Abstract
The rising incidence of obesity and insulin resistance to epidemic proportions has closely paralleled the surge in the prevalence of diabetes and outpaced therapeutic advances in diabetes prevention and treatment. Current evidence points to obesity induced oxidative stress and chronic inflammation as the common denominators in the evolution of insulin resistance and diabetes. Of all the hypoglycemic agents in the pharmacological arsenal against diabetes, thiazolidinediones, in particular pioglitazone, as well as metformin appear to have additional effects in ameliorating oxidative stress and inflammation; rendering them attractive tools for prevention of insulin resistance and diabetes. In addition to their hypoglycemic and lipid modifying properties, pioglitazone and metformin have been shown to exert anti-oxidative and anti-inflammatory effects in vascular beds, potentially slowing the accelerated atherosclerosis in diabetes, which is the major cause of morbidity and mortality in the affected population. The combination of pioglitazone and metformin would thus appear to be an effective pharmacological intervention in prevention and treatment of diabetes. Finally, this review will address the currently available evidence on diabetic cardiomyopathy and the potential role of combination therapy with pioglitazone and metformin.
Introduction
The recent escalation of obesity from an individual health problem to a major public health issue reaching epidemic proportions has drawn attention to a constellation of abnormalities (abdominal obesity, hypertension, dyslipidemia) collectively referred to as metabolic syndrome. As an indicator of insulin resistance and a harbinger of diabetes, this syndrome has been associated with major cardiovascular mortality and morbidity (CitationGalassi et al 2006). The surge in the prevalence of diabetes to epidemic proportions around the world has followed the same trends as the obesity and metabolic syndrome. The current global prevalence of type 2 DM is approximately 150 million with a predicted increase to 215 million by 2010 and 300 million by 2025 reported by CitationCDC (2006). Therefore, it would seem intuitive to blame excess body weight and excess adipose tissue mass for causing insulin resistance and diabetes. Yet, the exact pathophysiological events leading to the development of diabetes or cardiovascular disease in metabolic syndrome remain unknown. This review aims to go over the current literature on the pathogenesis of diabetes and effects of two different insulin sensitizers, on the treatment of diabetes and cardiovascular complications of diabetes.
Pathogenesis of insulin resistance and diabetes
Subjects with type 2 diabetes (T2DM) are characterized by chronic insulin resistance with poor β-cell compensation. Although there is much descriptive information on the pathogenesis of type 2 diabetes (T2DM), the underlying mechanisms remain unknown. The initial pathophysiologic event is usually insulin resistance associated with obesity and a sedentary lifestyle. Along with the development of insulin resistance, insulin secretory decompensation develops, leading to impaired glucose tolerance, and eventually T2DM (CitationMartin et al 1992). It is not well understood why obesity causes insulin resistance. However, the hypotheses linking obesity to insulin resistance generally fall into the following categories: inflammation, lipotoxicity, oxidative stress and endoplasmic reticulum stress.
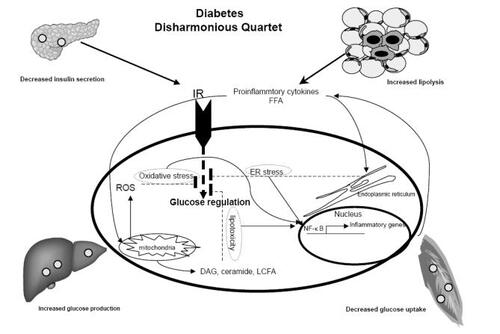
Obesity is associated with increased accumulation of macrophages in adipose tissues and increased levels of pro-inflammatory cytokines (CitationDi Gregorio et al 2005). In addition, obesity results in excess lipid accumulation not only in adipose tissue but also in tissues such as the liver, skeletal muscle, heart and pancreas (CitationUnger 2003). There is a strong correlation between intramyocellular fat content (IMCL) and insulin resistance (CitationPerseghin et al 1999; CitationFriedman 2002). Ectopic lipid accumulation with resultant functional impairment has been called lipotoxicity, a pathophysiologic event preceding diabetes (CitationFriedman 2002). Increased level of reactive oxygen species (ROS) has been also known as a culprit in the pathogenesis of insulin resistance and diabetic complications (CitationPetersen et al 2004; CitationSchrauwen and Hesselink 2004; CitationHoustis et al 2006; CitationMehta et al 2006). Insulin resistance and diabetes are associated with disruption and uncoupling of several key oxidative reactions that result in excessive production of ROS at mitochondrial and cellular levels (CitationNishikawa et al 2000). Increased production of reactive oxygen species in mitochondria, accumulation of mitochondrial DNA damage, and progressive respiratory chain dysfunction are associated with atherosclerosis or cardiomyopathy in human investigations and animal models of oxidative stress (CitationMehta et al 2006; CitationMadamanchi and Runge 2007). More recently, obesity-induced endoplasmic reticulum stress (increased unfolded protein response due to the disruption of the smooth operation of endoplasmic reticulum) has been demonstrated to result in peripheral insulin resistance (CitationHotamisligil 2005). Lipotoxicity, oxidative stress and endoplasmic reticulum stress are associated with activation of nuclear factor-κB (NF-κB), chronic inflammation and insulin resistance (). Interventions aimed at modulating any of the above mentioned pathways are potential therapies for diabetes and its complications.
