Abstract
While clinical trials demonstrate the benefits of blood pressure and cholesterol reduction, medication adherence in clinical practice is problematic. We hypothesized that a single-pill would be superior to a 2-pill regimen for achieving adherence. In this retrospective, cohort study based on pharmacy claims data, patients newly initiated on a calcium channel blocker (CCB) or statin simultaneously or within 30 days, regardless of sequence, were followed (N = 4703). Adherence was measured over 6 months as proportion of days covered (PDC). At baseline, mean age was 63.0 years, 51.6% were female, and mean number of other medications was 7.8. Overall, 16.9% of patients were on single-pill amlodipine/atorvastatin, 15.6% amlodipine + atorvastatin, 24.7% amlodipine + other statin, 13.9% other CCB + atorvastatin, 28.9% other CCB + other statin. Percentages of patients achieving adherence (PDC ≥ 80%) were: 67.7% amlodipine/atorvastatin; 49.9% amlodipine + atorvastatin; 40.4% amlodipine + other statin; 46.9% other CCB + atorvastatin; 37.4% other CCB +other statin. After adjusting for treatment selection and cohort differences, odds ratios for adherence with amlodipine/atorva-statin were 1.95 (95% confidence interval [CI], 1.80–2.13) vs amlodipine + atorvastatin, 3.10 (95% CI, 2.85–3.38) vs amlodipine + other statin, 2.06 (95% CI, 1.89–2.24) vs other CCB + atorvastatin, 2.85 (95% CI, 2.61–3.10) vs other CCB + other statin (all p <0.0001). Single-pill amlodipine/atorvastatin may provide clinical benefits through improving adherence, offering clinicians a practical solution for cardiovascular risk management.
Introduction
Recent clinical trials and meta-analyses have demonstrated the benefits of antihypertensive and lipid-lowering therapies for the prevention of cardiovascular events (CitationALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group 2002; CitationSever et al 2003; CitationStaessen et al 2003; CitationBaigent et al 2005) and guidelines support an integrated approach to the reduction of cardiovascular risk (CitationExpert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults 2001). However, few patients receive adequate antihypertensive and lipid-lowering treatment in clinical practice (CitationWong et al 2006). For example, only 9% of US adults with hypertension and dyslipidemia are at target levels for both blood pressure and low-density lipoprotein cholesterol (CitationWong et al 2006).
Among the key causes of this treatment gap may be poor patient adherence to cardiovascular medications (CitationWei et al 2002; CitationSokol et al 2005; CitationBramley et al 2006; CitationBurnier 2006; CitationHo et al 2006; CitationKulkarni et al 2006). Several studies have observed that patient adherence to antihypertensive and/or lipid-lowering therapy is low (CitationBenner et al 2002; CitationBlackburn et al 2005; CitationChapman et al 2005). The use of single-pill combination therapy may improve adherence by synchronizing therapies, so that medications are initiated at the same time (CitationChapman et al 2005; CitationAgarwal et al 2008), and by reducing pill burden (CitationDezii 2000; CitationVanderpoel et al 2004; CitationChapman et al 2005; CitationGerbino and Shoheiber 2007) and out-of-pocket costs to the patient (CitationPiette et al 2004; CitationSoumerai et al 2006; CitationThiebaud et al 2008).
A single-pill therapy combining the antihypertensive medication, amlodipine besylate, and the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor (statin), atorvastatin calcium, is the first fixed-dose combination therapy for the treatment of 2 major risk factors for cardiovascular disease (CitationBlank et al 2005). This study sought to test the hypothesis that this single-pill therapy would be superior to a multi-pill antihypertensive and lipid-lowering treatment regimen in the achievement of adherence.
Methods
Study design
This adherence study was a retrospective, longitudinal, cohort study of a diverse, insured, nationally representative US population. Pharmacy claims were used to identify patients using calcium channel blocker (CCB) and statin therapy during the 16-month study period between April 1, 2004 and July 30, 2005.
