Abstract
Rheumatic fever (RF) is a sequel of group A streptococcal throat infection and occurs in untreated susceptible children. Rheumatic heart disease (RHD), the major sequel of RF, occurs in 30%–45% of RF patients. RF is still considered endemic in some regions of Brazil and is responsible for approximately 90% of early childhood valvular surgery in the country. In this study, we present a 15-year clinical follow-up of 25 children who underwent surgical valvular repair. Histopathological and immunological features of heart tissue lesions of RHD patients were also evaluated. The patients presented severe forms of RHD with congestive symptoms at a very young age. Many of them had surgery at the acute phase of RF. Histological analysis showed the presence of dense valvular inflammatory infiltrates and Aschoff nodules in the myocardium of 21% of acute RHD patients. Infiltrating T-cells were mainly CD4+ in heart tissue biopsies of patients with rheumatic activity. In addition, CD4+ and CD8+ infiltrating T-cell clones recognized streptococcal M peptides and cardiac tissue proteins. These findings may open the possibilities of new ways of immunotherapy. In addition, we demonstrated that the surgical procedure during acute phase of the disease improved the quality of life of young RHD patients.
Introduction
Rheumatic fever (RF), a sequel of group A streptococcal throat infection occurs in untreated susceptible children and is a multisystem inflammatory disease. The clinical signs of RF are the same throughout the world. In the 1950s, Jones established the major criteria for diagnosing initial attacks of RF, which comprised polyarthritis, carditis, and chorea. These criteria were revised in 1965 and the second and latest revision done in 1992 remains useful to date (CitationDajani et al 1992). Arthritis is the earliest and most common feature of the disease, present in 60%–80% of patients. Carditis, the most serious manifestation of the disease, occurs a few weeks after Streptococcus pyogenes throat infection in 30%–45% of RF patients, and usually presents as pancarditis. Endocarditis is the most serious sequel and frequently leads to chronic rheumatic heart disease (RHD). Valvular lesions and mitral and aortic regurgitation are the most common events caused by repeated valvulitis.
RHD remains a major public health problem in developing countries, leading to 233,000 deaths/year (CitationCarapetis, McDonald et al 2005; CitationCarapetis, Steer et al 2005). The incidence of RHD in the world is at least 15.6 million cases and the highest documented prevalence of the disease among children from developing countries is 5.7 per 1,000 in sub-Saharan Africa (CitationCarapetis, McDonald et al 2005; CitationCarapetis, Steer et al 2005). Epidemiological data from many developing countries are still of poor quality, and the numbers of RHD cases are surely higher than those known. In Brazil, the incidence of acute RF has decreased by 75% in the last 10 years however acute RF is still high, reaching 5000 new cases in 2002 (data from the Brazilian Health Ministry).
The pathogenesis of RHD depends on several host factors that mediate a pathological autoimmune response triggered by a defensive immune response against S. pyogenes. Genetic predisposition is one of the leading factors contributing to the development of autoimmunity.
The association of certain HLA class II alleles with autoimmune diseases is frequent due to the fact that these molecules are expressed on the surface of antigen-presenting cells (APC) such as macrophages, dendritic cells, and B lymphocytes, which present pathogen antigens and trigger the activation of T and B cells.
Several HLA class II alleles have been associated with the development of RF/RHD in different countries (reviewed by; CitationGuilherme et al 2005). Among these alleles, HLA-DR7 is the most frequently associated with RF/RHD and seems to be related to the development of multiple valvular lesions. The TNFA gene related to inflammatory responses is located near HLA class II genes and has been associated with several autoimmune diseases. The presence of either one of the TNFA alleles (-308A and -238A) was associated with the development of RF and RHD (CitationHernandez-Pacheco et al 2003). We also observed the same association with patients that develop aortic valve lesions (Ramasawmy et al 2006).
Severe RHD is mediated mainly by T lymphocytes, which participate in a delayed-type hypersensitivity reaction leading to local inflammation. We have shown the significance of molecular mimicry between beta hemolytic streptococci and heart tissue proteins through an analysis of the T-cell repertoire leading to local tissue damage in RHD. We have also demonstrated the ability of T-cell clones infiltrating the heart lesions of severe RHD patients to recognize M protein peptides and heart tissue-derived proteins (CitationGuilherme et al 1995).
