Abstract
Diabetes mellitus is characterized by a lack of insulin causing elevated blood glucose, often with associated insulin resistance. Over time, especially in genetically susceptible individuals, such chronic hyperglycemia can cause tissue injury. One pathological response to tissue injury is the development of fibrosis, which involves predominant extracellular matrix (ECM) accumulation. The main factors that regulate ECM in diabetes are thought to be pro-sclerotic cytokines and protease/anti-protease systems. This review will examine the key markers and regulators of tissue fibrosis in diabetes and whether their levels in biological fluids may have clinical utility.
Introduction
Fibrosis is characterized by extracellular matrix (ECM) accumulation and often by a change in the quality of the ECM, as well as angiogenesis. It is a common pathological response to tissue insults such as hyperglycemia, dyslipidemia, and hypertension. This review will examine the extent and type of tissue fibrosis that occurs in experimental and human diabetes, with an emphasis on potential circulating and urinary predictors and markers of fibrosis in human diabetes. While the pathogenesis and nature of end-organ complications in type 1 and type 2 diabetes are similar, especially in glucose-dependent aspects of microvascular disease, where possible throughout the text the type of diabetes is referenced.
Fibrosis in tissues affected by diabetic complications
Microvessels
Long-standing diabetes leads to both structural and functional anomalies in the vasculature (CitationZimmet 2000; CitationKhan et al 2003) which characterize micro- and macrovascular diabetic complications: retinopathy, nephropathy, cardiomyopathy, peripheral vascular disease, cerebrovascular disorders, and atherosclerosis. Firstly described by CitationSiperstein and colleagues (1968), extracellular matrix (ECM) alterations and basement membrane (BM) thickening have been documented as structural hallmarks in all target organs of diabetic complications (CitationBrownlee et al 1979; CitationTsilibary 2003). The morphological and biochemical disturbances of the ECM are directly related to a loss of function in target organs (CitationFarquhar et al 1972; CitationScheinman et al 1974; CitationIkeda et al 1991; CitationMakino et al 1993). ECM comprises an insoluble network of collagens, elastins, structural glycoproteins, proteoglycans-hyaluronans and integrins, which provide not only mechanical support for the cells, but also mediate complex interactions between the cells or between cells and the ECM of vascular tissues (CitationHayden et al 2005). EC matrices differ qualitatively and quantitatively from tissue to tissue and within various organs.
Diabetic retinopathy
Expansion of ECM that occurs in diabetic complications can be due to increased synthesis of matrix proteins and/or an inhibition of ECM degradation. With respect to the increased synthesis of matrix, proteins that are normally present in these structures or proteins that are not present in normal tissue, or both, may be induced. Thus, collagen types I and IV as well as laminin and fibronectin are normal constituents of normal retinal vessels from large thick-walled vessels down to microvessels less than 10 microns in diameter, whilst types III and V collagen were seen to stain primarily the walls of the larger vessels. A preclinical hallmark of early diabetic retinopathy (DR) is the thickening of the capillary basement membrane (BM) resulting from increased production and/or decreased breakdown of collagen IV, laminin, fibronectin, and other proteins (CitationRoy et al 1990; CitationLjubimov et al 1996; CitationSpirin et al 1999; CitationLorenzi et al 2001). In proliferative diabetic retinopathy (PDR), the BM of the new vessels and the epiretinal membranes show significantly increased amounts of types VI, VIII, XII, and XIV collagen, as well as perlecan, bamacan (CitationLjubimov et al 1996), fibronectin, tenascin (CitationIoachim et al 2005), and vimentin (CitationHosoda et al 1993). While not present in normal retina (CitationJerdan et al 1986), type II collagen was found in epiretinal membranes (ERM) (CitationHosoda et al 1993). A positive relationship was found between fibronectin expression and ERM proliferative activity (CitationIoachim et al 2005). Downregulation of fibronectin synthesis could partially prevent retinal BM thickening along with a reduction of pericyte loss and acellular capillaries in animal model (CitationRoy et al 2003).