Data source
This study utilized national pharmacy administrative data from MedImpact Healthcare Systems, Inc. (San Diego, CA, USA) (CitationSullivan et al 2005; CitationThiebaud et al 2005, Citation2006, Citation2008; CitationPatel et al 2007). MedImpact provides pharmacy benefit management services to plan sponsors, including employer corporations, unions, managed care organizations, health plans, insurance carriers, third-party administrators, as well as local, state, and federal employee programs.
Patient selection
Patients included in the analysis were required to be ≥18 years of age and continuously enrolled in a plan sponsor throughout the study period. Patients were newly started on either a CCB or a statin during the study period, and filled prescriptions for both a CCB and statin simultaneously or within 30 days of each other, irrespective of initiation sequence. Five cohorts were specified depending on their CCB and statin therapy: 1. amlodipine/atorvastatin (single pill); 2. amlodipine + atorvastatin; 3. amlodipine + other statin (not including atorvastatin); 4. atorvastatin + other CCB (not including amlodipine); 5. other CCB + other statin (not including atorvastatin or amlodipine). The index date was defined as the first amlodipine/atorvastatin prescription for the single-pill combination cohort. For patients taking CCB + statin treatments as 2 pills, the index date was the prescription claim fill date of the second medication class. Patients were followed for 180 days from the index date to calculate study outcomes. In an additional analysis, patient adherence at 1 year was evaluated for a subset of patients from the primary analysis who had at least 1 full year of follow-up data. Patients’ pharmacy utilization in the 180 days prior to the index date was used to calculate baseline measures such as comorbidity indices.
Patient and regimen characteristics
Age and gender were collected for each patient, as well as several variables describing the patient’s pharmacy benefit and medication-taking history, as described below. Business type included commercial managed care organization (MCO), managed Medicare plans, managed Medicaid plans, and self-insured payers. Formulary type defined the pharmacy benefit plan as either open formulary, where all medications are available under a plan sponsor’s pharmacy benefit and members would pay a co-payment amount, or a closed formulary, where selected medications are not covered and patients would incur total out-of-pocket cost for these medications. Number of drugs used at baseline was defined by the unique number of medications utilized. Combination tablets including more than 1 drug were only counted as a single medication in this measure. Other specific medications considered at baseline were the continuing study medications (CCB or statin), angiotensin-converting enzyme inhibitors, beta-blockers, diuretics, angiotensin receptor blockers, coronary vasodilators, digoxin, platelet aggregation inhibitors, nitrates, antidiabetic agents, and antidepressants. Adherence patterns at baseline were estimated using prescription use prior to the index date. First DataBank (CitationFirst DataBank Inc. 2006) classification methodology was used to identify medications for chronic conditions. The prescription claims for these medications are flagged as being either new or refill prescriptions. Using these data, a proxy measure for prior adherence was obtained by calculating: (number of refills)/(number of refills + new claims), where patients with a greater proportion of refills for chronic medications are deemed more compliant at baseline.
Outcomes of interest
The primary outcome of interest was adherence, measured using the proportion of days covered (PDC). The PDC is the proportion of days in the study period that the treatment regimen is available to the patient, as observed from pharmacy claims data (CitationBenner et al 2002; CitationChapman et al 2005; CitationHo et al 2006). A ‘covered day’ for the purposes of the PDC requires the patient to have access to both drugs on a given day during the observation period (ie, for the 2-pill treatment cohorts, a day covered on both therapies is required). Using this method, in scenarios where an overlap in medication refills exists, the claims and respective days supply were shifted forward in the corresponding amount of overlap. Days supply falling beyond the 180-day study period were truncated and not used in the PDC calculation. For these cohorts, the days supply associated with switching to a different statin or CCB medication was credited to the initial respective study cohorts. Patients were considered adherent if PDC was ≥80% (over 180 days for the main analysis, or 360 days for the 1-year analysis) (CitationBenner et al 2002; CitationChapman et al 2005; CitationHo et al 2006).
