Abstract
Cardiovascular disease (CVD) remains the leading cause of death in Western society today. There is a striking gender difference in CVD with men predisposed to earlier onset and more severe disease. Following the recent reevaluation and ongoing debate regarding the estrogen protection hypothesis, and given that androgen use and abuse is increasing in our society, the alternate view that androgens may promote CVD in men is assuming increasing importance. Whether androgens adversely affect CVD in either men or women remains a contentious issue within both the cardiovascular and endocrinological fraternities. This review draws from basic science, animal and clinical studies to outline our current understanding regarding androgen effects on atherosclerosis, the major CVD, and asks where future directions of atherosclerosis-related androgen research may lie.
Introduction
Epidemiological studies have shown there is a striking gender difference in cardiovascular disease (CVD) with men having higher rates of clinical events than women (CitationKalin and Zumoff 1999). These findings, together with the increased incidence of CAD in women after menopause (CitationTracy 1966), have led to the dogma that female hormones protect against the development of CVD (CitationJeanes et al 2007). The opposite hypothesis, that male hormones may promote CVD in men, has been little investigated. With prospects of androgens being introduced widely for non-classical therapeutic applications, an important clinical question is: do androgens increase the risk or severity of CVD? Such androgen therapy is being targeted towards our aging population, a population that would have pre-existing CVD. The recent proposal that stems from the female hormone replacement therapy (HRT) studies is that HRT appears beneficial in females only when it is initiated before the development of significant atherosclerosis (CitationRossouw et al 2007). Whether this is also true for androgen-based therapies is unknown. This review explores what is known about androgens and their gender-specific effects on the pathogenesis of atherosclerosis to try and highlight those questions that need to be answered for the safe use of androgen-based HRT in both men and women.
Risk factors of atherosclerosis
Primary risk factors for atherosclerosis include elevated levels of low-density lipoprotein (LDL), increased levels of homocysteine, hypertension, diabetes mellitus, obesity, smoking, increasing age and also male gender (CitationRoss 1999). In all developed countries, men have an earlier and greater incidence of heart disease than women (CitationLiu et al 2003; CitationWu and von Eckardstein 2003; CitationIsidori et al 2005). This may be one of the oldest clues to the underlying pathogenic mechanisms of atherosclerosis and suggests that gender-related differences between men and women drives, at least in part, the disparate atherosclerotic plaque formation (CitationLiu et al 2003). There remains no clear evidence, to date, that there is a genetic contribution to the male predisposition to atherosclerosis, however the androgenic milieu may underlie plaque formation in men.
Molecular mechanisms of androgen action
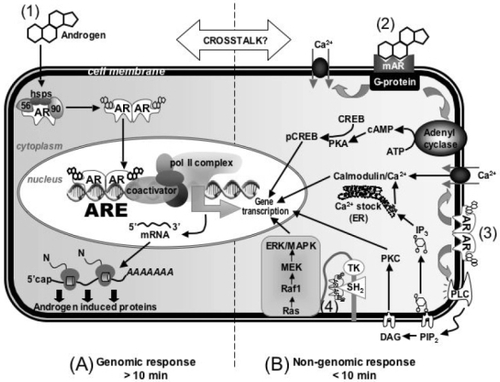
The molecular machinery mediating cellular responses to androgens is complex and involves both genomic and nongenomic effects that are still far from being clearly understood (). Genomic effects of androgens are mediated by a specific receptor, the androgen receptor (AR). In response to the binding of androgens to AR, it switches to a transcription factor that regulates target gene expression (CitationDavison and Bell 2006). Non-genomic effects of androgens occur independently of AR. Instead, membrane-bound receptors have been proposed to trigger rapid effects of androgens that lead to 2nd messenger signaling (CitationBenton et al 2004). This, in turn, triggers a variety of cell responses (CitationWierman 2007). These nongenomic pathways underlie the rapid vasodilation of coronary arteries by testosterone (CitationMalkin et al 2006; CitationCooper et al 2007; CitationSeyrek et al 2007).
Figure 1 Molecular mechanisms of androgen action. (1) Androgens mediate gene transcription via binding to the classical cytosolic AR in the genomic pathway; (2) Androgens mediate rapid effects through a novel membrane receptor; (3) Androgens interact with the classical cytosolic AR associated with the plasma membrane; (4) Androgens act through a multi-protein complex associated with the plasma membrane.
Regulation and tissue expression of AR
AR function and transactivation ability is regulated by post-translational modifications such as phosphorylation (CitationZhou et al 1995; CitationGioeli et al 2002), acetylation and sumoylation (CitationThomas et al 2004). AR expression itself is regulated at both the mRNA and protein levels by androgens (CitationLee and Chang 2002). Androgens predominantly decrease AR mRNA at the transcriptional level (CitationTrapman et al 1990; CitationKrongrad et al 1991) however, they simultaneously increase AR stability and translational efficiency thereby even in the presence of decreased AR mRNA levels, androgens increase AR protein levels in most cell types (CitationYeap et al 1999).