Diabetic renal disease
Type IV collagen collagen, fibronectin and laminin, which are normal constituents of the mesangium and glomerular basement membrane (GBM), are increased in diabetic kidney disease (CitationKim et al 1991; CitationMakino et al 1993; CitationKiryu et al 1994; CitationZhu et al 1994; CitationYagame et al 1995; CitationRazzaque et al 1997; CitationMoriya et al 2001). Accelerated matrix deposition (type IV collagen) can be present even in early stages of diabetic renal disease (microalbuminuria stages) in experimental models (CitationLiu Y et al 2007). In diabetic diffuse glomerulosclerosis deposition of collagen IV, V, laminin, and fibronectin is increased in the mesangial matrix and glomerular basement membranes (CitationNerlich et al 1991; CitationTsilibary 2003), whilst in nodular glomerulosclerosis normal BM components are decreased or absent (CitationOlgemoller et al 1993). Expressed only under pathological conditions, type I and III collagen appears in the late stages of glomerulosclerosis (CitationGlick et al 1992; CitationMakino et al 1994; CitationRazzaque et al 1994; CitationMakino et al 1995; CitationStokes et al 2000; CitationSchaefer et al 2001), and are associated with the development of Kimmelstiel-Wilson nodules rather than with the diffuse expansion of the mesangial matrix, which occurs in the early and moderately advanced stages of the disease. Decreased levels of proteoglycans (heparin sulphate, perlecan) found in diabetic kidney in the mesangial matrix, GBM, the endothelial and epithelial BM, and renal tubular cells have also been assigned a role in the development of diabetic micro- and macroalbuminuria (CitationSchaefer et al 2001). Interestingly, a substantial subset of type 2 diabetic patients, despite the presence of microalbuminuria or proteinuria, have normal glomerular structure with or without tubulointerstitial and/or arteriolar abnormalities (CitationFioretto et al 2007).
Diabetic cardiomyopathy
Both types I and III collagen are present in normal and diseased myocardial tissue. Type I collagen is predominant in the myocardium, but type III is more specific to cardiac tissue (CitationBishop et al 1995; CitationZannad et al 2000; CitationD’Armiento 2002). Myocardial biopsies from diabetic subjects revealed a significantly higher proportion of type III collagen compared with their nondiabetic counterparts, while the proportion of collagen type I did not differ between the groups (CitationShimizu et al 1993). Responsible for the increased left ventricle (LV) mass (Citationvan Hoeven et al 1990), diffuse myocardial fibrosis has a distribution in both interstitium and perivascular sites (CitationRegan et al 1977; CitationNunoda et al 1985; CitationGenda et al 1986; CitationDas et al 1987; Citationvan Hoeven and Factor 1990). Extensive myocyte necrosis and replacement of contractile fibers by connective tissue are likely to account for depressed cardiac performance, at least in advanced stages of diabetic cardiomyopathy (CitationFactor et al 1980). It appears that hypertrophy of myocardial cells and myocardial interstitial fibrosis may be present even in mild hyperglycemia in diabetes (CitationNunoda et al 1985).
Larger arteries
In animal models of type 2 diabetes it has been found that increased intimal proliferation and medial thickness as well as ECM deposition occur in vessels such as mesenteric arteries and aorta (CitationSong and Ergul 2006). Vascular remodeling and hypertrophy associated with augmented expression of dedifferentiation markers of vascular smooth muscle cells also occur in larger vessels like aorta (CitationVranes et al 1999). Fibronectin expression varied in different reports in large vessels (CitationFukuda et al 2005). Proteoglycans (PGs) such as versican, biglycan, and decorin have been involved in diabetic nephropathy pathogenesis (CitationSchaefer et al 2001). PGs accumulate in developing atherosclerotic and restenotic lesions, and thus contribute to plaque burden and influence cellular and extracellular events associated with the pathogenesis of vascular lesions, such as migration and proliferation, lipid metabolism and retention, and thrombosis (CitationShirk et al 2000; CitationEdwards et al 2004; CitationNakashima et al 2007; CitationTran et al 2007).