A secondary outcome was non-persistence or discontinuation of therapy, measured as the number of days until the first 30-day gap in therapy (CitationLarsen et al 2002). A sensitivity analysis was undertaken using the medication possession ratio (MPR), calculated as the average of the days supplied with a CCB and the days supplied with a statin divided by the study period (patients were considered adherent if MPR ≥80%) (CitationMcCombs et al 1994; CitationHalpern et al 2006). It should be noted that the definitions of PDC and MPR for the measurement of adherence with multiple drugs have not been firmly established in the literature. However, for the purposes of our study, MPR differed to the PDC measurement in that patients did not need to have access to both drugs on the same day to be considered adherent.
Statistical analyses
The primary end point was the adjusted likelihood of achieving 80% adherence with amlodipine/atorvastatin vs other study cohorts. The secondary end point was the adjusted hazard of discontinuing therapies with amlodipine/atorvastatin vs other study cohorts.
Data extraction and statistical analyses were performed using SAS® software, Version 9.1.3 (SAS Institute, Inc, Cary, NC, USA). T-tests, F-tests, or Chi-square tests were used to check for differences in baseline characteristics and outcomes between the amlodipine/atorvastatin cohort and all other cohorts. Adjustments for differences as observed in were performed using a propensity-score weighting technique to balance treatment groups and address the potential for treatment selection bias due to non-random assignment of treatment by physicians (CitationD’Agostino and Kwan 1995; CitationD’Agostino 1998; CitationHirano and Imbens 2001; CitationD’Agostino and D’Agostino 2007; CitationSchneeweiss et al 2008). Propensity score weighting was selected based on a recent comparative simulation that showed this method to be more robust at minimizing bias than other risk adjustment methods, even if the relationship between the observed outcomes and the treatment assignment mechanism was misspecified (CitationLunceford and Davidian 2004). To apply this method, the propensity score was calculated as the probability of patients receiving each treatment, conditional on the potential confounders listed in . A propensity score weight was created as the inverse of the propensity score. The primary outcome, odds ratio for achieving adherence ≥80%, was calculated using logistic regression, controlling for the 15 confounders as well as weighting each observation by the propensity score. Therefore, the propensity score weighting addresses the factors associated with the treatment selection process, and the multivariable logistic regression model measured the effect of treatment on adherence while controlling for baseline confounders.
Table 1 Patient baseline demographics
Adjusted results are presented as the odds ratio (OR) with 95% confidence intervals (CI) for the adherence outcome, and as the hazard ratio (HR) with 95% CI for the persistence outcome. In all analyses differences were considered significant at the level of p < 0.05.
Results
Study population
A total of 4703 patients met the study criteria. At baseline, the mean age was 63.0 years, 51.6% of patients were female, 10.7% took nitrate/coronary vasodilators, and 29.6% took antidiabetic medications. The mean number of baseline medications was 7.8. These characteristics varied between the 5 cohorts; patients in the amlodipine/atorvastatin cohort were younger than those in the other cohorts, and more were male. The amlodipine/atorvastatin cohort also appeared to be healthier than other cohorts, utilizing fewer drugs at baseline, eg, a lower proportion received angiotensin-converting enzyme inhibitors, diuretics, coronary vasodilators, digoxin, antidiabetic agents, and antidepressants (). The majority of all patients were enrolled in an MCO (56.4%–81.0%). Patients in the other CCB + atorvastatin cohort, and those in the amlodipine + atorvastatin cohort were slightly more likely to be Medicaid enrollees, while those patients in the other CCB + other statin cohort were more likely to be Medicare enrollees. Patients receiving amlodipine/atorvastatin were more likely to be enrolled in a self-insured employer population.
