (TI, nonselective inversion pulse) was adjusted manually between 180 and 300 ms to null the signal of normal myocardium. Depending on the field of view (FOV), the typical in-plane resolution was 1.6 × 1.3 mm2 for all sequences. Total imaging time including patient positioning was 45–60 minutes.
Image analysis, scoring, and calculating
All MRI examinations were interpreted by two radiologists experienced in cardiac MRI by consensus. TrueFISP images were reviewed as cine-loops on a workstation, whereas hardcopies were used for the read-out of the inversion recovery TurboFLASH images. All inversion recovery TurboFLASH data sets in long and short axis orientation were reviewed regarding LE.
Concerning segmental function, standardized myocardial segmentation and nomenclature for tomographic imaging of the heart has been suggested by the American Heart Association. A 17 segment model of the left ventricle was established as well as an assignment of left ventricular myocardial segments to coronary arterial topography (CitationCerqueira et al 2002). In basal and mid-ventricular portions of the LV, the myocardial circumference was additionally divided into 6 subsegments; in the apical third of the LV, four segments were evaluated plus the apex itself. This resulted in 49 to 61 segments per patient depending on LV dimensions: According to the AHA statement, a mean score was then calculated to assign the scores from all designated short axis images to the individual 17 AHA segments.
The 17 segments were distributed into 3 vessel territories, belonging to the 3 large epicardial arteries left anterior descending (LAD, 7 segments), circumflex artery (Cx, 5 segments) and right coronary artery (RCA, 5 segments). All 17 segments in each patient were assigned one of the major large epicardial arteries. A mean late enhancement score for each of these vessel territories was calculated. Based on the published data by CitationKim et al (2000), dysfunctional segments with LE score 1 or 2 are expected to recover after sufficient revascularization; those segments scored 3 or 4 are considered unlikely to improve function after restitution of perfusion.
A score 1 to 4 system was used to describe the transmural extent of LE in each of the turboFLASH short axis images: Score 1 = no LE; score 2 = subendocardial LE including less than 50% of the wall thickness; score 3 = nontransmural LE including 50% or more of the wall thickness; score 4 = transmural LE (CitationHunold et al 2002). The extent of segmental wall thickening (ie, the degree of wall motion or contractility) of the left ventricle was graded on a five-point scale in which a score of 0 indicated normal function, a score of 1 mild or moderate hypokinesia, a score of 2 severe hypokinesia, a score of 3 akinesia and a score of 4 dyskinesia (CitationKim et al 2000). Global LV function was assessed by slice summation in end diastole and end systole of the short axis slices after manual planimetry of the endocardial borders using the ARGUS® software (Siemens AG, Munich, Germany).
Surgical management
Surgical revascularization was performed as previously described (CitationThielmann et al 2006) in all patients using median sternotomy, standard CPB technique with ascending aortic and two-stage venous cannulation. During CPB, moderate hemodilution (hematocrit 20%–25%) with mild systemic hypothermia (>32 °C) was maintained. Myocardial protection was achieved using antegrade and optional retrograde cold crystalloid (Bretschneider) cardioplegic arrest and additional topical cooling. Patients were postoperatively monitored with respect to arterial pressure, pulmonary pressure, and central venous pressure.
Clinical follow-up
Patients were contacted 18 months after surgery. The clinical status was assessed using a simple questionnaire.
Statistical analysis
Results are expressed as mean ± SEM. For parametric data, differences of means between groups were evaluated using Student’s t-test. For categorical data, Fischer’s excact test was applied. Differences were considered to be significant with a P value < 0.05. A possible correlation between intraoperative graft flow and change in myocardial function from before surgery to after surgery was investigated using a bivariate correlation analysis (SPSS 13.0, SPSS Inc., Chicago, Il, USA).
Results
Three patients died perioperatively (hospital mortality 9%), during follow-up one patient died from stroke. Out of the remaining 29 patients, six patients had a contraindication to undergo MRI at the time of follow up because they had received cardiac resynchronization therapy using implantable pacemakers and defibrillators in the meantime. Three patients did not want to undergo MRI again, because they had experienced considerable claustrophobia at the time of the preoperative scan. In three patients, mobility was severely compromized for noncardiac reasons and one patient had moved abroad and was not available for MRI.
and show pre-, intra- and postoperative data of the remaining 16 patients. One patient had one vessel disease, one had two vessel disease, all others had three vessel disease. Volume (end systolic and end diastolic volume) and function (left ventricular ejection fraction) parameters, as determined by MRI, significantly improved from before to after surgery ( and ). As an example, shows pre- and postoperative MRI scans. In the respective patient, left ventricular function improved considerably ().