In this work, we presented a cohort of 25 young RHD patients that had been submitted to a surgery during an acute RF episode, an unusual procedure and we showed that they had a good prognosis. We also evaluated the histopathological and immunological features of this group of patients in order to verify the presence of inflammatory reactions in the heart tissue.
Patients and methods
We studied 25 severe RHD patients, 17 males and 8 females who underwent cardiac valvular surgery before 16 years of age, in which 14 (56%) presented an acute RF episode. The other 11 patients also have had recent acute RF episode. The patients were followed from 1990 to 2006 at the Heart Institute, University of São Paulo. Their age at the first clinical evaluation varied from 6 to 16 years (mean = 10.6 ± 2.6 years). The initial attack of RF had occurred from 3 to 16 years of age (mean = 9.5 ± 3.4 years) and was clinically defined by the presence of polyarthritis in 13 patients, carditis in 24 patients, and fever in one patient. Only one patient studied presented with Sydenham chorea during the clinical evolution of the disease. For immunological and histopathological evaluations, heart tissue fragments were collected during valve surgery. Fragment collection was approved by the Heart Institute Ethics Committee (HC-FMUSP) and informed consent was obtained from the parents of the patients participating in the study.
Histological analysis and immunohistochemistry
Histological sections from heart fragments obtained during valve surgery in twenty-four patients were stained with hematoxylin-eosin (HE) and were reviewed by two pathologists, in order to evaluate histological features of acute and chronic RHD, such as inflammatory infiltrates, rheumatic activity, neovascularization, fibrosis, and calcification.
Heart fragments included valvular tissue from aortic (AV; n = 7 patients) and mitral (MV; n = 7 patients) valves, myocardial tissue from the right atrium (RA; n = 5 patients), left atrium (LA; n = 18 patients) and papillary muscles (PM; n = 2 patients), and pericardium (PER; n = 5 patients).
Anti-CD4 (MT 310) and anti-CD8 (DK 25) (Dakopatts) monoclonal antibodies were used to define T-cell subpopulations and anti-CD20 (L26) (Dakopatts) for B cell populations. Peroxidase-coupled avidin (Dakopatts) was added later and the reaction was developed with diamino-benzidine (Sigma-Aldrich).
Establishment of heart infiltrating T-cell lines and T-cell clones
Heart infiltrating T-cell lines (HIL) were established from surgical fragments as described before (CitationGuilherme et al 1995). Briefly, heart tissue was finely minced, placed in flat-bottom 96-well plates (Becton and Dickson, Lincoln Park, NJ) with Dulbecco’s modified Eagle medium (DMEM) (Invitrogen, Life Technologies) supplemented with 2 mM L-glutamine (Invitrogen), 10% pooled normal human serum, antibiotics (Gentamycin 40 μg/ml and Peflacyn 20 μg/ml), and 40 IU/ml of human recombinant IL-2 (PeproTech Inc) on a HLA-DR-matched feeder layer of peripheral blood mononuclear cells (PBMC, 105 cells/well), irradiated at 5000 rad. All T-cell lines were further expanded with irradiated HLA-DR matched feeder cells and Phytohemagglutinin (PHA-P, 2.5 μg/ml). T-cell clones were obtained by the limiting dilution method in the presence of 105 HLA-DR matched PBMC, irradiated PHA-P (2.5 μg/ml), and IL-2 (20 IU/ml). For T-cell clones expansion, we also used IL-7 and IL-15 (2.5 ng/ml, PeproTech Inc).
Peptide synthesis
Streptococcal M5-derived peptides were synthesized by the “tea bag” method using t-BOC chemistry (CitationHoughten et al 1985), checked by mass spectrometry, and purified by high pressure liquid chromatography (HPLC). Fifty peptides of the light meromyosin (LMM) fragment provided by Dr. M.W. Cunningham were designed based on the cardiac myosin β-chain protein sequence (CitationDiederich et al 1989) and were synthesized as 18-mer peptides with a 5-amino acid overlap as previously described (CitationGalvin et al 2002). Twenty-two N-terminal overlapping M5 peptides were synthesized as 15- to 20-mers, based on the streptococcal M5 protein sequence (CitationPhillips et al 1981; CitationManjula et al 1985). See for sequences of peptides recognized by T-cells in a proliferation assay.