Unadjusted mean adherence levels
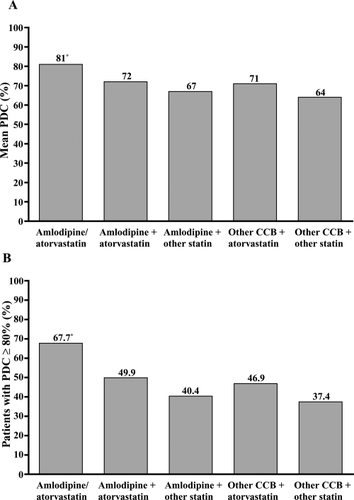
Pair-wise comparisons of unadjusted mean adherence levels showed significant differences between single-pill amlodipine/atorvastatin and all other cohorts. Adherence for amlodipine/atorvastatin patients was on average 9%–17% higher compared with the other cohorts (). The proportion of patients achieving adherence (PDC ≥80%) was higher in the amlodipine/atorvastatin cohort than in the other 4 cohorts ().
Adjusted proportion of patients achieving adherence
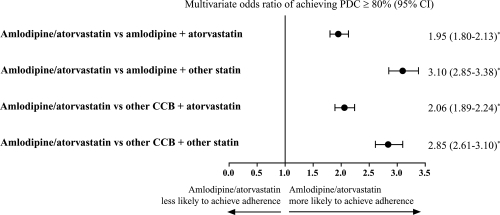
Adjustments were made for baseline cohort differences using the propensity score weighting models to account for potential treatment selection bias at baseline. Chi-square and ANOVA tests confirmed that the differences between cohorts on the 15 variables were not significantly different following adjustment. After adjustments at 180 days follow-up, patients taking single-pill amlodipine/atorvastatin were approximately twice as likely to be adherent as patients taking amlodipine + atorvastatin separately (OR, 1.95; 95% CI, 1.80–2.13, p < 0.0001; ). Additionally, patients taking amlodipine/atorvastatin were between 2.06 and 3.10 times more likely to be adherent than patients taking any of the other 3 drug treatment regimens (amlodipine + other statin, other CCB + atorvastatin, and other CCB + other statin; p < 0.0001; ).
Figure 2 Adjusted probability of achieving adherence (PDC ≥ 80%) at 180 days’ follow-up. Logistic regression model analysis adjusting for covariates including age, gender, business type, formulary type, baseline antihypertensive therapy, cardiovascular disease medications, antidiabetic medications, antidepressants, number of drugs, co-payments, and maintenance medication refill percentage.
*p < 0.0001 for group comparison parameter estimate in the regression.
Non-persistence/therapy discontinuation
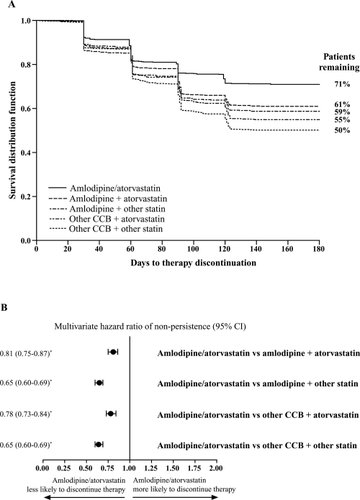
At 180 days, 71% of single-pill amlodipine/atorvastatin patients remained on therapy in comparison with 61% of patients receiving amlodipine and atorvastatin as separate pills and 50%–59% of patients receiving the other treatment combinations (). After adjusting for covariates, patients taking amlodipine/atorvastatin were 19% less likely to discontinue therapy than those taking atorvastatin + amlodipine separately (HR, 0.81; 95% CI, 0.75–0.87, p < 0.0001). Patients in the other 2-pill regimen cohorts were between 22% and 35% more likely to discontinue therapy compared with the amlodipine/atorvastatin cohort ().
Figure 3 (A) Estimated unadjusted Kaplan-Meier curves for time to therapy discontinuation, and (B) adjusted hazard ratio of discontinuing therapies. Proportional hazard model analysis adjusting for covariates including age, gender, business type, formulary type, baseline antihypertensive therapy, cardiovascular disease medications, antidiabetic medications, antidepressants, number of drugs, co-payments, and maintenance medication refill percentage.