Figure 1 Diabetes is characterized by decompensated insulin secretion for insulin resistance at target organs including adipose tissue, liver and muscle. Insulin resistance is associated with increased proinflammatory cytokines. Inflammatory pathways in insulin resistance can be initiated by extracellular mediators such as cytokines and free fatty acid (FFA) or by intracellular stresses such as ER stress, excess ROS production by mitochondria or lipotoxicity. Activation of NF-κB pathway leads to induction of chemokines that recruit inflammatory cells, such as macrophages. (FFA Free fatty acid, ER Endoplasmic reticulum, DAG diacylglycerol, LCFA long chain fatty acid, IR insulin receptor).
Pharmaceutical intervention to relieve insulin resistance (insulin sensitizers)
Because insulin resistance is the key feature of type 2 diabetes, much drug development has been focused on insulin sensitizers. At present, two classes of drugs are used as insulin sensitizers: biguanides (metformin), and thiazolidinediones (rosiglitazone and pioglitazone). The mechanism of action of these drugs is not well understood. Metformin is relatively more active in the suppression of hepatic glucose production (CitationDeFronzo et al 1991; CitationStumvoll et al 1995), and thiazolidinediones are more active in stimulation of muscle glucose uptake (CitationNolan et al 1994; CitationYki-Jarvinen 2004).
Metformin
Metformin is the only biguanide available in the U.S. and it is one of the most commonly used anti-diabetic medications. Several studies have demonstrated improved peripheral insulin sensitivity following metformin treatment in diabetic patients (CitationWiden et al 1992; CitationVelazquez et al 1994; CitationDorella et al 1996; CitationLi et al 1999; CitationMoghetti et al 2000). In one study, metformin produced an improvement in basal, but not insulin stimulated, glucose transport, and no effect on insulin secretion (CitationMorel et al 1999) yet in another study, metformin did not have any effect on peripheral insulin sensitivity (CitationRasouli et al 2005). The improvement in insulin sensitivity by metformin was less than that observed with thiazolidinedione in a recent study in diabetic patients receiving a sulfonylurea (CitationKim et al 2002; CitationRasouli et al 2005). Hence, the data suggest that metformin has a primary action on hepatic glucose production, either through direct activation of insulin receptor (CitationGunton et al 2003) or inhibition of key enzymes in the gluconeogentic pathways (CitationCusi et al 1996; CitationDorella et al 1996). Recent studies have shown that metformin stimulated AMP kinase activity (CitationZhou et al 2001), and enhanced the peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1α) expression (CitationSuwa et al 2006). Both AMP kinase and PGC are major regulators of fatty acid oxidation; hence, metfomin might improve lipotoxicity. Indeed metformin decreased muscle fat content in diabetic subjects (CitationMathieu-Costello et al 2003), however, we did not detect any change in IMCL in our study, with metformin (CitationRasouli et al 2005). Metformin has also favorable effects on some inflammatory markers such as CRP levels. However, the effect of thiazolidinedione or weight loss on lowering CRP was more pronounced (CitationChu et al 2002; CitationThe Diabetes Prevention Program Research Group 2005). We have previously reported no change in plasma levels of TNFα or adiponectin in subject with impaired glucose tolerance after treatment with metformin for 10 weeks, however, resistin level decreased significantly in that study (CitationRasouli et al 2006). In addition, metformin did not change the number of adipose tissue macrophages as markers of inflammation (CitationBodles et al 2006; CitationDi Gregorio et al 2005).