Nonalcoholic fatty liver disease
Type 2 diabetic patients also have an increased risk for developing chronic liver disease. Nonalcoholic fatty liver disease (NAFLD) represents a spectrum of conditions characterized histologically by excessive accumulation of hepatic fat in the absence of alcohol consumption. Obesity, type 2 diabetes, dyslipidemia, and hypertension contribute to the risk for liver disease and to disease progression. Two main histological patterns of NAFLD are described: fatty liver alone and nonalcoholic steatohepatitis (NASH). NASH is an increasingly recognized cause of liver-related morbidity and mortality (CitationAngulo 2002; CitationSanyal 2002; CitationCharlton 2004), with about a quarter of patients progressing to serious liver sequelae, including end-stage liver disease and hepatocellular carcinoma (CitationBugianesi et al 2002; CitationRatziu et al 2002). Those at highest risk include patients with significant hepatic necro-inflammation and fibrosis (CitationRatziu et al 2000; CitationSanyal 2002). Unlike other vascular beds, the normal hepatic sinusoids have no BM to become thickened. Sinusoidal (perisinusoidal) fibrosis with formation of BMs occurs in a variety of liver diseases, including chronic viral hepatitis, alcoholic hepatitis and NASH. In those diseases, the fibrosis is a result of the activation of the hepatic stellate cells with a phenotypic transition to collagen-producing myofibroblasts. Activated hepatic stellate cells are involved in the ECM degradation and remodeling that occur with fibrogenesis (CitationSugimoto et al 2005).
Effects of diabetes on regulators of ECM turnover
Metabolic and hemodynamic induction of fibrosis by hyperglycemia
Chronic hyperglycemia is a main factor in the onset of microvascular diabetic complications in both type 1 and 2 diabetes, as strict glycemic control reduces end-organ complication incidence and rate of progression (CitationThe Diabetes Control and Complications Trial Research Group 1993; CitationUK Prospective Diabetes Study Group 1998). This pathogenesis is shown schematically in . Specific biochemical pathways linking hyperglycemia to microvascular changes have been proposed: increased glucose flux through the polyol pathway (CitationGreene et al 1987), nonenzymatic glycation of proteins (CitationBrownlee et al 1988), glucose autooxidation, and oxidative stress (CitationHunt et al 1990), hyperglycemic pseudohypoxia (CitationWilliamson et al 1993), enhanced activation of protein kinase C isoforms (CitationLee et al 1989; CitationDeRubertis and Craven 1994), and alteration in cell signaling pathways (CitationBrownlee 2001; CitationSheetz et al 2002). As described in subsequent sections, experimental data support causative roles for hyperglycemia and these downstream biochemical pathways in causing alterations in ECM turn-over (CitationFukui et al 1992; CitationNahman et al 1992; CitationRoy et al 1994; CitationWahab et al 1996). Hyperglycemia can work through both metabolic and hemodynamic pathways to change growth factors and ECM turn-over. This is shown schematically in .
Figure 1 The linear pathway leading from insulin deficiency, through hyperglycemia to diabetes complications.
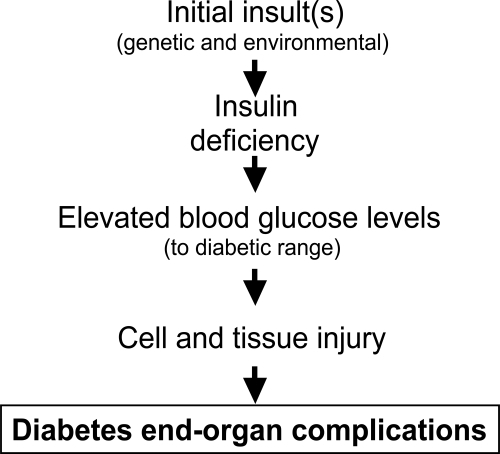
Figure 2 Schematic diagram indicating how hemodynamic and metabolic factors, and growth factors, can network to cause tissue damage. Inflammation and fibrosis occur variably in tissue at different stages of diabetes complications.
Advanced glycation end-products (AGEs)
Hyperglycemia is responsible for the presence of high levels of nonenzymatically produced AGEs in patients with diabetes (CitationGoldin et al 2006). AGEs are able to stimulate directly the production of ECM. Nonenzymatic glycosylation of collagens produces cross-linkages and hence may produce physical alterations in the properties of the ECM. AGEs modification of matrix proteins is able to disrupt matrix-matrix and matrix-cell interactions, contributing to their profibrotic action. In addition, AGEs significantly interact with the renin-angiotensin system. AGEs play important roles in cell signaling by interacting with specific receptors, receptor for advanced glycation end products (RAGE), that link to the activation of adhesion molecules, proinflammatory cytokines and growth factors, thus contributing to the pathogenesis of diabetic complications (CitationMason et al 2003; CitationMcLennan et al 2004). AGEs have extracellular effects, such as protein cross-linking, that appear to inhibit ECM degradation and promote the expansion of the glomerular mesangial matrix and BM in diabetic kidney disease. Drugs that either inhibit the formation of AGE or break AGE-induced cross-links have been shown to be renoprotective in experimental models of diabetic nephropathy (CitationForbes et al 2002).