*p < 0.0001 for group comparison parameter estimate in the regression.
Patient adherence after 1 year of therapy
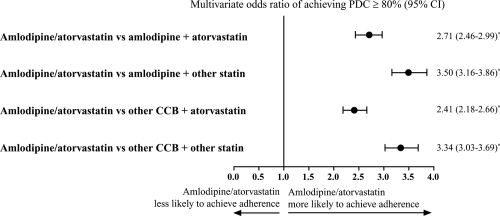
In a subset analysis, patient adherence was evaluated over 1 year among patients who were enrolled for an additional 6 months’ follow-up (N = 3561; 24% drop in patient count from the original cohort). The baseline characteristics for this subset of patients were comparable to the overall patient population. At 1 year, in the single-pill amlodipine/atorvastatin cohort, 63.9% of patients achieved adherence. In comparison, the proportion of patients on a 2-pill regimen who were adherent with both antihypertensive and lipid-lowering medications ranged from 33.1% to 43.6%. After adjusting for cohort differences at baseline for this 1-year subgroup, patients given single-pill amlodipine/atorvastatin were found to be approximately 3-times more likely to achieve adherence over 1 year of follow-up ().
Figure 4 Adjusted probability of achieving adherence (PDC ≥ 80%), over 1-year follow-up. Logistic regression model analysis adjusting for covariates including age, gender, business type, formulary type, baseline antihypertensive therapy, cardiovascular disease medications, antidiabetic medications, antidepressants, number of drugs, co-payments, and maintenance medication refill percentage.
*p < 0.0001 for group comparison parameter estimate in the regression.
Sensitivity analysis
In order to determine the effect of the method of measuring adherence used on the observed differences in adherence between patients receiving single-pill amlodipine/atorvastatin and the other treatment cohorts, we conducted a sensitivity analysis for which we used MPR in place of PDC as the measurement of adherence. This analysis found a consistent trend for greater adherence with amlodipine/atorvastatin vs 2-pill CCB + statin regimens at both 6 months (adjusted ORs, 1.19–1.82, p < 0.0001) and 1 year (adjusted ORs, 1.59–1.92, p < 0.0001). However, the magnitude of the adherence benefit was smaller here than with the PDC measurement.
Discussion
This adherence study demonstrated that patients taking single-pill combination amlodipine/atorvastatin were more likely to be adherent and persistent with therapy compared with patients taking CCBs and statins as 2 separate pills, over both 6 months and 1 year.
The probability of achieving adherence using single-pill amlodipine/atorvastatin was 1.95-times greater than with the parent compounds amlodipine and atorvastatin administered separately (p < 0.0001), and ranged from 2.06- to 3.10-times greater than for any of the other CCB and statin regimens studied (p < 0.0001). For the additional 1-year analysis, patients receiving amlodipine/atorvastatin were 2.41- to 3.50-times more likely to achieve adherence than patients on a 2-pill CCB and statin regimen. For the comparison of each of the 2-pill regimen cohorts with amlodipine/atorvastatin, the magnitude of the adherence difference was greater after 1-year follow-up (for those patients remaining on therapy over this time) than at 6 months. Furthermore, patients taking single-pill amlodipine/atorvastatin were less likely to discontinue therapies compared with those taking the parent compounds or other CCB and statin combinations.
The adherence benefit of single-pill amlodipine/atorvastatin may at least in part be due to the reduction in pill burden, and the synchronization of therapy initiation, given that previous observations have shown these factors to increase patient adherence to multiple therapies (CitationDezii 2000; CitationVanderpoel et al 2004; CitationChapman et al 2005; CitationGerbino and Shoheiber 2007).
