Abstract
The effect of thiazolidinediones (TZDs) on the progression of atherosclerosis in diabetes patients remains unclear. There has been heightened interest in recent years in this class of diabetes medications due to the non-glycemic lowering effects, such as altering lipids, inflammation and hematologic profiles. There have been several exciting studies over the past few years focused on the mechanism of action of the TZDs with respect to alteration in the cardio-metabolic profile in diabetes patients. New tools such as intravascular ultrasound have been used to follow plaques characteristics over time on a much more sensitive scale than has ever been possible in the past by coronary angiograms. These advances have enabled researchers to follow closely the macrovascular effects of different anti-atherosclerotic medications such as statins and TZDs. This article reviews the pathophysiology of atherosclerosis in diabetes, the role that TZDs play in this process and the imaging trials looking at the progression or regression of atherosclerosis in patients treated with TZDs.
Introduction
The purpose of this review is to address many of the controversies surrounding the affects of glitazones and coronary atherosclerosis. The impact of thiazolidinediones (TZDs) and their ability to alter the progression of atherosclerosis in diabetics remains unclear. As the prevalence of diabetes increases, the burden of atherosclerosis is increasing at an alarming rate. The Center for Disease Control (CDC) now projects that by 2050 nearly 50 million people will be diagnosed with diabetes, up from their prior estimate of 39 million released just 2 years earlier. The largest impact will be seen in minority groups, with an expected increase of 481% in Hispanics and 208% in blacks.Citation1 An analysis of the Framingham Heart Study by Fox et al demonstrated that the lifetime risk for cardiovascular disease is increased among individuals with obesity and diabetes, with a 78.8% lifetime risk in women and an 86.9% risk in men.Citation2 Overall, obesity alone was found to carry an additional 20% risk for the development of cardiovascular disease (CVD), compared to normal weight individuals. Prior studies have estimated that the lifetime risk of diabetes increases in proportion to BMI, ranging from 7.6% among underweight individuals to as high as 74.4% among individuals with stage 2 obesityCitation3 ().
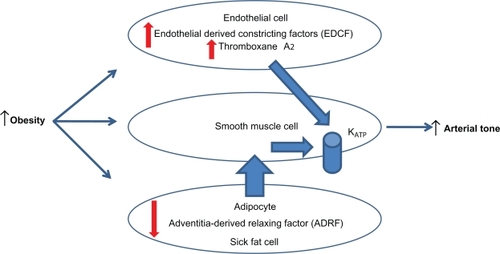
Figure 1 Endothelial dysfunction-obesity hypothesis.Citation10
Patients with diabetes have a significant increase in the risk of advanced cardiovascular disease. Over a period of 18 years, Juutilainen et al studied 2301 patients with and without diabetes who were all free of CVD at baseline.Citation4 50% of subjects with type 1 diabetes, 68% of subjects with type 2 diabetes, and 19% of nondiabetes subjects died during the follow-up period. The adjusted hazard ratio for type 1 diabetics vs nondiabetics was 3.6 (95% CI 2.2–5.7) in men and 13.3 (95% CI 6.9–22.5) in women. For type 2 diabetics vs nondiabetics, the adjusted hazard ratio was 3.3 (95% CI 2.5–4.5) in men and 10.1 (95% CI 6.7–17.4) in women. Similar results were found by the Renfrew Paisley survey with 25 years of follow-up of 15,406 patients.Citation5 Goraya et al in a population based autopsy study, noted similar cardiovascular autopsy findings in non-diabetes patients with known ischemic heart disease compared to diabetes patients with no known ischemic heart disease, further emphasizing the role of diabetes as a coronary artery disease risk factor equivalent.Citation6
Given these findings, there has been much interest in altering the inherent CV risk in diabetics through alteration of their metabolic profile. TZDs have been of particular interest due to the recognized pleiotrophic effects above and beyond lowering plasma glucose levels. In this article, we will review the effect of diabetes in the pathobiology of atherosclerosis, molecular and vascular biology of TZDs, as well as the clinical imaging studies looking at progression or regression of atherosclerosis in patients treated with glitazones.