The impact of metformin on oxidative stress has also been investigated in several studies. It has been shown that metformin can quench the surge in reactive oxidant species in insulin resistance and diabetes. In a study by Rosen et al on Goto-Kakizaki (GK) rat model of type 2 diabetes, Metformin was noted to ameliorate mitochondrial oxidative stress as demonstrated by a reduction in mitochondrial aconitase activity. In addition, metformin prevented the rise in oxidized proteins (carbonyl moieties) and lipid peroxides that are usually observed in this model of type 2 diabetes (CitationRosen and Wiernsperger 2006). Furthermore, metformin has been shown to reduce ROS levels in human leukocytes by either directly scavenging the free radicals or modulating their intracellular production (CitationBonnefont-Rousselot et al 2003).
Thiazolidinediones
The thiazolidinedione derivatives, pioglitazone and rosiglitazone, are synthetic ligands for peroxisome proliferative-activated receptorγ (PPARγ) which improve insulin sensitivity. PPARγ is mainly expressed in adipose tissue; however, improved insulin sensitivity is a function that primarily involves glucose transport into skeletal muscle. This effect is poorly understood, but it is thought to be due to redistribution of lipid from ectopic sites to subcutaneous adipose tissue. PPARγ agonists promote adipocyte differentiation, and they promote FFA uptake and storage in subcutaneous adipose rather than visceral adipose tissue or ectopic sites (muscle or liver) (CitationBajaj et al 2003; CitationRasouli et al 2005). In addition to the favorable effect on lipotoxicity, we have previously reported that thiazolidinediones decreased inflammatory responses in insulin resistance (CitationDi Gregorio et al 2005; CitationBodles et al 2006) by reducing the number of adipose tissue macrophage. Pioglitazone promotes apoptosis in adipose tissue macrophages along with improvement in insulin sensitivity (CitationBodles et al 2006). PPAR ligands have been shown to regulate inflammatory cytokines like TNFα, CRP, PAI and adiponectin as well (CitationChu et al 2002; CitationYki-Jarvinen 2004; CitationRasouli et al 2005, Citation2006). Thiazolidinediones have also been shown to exert potent antioxidant effects. In a study by Hwang and colleagues, rosiglitazone reduced NADPH oxidase activity and superoxide ion O2− generation in the vascular tissues obtained from obese, diabetic, leptin receptor-deficient (db(−)/db(−)) mice (CitationHwang et al 2007). Similarly, pioglitazone has been found to reduce superoxide ion O2− generation in human coronary artery endothelial cells (CitationMehta et al 2003).
Cardiovascular complications of diabetes
Cardiovascular disease is the major cause of mortality and morbidity in diabetes and is usually due to atherosclerosis in the coronary, cerebral and other peripheral vascular beds (CitationTziakas et al 2005; CitationPambianco et al 2006). Yet, myocardial dysfunction in the absence of epicardial coronary atherosclerosis has also been described and accounts for some cases of congestive heart failure in diabetics (CitationRubler et al 1972).
Accelerated atherosclerosis in diabetes
Several lines of evidence point to endothelial dysfunction and loss of endothelial vasoreactivity as one of the initial events in atherosclerotic plaque formation (CitationDavignon and Ganz 2004). The dysfunctional endothelium subsequently expresses a host of cell surface receptors including intercellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1) as well as receptors for oxidized lipid particles such as leptin-like oxLDL receptor (LOX-1) which facilitate inflammatory cell recruitment and oxidized LDL transport to the intimal layer. The ingestion of oxidized lipid particles by these cells results in further activation of inflammatory cells and release of cytokines and growth factors which perpetuate the vicious cycle of inflammation, cell recruitment, lipid particle ingestion and growth; ultimately leading to an atherosclerotic plaque. The acceleration of this process in diabetes could be attributed to several potential mechanisms. There is growing evidence that excessive ROS formation in diabetes leads to endothelial dysfunction (CitationVincent and Taylor 2006). The first evidence of endothelial dysfunction in human type 1 and type 2 diabetes were reported from examination of penile corpura cavernosa (CitationSaenz de Tejada et al 1989). Subsequent studies confirmed loss of endothelial vasoreactivity in other vascular beds including coronary arteries (CitationNitenberg et al 1993). Although the exact mechanism of endothelial dysfunction in diabetics has not been elucidated, several studies point to reduced NO bioavailability and that tight glycemic control can improve endothelial reactivity (CitationRodriguez-Manas et al 2003). Our laboratory data indicates that increased ROS induces LOX-1 expression which not only facilitates ox-LDL incorporation into endothelium but also enhances expression of adhesion molecules ICAM-1 and VCAM (CitationLi and Mehta 2000; CitationChen et al 2001; CitationLi et al 2003). The dyslipidemic milieu of diabetes ensures a continued flux, oxidation and uptake of LDL particles into the eventual atherosclerotic plaque.