The renin-angiotensin-aldosterone system
The renin-angiotensin-aldosterone system (RAAS) is an important contributor to the pathogenesis of diabetic micro- and macrovascular complications by inducing various tissue responses, such as vasoconstriction, inflammation, oxidative stress, cell hypertrophy and proliferation, angiogenesis and fibrosis. RAAS effects can be locally generated in many organs (CitationPaul et al 1993; CitationMorgan et al 1994; CitationWagner et al 1996; CitationEngeli et al 1999; CitationBataller et al 2003).
Angiotensin II (Ang II), the main physiological effector molecule of RAAS, mediates fibrosis by stimulating the synthesis of ECM components (CitationKagami et al 1994; CitationGomez-Garre et al 1996; CitationBrilla et al 1997) apoptosis/proliferation (CitationEfrati et al 2007), infiltration of inflammatory cells, and the release of inflammatory cytokines and growth factors such as transforming growth factor (TGF)-β1 (CitationWu et al 1997), monocyte chemoattractant protein (MCP)-1 (CitationRuiz-Ortega et al 1998), vascular endothelial growth factor (VEGF) (CitationOtani et al 2000), platelet derived growth factor (PDGF) (CitationNaftilan et al 1989), and connective tissue growth factor (CTGF) (CitationRuperez et al 2003a; CitationFinckenberg et al 2003). Ang II is up-regulated under the diabetic conditions (CitationSingh et al 1999) and exerts its deleterious effects through the angiotensin type I receptor (AT1R). RAS blocking with either angiotensin converting enzyme inhibitors (ACEI) or angiotensin type 1 receptor blockers (ARB) has clearly demonstrated positive outcomes on diabetic complications, with benefits beyond those derived from lowering blood pressure. Thus ACEI and/or ARB treatment can slow the progression of diabetic renal disease (CitationThe EUCLID Study Group 1997; CitationHeart Outcomes Prevention Evaluation Study Investigators 2000), decrease cardiovascular events (CitationHeart Outcomes Prevention Evaluation Study Investigators 2000), may decrease retinopathy in T1DM (CitationChaturvedi et al 1998) and improves diastolic dysfunction in diabetic patients (CitationKawasaki et al 2007).
Another component of the RAAS, aldosterone, plays also a role in the development of hypertension, endothelial dysfunction, vascular structure damage, proteinuria, myocardial fibrosis, collagen synthesis (CitationCha et al 2005). Spironolactone, an aldosterone antagonist, associated to ACEI and/or ARB treatment may offer additional renoprotection in diabetic nephropathy (CitationSato et al 2003; CitationSchjoedt et al 2006; Citationvan den Meiracker et al 2006).
Growth factors
Under normal circumstances ECM undergoes continuous synthesis and degradation and ECM turn-over is a requisite for normal structure and function of organs and tissues (CitationTyagi et al 1995). ECM turn-over is characterized by a balance between matrix formation and matrix degradation. Factors that regulate ECM formation include multiple forms of growth factor such as such as TGF-β (CitationMcClain et al 1992; CitationKolm et al 1996; CitationRiser et al 1998), CTGF (CitationTwigg and Cooper 2004; CitationTwigg et al 2001; CitationMcLennan et al 2004; CitationParadis et al 2001; CitationLiu X et al 2007), and insulin-like growth factor I (IGF-I), fibroblast growth factor (FGF), epidermal growth factor (EGF) and PDGF, (CitationLembach 1976; CitationTseng et al 1982; CitationDresow et al 1984; CitationRoberts et al 1986; CitationQi et al 2005). Enzymes responsible for ECM degradation and remodeling include the matrix metalloproteinases (MMPs) (CitationMcLennan et al 1998, Citation2000; CitationDeath et al 2003), and serine proteases (CitationGeiger et al 1988), as well as their respective tissue inhibitors, the TIMPs (CitationNakamura et al 1994; CitationShankland et al 1996; CitationGomez et al 1997), and PAI-1 (CitationFisher et al 1997; CitationMcLennan et al 2000). These regulators of ECM will now be explored in detail in diabetic complications.