AR has been detected in the majority of tissues throughout the body (CitationQuigley et al 1995). AR is evident in vascular cells and gender-specific expression of AR has been shown in monocyte-derived macrophages (CitationNg et al 2003), endothelial cells (CitationDeath et al 2004) and vascular tissue (CitationDeath et al 2004), where cells or tissue from male donors had significantly higher AR protein levels. The gender dichotomy in AR expression may underlie gender-specific effects of androgens on atherosclerosis.
Metabolic activation of testosterone
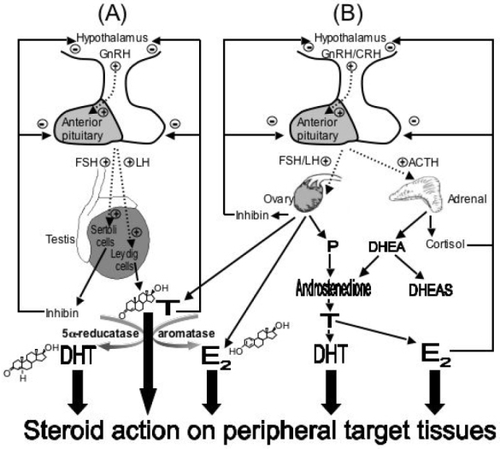
Testosterone and other androgens can mediate effects via metabolic activation (). This involves the conversion of testosterone at peripheral nongonadal tissues to active metabolites, estradiol or DHT. Conversion of testosterone to estradiol involves a P450-dependent aromatase enzyme (CYP19) and acts to diversify androgen action, since estradiol binds to the estrogen receptor (ER), and not AR, thereby regulating the expression of a completely different set of genes. The conversion of testosterone to DHT is catalyzed by 5α-reductases. DHT has greater binding affinity for AR than testosterone and a slower dissociation rate, therefore has a higher molar potency (CitationGrino et al 1990). Hence, the conversion of testosterone to DHT effectively amplifies AR action.
Figure 2 Schematic representation of the major sources of androgens in men and women. (MEN) The hypothalamic-pituitary-gonadal axis in men. The dotted lines represent the pulsatile release of gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone (FSH) and luteinising hormone (LH). LH serves to stimulate the testis to produce testosterone (T) or FSH stimulates production of inhibin. In turn, these exert a negative feedback on both the pituitary and hypothalamus regulating LH and FSH secretion. T can be reduced to the more active dihydrotestosterone (DHT) or aromatised to estradiol (E2) in target tissues. (WOMEN) The hypothalamic-pituitary-ovarian and hypothalamic-pituitary-adrenal axis represents the major sources for androgen synthesis in women. In addition to inhibin and cortisol, high levels of E2, progesterone (P) and adrenal androgens exert a negative feedback to regulate the secretions of GnRH, corticotropin-releasing hormone (CRH), FSH, LH and adrenocorticotropic hormone (ACTH).
Androgens and vascular cell effects
Androgens have been shown to promote-, and suppress-, pro-atherogenic, pro-inflammatory effects on all cell types involved in atherogenesis. Given current evidence it would appear that androgen effects are dependent on cell type, dose, type of androgen, and time of exposure. For example, T suppresses vascular cell adhesion molecule-1 (VCAM-1) expression in human endothelial cells, via an aromatase/estrogen receptor-dependent mechanism (CitationHatakeyama et al 2002; CitationMukherjee et al 2002); however, DHT, a non-aromatisable androgen, induces VCAM-1 expression in human endothelial cells (CitationDeath et al 2004). Similarly, T has been shown to enhance reverse cholesterol transport (CitationLanger et al 2002) whilst DHT promotes cholesteryl ester accumulation in monocyte derived macrophages (CitationNg et al 2003). In addition, T has been shown to inhibit nitric oxide release from monocytes via inhibition of inducible nitric oxide synthase (CitationFriedl et al 2000). This decrease in NO potentially increases thrombosis risk via increased platelet aggregation. Additionally, T has adverse effects stimulating the proliferation of rat vascular smooth muscle cells (CitationFujimoto et al 1994), inducing proteoglycan synthesis and the elongation of glycosaminoglycans (GAG) chains on these proteoglycans (CitationHashimura et al 2005) and T increases apoptotic damage of vascular smooth muscle cells (CitationLing et al 2004). Importantly, some of the effects on both endothelial cells and MDMs were gender-specific, occurring in cells derived from males but not females, and associated with increased AR expression in male-derived cells (CitationNg et al 2003; CitationDeath et al 2004). This suggests that steps in atherogenesis could be markedly different between genders, mediated by androgen exposure and AR expression levels.