Pathobiology of atherosclerosis
Mechanisms leading to increased atherosclerotic risk in patients with diabetes continue to be discovered. Obesity frequently precedes the development of insulin resistance and diabetes.Citation7 High fat diets in animal models potentiate endothelial derived contracting factor (EDCF) mediators leading to the formation of increased levels of free radicals and pronounced up regulation of vascular thromboxane prostanoid receptor gene. These two changes weaken the protective role of the endothelium with reduced nitric oxide production and enhanced responsiveness to endothelin.Citation8,Citation9,Citation10 Summarizing these findings, Traupe et al advocate that obesity related increases in production of EDCFs may contribute to the development of vascular diseases. As insulin resistance advances and patients develop diabetes, comparable vascular abnormalities are present at the endothelial levelCitation11,Citation12 (see ).
Subjects with type 2 diabetes mellitus are at high risk for the development of atherosclerosis. An essential event in the growth of atherosclerosis is macrophage foam cell formation. Burke et al studied the morphology of diabetes plaques postmortem and found a strong positive association of increased macrophage infiltrate that was independent of cholesterol levels and patient age.Citation13 The necrotic core size or macrophage infiltrate was significantly increased in diabetic subjects compared to nondiabetics with or without hyperlipidemia (P < 0.002). In addition, the removal of cholesterol by macrophages plays a vital role in macrophage foam cell. Mauldin et al studied blood samples collected from diabetes patients for regulation of cholesterol efflux by ATP-binding cassette (ABC) transporters ABCA1 and ABCG1. Macrophages from subjects with type 2 diabetes mellitus had a 30% diminution in cholesterol efflux to high density lipoprotein (HDL) or Apo A1 with an analogous 60% increase in cholesterol accumulation relative to control subjects. This reduction in cholesterol efflux in type 2 diabetes patients is additive to the already amplified risk of atherosclerosis.
Intravascular ultrasound studies (IVUS) from Hong et al found that diabetes patients presenting with acute coronary syndrome (ACS), had a higher incidence of multiple plaque ruptures (60% vs 29% non-diabetes, P < 0.001) and thrombus (72% vs 52% non-diabetes, P < 0.032) by IVUS than non-diabetes patients. A significant correlation with increased necrotic core volumes and thin cap fibroatheroma in the diabetes subset.Citation14
Basic research is very important and its ties to clinical practice always are highly sought after. Recently, the ATHEROMA trial studied macrophages using iron oxide in humans given atorvastatin.Citation15 Historically, increases in macrophage infiltration increase the risk of plaque rupture consequently detecting macrophage activity and inflammation within the atheroma could help discriminate stable plaque from vulnerable plaques. To evaluate macrophage activity, Tang et al randomized 47 patients with carotid stenosis by carotid ultrasound who had plaque accumulation on MRI to atorvastatin 10 mg or 80 mg for 12 weeks. The primary end point definition was a change from baseline in signal intensity on the ultrasmall superparamagnetic iron-oxide-(USPIO)-enhanced MRI. Twenty patients completed the study, finding a significant reduction from baseline in signal intensity, the USPIO-enhanced MRI definition of plaque inflammation, in the high-dose atorvastatin arm at 6 and 12 weeks, while the patients treated with atorvastatin 10 mg showed no significant difference. This type of research is extremely important to further understanding of types of treatments that may regress plaque and reduce clinical events.
In summary, histology, metabolic changes and imaging studies all point toward a much higher risk for atherosclerosis development and CVD risk in patients with diabetes.
Molecular and vascular biology of glitazones
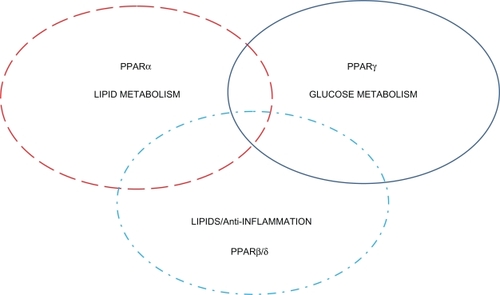
By definition the TZDs (glitazones) act by binding to peroxisome proliferator-activated receptors (PPARs), a group of receptor molecules inside the cell nucleus, specifically PPARγ/α/β/δ. The ligands for these receptors are free fatty acids (FFAs) and eicosanoids. When activated, the receptor migrates to the DNA, activating transcription of a number of specific genes. These nuclear changes have far reaching affects on metabolic status in patients. Issemann and Green discovered the mechanism by which peroxisome proliferation in the liver was induced by hypolipidemic drugs.Citation16 The discovery of this mechanism in 1990 led to the rapid development of many compounds in an attempt to improve the metabolic profile of primarily diabetes patients ().