Table 5 Cross reactive intralesional T-cell clones from RHD patients
Preparation of heart tissue proteins
Heart tissue fractions were obtained from lysates of postmortem normal human myocardium and aortic valve tissue, and then separated by SDS-PAGE (1D-electrophoresis). Mitral valve-derived proteins were separated by 2D-electrophoresis that combined urea isoelectrofocusing electrophoresis (IEF) using a broad range carrier ampholytes mixture (pH = 3.6–9.2) in the first dimension, with SDS-PAGE (5%–15% polyacrylamide gel) electrophoresis in the second dimension in order to resolve valvular tissue proteins by isoelectric point and molecular weight as previously described (CitationO’Farell et al 1975). All proteins isolated by both 1D- and 2D-electrophoresis were blotted onto nitrocellulose membranes (CitationAbou-Zeid et al 1987). The blots were divided into several horizontal strips with approximately the same amount of protein. A nitrocellulose strip without protein was used as negative control. The strips were solubilized in dimethyl sulfoxide (E. Merck), reprecipitated in sodium carbonate/sodium bicarbonate buffer 0.05 mol/L, pH 9.6, and washed with RPMI 1640 medium (Sigma), yielding a fine suspension of protein-loaded nitrocellulose particles that were used as antigens for the proliferation assay.
Proliferation assay
Proliferation assays were performed in 96-well plates incubating 3 × 104 T-cell clones with 105 irradiated (5000 rad) HLA-DR matched mononuclear cells or 105 peripheral blood mononuclear cells (PBMC) with 5 μg/ml of streptococcal M5 synthetic peptides, 3 μM of light meromyosin peptides (LMM), and 50 μl/well of heart tissue proteins for 96h at 37 °C in a humidified 5% CO2 incubator. Negative controls were a suspension of lymphoblasts and irradiated PBMC in DMEM for the peptides and 50 μl of a protein-free nitrocellulose suspension for valve protein experiments. PHA-P (2.5 μg/ml) was used as the positive control for proliferative responses. All antigens were tested in triplicate and pulsed-labeled with 0.5 μCi/well of tritiated thymidine (Amersham) for the final 18h of culture. Cells were then harvested and analyzed in an automated beta counter (Beta plate 1205-LKB). A proliferative response was considered positive when the stimulation index (SI) was ≥2.5. Negative controls presented less than 500 cpm.
Statistical analysis
The statistical analysis was accomplished with software SAS and GraphPad Prism v. 4.00 and a p value <0.05 was considered statistically significant. Means and standard deviations were calculated for quantitative variables. Paired and unpaired Student’s t-Test were used when appropriated. Kaplan-Meyer was applied for survival analysis. The Mann-Whitney test was employed to evaluate the relation between the numbers of infiltrating T lymphocytes and rheumatic activity.
Results
All patients (100%) had mitral valve disease. Isolated mitral disease was observed in 9 patients, and mitral combined with aortic valve disease in 8 patients. Mitral and tricuspid valve was seen in one patient and trivalvular valve disease (aortic, mitral, and tricuspid) in 7 patients (). Tricuspid valve regurgitation secondary to mitral valve disease and pulmonary hypertension was seen in 16 patients.
Table 1 Clinical data of RHD patients
At the time of the first surgery, the majority of patients had advanced heart failure due to severe valvular disease. Two patients presented in New York Heart Association (NYAH) functional class II, 7 in class III, and 16 in class IV ().
Ten patients underwent heart surgery during an episode of acute rheumatic carditis associated with severe heart failure. Moreover, 4 patients who underwent valve surgery soon after had been treated for acute carditis. Of those, 2 had recurrence of acute carditis after surgery, resulting in worsening of valve and myocardium disease.
Isolated mitral disease or disease associated with another valvular dysfunction was the main cause of heart failure leading to heart surgery in 22 out of 25 patients (88%). Three patients (#1, 5 and 19) (mean age = 14.3 ± 0.6) underwent surgery due to mitral stenosis. Among the 22 patients with mitral valve dysfunction, isolated mitral valve regurgitation occurred in 3 patients, mitral regurgitation combined with tricuspid regurgitation in 3, combined with aortic regurgitation in 4, and associated with aortic and tricuspid valve in 12.