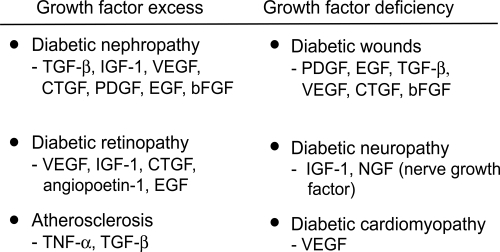
The important role of growth factors in the pathogenesis of diabetic long-term complications was suggested by their increased concentrations in target tissues (CitationYamamoto et al 1993; CitationTikellis et al 2004; CitationRoestenberg et al 2006; CitationUmezono et al 2006). In diabetic nephropathy, IGF-I seems to be implicated in the earlier stages of the disease, while TGF-β and CTGF are involved both in the early and later stages, being responsible, at least in part, for ECM accumulation (CitationPark et al 1997; CitationGilbert et al 1998; CitationRiser et al 2000). VEGF and FGF play a pivotal role both in nonproliferative and proliferative retinopathy (CitationWells et al 1996; CitationMathews et al 1997).
indicates the main growth factors involved in diabetic complications, based on tissue levels measured in human and animal diabetes, and also on intervention studies mainly in animals. An excess of growth factor is implicated in tissues where fibrosis predominates, whereas a lack of growth factors occurs in diabetic neuropathy and wound healing.
Figure 3 The major growth factors implicated in diabetes complications. The prosclerotic ones involved in human diabetic fibrosis are currently thought to be TGF-β and CTGF.
TGF-β
TGF-β is generally accepted to be the main pro-fibrotic factor in diabetic nephropathy. Several lines of experimental and clinical evidence support a major role for TGF-β in development of glomerulosclerosis and interstitial fibrosis in diabetes. Diabetic environment up-regulated TGF-β1 expression and bioactivity in glomerular mesangial cells and proximal tubule cells (CitationZiyadeh et al 1994, Citation1998; CitationSharma et al 1995). With the development of incipient diabetic nephropathy TGF-β mRNA increased in mesangial cells, podocytes and tubular epithelial cells. Progression to manifest diabetic nephropathy was associated with further increase in TGF-β mRNA, especially in the glomeruli (CitationWahab et al 2005). The kidney of a diabetic patient actually elaborates TGF-β1 protein into the circulation whereas the kidney of a nondiabetic subject extracts TGF-β1 from the circulation (CitationSharma et al 1997). Inhibition of TGF-β1 with neutralizing antibodies prevented glomerular enlargement, and attenuated the excess matrix expression by reducing type IV collagen and fibronectin mRNA (CitationSharma et al 1996; CitationZiyadeh et al 2000).
Up-regulation of TGF-β protein was demonstrated in the myocardium of rodents with diabetic cardiomyopathy. ARB treatment reduced its expression and decreased cardiac fibrosis (CitationWestermann et al 2007). TGF-β is also involved in liver fibrosis (CitationRoth et al 1998), by activating the hepatic stellate cells and inducing apoptosis of hepatocytes in liver injury (CitationOberhammer et al 1992). However, due to its important anti-proliferative and anti-inflammatory effects, TGF- β is not a suitable target for therapeutic intervention.