Figure 2 Peroxisome proliferative activated receptors (PPARs) clinical overview. This figure shows the overlapping activity of the PPARs. Clinically the current glucose-lowering agents and insulin sensitizers are PPARγ. PPARα compounds are primarily fibrates that reduce triglyceride levels and increase high-density lipoprotein cholesterol. PPARβ/δ is still experimental.
In general PPARα agonist (fibrates) improved the dyslipidemic profile in patients by lowering triglycerides (TG) and increasing HDL. Unfortunately, large pivotal trails (Fenofibrate Intervention and Event Lowering in Diabetes (FIELD)) did not demonstrate positive primary endpoints, although secondary cardiovascular endpoints were more favorable.Citation17 Fenofibrate failed to significantly reduce the risk of the primary outcome, coronary events. It did reduce total cardiovascular events, mainly due to fewer non-fatal myocardial infarctions and revascularizations. The placebo arm had an increased rate of statin initiation, which might have masked a moderately larger treatment benefit.
Other basic science studies have found that fibrates could increase plasma homocysteine levels that occur through a PPARα-dependent mechanism and could reduce hepatic apo-AI production, by decreasing PPARα activity.Citation18,Citation19 On the contrary other indirect beneficial effects of PPARα are suggestive from pioglitazone in the PROactiveCitation36 and PERISCOPE studies that demonstrated increases in HDL with reduction in triglycerides.Citation20 In a head-to-head clinical trial and meta-analysis, pioglitazone decreased TG significantly while this was not seen with rosiglitazone.Citation21,Citation22 Recently reported, pioglitazone increased IkBa expression in a PPARα-dependent manner potentially increasing expression of PPARα target genes in subcutaneous fat.Citation23,Citation24 Rosiglitazone had no effects on PPARα activation.Citation22 However rosiglitazone has shown a reduction in restenosis after coronary artery stenting and MMP-9 in type 2 diabetes patients.Citation25,Citation26
PPAR gamma ligands (thiazolidinediones (TZDs)) are the primary drugs used in type 2 diabetes for improving insulin sensitivity in skeletal muscle. The beneficial effects of TZDs on insulin sensitization effects are via the activation of PPARγ in adipose tissue, resulting in improved insulin sensitivity in skeletal muscle and liver. Human studies support PPARγ mRNA expression in human skeletal muscle is acutely regulated by insulin and is augmented in both obese non-diabetes and type II diabetes subjects in direct relation to BMI and fasting insulin levels.Citation27 These abnormalities of PPARγ are in part involved in skeletal muscle insulin resistance of obesity and type II diabetes. PPARγ induces subcutaneous adipocyte differentiation and reduces the visceral-to-subcutaneous adipose tissue ratio, which help to store free fatty acids in a less harmful subcutaneous location.
PPARβ/δ is a new exciting area of research. This PPAR isoform has only recently been studied because of its ubiquitous nature. Its major efforts are focused in lipid uptake and anti-inflammatory roles. In several animal models of obesity and diabetes, these compounds increase HDL cholesterol and decrease white adipose tissue fat deposits. They have been found to reduce triglycerides, small dense LDL, and improve fasting insulin.Citation28,Citation29
In summary, in vitro research suggests that PPARs exert anti-atherogenic effects by inhibiting the expression of pro-inflammatory genes and enhancing cholesterol efflux via activation of the liver X receptor–ABCA1 (LXR-ABCA1) pathway. Foam cell formation is a major therapeutic target in atherosclerosis. Basic cell research by Li et al found that PPARα and PPARγ agonists inhibited foam-cell formation in vivo through distinct ABCA1-independent pathways with stimulation of HDL cholesterol efflux.Citation30 These findings from basic science would suggest a clinical benefit of TZDs in reducing atherosclerosis.