In order to achieve maximum benefits in terms of clinical outcomes, it is important that clinicians consider patients’ global cardiovascular risk, and address the management of all coexisting cardiovascular risk factors (CitationExpert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults 2001; CitationSever et al 2003; CitationLopez et al 2007). This is reflected in current guidelines and recommendations aimed at reducing the risk of cardiovascular disease in the population (CitationExpert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults 2001). In particular, the use of both antihypertensive and statin therapies for the management of concomitant hypertension and dyslipidemia, or hypertension in the presence of multiple cardiovascular risk factors, is recognized as providing significant benefits for lowering the risk of cardiovascular events (CitationHeart Protection Study Collaborative Group 2002; CitationSever et al 2003). However, lack of patient adherence to multiple therapies compromises this treatment strategy and is associated with poor clinical outcomes (CitationBenner et al 2002; CitationWei et al 2002; CitationBlackburn et al 2005; CitationChapman et al 2005; CitationSokol et al 2005; CitationBramley et al 2006; CitationBurnier 2006; CitationHo et al 2006; CitationKulkarni et al 2006). As a result, concerns that increasing a patient’s pill burden will decrease adherence may discourage physicians from adding further medications to a patient’s existing regimen, despite potential therapeutic benefits. The combination of amlodipine and atorvastatin in a single pill may help to allay such concerns with regard to the addition of statin therapy in hypertensive patients with high cardiovascular risk, or an antihypertensive in those with elevated blood pressure who are already receiving a statin. In support of this, the present study provides preliminary indications that single-pill amlodipine/atorvastatin may help address issues of poor adherence to antihypertensive and lipid-lowering medications. Additional studies assessing whether increased adherence and persistence with single-pill amlodipine/atorvastatin translates to improved attainment of blood pressure and lipid goals and, more importantly, a reduced incidence of cardiovascular events and improved health outcomes for the patient, are warranted.
The study limitations include the potential for bias that may have occurred due to the retrospective and non-randomized nature of the design, and that possible drug treatment selection bias may not be fully taken into account by the propensity weighting methodology used. However, Chi-square and ANOVA tests of differences between cohorts on the 15 independent variables used for adjustment found no significant differences after adjustment methods were applied. Indications for single-pill amlodipine/atorvastatin may include an expected lack of adherence to medications, which may be a confounding factor in this analysis, although previous adherence patterns were included as a covariate for propensity score weight. In addition, while patients in the single-pill cohort were younger, took fewer baseline medications, and were more likely to be enrolled in an MCO than in the other cohorts (characteristics which could potentially influence the likelihood of being adherent) these factors were also adjusted for in the proportional hazards analysis. Limited variables were available due to the lack of data related to medical diagnoses and severity of illness.
Other important confounding factors that could not be considered in this administrative claims analysis are the quality of care, intensity of counseling, patient’s perception and beliefs towards the effectiveness of treatment (ie, potential for self-efficacy), the health care resources available, and the effect on clinical end points (eg, blood pressure and cholesterol). However, this study was specifically designed to assess differences in observed adherence between treatment regimens in a real-world setting, and was not intended to assess the influence of these other factors.
Assessing adherence based on prescription refill rates is a proxy measure only and a patient’s true pattern of medication taking may still be unknown. In addition, the timing of prescription refill patterns was assumed to correlate with medication consumption, which may amplify the magnitude of adherence differences observed using PDC vs MPR. However, it has been noted that most studies of the validity of prescription refill rates have shown that measures of prescription refill rates are significantly associated with other measures of adherence, serum drug levels, or physiologic drug effects (CitationSteiner and Prochazka 1997). The observed benefits on adherence of single-pill amlodipine/atorvastatin compared with 2-pill CCB and statin regimens were consistent with the sensitivity analysis using the MPR as an alternative method of measuring adherence, although the magnitude of the difference was lower.
Despite the limitations detailed above, the benefits on adherence of single-pill amlodipine/atorvastatin compared with a 2-pill CCB and statin regimen were robust in that they were observed using 2 methods of measurement (PDC and MPR), at 2 points in time, and are consistent with the evaluation of persistence. Given the high level of significance observed in these comparisons, additional statistical adjustments for multiple comparisons were not necessary as they would have remained significant under such adjustments.