Effects of thiazolidinediones and metformin in decelerating atherosclerotic plaque formation in diabetes
Thiazolidiones and in particular pioglitazone have been shown to improve endothelial function in diabetes. In a randomized controlled trial, Martens and colleagues demonstrated that four weeks of therapy with pioglitazone improves shear stress-induced flow mediated vasodilation (an endothelial dependent phenomenon) of brachial artery in patients with type 2 diabetes with no effect on endothelium independent vasodilation (CitationMartens et al 2005). In addition, pioglitazone has also been shown to reduce LOX-1 as well as VCAM expression which can potentially prevent plaque formation (CitationImamoto et al 1920; CitationMehta et al 2003). The metabolic effects of pioglitazone in improving insulin sensitivity, reducing blood glucose, LDL, triglyceride and certain inflammatory mediators such as C-reactive protein levels further modulates the substrate for plaque formation and halts atheroma progression (CitationBerhanu et al 2006; CitationSzapary et al 2006). Metformin has also been noted to effectively reduce oxidative stress through inhibition of the PKC and NADPH oxidase pathways (CitationOuslimani et al 2005; CitationMahrouf et al 2006). While reducing the oxidative stress burden, metformin can ultimately improve endothelial function (CitationDe et al 2005). Metformin has also been shown to exert antiproliferative effects on vascular smooth muscle cells through inhibition of PKC pathway which can also slow the pace of plaque formation (CitationLi et al 2005). Several human studies have indeed indicated that macrovascular complications of diabetes are reduced by metformin (CitationJohnson et al 2005). Given the evidence, it appears that thiazolidinediones and metformin modulate endothelial function and plaque formation through distinctly separate pathways rendering their combination an attractive agent in decelerating diabetic atherosclerosis.
Diabetic cardiomypcarthy and cardiac lipotoxicity
Approximately 34 years ago, Rubler and colleagues reported a series of four diabetic patients with congestive heart failure, normal epicardial coronary arteries and no other known causes of congestive heart failure in association with diabetic glomerulosclerosis (CitationRubler et al 1972). This new entity termed diabetic cardiomyopathy is frequently associated with diastolic dysfunction, left ventricular hypertrophy and fibrosis (CitationDi Bonito et al 1996). Subsequent attempts in elucidating the putative mechanisms in animal models indicated a direct correlation between myocardial fat content and systolic and diastolic dysfunction. In a transgenic mice model which over expressed long chain Acyl-CO A synthase (ACS), Chiu and Schaffer were able to demonstrate dilated cardiomyopathy by sequential echocardiography along with excessive triglyceride accumulation in cardiomyocytes by both light and electron microscopy (CitationChiu et al 2001). In addition, incubation of cardiomyocytes with long-chain fatty acids resulted in DNA fragmentation and cardiomyocyte apoptosis (CitationKong and Rabkin 2000). The obese (fa/fa) rat which develops diabetes with aging is the closest animal model to human metabolic syndrome. A recent study by Zhao and colleagues demonstrated that the obese (fa/fa) rats will accumulate significantly higher amounts of cardiac triglycerides as early as 7 weeks of age as compared to the normal lean rats. Further evaluation of myocardial performance by echocardiography at 20 weeks of age revealed a dilated cardiomypoathy along with systolic and diastolic dysfunction in the obese (fa/fa) rats while the lean rats maintained the normal cardiac function at 20 weeks in this study (CitationZhou et al 2000). Despite these well-described animal models of myocardial lipotoxicity, the prevalence and mechanisms of cardiac lipotoxicity in humans remain unknown.