The mean age at the time of first surgery was 12.0 ± 2.8 years. Eighteen patients underwent conservative mitral valve surgery (15 repairs and 3 commissurotomies). There were 4 repairs of the tricuspid valve and 7 of the aortic valve. Additionaly, there were 12 implants of biological prostheses (6 mitral and 6 aortic), and one patient received two mechanical prostheses (in aortic and mitral positions).
After surgery, we noticed a reduction of left ventricle diastolic and left atrium diameters (). In addition, the left ventricular shortening fraction had a mild reduction ().
Table 2 Echocardiography data before surgery and until seven days post surgical treatment
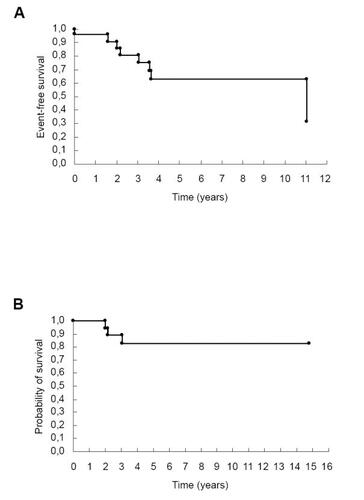
During follow-up, 7 (28%) patients underwent reoperations. Bioprosthesis stenosis was the main cause of reoperation, occurring in 4 cases. Anterior chordal rupture and acute mitral valve regurgitation occurred in one patient. Mitral valve infective endocarditis and heart failure occurred in 2 cases.
Three patients died during long-term follow-up, all of them with a bioprosthesis. One patient had infective endocarditis two years after heart surgery. Another patient had bioprosthesis stenosis with severe heart failure and died due to cardiogenic shock after surgery. The last patient died one year after the second valvular surgery, also due to cardiogenic shock. The remaining patients had good clinical evolution and are in NYHA class I or II ().
Figure 1 Kaplan-Meier survival curve: follow-up of 25 rheumatic valve disease patients. (A) Event-free survival (death and reoperation); (B) probability of survival.
Heart biopsies were retrieved from all patients except one (#22). Histopathologic data from valvular and myocardial tissues are summarized in . Both myocardium and valve samples were analyzed in nine patients (#1, 2, 3, 4, 6, 9, 11, 12, 13). Only valvular or myocardium fragments were obtained from 3 (#8, 10, and 14) and 12 patients (#5, 7, 15, 16, 17, 18, 19, 20, 21, 23, 24, 25), respectively. Inflammatory reactions were observed in valvular tissues from 12 patients, varying from severe to mild intensity (), and in 10 out of 12 myocardium fragments analyzed, mainly with mild intensity (). Rheumatic activity was confirmed by the presence of fibrin deposits (verrucae) or Aschoff bodies (AB) in valvular tissue (#2, 3, 8, 10, and 11), myocardium (#2, 3, 11, 18, and 20), or pericardium (#12, 15 and 23, data not shown). Valvular neovascularization and fibrosis were observed in 10 and 11 patients, respectively, and graded according to intensity as mild, moderate, or severe. Calcification was observed in only one valvular tissue analyzed (#1) ().
Table 3 Histopathological data from valvar and myocardial tissues of rheumatic heart disease patients
Visceral and both visceral and parietal pericardium from patients #15 and #12, 21, 23, 24, respectively, presented moderate fibrosis (data not shown). Pericardial inflammation was observed in 4 patients (#12, 15, 21 and 23), three of them with fibrin deposits in the connective tissue (#12, 15, 23) (data not shown).
Immunohistochemical staining of heart tissue fragments (9 from valvular tissue and 14 from myocardium) of 18 patients showed the predominance of infiltrating CD4+ T-cells (), except for two patients, one that we could not identify infiltrating T-cells (#19) and the other that exhibited only a few CD8+ T-cells (#25) ().