Therefore, both T and DHT can have effects that could lead to the development of atherosclerosis, associated with male-dependent AR expression. However, T can have equally anti-atherogenic effects, associated with aromatisation. Obviously, more work is required for us to understand how androgens act at the cellular level. One of the major questions that has recently emerged is whether aromatisation is an important protective mechanism? There is now a real need to study and understand the metabolic activation pathways of T, in those cell types associated with atherosclerosis, and to determine if manipulating those pathways can switch between the atheroprotective versus atherogenic effects of T?
Androgens and atherosclerosis: evidence from animal model studies
As with the cellular studies, androgen treatment has been shown to both promote and retard lesion formation in animal studies of atherosclerosis (). The effects of T appear to be gender-, steroid/dose/administration-, and/or species- specific. For the most part, T treatment of male animals has led to a decrease in atherosclerotic lesion size or the atherosclerosis-related end point studied (e.g. aortic cholesterol content) (CitationBruck et al 1997; CitationElhage et al 1997; CitationAlexandersen et al 1999; CitationNathan et al 2001). Similarly, DHEA treatment of male animals has led to a decrease in atherosclerosis (CitationGordon et al 1988; CitationArad et al 1989; CitationEich et al 1993). Both T and DHEA are readily aromatisable to estradiol, and aromatase inhibition has been shown to block the atheroprotective effects of T (CitationNathan et al 2001). In keeping with the importance of aromatase to mediate the atheroprotective effects of T, a study showed that a 3-month treatment with an anabolic androgenic steroid, stanozolol, had no effect on atherosclerosis or blood lipids in cholesterol-fed rabbits (CitationFogelberg et al 1990). Stanozolol is a 5α-reduced substrate so it cannot be converted to estrogen by aromatase and therefore, only has androgenic effects. Interestingly, 2/10 stanozolol-treated normal diet fed rabbits developed atherosclerosis versus 0/72 control rabbits, thereby suggesting that stanozolol may increase the propensity for atherosclerotic lesion development. This was not followed up by these investigators.
Table 1 Effects of androgens on atherosclerosis in animal models
In contrast to the studies that showed T was atheroprotective, two studies (out of 12) demonstrated increased atherosclerotic plaque formation after exogenous T treatment (CitationToda et al 1984; Citationvon Dehn et al 2001). However, both of these studies used an experimental approach that differed from usual practice. The first studied chicks, rather than rodents, and they only observed increased atherosclerotic lesion when T was administered at 150 mg for 7 weeks (compared to 50 mg/day/rabbit for 3 months, CitationBruck et al 1997). No studies have subsequently been performed in chicks to confirm the original findings. The second study demonstrating adverse effects of T used chemical, rather than surgical, castration of apoE-deficient mice. In this model, T treatment (35 mg dosage) was observed to increase atherosclerotic lesion area by a significant, but small, extent. This study did not examine the aromatase pathway so in this animal model it is not clear if aromatase would be expressed, which could help explain the disparate result.
As with the male animal model data, testosterone effects on atherosclerotic plaque formation in female animal models is also contentious. The data that exists is very limited with only 5 studies in total. Three of these studies were performed in rodents and testosterone was found to decrease lesion size in 2/3 of them (CitationElhage et al 1997; Citationvon Dehn et al 2001). The third rodent study showed no effect of testosterone treatment on atherosclerotic plaque development (CitationChen et al 1996). The other two female animal studies were performed with primates and showed that testosterone or the anabolic androgen, nandrolone, induced atherosclerosis over a 2-year treatment period (CitationAdams et al 1995; CitationObasanjo et al 1996). These primate studies, whilst only have a small number of animals in the experimental groups, remains the strongest evidence that exogenous androgen treatment may be atherogenic in females.
Importantly, all of the animal studies have targeted the effects of androgens on atherosclerotic plaque development without examining an effect of T on existing plaque. As has now been highlighted by the recent estrogen therapy trials, the timing of hormone therapies can have different outcomes on CVD. Although contentious, and remains to be proven, estrogen-based therapies given after plaque has developed leads to adverse effects (increased myocardial infarction, stroke) in the short-term. Whether androgen-based therapies have similar outcomes dependent on timing and age of patient has not been investigated.