CTGF
CTGF is another prominent growth factor in the pathogenesis of diabetic nephropathy. High glucose conditions, TGF-β, AGEs, RAS, TNF-α, mechanical strain or CTGF itself promote CTGF expression by mesangial cells (CitationWahab et al 1996; CitationMurphy et al 1999; CitationRiser et al 2000; CitationTwigg et al 2001, Citation2002a; CitationCooker et al 2007; CitationHughes et al 2007). CTGF can also be produced by podocytes (CitationRoestenberg et al 2006), parietal epithelial cells (CitationUmezono et al 2006) and proximal tubular cells (Wang et al 2001; CitationRoestenberg et al 2006). In experimental type 1 and type 2 diabetes CTGF mRNA and protein was up-regulated in various organs: kidney, heart, liver, retina (CitationRoestenberg et al 2006). Glomerular CTGF mRNA levels were found to be upregulated in diabetic patients with microalbuminuria as well as in overt nephropathy (CitationUmezono et al 2006). In addition, CTGF mRNA levels were found to correlate with the degree of albuminuria (CitationAdler et al 2001). We have recently found that renal tubular CTGF protein by renal biopsy at 5 years predicts albuminuria at 10 years, in a diabetic baboon model of type 1 diabetes (CitationThomson et al 2007). The role of CTGF in DN pathogenesis in both type 1 and type 2 diabetes has been confirmed by a recent study showing that a CTGF antisense oligonucleotide may attenuate progression of nephropathy in mice (CitationGuha et al 2007). CTGF overexpression in podocytes was critically involved in diabetes-related GBM thickening (CitationRoestenberg et al 2006). However, a site-specific downregulation of CTGF accompanied by a reduced VEGF-A mRNA in glomeruli from diabetic patients can be evidenced in DN and is a result of podocyte loss (CitationBaelde et al 2007).
Relative abundance of myocardial mRNA for CTGF compared with brain natriuretic peptide (BNP) was positively correlated with diastolic dysfunction, myocardial fibrosis area, and procollagen type 1 mRNA expression in a rat pressure overload cardiac hypertrophy model. Exogenous BNP prevented the production of CTGF in cardiac myocytes (CitationKoitabashi et al 2007).
CTGF has been shown to be up-regulated in the retina of diabetic rats (CitationTikellis et al 2004). It appears that in diabetes CTGF expression shifts from microglia to microvascular pericytes (CitationKuiper et al 2004). CTGF was also expressed in endothelial cells and myofibroblast in PDR membranes, and in myofibroblast in proliferative vitreoretinal membranes (CitationAbu El-Asrar et al 2007). CTGF is overexpressed in pericytes in the human diabetic retina, irrespective of changes related to clinical DR like vascular leakage (CitationKuiper et al 2004). Overexpression of CTGF in cultured human aortic smooth muscle cells, a cell type closely related to pericytes and mesangial cells, induced apoptosis by activating caspase 3 (CitationHishikawa et al 1999).
In fibrotic liver, CTGF mRNA and protein are produced by hepatocytes, fibroblasts, myofibroblasts, hepatic stellate cells (HSCs), endothelial cells, and bile duct epithelial cells (CitationRachfal et al 2003). Interestingly, whilst in HSCs CTGF was only marginally stimulated by TGF-β, in cultured hepatocytes it was strongly upregulated by TGF-β (CitationGressner et al 2007). CTGF could be reduced in liver by antisense RNA of CTGF recombinant or slicing through siRNA, which decreased activation of HSCs, prevented the upregulation of CTGF and TGF-beta1 gene expression and inhibited accumulation of connective tissue proteins in the liver (CitationGeorge et al 2007; CitationLu et al 2007).
Once induced, CTGF can up-regulate its own gene expression (CitationRiser et al 2000; CitationTwigg et al 2001; CitationWahab et al 2001). It is also able to initiate changes in ECM composition: it increased expression of fibronectin (CitationWahab et al 2001; CitationRuperez et al 2003a; CitationWeston et al 2003) and enhanced fibronectin assembly into an insoluble matrix (CitationWeston et al 2003), increased in type IV (CitationZhou et al 2004), type III (CitationLam et al 2004) and type I collagen production (CitationGore-Hyer et al 2002), and up-regulated integrins on the cell surface, facilitating the deposition and assembly of ECM proteins (CitationWeston et al 2003). Furthermore, CTGF caused induction of plasminogen activator inhibitor-1 (CitationWahab et al 2001), rearrangement of the actin cytoskeleton (CitationCrean et al 2002) and an increase in TIMP-1 with subsequent decrease in matrix degradation (CitationMcLennan et al 2004). CTGF also exerted a strong chemotactic effect on peripheral blood mononuclear cells in vitro (CitationCicha et al 2005), which may then contribute to tissue inflammation and late fibrosis (CitationFrazier et al 1996).