Effects of thiazolidinediones and metformin on diabetic cardiomyopathy
Animal studies indicate that pioglitazone can prevent diastolic dysfunction in advanced diabetes by improving myocardial fatty acid metabolism (CitationKim et al 2003). While these studies supported the cardiac lipotoxicity hypothesis and pointed to an emerging potential therapy, early use of thiazolidinediones in humans was associated with several reports of fluid retention and exacerbation of congestive heart failure (CHF) (CitationKermani and Garg 2003; CitationCheng and Fantus 2004). A large multicenter clinical trial on 16,000 individuals with CHF aimed to assess potential benefits of pioglitazone in preventing complications of diabetes yielded mixed results; indicating that the favorable effects of pioglitaozne in reducing mortality are offset by increased number of hospital admissions for CHF exacerbations (CitationDormandy et al 2005). Therefore, further clinical studies are needed to elucidate the role of Thiazolidinediones in diabetic cardiomyopathy. Similarly, the initial concerns that metformin can cause lactic acidosis in patients with diabetes and congestive heart failure were revisited in a large retrospective study of over 12,000 patients (CitationEurich et al 2005). This study indicated that metformin alone or in combination therapy is associated with lower morbidity and mortality in patients with heart failure and type 2 diabetes as compared to sulfonylureas (CitationEurich et al 2005).
Summary and future directions
The increasing incidence of diabetes has paralleled the epidemic of obesity and insulin resistance. The exact mechanism(s) linking obesity to insulin resistance and diabetes remain to be explored but the evidence points to the three axes of oxidative stress, lipotoxicity and ER stress. Given the multiple putative pathways involved in the pathogenesis of diabetes and its complications, the treatment of this condition calls for simultaneous disruption of such pathologic phenomena. Therefore, it is anticipated that combination therapy with two or more agents will surpass the currently accepted indication of achieving optimal glucose control and expand to include most patients with diabetes. Pioglitazone and metformin are two insulin sensitizers with different mechanisms of action and distinctly separate effects on oxidative stress and lipotoxicity rendering their combination a potentially powerful tool in treating diabetes. In addition, the favorable effects of pioglitazone on endothelial function and cell surface receptor expression are in synergy with some of the anti-atherosclerotic effects of metformin; thus rendering combination therapy an attractive tool against accelerated atherosclerosis of diabetes.
Acknowledgements
This work was supported by the Research Service of the Department of Veterans Affairs through VA Merit Review grant to NR, and by grant M01RR14288 from National Center for Research Resources (National Institutes of Health) to the University of Arkansas for Medical Sciences to fund the General Clinical Research Center.
References
- BajajMSuraamornkulSPratipanawatrTPioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetesDiabetes20035213647012765945
- BerhanuPKipnesMSKhanMAEffects of pioglitazone on lipid and lipoprotein profiles in patients with type 2 diabetes and dyslipidaemia after treatment conversion from rosiglitazone while continuing stable statin therapy.Diabetes and Vascular Disease Research200633944 [erratum appears in Diab Vasc Dis Res. 2006; 3(2):71]16784180
- BodlesAMVarmaVYao-BorengasserAPioglitazone induces apoptosis of macrophages in human adipose tissueJournal of Lipid Research2006M600235MJLR200
- Bonnefont-RousselotDRajiBWalrandSAn intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stressMetabolism: Clinical and Experimental200352586912759888
- [CDC] Centers for Disease Control and PreventionDiabetes data and trends [online]2006 URL: http://www.cdc.gov/diabetes/statistics
- ChenMNagaseMFujitaTDiabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: possible role of LOX-1 ligand and AGEBiochemical and Biophysical Research Communications2001287962811573959
- ChengAYFantusIGThiazolidinedione-induced congestive heart failureAnnals of Pharmacotherapy2004388172015039476
- ChiuHCKovacsAFordDAA novel mouse model of lipotoxic cardiomyopathyJournal of Clinical Investigation20011078132211285300
- ChuNVKongAPSKimDDDifferential effects of metformin and troglitazone on cardiovascular risk factors in patients with type 2 diabetesDiabetes Care200225542911874944
- CusiKConsoliADeFronzoRAMetabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitusJournal of Clinical Endocrinology Metabolism1996814059678923861