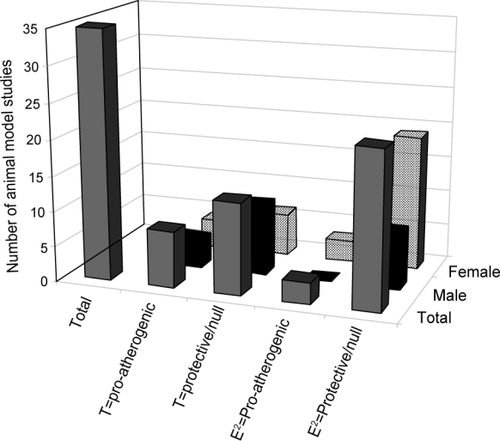
summarizes the primary effect of androgen and estrogen treatment on atherosclerotic lesion development, as measured in animal studies. Note that many more studies have targeted estradiol effects and the number of androgen studies are relatively small in comparison, especially those that focus on T effects in females. Therefore, it remains unclear whether T is anti- or pro-atherogenic in female animal models, whilst in males T is atheroprotective, most probably via aromatisation to estradiol. Direct T or stanozolol effects appear to be atherogenic, although the limited number of studies do not allow for any firm conclusions to be made. Therefore, androgens, atherosclerosis, and gender-specific effects remains an important area for future research development.
Figure 3 Animal model studies examining the effects of androgens on atherosclerosis. Total number of animal model studies are represented by the solid grey bar. Of the total number of studies, male studies are represented by the black solid bar. Of the total number of studies, female studies are represented by the hatched bar. T=pro-atherogenic – represents the number of animal model studies showing pro-atherogenic effects of T treatment or castration; T=protective/null – represents the number of animal model studies showing protective or null effects of T treatment or castration; E2=pro-atherogenic – represents the number of animal model studies showing pro-atherogenic effects of T treatment or castration; E2=protective/null – represents the number of animal studies showing protective or neutral effects of T treatment or castration.
Androgens and atherosclerosis: evidence from clinical studies
To date, the major clue that androgens may drive CAD in men remains the gender dichotomy in the earlier incidence of atherosclerosis. However, epidemiologic studies report no association between high physiologic androgen levels and atherosclerosis (CitationEnglish et al 2000; CitationHak et al 2002; CitationMuller et al 2004). Instead, the inverse has been reported namely that hypoandrogenemia associates with CAD (CitationMalkin et al 2003), or an atherogenic lipid profile (CitationTchernof et al 1997; CitationZmuda et al 1997), metabolic syndrome (CitationKupelian et al 2006), type 2 diabetes (CitationHaffner et al 1996; CitationStellato et al 2000), systolic and diastolic hypertension (CitationSvartberg et al 2004), visceral obesity (CitationKhaw and Barrett-Connor 1992), increased fibrinogen (CitationBonithon-Kopp et al 1988), arterial stiffness (CitationHougaku et al 2006) and all-cause or cardiovascular deaths (CitationBarrett-Connor and Khaw 1988). Hypoandrogenemia is common and it has been reported that 10% of men between 40 and 60 years of age and 25% between 60 and 80 years of age have low levels of free T (CitationVermeulen and Kaufman 2002) therefore hypo- rather than hyper- androgenemia may be the gender-specific factor driving atherosclerosis. However, not all studies have found an association between hypoandrogenemia and increased CVD (CitationContoreggi et al 1990) therefore it is difficult to draw any firm conclusions. It is of interest that castration of male rodents has been shown to increase atherosclerosis in animal models of atherosclerosis (CitationNathan et al 2001).
Given that low T levels appear harmful for CVD and its important risk factors, T supplementation would be expected to be beneficial. Meta-analysis review of cardiovascular safety of T replacement therapy has reported that T supplementation was relatively safe in terms of cardiovascular health (CitationHaddad et al 2007). However, this meta-analysis needs to be interpreted with caution as none of the randomized controlled trials that were included in the analysis were designed to assess cardiovascular safety and therefore adverse outcomes may have been censored and/or not reported, therefore, weakening the meta-analysis conclusions. However, other studies have shown that T replacement therapy has demonstrable beneficial effects on CVD risk factors, including waist measurements (CitationMarin et al 1992), visceral abdominal fat mass (CitationMarin et al 1992), as well as positive effects on numerous metabolic parameters including insulin sensitivity, glucose control, and hyperlipidemia (CitationEnglish et al 2000; CitationMalkin et al 2006). Direct effects on the arterial tree have also been described with consistent improvement in both anginal symptoms and ischemia on electrocardiograms in men treated with injectable T preparations (CitationRosano et al 1990; CitationWebb et al 1999; CitationEnglish et al 2000; CitationPugh et al 2003).
However, while the observations above would suggest T supplementation improves CVD risk factors, meta-analysis by CitationWhitsel et al (2001) found a dose-dependent decrease in HDL-C and total cholesterol levels with T use in hypogondal men. Similarly, small intervention trials have demonstrated that exogenous T supplementation in young men lowers HDL (CitationMeriggiola et al 1995; CitationWu et al 1996) but in older men T did not affect HDL (CitationSnyder et al 2001; CitationPage et al 2005). The disparite findings may indicate that the effect of T replacement on HDL may be age-dependent. Any effect of T on lowering HDL-C needs to be considered as low HDL levels are a strong risk factor for CVD (CitationGordon et al 1997).