Glitazones and CV imaging of atherosclerosis
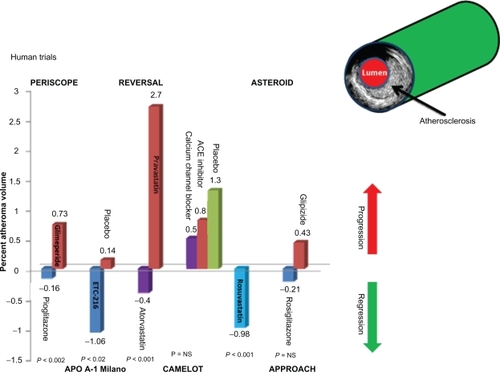
There are few things that influence humans more than a good illustrative picture. IVUS or imaging of the human coronary for atherosclerosis is probably very similar. One of the most impressive changes that come to mind is the research trial REVERSAL. The trial evaluated intensive lipid-lowering treatment (atorvastatin) vs moderate lipid-lowering regimen (pravastatin). The primary end point of the REVERSAL study was percent change in atheroma volume, which showed a 2.7% significant increase in the pravastatin group and a 0.4% nonsignificant reduction in the atorvastatin group (P < 0.02). This changed, to a large degree, many physicians view of statins in atherosclerosis treatment. The visual impact from the IVUS image was very impressive.
Studies with the glitazones are equally impressive. The studies to follow are completed in patients with type 2 diabetes who are considered as a coronary heart disease risk equivalent.
An important factor in the trials to follow is that in most of these trials patients were on statins and antiplatelet agents representative of the current high level of cardiovascular and diabetes care. The addition of the glitazones to these patients already on maximal cardiovascular treatment will be important when considering the results.
PERISCOPE was one of the first large randomized landmark glitazones IVUS trials. It enrolled 543 patients with type 2 diabetes who had baseline IVUS of the coronary artery and then were randomized to receive either glimepiride (1–4 mg) or pioglitazone (15–45 mg) for 18 months, at which time IVUS studies were repeated. The primary endpoint found that the mean percent atheroma volume decreased by 0.16% in pioglitazone-treated subjects but increased by 0.73% in glimepiride-treated patients. The use of statins in this trial was greater than 80% with 90% aspirin use. Both agents reduced HbA1c and fasting insulin levels, although pioglitazone’s effects on these end points were statistically greater. Pioglitazone also produced statistically beneficial changes in HDL and TG levels. In the pioglitazone group, compared with glimepiride, HDL levels increased 5.7 mg/dL (95%CI, 4.4 to 7.0 mg/dL; 16.0%) vs 0.9 mg/dL (95% CI, −0.3 to 2.1 mg/dL; 4.1%), and median TG levels decreased 16.3 mg/dL (95% CI, −27.7 to −11.0 mg/dL; 15.3%) vs an increase of 3.3 mg/dL (95% CI,−10.7 to 11.7 mg/dL; 0.6%) (P < 0.001 for both comparisons).Citation31
APPROACH randomized 672 patients with type 2 diabetes and indications for coronary angiography or PCI, at least 1 clinically significant coronary lesion, and 10% to 50% narrowing of at least 1 untreated coronary artery. 333 were randomized to rosiglitazone at up to 8 mg/day and 339 patients who received glipizide at 15 mg/day. Aspirin use was about 84%, beta blockers in 67%, angiotensin-converting enzyme (ACE) inhibitors or angiotensin-receptor blockers in about 74%, and statins in about 80%. The primary analysis from baseline to 18 months follow up found the percent atheroma volume was not significantly different between the treatment groups. A decrease of atheroma volume was seen by −0.21% in rosiglitazone-treated subjects with an increase by +0.43% in glipizide-treated patients (P = 0.12). Hypoglycemia was more common the glipizide arm 28% vs 8% with rosiglitazone (P < 0.0001). In summary, the primary endpoint was unremarkable, however the results imply that rosiglitazone could be antiatherosclerotic but it did not appear to be proatherosclerotic.Citation32,Citation33
What accounts for the difference between these two imaging trials is unclear at present. Metabolics of the two compounds are different in relation to HDL and triglycerides from Goldberg et al and may affect plaque biology.Citation34 The comparative drugs used in the trial are different and the duration of diabetes is different (see and ). The anti-inflammatory aspects could play a role but at present there is no clear explanation why the results were different. Two different IVUS control labs were used, both very well known with high quality work. Amount of atherosclerotic burden and type of plaque biology could be different but unknown at present. Longstanding prior statin treatment in some groups may have already reduced soft plaque that more easily is removed. More basic research on plaque biology is required. Another important comment concerning these two trials is a possible lack of significant difference between the rosiglitazone and comparator in the APPROACH was due to a better outcome in the group treated with comparator (glipizide), as opposed to the effect observed in PERISCOPE, where the group treated with glimepiride fared worse. The mean percent atheroma volume decrease 0.16% with pioglitazone and 0.21% with rosiglitazone appear rather comparable. It should be noted that in PERISCOPE glimeperide increased it by 0.73% while in APPROACH glipizide increased it only 0.43%. Thus it is possible for the difference between the two studies was in the different effects of the comparator drugs rather than the TZDs.