- DavignonJGanzPRole of endothelial dysfunction in atherosclerosisCirculation200410923 Suppl 1III273215198963
- DeJJKooyALehertPEffects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized,placebo-controlled trialJournal of Internal Medicine2005257100915606381
- DeFronzoRABarzilaiNSimonsonDCMechanism of metformin action in obese and lean noninsulin-dependent diabetic subjectsJournal of Clinical Endocrinology and Metabolism19917312943011955512
- Di BonitoPCuomoSMoioNDiastolic dysfunction in patients with non-insulin-dependent diabetes mellitus of short durationDiabetic Medicine19961332149162606
- Di GregorioGBYao-BorengasserARasouliNExpression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazoneDiabetes20055423051316046295
- DorellaMGiustoMDaTVImprovement of insulin sensitivity by metformin treatment does not lower blood pressure of nonobese insulin-resistant hypertensive patients with normal glucose toleranceJournal of Clinical Endocrinology and Metabolism1996811568748636369
- DormandyJACharbonnelBEcklandDJSecondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trialLancet200536612798916214598
- EurichDTMajumdarSRMcAlisterFAImproved clinical outcomes associated with metformin in patients with diabetes and heart failureDiabetes Care20052823455116186261
- FriedmanJFat in all the wrong placesNature2002415268911796987
- GalassiAReynoldsKHeJMetabolic syndrome and risk of cardiovascular disease: a meta-analysisThe American Journal of Medicine20061198121917000207
- GuntonJEDelhantyPJDTakahashiSIMetformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2Journal of Clinical Endocrinology Metabolism20038813233212629126
- HotamisligilGSRole of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetesDiabetes200554Suppl 2S73816306344
- HoustisNRosenEDLanderESReactive oxygen species have a causal role in multiple forms of insulin resistanceNature2006440944816612386
- HwangJKleinhenzDJRupnowHLThe PPARgamma ligand, rosiglitazone, reduces vascular oxidative stress and NADPH oxidase expression in diabetic miceVascular Pharmacology2007464566217337254
- ImamotoEYoshidaNUchiyamaKInhibitory effect of pioglitazone on expression of adhesion molecules on neutrophils and endothelial cellsBiofactors192013747
- JohnsonJASimpsonSHTothELReduced cardiovascular morbidity and mortality associated with metformin use in subjects with Type 2 diabetesDiabetic Medicine20052249750215787679
- KermaniAGargAThiazolidinedione-associated congestive heart failure and pulmonary edemaMayo Clinic Proceedings20037810889112962163
- KimSKZhaoZSLeeYJLeft-ventricular diastolic dysfunction may be prevented by chronic treatment with PPAR-alpha or -gamma agonists in a type 2 diabetic animal modelDiabetes/Metabolism Research Reviews20031948793
- KimYBCiaraldiTPKongATroglitazone but not metformin restores insulin-stimulated phosphoinositide 3-kinase activity and increases p110beta protein levels in skeletal muscle of type 2 diabetic subjectsDiabetes200251443811812753
- KongJYRabkinSWPalmitate-induced apoptosis in cardiomyocytes is mediated through alterations in mitochondria: prevention by cyclosporin ABiochimica et Biophysica Acta20001485455510802248
- LiCLPanCYLuJMEffect of metformin on patients with impaired glucose toleranceDiabetic Medicine1999164778110391395
- LiDLiuLChenHLOX-1, an oxidized LDL endothelial receptor, induces CD40/CD40L signaling in human coronary artery endothelial cellsArteriosclerosis, Thrombosis and Vascular Biology20032381621
- LiDMehtaJLAntisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cellsCirculation200010128899510869259
- LiLMamputuJCWiernspergerNSignaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: inhibitory effect of metforminDiabetes20055422273415983226
- MadamanchiNRRungeMSMitochondrial dysfunction in atherosclerosisCirculation Research20071004607317332437
- MahroufMOuslimaniNPeynetJMetformin reduces angiotensin-mediated intracellular production of reactive oxygen species in endothelial cells through the inhibition of protein kinase CBiochemical Pharmacology2006721768316730666
- MartensFMVisserenFLde KoningEJShort-term pioglitazone treatment improves vascular function irrespective of metabolic changes in patients with type 2 diabetesJournal of Cardiovascular Pharmacology200546773816306801
- MartinBCWarramJHKrolewskiASRole of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up studyLancet199234092591357346
- Mathieu-CostelloOKongACiaraldiTPRegulation of skeletal muscle morphology in type 2 diabetic subjects by troglitazone and metformin: relationship to glucose disposalMetabolism200352540612759881