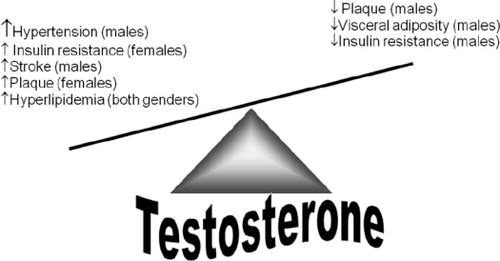
Other cardiovascular diseases that often coexist with CVD, including hypertension and ischemic stroke, also show a similar gender bias, with males at higher risk. Androgens have been reported to adversely affect both conditions, with reports of prohypertensive effects (CitationJenkins et al 1994; CitationReckelhoff 2005) and to worsen the acute phase of stroke (CitationHawk et al 1998). Therefore, when considering T replacement therapy in aging men, the effect on atherosclerosis cannot be considered without simultaneously investigating hypertension and stroke ().
Anabolic androgenic steroid (AAS) use has been anecdotally associated with various forms of cardiovascular disease. Self-administration of AAS has been linked with sudden cardiac death, androgen-induced vasospasm, platelet aggregation, activation of the coagulation cascade, and abnormal left ventricular function and hypertrophy (CitationMaron et al 1996). It has also been reported that self-administration of several AAS simultaneously for 8 or 14 weeks produces profound unfavorable effects on lipoproteins and lipids, leading to an increased atherogenic profile (CitationHartgens et al 2004). However, any link between the adverse lipid profile induced by AAS use and increased atherosclerosis remains to be established.
In women, it is much clearer that androgen excess is linked to the burden of CVD risk factors. The most well studied of such risk factors is insulin resistance. It has been hypothesized that insulin resistance is a consequence of androgen effects. Excessive androgenic steroid exposure of female rats (CitationHolmang et al 1990), normal females (CitationPolderman et al 1994), transsexual females (CitationBjorntorp 1993), and patients with aplastic anemia (CitationWoodard et al 1981) can lead to insulin resistance and may at least be partly reversed by estrogen administration (CitationAndersson et al 1997). In keeping with this hypothesis, it was recently demonstrated that post-menopausal women with well-controlled type 2 diabetes that are insulin resistant show evidence of biochemical and clinical androgen excess, compared to non-diabetic, post-menopausal women with no known risk factors for diabetes other than obesity (CitationKorytkowski et al 2005). Further evidence of a link between high androgen levels and CVD or CVD risk factors is observed in women with polycystic ovary syndrome (PCOS). Women with PCOS have a sustained exposure to high physiologic androgen levels. This condition is associated with endothelial dysfunction, obesity and metabolic abnormalities such as insulin resistance and dyslipidaemia, all of which may predispose PCOS women to premature atherosclerosis (CitationParadisi et al 2001; CitationKrentz et al 2007).
However, despite the association between excess androgen in women and insulin resistance, CVD risk factors and angiographical evidence of atherosclerosis, there remains no evidence of increase cardiovascular mortality in these women. Additionally, in female-to-male transsexuals, testosterone therapy has not been linked to excess cardiovascular mortality or morbidity (Citationvan Kesteren et al 1997). Therefore, the question of the cardiovascular safety of androgen therapy in women remains unanswered. Based on existing observations, androgen use may increase insulin resistance in women with a consequent sequalae of cardiovascular effects however apart from the observations in women with PCOS and type 2 diabetes, and animal data suggesting androgens promote atherosclerosis in females, there is no solid data to support the claim (). More work is necessary to establish a real link between androgens, insulin resistance and atherosclerosis in women.
Summary
From our current understanding of the effects of androgens on atherosclerosis, it has become apparent that the view androgens are harmful is too simplistic. This is made most evident by the erratic nature of the findings reported in cellular, animal and clinical studies. Clearly, much more work is needed in both the basic science and clinical arenas to fully elucidate the effects of androgens on the development of atherosclerosis. For men, exogenous T treatment appears largely beneficial, at least in part via aromatization of T to estradiol, especially if physiological T levels are deficient. However, self-administered AAS usage remains a major CVD safety concern, especially given reported adverse lipid profile effects. As clinicians consider the use of T in management of symptoms associated with the aging male, there remains inconsistent and poorly reported data on cardiovascular risk of long-term T use. For T treatment in aging women, the current data would suggest androgen excess has adverse effects on CVD risk factors, especially in women with diabetes. In summary, there remains limited knowledge about exogenous androgen treatments in both men and women. Despite this, androgen use and abuse is increasing in our society, either for therapeutic or recreational reasons. Whether androgens adversely affect CVD in either men or women remains a contentious issue that is in desperate need of more research.