Table 1 Trial demographics and imaging results
Other considerations of vascular imaging of the human carotid are helpful in evaluation of atherosclerosis. Two large trials related to the glitazones have been completed.
The most recent study reported was involving patients with pre-diabetes but without clinical evidence for CVD. STARR (STudy of Atherosclerosis with Ramipril and Rosiglitazone), a sub-study of the DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) trial with 1425 subjects intended at assessing the effects of the ACE inhibitor ramipril and the TZD rosiglitazone on the progression of carotid intima-media thickness (CIMT). Using a 2 × 2 factorial design subjects were randomized to ramipril 15 mg/day or its placebo and to rosiglitazone 8 mg/day or its placebo. The primary study outcome was the annualized change of the aggregate maximum CIMT, computed as the average of the maximum CIMTs across 12 carotid arterial segments. A secondary endpoint of annualized change of the mean far wall left and right common CIMT. Median follow-up was 3 years and carotid ultrasound examinations were obtained at baseline and yearly thereafter. Rosiglitazone decreased glycemia with a mild reduction in blood pressure and modest favorable effects on CIMT progression. Compared with placebo, rosiglitazone reduced the primary CIMT outcome, but the difference was not statistically significant. The primary outcome aggregate maximum CIMT was 0.00063 mm/year for the rosiglitazone arm and 0.0090 mm/year for placebo (difference −0.0027 P = 0.08).
The CHICAGO study was a randomized, double-blind, comparator-controlled trial from the Chicago metropolitan area at 28 clinical sites. 462 patients with type 2 diabetes were randomized to receive 72 weeks of treatment with pioglitazone, or glimepiride, titrated to the HbA1c target. Patients had a mean duration of diabetes of 7.7 years and a mean HbA1c value of 7.4%. 55% of both groups were receiving statins. CIMT images were captured by a single ultrasonographer at one center and read by a single reader blinded to treatment assignment using automated edge-detection technology. The main outcome measure was the absolute change from baseline to the final visit at 72 weeks in the mean posterior-wall CIMT of the left and right common carotid arteries. The results found the CIMT was less with pioglitazone than it was with glimepiride at all time points – weeks 24, 48, and 72. The primary end point of progression of mean CIMT was less with pioglitazone, with a difference between groups of 0.013 mm, favoring the pioglitazone group (95% CI −0.013 [–0.024 to 0.002]; P < 0.02).Citation35
The CIMT results between STARR and CHICAGO are different. The types of patients are different between these two studies, CHICAGO used type 2 diabetes patients and STARR was pre-diabetes patients. Use of other medication may impact the results and drug combinations choices can affect plaque biology. The consistent beneficial findings with pioglitazone from PERISCOPE and CHICAGO merits a closer look at this type of TZD. The inconsistency from rosiglitazone in the trials is disturbing; however the trials are different in design, patient characteristics, and drug combinations used. All of these differences make it very hard to make strong statements or recommendations.