Table 4 Evaluation of heart-tissue infiltrating lymphocytes subsets
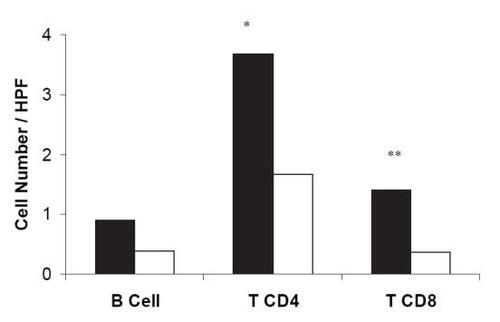
Patients (#2, 3, 8, 10, 11, and 20) which presented histological features of rheumatic activity, showed an increased number of heart tissue infiltrating CD4+ (mean = 3.7 ± 2.9 cells, p = 0.03) and CD8+ T-cells (mean = 1.4 ± 1.2 cells, p = 0.03) when compared with patients without rheumatic activity (). In addition, we observed a higher number of CD4+ T-cells than CD8+ T-cells in the group with rheumatic activity.
Figure 2 The presence of heart tissue T lymphocytes is related with rheumatic activity. The number of infiltrating T and B lymphocytes and their relation with rheumatic activity in both valve and myocardium tissues are presented. Black bars, mean tissue with rheumatic activity and white bars, without rheumatic activity. *P = 0.03; **P = 0.03. HPF, high power microscopic field.
We established T-cell lines from heart tissue fragments (valves and/or myocardium) from 18 out 25 of patients that allowed us to obtain 72% of infiltrating T-cells growing (). Among the T-cell lines established, we also observed a predominance of CD4+ T-cells (data not shown).
Figure 3 Heart infiltrating T-cells. Heart tissue biopsies were cut in small fragments and kept in culture until establishment of T-cell lines. The picture represents lymphoblasts cultivated for 15 days in Dulbecco’s modified Eagle medium supplemented with IL-2. Photograph from Immunology Laboratory, Heart Institute; original magnification ×200.
Intralesional T-cell clones from the valves and myocardium were established from T-cell lines of 7 patients (#1, 3, 4, 5, 7, 8, and 10). shows the reactivity of heart tissue-derived T-cell clones from each patient. Several heart tissue proteins from myocardium and/or valvular tissue and streptoccal M5 synthetic peptides were recognized by these T-cell clones as well as some cardiac myosin synthetic peptides (). Five T-cell clones (3.1.79, 4.1.18, 5.1.31, 7.1.1, and 8.1.2) were CD4+ and T-cell clones 1.1.27 and 10.2.13 were CD8+.
Peripheral blood reactivity against M5 peptides and cardiac proteins were analyzed for 12 patients (#2, 3, 4, 5, 7, 9, 11, 13, 15, 17, 19, and 20) at the time of the surgery. Eight patients (#3, 4, 5, 11, 13, 15, 17, and 19) presented T-cells in the periphery able to recognize M5 peptides and/or cardiac proteins (). The M5 peptides and cardiac proteins recognized by peripheral T-cells were the same recognized by intralesional T-cell clones ().
Table 6 Peripheral blood reactivity against streptococcal M5 peptides and cardiac proteins
Discussion
Rheumatic fever is still considered endemic in some regions of Brazil and RHD is responsible for approximately 90% of early childhood valvular surgeries in the country. In this study, we present a 15-year follow-up of 25 children that underwent valvular surgery repair.
We describe a group of rheumatic heart disease patients that have developed congestive symptoms at a very young age, a particularly severe form of the disease. Most patients with RHD do not develop congestive symptoms until adulthood. The severity of the disease of these young patients is related to recurrent outbreaks of acute rheumatic fever or a particularly severe first attack of rheumatic fever (CitationVelloso et al 1991; CitationTarasoutchi et al 2005).
Surgery after a single attack of rheumatic fever is related to very intense valve damage, usually with ruptured mitral valve chordae due to acute valvulitis (CitationHillman et al 2004). Many of our patients had surgery during or soon after an acute phase of rheumatic fever. These patients usually presented myocarditis at the time of the surgery, leading to a turbulent post-operatory period because of myocarditis-induced ventricular dysfunction. Even so, we could not relate surgery in the acute phase of rheumatic fever with an increased possibility of reoperation (p = 0.362) or excess of mortality. It is possible that these patients have a larger number of cross-reactive lymphocyte clones or that these clones may be more active in inducing inflammation and damage (CitationGuilherme et al 1995).