References
- AdamsMRWilliamsJKKaplanJREffects of androgens on coronary artery atherosclerosis and atherosclerosis-related impairment of vascular responsivenessArterioscler Thromb Vasc Biol199515562707749870
- AlexandersenPHaarboJByrjalsenINatural androgens inhibit male atherosclerosis: a study in castrated, cholesterol-fed rabbitsCirc Res1999848131910205149
- AnderssonBMattssonLAHahnLEstrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitusJ Clin Endocrinol Metab199782638439024268
- AradYBadimonJJBadimonLDehydroepiandrosterone feeding prevents aortic fatty streak formation and cholesterol accumulation in cholesterol-fed rabbitsArteriosclerosis19899159652522296
- Barrett-ConnorEKhawKTEndogenous sex hormones and cardiovascular disease in men. A prospective population-based studyCirculation198878539453409497
- BentonWPMGuoZKruckenJWunderlichFRapid effects of androgens in macrophagesSteroids2004695859015288774
- BjorntorpPHyperandrogenicity in women-a prediabetic condition?J Intern Med1993234579838258749
- Bonithon-KoppCScarabinPYBaraLRelationship between sex hormones and haemostatic factors in healthy middle-aged menAtherosclerosis1988717163377881
- BruckBBrehmeUGugelNGender-specific differences in the effects of testosterone and estrogen on the development of atherosclerosis in rabbitsArterioscler Thromb Vasc Biol199717219299351389
- ChenSJLiHBDurandJEstrogen reduces myointimal proliferation after balloon injury of rat carotid arteryCirculation199693577848565178
- ContoreggiCSBlackmanMRAndresRPlasma levels of estradiol, testosterone, and DHEAS do not predict risk of coronary artery disease in menJ Androl199011460702147671
- CooperBCGokinaNIOsolGTestosterone replacement increases vasodilatory reserve in androgen-deficient female ratsFertility and Sterility200787422517081525
- DavisonSLBellRAndrogen physiologySeminars in Reproductive Medicine20062471716633980
- DeathAKMcGrathKCYSaderMADihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-(B-dependent pathwayEndocrinology200414518899714684616
- EichDMNestlerJEJohnsonDEInhibition of accelerated coronary atherosclerosis with dehydroepiandrosterone in the heterotopic rabbit model of cardiac transplantationCirculation19938726158419015
- ElhageRArnalJFPieraggiMT17(-estradiol prevents fatty streak formation in apolipoprotein E-deficient miceArterioscler Thromb Vasc Biol1997172679849409242
- EnglishKMMandourOSteedsRPMen with coronary artery disease have lower levels of androgens than men with normal coronary angiogramsEur Heart J200021890410806012
- EnglishKMSteedsRPJonesTHLow-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled studyCirculation200010219061111034937
- FogelbergMBIDicsfalusyUHenrikssonPStanozolol and experimental atherosclerosis: atherosclerosis development and blood lipids during anabolic steroid therapy of New Zealand white rabbitsScand J Clin Lan Invest199050693700
- FriedlRBrunnerMMoeslingerTTestosterone inhibits expression of inducible nitric oxide synthase in murine macrophagesLife Sci2000684172911205891
- FujimotoRMorimotoIMoritaEAndrogen receptors, 5(-reductase activity and androgen-dependent proliferation of vascular smooth muscle cellsJ Steroid Biochem Mol Biol199450169748049146
- GioeliDFicarroSBKwiekJJAndrogen receptor phosphorylation. Regulation and identification of the phosphorylation sitesJournal of Biological Chemistry2002277293041412015328
- GordonGBBushDEWeismanHFReduction of atherosclerosis by administration of dehydroepiandrosterone: a study in the hypercholesterolemic New Zealand white rabbit with aortic intimal injuryJ Clin Invest1988825864
- GordonTCastelliWPHjortlandMCHigh density lipoprotein as a protective factor against coronary artery disease: the Framingham StudyAm J Med19976270714193398
- GrinoPBGriffinJEWilsonJDTestosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosteroneEndocrinology19901261165722298157
- HaddadRMKennedyCCCaplesSMTestosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled studiesMayo Clinic Proc2007822939
- HaffnerSMShatenJSternMPLow levels of sex hormone binding globulin and testosterone predict the development of non insulin dependent diabetes mellitus in menAmerican Journal of Epidemiology1996143889978610702
- HakAEWittemanJCde JongFHLow levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam studyJ Clin Endocrinol Metab2002873632912161487
- HartgensFRietjensGKeizerHAEffects of androgenic-anabolic steroids on apolipoproteins and lipoprotein (a)Br J Sports Med200438253915155420