In summary, imaging trials are not perfect for answering many of our questions concerning glitazones in patients with pre-diabetes or type 2 diabetes. Most importantly they do not fully answer the main question: do they reduce cardiovascular clinical events, or regression of atherosclerosis will remain unanswered for now, requiring future studies. In closing two large studies have attempted to answer the cardiovascular question related to glitazones. PROactive trialCitation36 enrolled 5238 patients with type 2 diabetes who had evidence of macrovascular disease in a prospective, randomized study. The primary endpoint included a composite of endovascular or surgical intervention in the coronary or leg arteries, and amputation above the ankle, all-cause mortality, non-fatal myocardial infarction (including silent myocardial infarction), stroke, acute coronary syndrome. The primary endpoint was not met (hazard ratio 0.90, 95% CI 0.80–1.02, P = 0.095) primarily due to leg revascularization. The principle secondary endpoint was a composite of all-cause mortality, non-fatal myocardial infarction, and stroke (0.84, 0.72–0.98, P = 0.027). This study has been one of the most controversial studies related to TZDs and still is unresolved from main points of view. Many of the sub-studies are very positive toward pioglitazone, with consistent trends in a favorable direction; however the primary study missed its endpoint. On the heels of this large TZD trial is RECORD,Citation37 which was a multicenter, open-label trial, in which 4447 patients with type 2 diabetes on metformin or sulfonylurea monotherapy with mean HbA1c of 7.9% were randomly assigned to addition of rosiglitazone (n = 2220) or to a combination of metformin and sulfonylurea (active control group, n = 2227). The primary endpoint was cardiovascular hospitalization or cardiovascular death, with a hazard ratio non-inferiority margin of 1.20. Analysis was by intention to treat. The primary outcome during a mean 5·5-year follow-up, met non-inferiority criteria (hazard ratio 0.99, 95% CI 0.85–1.16). Specific to cardiovascular death the HR was 0.84 (0.59–1.18), and for myocardial infarction was 1.14 (0.80–1.63), and 0.72 (0.49–1.06) for stroke. Both of these trials reported increased heart failure concerns and bone fractures that made things even more difficult with these compounds. Currently, the controversy still continues to be a hotly debated topic on both sides and unlikely to be resolved in time.
In closing, the future is bright for many other imaging modalities from research that have not been included due to the current studies did not use this type of equipment. For imaging from inside the coronary tree, the current resolution of IVUS is around 150 microns; however soon, with radiolabeling, the use of optical coherence tomography at 5 microns may offer substantial advantage to our understanding of atherosclerosis.Citation38
Disclosures
RC lectures for Pfizer, Takeda, GSK and MSD. The other authors declare no conflicts of interest.
References
- NarayanKMBoyleJPGeissLSSaaddineJBThompsonTJImpact of recent increase in incidence on future diabetes burden: US, 2005–2050Diabetes Care20062992114211616936162
- FoxCSPencinaMJWilsonPWPaynterNPVasanRSD’AgostinoRBSrLifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart studyDiabetes Care20083181582158418458146
- NarayanKMBoyleJPThompsonTJGreggEWWilliamsonDFEffect of BMI on lifetime risk for diabetes in the USDiabetes Care20073061562156617372155
- JuutilainenALehtoSRönnemaaTPyöräläKLaaksoMSimilarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjectsDiabetes Care200831471471918083789
- WhiteleyLPadmanabhanSHoleDIslesCShould diabetes be considered a coronary heart disease risk equivalent?: results from 25 years of follow-up in the Renfrew and Paisley surveyDiabetes Care20052871588159315983305
- GorayaTYLeibsonCLPalumboPJCoronary atherosclerosis in diabetes mellitus: a population-based autopsy studyJ Am Coll Cardiol200240594695312225721
- XiangLDearmanJAbramSRCarterCHesterRLInsulin resistance and impaired functional vasodilation in obese Zucker ratsAm J Physiol Heart Circ Physiol20082944H1658H66618296567
- TraupeTD’UscioLVMuenterKMorawietzHVetterWBartonMEffects of obesity on endothelium-dependent reactivity during acute nitric oxide synthase inhibition: modulatory role of endothelinClin Sci (Lond)2002103Suppl 4813S15S12193045
- TraupeTLangMGoettschWObesity increases prostanoid-mediated vasoconstriction and vascular thromboxane receptor gene expressionJ Hypertens200220112239224512409963
- GollaschMEndothelium-derived contracting factor: a new way of looking at endothelial function in obesityJ Hypertens200220112147214912409950