Figure 1 Volume parameters obtained by using magnetic resonance imaging before (crosshatched bars) and after CABG (hatched bars). Data are mean ± SEM. ESV, Endsystolic volume; EDV, Enddiastolic volume. Numerical values above error bars indicate level of statistical significance between the groups (Student’s t-test for unpaired samples).
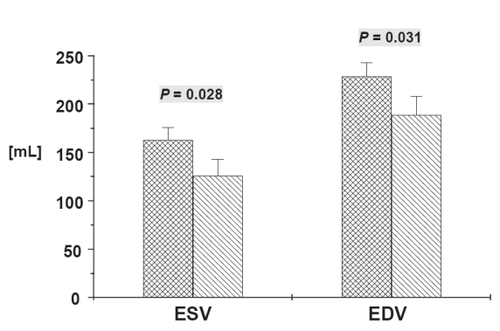
Figure 2 Left ventricular ejection fraction (LVEF) obtained by using magnetic resonance imaging before and after surgery. Data are mean ± SEM. Numerical value above error bar indicates level of statistical significance between groups (Student’s t-test for unpaired samples).
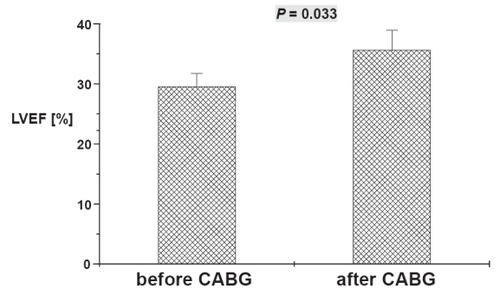
Figure 3 MR Imaging of a 64 year old male before (top line) and after (bottom line) 3-fold CABG. SSFP Cine images in enddiastole (A) and endsystole (B) reveal the severely impaired global LV function before surgery (EF 30%) with akinesia in the apical inferoseptal and anterolateral wall and the apex (segments 14, 16, and 17) and hypokinesie in the basal and mid-ventricular lateral wall. The contrast-enhanced TurboFLASH image (C) shows broad subendocardial late enhancement (bright signal) in the apical septum, thin LE in the lateral wall and transmural LE in the apex meaning chronic scar. LV function after surgery (D, E) shows no improvement in the apical septum and the apex, whereas the complete lateral wall improved and became normokinetic. No changes in scar extent (F). Global LV function improved to EF 40%, LV volumes decreased.
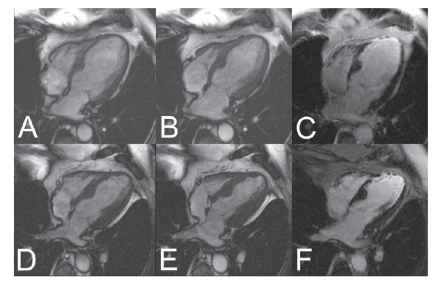
Table 1 Preoperative patient data
Table 2 Intra- and postoperative patient data
In parallel to global functional improvement, there was functional improvement on a segmental basis. Before surgery, 417 out of 875 segments (48%) in 16 patients showed evidence of late enhancement. 42 segments (4.8%) were classified to be normokinetic (functional score = 0). After surgery, 177 segments (20.4%) were normokinetic (p < 0.05). Of 388 segments preoperatively classified to be severely hypokinetic (functional score = 2), 141 (36.3%) improved by at least one score point. Of 193 segments classified as akinetic before surgery (functional score = 3), 21 segments (10.8%) improved by at least one score point at the time of follow-up.
Myocardial segments assigned to the LAD territory had a mean score of 3.2 ± 0.1 before surgery and a score of 2.8 ± 0.2 at follow up (p < 0.05). Myocardial segments assigned to the circumflex artery had a mean functional score of 3.2 ± 0.2 before and 2.9 ± 0.2 after surgery (p = 0.1). Left ventricular myocardial segments assigned to the right coronary artery territory had a mean score of 2.6 ± 0.2 before and 2.4 ± 0.3 after surgery (p = 0.10).
There was also significant clinical improvement. Before surgery patients reported a mean NYHA class of 3.0 ± 0.1. Eighteen months after surgery mean NYHA class was 2.2 ± 0.2 (p < 0.01).