The remaining patients were submitted to surgery in the chronic phase of RHD. This population differs from the patients operated in the acute phase because they usually have severe valve damage secondary to repeated bouts of acute rheumatic fever. This population more closely resembles the adult population with rheumatic valve disease, usually having mild symptomatic or asymptomatic acute carditis, but severe sequelae due to chronic RHD. The lack of acute symptoms in this population leads to a delayed diagnosis of rheumatic fever, and thus delayed access to secondary prophylaxis and medical care (CitationRizvi et al 2004). The first symptoms that lead the patient to seek medical care are usually heart failure, not necessarily related to the acute phase of rheumatic fever. In both acute and chronic patients, we observed that histopathological and immunological analysis presented similar numbers of cross-reactive T-cells. However, the group that had surgery in the acute phase of the disease probably exhibited a large number of cross-reactive lymphocytes secondary to a single throat infection while patients in the chronic phase of the disease exhibited small numbers of cross-reactive lymphocytes that emerged from repeated streptococcal throat infections (CitationGuilherme et al 2001).
Many of our patients needed cardiac surgery immediately after the first attack of rheumatic fever due to the severity of this first episode. In these cases the prevention strategy using primary prophylaxis could be effective. Secondary prophylaxis could prevent the development of lesions due to a new acute rheumatic fever episode. There are a consensus among physicians that secondary prophylaxis for rheumatic fever is more cost-effective to prevent new RF episodes (CitationCarapetis, Steer et al 2005; CitationCarapetis et al 2006).
Histological evaluation of the heart valves in 24 RHD patients were completed and provided evidence about the severity of involvement as well as the phase of the disease. Features like fibrosis and neovascularization were observed in most of valves analyzed, indicating that the valve has gone through previous episodes of rheumatic activity. Usually, the degree of fibrosis is also proportional to the number of acute episodes. Calcification, a feature that points to chronic valvular involvement, was observed in only one patient. Inflammatory infiltrates may be sparse and patchy in the chronic lesions, but are usually dense in the acute phase of the disease. The concomitant presence of fibrin deposits (verrucae) on the endocardial surface implies that there is rheumatic activity. In our patients we observed dense valvular inflammatory infiltrates characterized mainly as CD4+ T-cells.
On the other hand, the analysis of myocardial biopsies may not bring significant contribution to the diagnosis of RHD, since the finding of Aschoff nodules is rare: present in 30% of acute RHD biopsies and in 40% of patients with recurrent RF attacks (CitationNarula et al 1993). We observed Aschoff nodules in 21% of the acute RHD patients studied. Although interstitial inflammatory mononuclear cells may be present, myocardial cell damage, the main criterion for other types of myocarditis, is not a striking feature in RHD. However, there is strong evidence that the nature of myocardial infiltrating cells is different from those present in valvular lesions (CitationGuilherme et al 2004). We showed that mononuclear cells from heart lesions predominantly secrete IFNγ and TNFα, Th1-type cytokines, in both acute RF and chronic RHD patients. Previous results from our group showed sparse production of IL-4 by valve-infiltrating cells (under 10% IL-4 positive cells) in 82% of the valve fragments analyzed and large numbers of IL-4 positive cells (over 50%) in 78% of the myocardium fragments analyzed. This suggests that low numbers of IL-4 producing cells in the valvular tissue may contribute to the progression of valvular RHD lesions. These observations suggest the involvement of different mononuclear cells in the myocardium and the valves. The fact that different types of cytokine producing cells were found reinforces the putative role of regulatory cytokines in myocardium healing in RHD and in the induction of progressive and permanent valve damage (CitationGuilherme et al 2004).
The molecular mimicry mechanism mediating RF/RHD is the process in which T-and B lymphocytes recognize self-antigens showing some degree of homology with streptococcal antigens. Although several cross-reactive antibodies have been described (reviewed by; CitationCunningham 2000 and Citation2003), their potential role in the development of RHD has only recently been clarified. Studies conducted by Cunningham’s group describe cross-reactive antibodies able to bind to the endothelial surface, leading to inflammation, cellular infiltration, and valve scarring (CitationGalvin et al 2000). The upregulation of the adhesion molecule VCAM-1 after binding of cross-reactive antibodies to the valvular endothelium facilitates cellular infiltration (CitationRoberts et al 2001). Our results showed that 91% of heart biopsies exhibited cellular infiltration with a predominance of CD4+ T-cells, in agreement with previous results (CitationGuilherme et al 1995). In addition, in the present study we observed the highest number of both CD4+ and CD8+ T-cells in patients that presented rheumatic activity. These results confirmed previous date (CitationGuilherme et al 2001) in which we analyzed the reactivity of intralesional T-cells of three RHD patients, one in acute phase, one six month after acute phase and, one in chronic phase of the disease. We observed the presence of 67% of auto reactive T-cell clones in the valve of patient in acute phase of the disease while the other two patients presented around 25%–30% of auto reactive T-cell clones (CitationGuilherme et al 2001). The date presented here indicated that during acute episode there are expansions of auto reactive T-cells in the heart and after the acute episode these auto reactive T-cells decreased in numbers but it is still important to maintain the heart lesions.