- MehtaJLHuBChenJPioglitazone inhibits LOX-1 expression in human coronary artery endothelial cells by reducing intracellular superoxide radical generationArteriosclerosis, Thrombosis and Vascular Biology20032322038
- MehtaJLRasouliNSinhaAKOxidative stress in diabetes: A mechanistic overview of its effects on atherogenesis and myocardial dysfunctionThe International Journal of Biochemistry and Cell Biology200638794803
- MoghettiPCastelloRNegriCMetformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluationJournal of Clinical Endocrinology and Metabolism2000851394610634377
- MorelYGolayAPernegerTMetformin treatment leads to an increase in basal, but not insulin-stimulated, glucose disposal in obese patients with impaired glucose toleranceDiabetic Medicine199916650510477209
- NishikawaTEdelsteinDDuXLNormalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damageNature20004047879010783895
- NitenbergAValensiPSachsRImpairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic functionDiabetes1993421017258513969
- NolanJJLudvikBBeerdsenPImprovement in glucose tolerance and insulin resistance in obese subjects treated with troglitazoneNew England Journal of Medicine19943311188937935656
- OuslimaniNPeynetJBonnefont-RousselotDMetformin decreases intracellular production of reactive oxygen species in aortic endothelial cellsMetabolism: Clinical and Experimental2005548293415931622
- PambiancoGCostacouTEllisDThe 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experienceDiabetes2006551463916644706
- PerseghinGScifoPDe CobelliFIntramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H–13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parentsDiabetes1999481600610426379
- PetersenKFDufourSBefroyDImpaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetesThe New England Journal of Medicine20043506647114960743
- RasouliNRaueUMilesLMPioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissueAJP – Endocrinology and Metabolism2005288E9304
- RasouliNYao-BorengasserAMilesLMIncreased plasma adiponectin in response to pioglitazone does not result from increased gene expressionAJP – Endocrinology and Metabolism2006290E426
- Rodriguez-ManasLLopez-DorigaPPetidierREffect of glycaemic control on the vascular nitric oxide system in patients with type 1 diabetes [see comment]Journal of Hypertension20032111374312777950
- RosenPWiernspergerNFMetformin delays the manifestation of diabetes and vascular dysfunction in Goto-Kakizaki rats by reduction of mitochondrial oxidative stressDiabetes/Metabolism Research Reviews20062232330
- RublerSDlugashJYuceogluYZNew type of cardiomyopathy associated with diabetic glomerulosclerosisAmerican Journal of Cardiology1972305956024263660
- Saenz de TejadaIGoldsteinIAzadzoiKImpaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotenceNew England Journal of Medicine19893201025302927481
- SchrauwenPHesselinkMKOxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetesDiabetes20045314121715161742
- StumvollMNurjhanNPerrielloGMetabolic effects of metformin in non-insulin-dependent diabetes mellitusNew England Journal of Medicine199533355047623903
- SuwaMEgashiraTNakanoHMetformin increases the PGC-1{alpha} protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivoJournal of Applied Physiology200610116859216902066
- SzaparyPOBloedonLTSamahaFFEffects of pioglitazone on lipoproteins, inflammatory markers, and adipokines in nondiabetic patients with metabolic syndromeArteriosclerosis, Thrombosis and Vascular Biology2006261828
- The Diabetes Prevention Program Research GroupIntensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose toleranceDiabetes20055415667215855347
- TziakasDNChalikiasGKKaskiJCEpidemiology of the diabetic heartCoronary Artery Disease200516Suppl 1S31016340402
- UngerRHMinireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndromeEndocrinology200314451596512960011
- VelazquezEMMendozaSHamerTMetformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancyMetabolism: Clinical and Experimental199443647548177055
- VincentHKTaylorAGBiomarkers and potential mechanisms of obesity-induced oxidant stress in humansInternational Journal of Obesity2006304001816302012
- WidenEIErikssonJGGroopLCMetformin normalizes nonoxidative glucose metabolism in insulin-resistant normoglycemic first-degree relatives of patients with NIDDMDiabetes19924135481551495
- Yki-JarvinenHThiazolidinedionesThe New England Journal of Medicine200435111061815356308
- ZhouGMyersRLiYRole of AMP-activated protein kinase in mechanism of metformin actionJ Clin Invest200110811677411602624
- ZhouYTGrayburnPKarimALipotoxic heart disease in obese rats: implications for human obesityProceedings of the National Academy of Sciences of the United States of America2000971784910677535