Following the findings from this study, physicians who prescribe antihypertensive and lipid-lowering therapies for the management of cardiovascular risk should consider the potential benefits of therapy adherence that may be afforded by a single-pill, such as amlodipine/atorvastatin, as a means of simplifying their patient’s treatment regimen, reducing pill burden, and synchronizing antihypertensive and statin therapy (CitationBangalore et al 2007). Single-pill amlodipine/atorvastatin therapy provides a potential opportunity for improving adherence over a multi-pill treatment regimen, although further studies are required to determine whether this will result in improvements in therapeutic goal attainment and patients’ long-term clinical outcomes, and in lower health-service utilizations.
Acknowledgements
This study was sponsored by Pfizer Inc. MedImpact Healthcare Systems, Inc. was primarily responsible for the study design, data analysis, and interpretation of the study, and received funding from Pfizer Inc. Bimal Patel at MedImpact was a paid consultant to Pfizer Inc in relation to this study. Editorial support was provided by Jon Edwards and Elizabeth Harvey of Envision Pharma, and funded by Pfizer Inc.
Disclosures
Bimal V Patel: consultancy fees from Pfizer Inc. R Scott Leslie and Patrick Thiebaud: no conflicts of interest. Michael B Nichol: research grants and consultancy fees from Pfizer Inc. Simon SK Tang, Henry Solomon, and Dennis Honda: employees of, and stock options in, Pfizer Inc. JoAnne M Foody: Speaker’s Bureau for Merck, Merck-Schering Plough, Millennium Pharmaceuticals, Novartis, and Pfizer Inc, and consultancy fees from Merck, Merck-Schering Plough, and Pfizer Inc.
References
- AgarwalSTangSSKRosenbergNDoes synchronizing initiation of therapy affect adherence to concomitant use of antihypertensive and lipid-lowering therapy?Am J Ther2008In press
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research GroupMajor outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)JAMA200228829819712479763
- BaigentCKeechAKearneyPMEfficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statinsLancet200536612677816214597
- BangaloreSKamalakkannanGParkarSFixed-dose combinations improve medication compliance: a meta-analysisAm J Med2007120713917679131
- BennerJSGlynnRJMogunHLong-term persistence in use of statin therapy in elderly patientsJAMA20022884556112132975
- BlackburnDFDobsonRTBlackburnJLAdherence to statins, beta-blockers and angiotensin-converting enzyme inhibitors following a first cardiovascular event: a retrospective cohort studyCan J Cardiol200521485815917876
- BlankRLaSalleJReevesRSingle-pill therapy in the treatment of concomitant hypertension and dyslipidemia (the amlodipine/atorvastatin Gemini study)J Clin Hypertens (Greenwich)200572647315886529
- BramleyTJGerbinoPPNightengaleBSRelationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizationsJ Manag Care Pharm2006122394516623608
- BurnierMMedication adherence and persistence as the cornerstone of effective antihypertensive therapyAm J Hypertens2006191190617070434
- ChapmanRHBennerJSPetrillaAAPredictors of adherence with antihypertensive and lipid-lowering therapyArch Intern Med200516511475215911728
- D’AgostinoRBJrPropensity score methods for bias reduction in the comparison of a treatment to a non-randomized control groupStat Med1998172265819802183
- D’AgostinoRBJrD’AgostinoRBSrEstimating treatment effects using observational dataJAMA2007297314617227985
- D’AgostinoRBKwanHMeasuring effectiveness. What to expect without a randomized control groupMed Care199533AS951057723466
- DeziiCMA retrospective study of persistence with single-pill combination therapy vs. concurrent two-pill therapy in patients with hypertensionManag Care200092611729417
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in AdultsExecutive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III)JAMA200128524869711368702