Intralesional T-cell clones able to recognize streptococcal M peptides and heart tissue-derived proteins pointed out the molecular mimicry mechanism that mediates the autoimmune reactions leading to valvular rheumatic lesions (CitationGuilherme et al 1995; CitationFaé et al 2006). The fact that some patients also exhibited T-cell reactivity in the periphery against streptococcal M peptides and heart tissue-derived proteins gives support to the idea that streptococci-primed T-cells migrate from the periphery to the heart, triggering valvular lesions (CitationGuilherme et al 2000; CitationFaé et al 2004).
Taken together, our results show that although the majority of patients had advanced heart failure due to severe valve disease and presented NYHA functional class (III/IV), they had different immunological patterns of responses against streptococcal and cardiac tissue proteins. These patterns could be illustrated by the immunological reactivity of patients 3 and 11 that exhibited rheumatic activity with a dense inflammatory infiltrate of CD4+ T-cells in the heart tissue. Both patients displayed a positive reactivity in the peripheral blood against M5 peptides and cardiac proteins. One of them (#3) also presented cross-reactive intralesional T-cell clones. On the other hand, patient 2 did not present reactivity in the periphery despite the presence of dense inflammatory infiltrates of CD4+ T-cells in the heart tissue, probably due to the low frequency of autoreactive T-cells in the periphery.
Our data illustrate that patients that underwent cardiac surgery mostly of them due to severe mitral regurgitation and severe heart failure during acute rheumatic fever episode were associated with a good late prognosis. Thus, when confronted with a patient with acute rheumatic carditis with expressive valvular dysfunction and refractory heart failure the clinician may consider surgery to treat the hemodynamical problem.
In conclusion, this work represents a well established, 15-year follow-up of a child RHD cohort that underwent surgical treatment and demonstrated that the surgical procedure during acute phase of the disease improved the quality of life of young RHD patients.
Most of the patients as mentioned before presented an inflammatory reaction in the heart tissue and displayed peripheral and intralesional cross-reactive CD4+ T-cells against streptococcal M peptides and heart tissue proteins that may lead to the progression of the disease. In view to prevent this progression new ways of immunotherapy could be proposed. Young RHD patients could be benefit by the use of the T-cell vaccination therapy (Cohen, I, 2001, reviewed) in which autologous primed T-cells could induce the deletion of pathological auto reactive T-cells and/or induce regulatory T-cells.
Acknowledgements
This work was supported by grants from CNPq and FAPESP.