- HashimuraKSudhirKNigroJAndrogens stimulate human vascular smooth muscle cell proteoglycan biosynthesis and increases lipoprotein bindingEndocrinology200514620859015661861
- HatakeyamaHNishizawaMNakagawaATestosterone inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human aortic endothelial cellsFEBS Letters20025301293212387879
- HawkTZhangYQRajakumarGTestosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male ratsBrain Res199879629689689481
- HolmangASvedbergJJennischeEEffects of testosterone on muscle insulin sensitivity and morphology in female ratsAm J Physiol1990259E555602221057
- HougakuHFlegJLNajjarSSRelationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurementsAm J Physiol Endocrinol Metab2006290E2344216159908
- IsidoriAMGiannettaEPozzaCAndrogens, cardiovascular disease and osteoporosisJournal of Endocrinological Investigation20052873916550728
- JeanesHNewbyDGrayGACardiovascular risk in women: the impact of hormone replacement therapy and prospects for new therapeutic approachesExpert Opinion on Pharmacotherapy200782798817266463
- JenkinsCSalisburyRElyDCastration lowers and testosterone restores blood pressure in several rat strains on high sodium dietClin Exp Hypertens199416611257951166
- KalinMFZumoffBSex hormones and coronary disease: a review of the clinical studiesSteroids199955330522237942
- KhawKTBarrett-ConnorELower endogenous androgens predict central adiposity in menAnn Epidemiol19922675821342319
- KorytkowskiMTKrugEIDalyMADoes androgen excess contribute to the cardiovascular risk profile in postmenopausal women with type 2 diabetes?Metabolism Clinical and Experimental20055416263116311096
- KrentzAJvon MuhlenDBarrett-ConnorESearching for polycystic ovary syndrome in postmenopausal women: evidence of a dose-effect association with prevalent cardiovascular diseaseMenopause2007142849217245231
- KrongradAWilsonCMWilsonJDAndrogens increase androgen receptor protein while decreasing receptor mRNA in LNCaP cellsMolecular and Cellular Endocrinology19917679881820979
- KupelianVPageSTAraujoABLow sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese menJ Clin Endocrinol and Metab2006918435016394089
- LangerCGanszBGoepfertCTestosterone up-regulates scavenger receptor B1 and stimulates cholesterol efflux from macrophagesBiochem Biophys Res Commun20022961051712207878
- LarsenBANordestgaardBGStenderSEffect of T on atherogenesis in cholesterol-fed rabbits with similar plasma cholesterol levelsAtherosclerosis19939979868461063
- LeeHJChangCRecent advances in androgen receptor actionCellular and Molecular Life Sciences20026016132214504652
- LingSDaiADilleyRJEndogenous estrogen deficiency reduces proliferation and enhances apoptosis-related death in vascular smooth muscle cells: insights from the aromatase knock-out mouseCirculation200453943
- LiuPYDeathAKHandelsmanDJAndrogens and Cardiovascular diseaseEndocrine Reviews2003243134012788802
- MalkinCJPughPJJonesTHChannerKSTestosterone for secondary prevention in men with ischaemic heart diseaseQuart J Med2003965219
- MalkinCJJonesRDJonesTHChannerKSEffect of testosterone on ex vivo vascular reactivity in manClin Sci20061112657416722820
- MalkinCJPughPJWestJNTestosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trialEur Heart J200627576416093267
- MarinPHolmangSJonssonLThe effects of testosterone treatment on body composition and metabolism in middle-aged obese menInt J Obes Relat Metab Disord19921699171335979
- MaronBJShiraniJPoliacLCSudden death in young competitive athletes Clinical, demographic and pathological profilesJAMA19962761992048667563
- MeriggiolaMCMarcovinaSPaulsenCATestosterone enanthate at a dose of 200 mg/week decreases HDL-cholesterol levels in healthy menInt J Androl199518237428567093
- MukherjeeTKDinhHChaudhuriGTestosterone attenuates expression of vascular cell adhesion molecule-1 by conversion to estradiol by aromatase in endothelial cells: implications in atherosclerosisPNAS20029940556011904449
- MullerMvan den BeldAWBotsMLEndogenous sex hormones and progression of carotid atherosclerosis in elderly menCirculation20041092074915096452
- NathanLShiWDinhHTestosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromataseProc Natl Acad Sci USA20019835899311248122
- NgMQuinnCMMcCrohonJAAndrogens up-regulate atherosclerosis-related genes in macrophages from males but not females: molecular insights into gender differences in atherosclerosisJ Am Coll Cardiol20034213061314522500
- ObasanjoIOClarksonTBWeaverDSEffects of the anabolic steroid nandrolone decanoate on plasma lipids and coronary arteries of female cynomolgus macaquesMetabolism19964546388609832
- PageSTAmoryJKBowmanFDExogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum TJ Clin Endocrinol Metab20059015021015572415
- ParadisiGSteinbergHOHempflingAPolycystic ovary syndrome is associated with endothelial dysfunctionCirculation200110314101511245645
- PoldermanKHGoorenLJAsschemanHInduction of insulin resistance by androgens and estrogensJ Clin Endocrinol Metab199479265718027240
- PughPJJonesTHChannerKSAcute haemodynamic effects of testosterone in men with chronic heart failureEur Heart J2003249091512714022
- QuigleyCADeBellisAMarschkeKBAndrogen receptor defects: historical, clinical and molecular perspectivesEndocrinology Reviews199516271321
- ReckelhoffJFSex steroids, cardiovascular disease and hypertensionHypertension200545170415583070
- RosanoGMLeonardoFPagnottaPAcute anti-ischemic effect of testosterone in men with coronary artery diseaseCirculation19909916667010190874
- RossRAtherosclerosis- an inflammatory diseaseN Engl J Med1999340115269887164
- RossouwJEPrenticeRLMansonJEWuLPostmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopauseJAMA200729714657717405972
- SeyrekMYildizOUlusoyHBYildrimVTestosterone relaxes isolated human radial artery by potassium channel opening actionJ Pharmocological Sciences200710330916
- SnyderPJPeacheyHBerlinJAEffect of transdermal testosterone treatment on serum lipid and apolipoprotein levels in men more than 65 years of ageAm J Med20011112556011566454
- StellatoRKFeldmanHAHambyOTestosterone, sex hormone binding globulin and the development of type 2 diabetes in middle aged menDiabetes Care200023490410857940
- SvartbergJvon MuhlenDSchirmerHAssociation of endogenous testosterone with blood pressure and left ventricular mass in men: the Tromso StudyEur J Endocrinol2004150657114713281
- TchernofALabrieFBelangerARelationships between endogenous steroid hormone, sex hormone-binding globulin and lipoprotein levels in men: contribution of visceral obesity, insulin levels and other metabolic variablesAtherosclerosis1997133235449298684
- ThomasHDadgarNAphaleAAndrogen receptor acetylation site mutations cause trafficking defects, misfolding, and aggregation similar to an expanded glutamine tractsJournal of Biological Chemistry200427983899514670946
- TodaTTodaYChoBHUltrastructural changes in the comb and aorta of chicks fed excess testosteroneArteriosclerosis1984514753
- TracyRESex differences in coronary disease: two opposing viewsJ Chronic Dis1966191245515966017
- TrapmanJRis-StalpersCvan der KorputJAThe androgen receptor: functional structure and expression in transplanted human prostate tumors and prostate tumor cell linesJournal of Steroid Biochemistry and Molecular Biology199037837422285596
- van KesterenPAsschemenHMegensJAJMortality and morbidity in transsexual subjects treated with cross-sex hormonesClin Endocrinol19974733742
- VermeulenAKaufmanJMDiagnosis of hypogonadism in the aging maleAging Male200252706
- von DehnGvon DehnOVolkerWAtherosclerosis in apolipoprotein E-deficient mice is decreased by the suppression of endogenous sex hormonesHorm Metab Res200133110411294492
- WebbCMAdamsonDLde ZeiglerDEffect of acute testosterone on myocardial ischemia in men with coronary artery diseaseAm J Cardiol199983437910072236
- WhitselEABoykoEJMatsumotoAMIntramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysisAm J Med2001111261911566455
- WiermanMESex steroid effects at target tissues: mechanisms of actionAdvances in Physiology Education200731263317327579
- WoodardTLBurghenGAKitabchiAEWilimasJAGlucose intolerance and insulin resistance in aplastic anemia treated with oxymetholoneJ Clin Endocrinol Metab19815390587026595
- WuFCFarleyTMPeregoudovAWaitesGMEffects of testosterone enanthate in normal men: experience from a multicenter contraceptive efficacy study. World Health Organization Task Force on Methods for the Regulation of Male FertilityFertil Steril199665626368774299
- WuFCvon EckardsteinAAndrogens and coronary artery diseaseEndocrine Reviews20032418321712700179
- YeapBBKruegerRGLeedmanPJDifferential posttranscriptional regulation of androgen receptor gene expression by androgen in prostate and breast cancer cellsEndocrinology199914032829110385425
- ZhouZXKemppainenJAWilsonEMIdentification of three proline-directed phosphorylation sites in the human androgen receptorMolecular Endocrinology19959605157565807
- ZmudaJMCauleyJAKriskaALongitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participantsAm J Epidemiol1997146609179345114