CTGF has been described as a downstream mediator of TGF-β in the fibrotic process (CitationIgarashi et al 1993; CitationGrotendorst et al 1996; CitationDuncan et al 1999; CitationWeston et al 2003; CitationKobayashi et al 2005). TGF-β1-induced effects can be blocked by CTGF antisense oligonucleotides (CitationDuncan et al 1999; CitationAbdel-Wahab et al 2002; CitationWeston et al 2003). However, CTGF can also exert its pro-fibrotic effects via TGF-β1-independent pathways (CitationMurphy et al 1999; CitationBlom et al 2001; CitationTwigg et al 2001; CitationMcLennan et al 2004; CitationChaqour et al 2006) as seen for induction of CTGF by AGEs which is TGF-β independent (CitationTwigg et al 2001). Glucose-induced collagen production was reduced by CTGF anti-sense nucleotide (CitationWahab et al 2001; CitationAbdel-Wahab et al 2002; CitationRuperez et al 2003a; CitationWeston et al 2003; CitationGuha et al 2007), ACEI or ARB (CitationRuiz-Ortega et al 1995; CitationWu et al 1997; CitationBorder et al 1998; CitationRuperez et al 2003b; CitationTsutsui et al 2007), or by treatment with an AGE inhibitor (CitationTwigg et al 2002b; CitationCandido et al 2003). CTGF can interact with, and influence the signaling of IGF-I (CitationLam et al 2003), VEGF (CitationBrigstock 2002), TGF-β (CitationGrotendorst et al 1996) and bone morphogenic proteins (BMPs) (CitationAbreu et al 2002). Moreover, CTGF can be cleaved by metalloproteases (MMPs) and other proteases (CitationHashimoto et al 2002).
PDGF-β
PDGF-β is involved in structural alterations at the glomerular level. It seems that high glucose induces an early activation of a PDGF loop that in turn causes an increase of TGF-β1 gene expression, thus modulating both human mesangial cell proliferation and mesangial matrix production (CitationDi Paolo et al 1996).
VEGF
VEGF appears to be another mediator for these early and late vascular changes. Neutralizing antibodies directed against VEGF blocked vascular permeability and blood flow changes induced by elevated tissue glucose and sorbitol levels in a dosage-dependent manner (Citationde Vriese et al 2001). VEGF signaling affected GBM thickening, slit pore density, and nephrin quantity, all of which were associated with the extent of diabetic albuminuria. These effects could be blocked by a VEGF receptor inhibition (CitationSung et al 2006).
FGF
FGF is secreted by fibroblasts, macrophages and in particular endothelial cells (EC) in response to tissue injury and is important in promotion of neovascularization. Produced and stored in epiretinal membranes (CitationHueber et al 1996; CitationSchneeberger et al 1997), FGF is a potent endothelial cell mitogen that has been proposed to play a role in proliferative diabetic retinopathy and other neovascular processes (CitationHanneken et al 1991).
Protease systems and their regulators
The MMP system
Metalloproteinases (MMPs) are a family of zinc-dependent enzymes with the combined ability to digest all ECM proteines: native and partially degraded fibrillar collagens, basement membrane collagens, proteoglycans, elastin, fibronectin. The gelatinase (MMP-2 and MMP-9) are two proteinases primarily responsible for breaking down type IV collagen from the BMs. These are produced by multiple vascular cell types, such as pericytes, podocytes, vascular smooth muscles cells, renal mesangial cells, fibroblasts, macrophages. The MMPs are synthesized as inactive zymogens with a pro-peptide domain that must be removed before the enzyme is active. Activation of MMPs can be induced by urokinase type (uPA) and tissue-type (tPA) plasminogen activators that cleave plasminogen into active plasmin. MMP-9, but not other MMPs, is able to upregulate biologically active proteins such as the profibrotic growth factor TGF-β (CitationRutschow et al 2006).