Conclusion
In patients with significant coronary artery disease and left ventricular dysfunction, survival after coronary artery bypass grafting has been shown to be better than with medical therapy alone (CitationLytle et al 2003). However, only those patients benefit from surgical revascularisation who displays the presence of dysfunctional but viable myocardium. Patients without viable myocardium do not benefit from myocardial revascularisation, irrespective of treatment strategy (CitationAllman et al 2002). The present study shows favorable results at 20 months follow-up after surgical revascularization. Reverse remodelling of the dysfunctional left ventricle could be demonstrated by an improvement of global and segmental function and patients were in a lower NYHA class.
The transmural extent of hyperenhancement was reported to significantly relate to the likelihood of improvement in contractility after revascularization in a reversed way (CitationKim et al 2000). Contractility improved in 78% of segments with no hyperenhancement but in only 1 of 58 segments with hyperenhancement of more than 75%. Myocardium that shows transmural late enhancement, which represents scare tissue, is not believed to be improved by revascularisation (CitationKim et al 1999, Citation2000).
The postoperative dropout concerning magnetic resonance follow up scan was considerable because of many reasons (see Results section), so that only 16 of 29 surviving patients could be investigated. Postoperative implantation of defibrillators and pacemakers has been described to lead to a loss of follow up in patients due to undergo postoperative MRI (CitationKim et al 2000). In recent years, cardiac resynchronization therapy has further increased the indication for pacemaker/defibrillator implantation in patients with compromized left ventricular function.
In conclusion, the present study clearly confirms the results of several recent clinical trials that noninvasive myocardial viability testing with magnetic resonance imaging detect those patients with severely compromized left ventricular function, in whom CABG surgery can significantly improve global left ventricular function (CitationAllman et al 2002; Bax et al 1997). Moreover, the present study could also demonstrate a significant reverse remodelling and improvement of left ventricular function after CABG surgery on a myocardial segmental basis using MRI viability testing. After surgery, left ventricular function improves along with a considerable clinical benefit of the patients.
Limitations
Our study encompasses the experience at a single tertiary medical center; therefore, the generalizability of our findings may not extend to all of the clinical centers performing CABG. One of the main limitations of the present study is the high drop-out rate during the follow-up period. Only 16 out of 29 surviving patients were eligible for postoperative MRI follow-up because of several limitations and contraindications to undergo MRI scan, having received cardiac resynchronization therapy in the meantime, had claustrophobia, were unavailable or had a severely compromized mobility for noncardiac reasons.
References
- AllmanKCShawLJHachamovitchRMyocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysisJ Am Coll Cardiol2002391151811923039
- BaxJJde RoosAvan der WallEEAssessment of myocardial viability by MRIJ Magn Reson Imaging1999104182210508304
- BonowROMyocardial viability and prognosis in patients with ischemic left ventricular dysfunctionJ Am Coll Cardiol20023911596211923040
- CerqueiraMDWeissmannNJDilsizianVStandardized myocardial segmentation and nomenclature for topographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart AssociationCirculation20021055394211815441
- HunoldPBrandt-MainzKFreudenbergLEvaluation of myocardial viability with contrast-enhanced magnetic resonacne imaging – comparison of the late enhancement technique with positronemmission tomographyRofo20021748677312101477
- HunoldPSchlosserTVogtFMMyocardial late enhancement in contrast-enhanced cardiac MRI: distinction between infarction scar and non-infarction-related diseaseAm J Roentgenol20051841420615855089
- KimRJFienoDSParrishTBRelationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile functionCirculation19991001992200210556226
- KimRJWuERafaelAThe use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunctionN Engl J Med200034314455311078769
- KleinCNekollaSGBengelFMAssessment of myocardial viability with contrast-enhanced magnetic resonance imaging. Comparison with positron emission tomographyCirculation2002105162711790695
- LytleBThe role of coronary revascularization in the treatment of ischemic cardiomyopathyAnn Thorac Surg200375S2S512820728
- SelvanagayamJBKardosAFrancisJMValue of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularizationCirculation200411015354115353496
- SimonettiOPKimRJFienoDSAn improved MR imaging technique for the visualization of myocardial infarctionRadiology20012182152311152805
- ThielmannMMassoudyPNeuhauserMPrognostic value of preoperative cardiac troponin I in patients undergoing emergency coronary artery bypass surgery with non-ST-elevation or ST-elevation acute coronary syndromesCirculation2006114I–44853
- VeenhuyzenGDSinghSNMcAreaveyDPrior coronary artery bypass surgery and risk of death among patients with ischemic left ventricular dysfunctionCirculation200110414899311571241
- WestenbergJJMvan der GeestRJLambHJMRI to evaluate left atrial and ventricular reverse remodelling after restrictive mitral annuloplasty in dilated cardiomyopathyCirculation2005112I–43742