- First DataBank IncNDDF File2006San Bruno, CAThe Hearst Corporation
- GerbinoPPShoheiberOAdherence patterns among patients treated with fixed-dose combination versus separate antihypertensive agentsAm J Health Syst Pharm20076412798317563050
- HalpernMTKhanZMSchmierJKRecommendations for evaluating compliance and persistence with hypertension therapy using retrospective dataHypertension20064710394816651464
- Heart Protection Study Collaborative GroupMRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trialLancet200236072212114036
- HiranoKImbensGWEstimations of causal effects using propensity score weighting: an application to data on right heart catheterizationHealth Serv Outcome Res Meth2001225978
- HoPMRumsfeldJSMasoudiFAEffect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitusArch Intern Med200616618364117000939
- KulkarniSPAlexanderKPLytleBLong-term adherence with cardiovascular drug regimensAm Heart J20061511859116368315
- LarsenJAndersenMKragstrupJHigh persistence of statin use in a Danish population: compliance study 1993–1998Br J Clin Pharmacol200253375811966668
- LopezVAFranklinSSTangSCoronary heart disease events preventable by control of blood pressure and lipids in US adults with hypertensionJ Clin Hypertens (Greenwich)200794364317541329
- LuncefordJKDavidianMStratification and weighting via the propensity score in estimation of causal treatment effects: a comparative studyStat Med20042329376015351954
- McCombsJSNicholMBNewmanCMThe costs of interrupting antihypertensive drug therapy in a Medicaid populationMed Care199432214268145599
- PatelBVRemigio-BakerRAMehtaDEffects of initial antihypertensive drug class on patient persistence and compliance in a usual-care setting in the United StatesJ Clin Hypertens (Greenwich)2007969270017786070
- PietteJDHeislerMWagnerTHCost-related medication underuse among chronically ill adults: the treatments people forgo, how often, and who is at riskAm J Public Health2004941782715451750
- SchneeweissSSeegerJDLandonJAprotinin during coronary-artery bypass grafting and risk of deathN Engl J Med20083587718318287600
- SeverPSDahlöfBPoulterNRPrevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trialLancet200336111495812686036
- SokolMCMcGuiganKAVerbruggeRRImpact of medication adherence on hospitalization risk and healthcare costMed Care2005435213015908846
- SoumeraiSBPierre-JacquesMZhangFCost-related medication nonadherence among elderly and disabled medicare beneficiaries: a national survey 1 year before the medicare drug benefitArch Intern Med200616618293517000938
- StaessenJAWangJGThijsLCardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003J Hypertens20032110557612777939
- SteinerJFProchazkaAVThe assessment of refill compliance using pharmacy records: methods, validity, and applicationsJ Clin Epidemiol199750105169048695
- SullivanPWNairKVPatelBVThe effect of the Rx-to-OTC switch of loratadine and changes in prescription drug benefits on utilization and cost of therapyAm J Manag Care2005113748215974556
- ThiebaudPPatelBVNicholMBImpact of rofecoxib withdrawal on cyclooxygenase-2 utilization among patients with and without cardiovascular riskValue Health20069361817076866
- ThiebaudPPatelBVNicholMBThe demand for statin: the effect of copay on utilization and complianceHealth Econ200817839717585395
- ThiebaudPPatelBVNicholMBThe effect of switching on compliance and persistence: the case of statin treatmentAm J Manag Care200511670416268750
- VanderpoelDRHusseinMAWatson-HeidariTAdherence to a fixed-dose combination of rosiglitazone maleate/metformin hydrochloride in subjects with type 2 diabetes mellitus: a retrospective database analysisClin Ther20042620667515823770
- WeiLWangJThompsonPAdherence to statin treatment and readmission of patients after myocardial infarction: a six year follow up studyHeart2002882293312181210
- WongNDLopezVTangSPrevalence, treatment, and control of combined hypertension and hypercholesterolemia in the United StatesAm J Cardiol200698204816828593