- ShiYSoKFManRYVanhouttePMOxygen-derived free radicals mediate endothelium-dependent contractions in femoral arteries of rats with streptozotocin-induced diabetesBr J Pharmacol200715271033104117767168
- TesfamariamBBrownMLCohenRAElevated glucose impairs endothelium-dependent relaxation by activating protein kinase CJ Clin Invest1991875164316482022734
- BurkeAPKolodgieFDZieskeAMorphologic findings of coronary atherosclerotic plaques in diabetics:a postmortem studyArterioscler Thromb Vasc Biol200424712661267115142859
- HongYJJeongMHChoiPlaque characteristics in culprit lesions and inflammatory status in diabetic acute coronary syndrome patientsJACC Cardiovasc Imaging20092333934919356580
- TangTYHowarthSPMillerSRThe ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid diseaseJ Am Coll Cardiol20092532220392035019477353
- IssemannIGreenSActivation of a member of the steroid hormone receptor superfamily by peroxisome proliferatorsNature199034762946456502129546
- KeechASimesRJBarterPFIELD study investigatorsEffects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trialLancet2005366950018491861 Erratum in: Lancet. 2006;368(9545):1415. Lancet. 2006;368(9545):142016310551
- de LorgerilMSalenPPaillardFLacanPRichardGLipid-lowering drugs and homocysteineLancet199935391482092109923885
- MikaelLGGenestJJrRozenRElevated homocysteine reduces apolipoprotein A-I expression in hyperhomocysteinemic mice and in males with coronary artery diseaseCirc Res200698456457116439690
- NissenSENichollsSJWolskiKPERISCOPE InvestigatorsComparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trialJAMA20082991315611507318378631
- ChiquetteERamirezGDefronzoRA meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factorsArch Intern Med2004164192097210415505122
- GoldbergRBKendallDMDeegMAGLAI Study InvestigatorsA comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemiaDiabetes Care20052871547155415983299
- OrasanuGZiouzenkovaODevchandPRThe peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in miceJ Am Coll Cardiol2008521086988118755353
- BogackaIXieHBrayGASmithSRThe effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivoDiabetes Care20042771660166715220243
- OsmanAOteroJBrizolaraAEffect of rosiglitazone on restenosis after coronary stenting in patients with type 2 diabetesAm Heart J20041475e2315131558
- MarxNFroehlichJSiamLAntidiabetic PPAR gamma-activator rosiglitazone reduces MMP-9 serum levels in type 2 diabetic patients with coronary artery diseaseArterioscler Thromb Vasc Biol200323228328812588772
- ParkKSCiaraldiTPAbrams-CarterLMudaliarSNikoulinaSEHenryRRPPAR-gamma gene expression is elevated in skeletal muscle of obese and type II diabetic subjectsDiabetes1997467123012349200661
- BergerJLeibowitzMDDoebberTWNovel peroxisome proliferator-activated receptor (PPAR) gamma and PPARdelta ligands produce distinct biological effectsJ Biol Chem1999274106718672510037770
- OliverWRJrShenkJLSnaithMRA selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transportProc Natl Acad Sci U S A20019895306531111309497
- LiACBinderCJGutierrezADifferential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha,beta/delta, and gammaJ Clin Invest2004114111564157615578089
- NissenSENichollsSJWolskiKPERISCOPE InvestigatorsComparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trialJAMA2008299131561157318378631
- RatnerRECannonCPGersteinHCAPPROACH Study GroupAssessment on the Prevention of Progression by Rosiglitazone on Atherosclerosis in diabetes patients with Cardiovascular History (APPROACH): study design and baseline characteristicsAm Heart J200815661074107919033001
- NestoRWAssessment on the Prevention of Progression by Rosiglitazone on Atherosclerosis in Type 2 Diabetes patients with Cardiovascular History (APPROACH) American Heart Association 2008 Scientific Sessions; November 12, 2008; New Orleans, LA. Late Breaking Clinical Trials Session 4
- GoldbergRBKendallDMDeegMAGLAI Study InvestigatorsA comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemiaDiabetes Care20052871547155415983299
- MazzoneTMeyerPMFeinsteinSBEffect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trialJAMA2006296212572258117101640
- DormandyJACharbonnelBEcklandEJASecondary prevention of macrovascular events in patients with type 2 diabetes: a randomized trial of pioglitazone. The PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events)Lancet200536694931279128916214598
- HomePDPocockSJBeck-NielsenHRosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomized, open-label trialLancet200937396812125213519501900
- VillardJWParanjapeASVictorDAFeldmanMDApplications of optical coherence tomography in cardiovascular medicineJ Nucl Cardiol200916462063919479314