References
- Abou-ZeidCFilleyESteeleJA simple new method for using antigens separeted by polyacrilamide gel electrophoresis to stimulate lymphocites “in vitro” after converting bands cut from Western blots into antigen-bearing particlesJ Immunol Methods1987985103104476
- CarapetisJRMcDonaldMWilsonNJAcute rheumatic feverLancet20053661556816005340
- CarapetisJRSteerACMulhollandEKThe global burden of group A streptococcal diseasesLancet Infect Dis200556859416253886
- CarapetisJRMayosiBMKaplanELControlling rheumatic heart disease in developing countriesCardiovasc JS Afr20061716465
- CohenIRT-cell vaccination for autoimmune disease: a panoramaVaccine2002207061011738733
- CunninghamMWPathogenesis of group A streptococcal infectionsClin Microbiol Rev20001347051110885988
- CunninghamMWAutoimmunity and molecular mimicry in the pathogenesis of post-streptococcal heart diseaseFront Biosci20038s5334312700052
- DajaniASAyoubEBiermanFZGuidelines for the diagnosis of Rheumatic Fever: Jones criteria, 1992 uptadeJAMA19922682069731404745
- DiederichKWEiseleIRiedTIsolation and characterization of the complete human beta-myosin heavy chain geneHum Genet1989812142202522082
- FaeKKalilJToubertAHeart infiltrating T-cell clones from a rheumatic heart disease patient display a common TCR usage and a degenerate antigen recognition patternMol Immunol20044011293515036919
- FaeKCda SilvaDDOshiroSEMimicry in recognition of cardiac myosin peptides by heart-intralesional T-cell clones from rheumatic heart diseaseJ Immunol200617656627016622036
- GalvinJEHemricMEKosankeSDInduction of myocarditis and valvulitis in Lewis rats by different epitopes of cardiac myosin and its implications in rheumatic carditisAm J Pathol200216029730611786423
- GalvinJEHemricMEWardKCytotoxic mAb from rheumatic carditis recognizes heart valves and lamininJ Clin Invest20001062172410903337
- GuilhermeLCunha-NetoECoelhoVHuman heart-infiltrating T-cell clones from rheumatic heart disease patients recognize both streptococcal and cardiac proteinsCirculation199592415207634457
- GuilhermeLCunha-NetoETanakaACHeart-directed autoimmunity: the case of rheumatic feverJ Autoimmun200116363711334505
- GuilhermeLDulphyNDouayCMolecular evidence for antigen-driven immune responses in cardiac lesions of rheumatic heart disease patientsInt Immunol20001210637410882418
- GuilhermeLOshiroSEFaeKCT-cell reactivity against streptococcal antigens in the periphery mirrors reactivity of heart-infiltrating T-lymphocytes in rheumatic heart disease patientsInfect Immun20016953455111500404
- GuilhermeLCuryPDemarchiLMRheumatic heart disease: proinflammatory cytokines play a role in the progression and maintenance of valvular lesionsAm J Pathol200416515839115509528
- GuilhermeLFaeKOshiroSEMolecular pathogenesis of rheumatic fever and rheumatic heart diseaseExpert Rev Mol Med2005711516336741
- Hernandez-PachecoGFlores-DominguezCRodriguez-PerezJMTumor necrosis factor-alpha promoter polymorphisms in Mexican patients with rheumatic heart diseaseJ Autoimmun200321596312892736
- HillmanNDTaniLYVeasyLGCurrent Status of Surgery for Rheumatic Carditis in ChildrenAnn Thorac Surg2004781403815464505
- HoughtenRAGeneral method for the rapid solid-phase synthesis of large numbers of peptides: Specificity of antigen-antibody interaction at the level of individual amino acidsProc Natl Acad Sci USA198582513152410914
- ManjulaBNTrusBLFischettiVAPresence of two distinct regions in the coiled-coil structure of the streptococcal Pep M5 protein: relationship to mammalian coiled-coil proteins and implications to its biological propertiesProc Natl Acad Sci USA1985821064683856248
- NarulaJChopraPTalwarKKDoes endomyocardial biopsy aid in the diagnosis of active rheumatic carditis?Circulation19938821982058222115
- O’FarellPHHigh resolution two-dimensional electrophoresis proteinsJ Biol Chemist1975250400721
- PhillipsJGNFlickerPFCohenCStreptococcal M protein: Alpha-helical coiled-coil structure and arrangement on the cell surfaceProc Natl Acad Sci USA1981784689937029524
- RamasawmyRFaeKCSpinaGAssociation of polymorphisms within the promoter region of the tumor necrosis factor-alpha with clinical outcomes of rheumatic feverMol Immunol20074418838
- RizviSFKhanMAKundiAStatus of rheumatic heart disease in rural PakistanHeart200490394915020513
- RobertsSKosankeSTerrence DunnSPathogenic mechanisms in rheumatic carditis: focus on valvular endotheliumJ Infect Dis20011835071111133385
- TarasoutchiFSpinaGSNobreFernandoSerranoCarlos VicenteJrProfilaxia da Febre ReumáticaTratado de Cardiologia SOCESP20051stManoleSão Paulo914
- VellosoLGMansurAJGrinbergMFatal active rheumatic disease. Study of 13 necropsy casesArq Bras Cardiol199156269731888299