A major control point in the regulation of active enzyme is inhibition of the active form by their tissue family of inhibitors. TIMPs comprise a family of four protease inhibitors (TIMP-1 to TIMP-4), which are expressed in a tissue specific pattern and regulate the function of MMPs either by inhibiting active MMPs or by controlling their activation process. Overall, all MMPs are inhibited by TIMPs once they are activated, with most of the MMPs being inhibited by TIMP-1. The gelatinases (MMP-2 and MMP-9) can form complexes with TIMPs when the enzymes are in the latent form. The complex of latent MMP-2 (pro-MMP-2) with TIMP-2 serves to facilitate the activation of pro-MMP-2 at the cell surface by MT1-MMP (MMP-14), a membrane-anchored MMP. The role of the pro-MMP-9/TIMP-1 complex is unknown.
An imbalance between MMPs and TIMPs plays an important role in ECM modeling that favors tissue fibrosis. For example, the imbalance between the MMP-2 and TIMP-2, caused primarily by an increase in TIMP-2 activity, may contribute to the pathogenesis of diabetic nephropathy (CitationHan et al 2006). Decreased MMP-2 expression and activity, and up-regulated MMP-9 protein were found in the myocardium of diabetic mice with STZ-induced DCM. These alterations were corrected by ARB treatment (CitationWestermann et al 2007). Myofibroblasts and vascular endothelial cells in PDR membranes expressed an increase in MMP-9 protein and activity (CitationAbu El-Asrar et al 2007). ProMMP-9 and activated MMP-9 levels were also significantly increased in vitreous samples in PDR patients. In addition, TIMP-1 levels were significantly increased in PDR patients. Activated MMP-9 levels in vitreous samples of PDR patients with hemorrhage were higher than those in PDR patients without hemorrhage, suggesting that activated MMP-9 might be involved in hemorrhagic transformation in these patients (CitationDescamps et al 2006).
The plasminogen system
Plasminogen activator (PA)/plasmin/PA inhibitor (PAI) system is involved in ECM degradation. PAI-1 may promote ECM build-up by preventing plasmin and MMPs activation (CitationMcLennan et al 2000). PAI-1 can regulate TGF-beta expression by binding to uPAR and activating the extracellular-regulated signal kinase (ERK)/MAPK pathway (CitationYang et al 2007). PAI-1 plays a critical role in ECM remodeling in the kidney. Normal human kidneys do not express PAI-1 but PAI-1 is overexpressed in pathologic conditions associated with renal fibrosis including diabetic nephropathy (CitationPaueksakon et al 2002; CitationHagiwara et al 2003). Reactive oxygen species mediate PAI-1 up-regulation in renal cells cultured under high glucose, hypoxia, and with TGF-beta1 (CitationLee and Ha 2005). PAI-1 induced ECM deposition in diabetic kidney through increased ECM synthesis by TGF-beta1 up-regulation as well as through decreased ECM degradation by suppression of plasmin and MMP-2 activity (CitationLee and Ha 2005). Impaired fibrinolysis resulting from high plasma PAI-1 can lead to excessive fibrin accumulation within vessels, resulting in atherothrombosis. Increased expression of PAI-1 was found in the arterial wall in patients with type 2 diabetes (CitationPandolfiet al 2001). This increased vascular expression of PAI-1 promotes neointima formation via accumulation of fibrin or fibrinogen as a result of inhibited clearance of platelet-fibrin thrombi. PAI-1, an acute phase protein, also has been involved in vascular inflammation (CitationAlessi et al 2004).
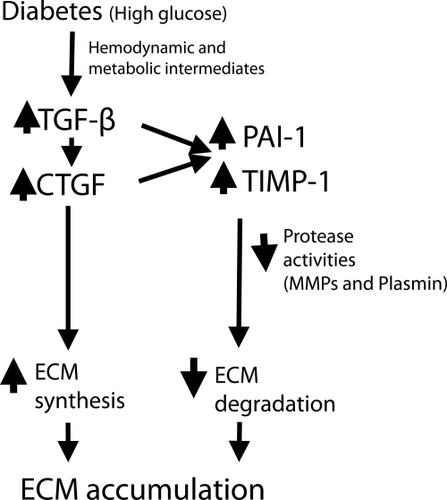
shows methods in diabetes by which pro-fibrotic growth factors may link with the protease and anti-protease systems to dysregulate ECM turnover and thus cause ECM accumulation.
Figure 4 One pathogenic pathway by which high glucose in diabetes and hypertension work through prosclerotic growth factors to dysregulate ECM turnover. Both TGF-β and CTGF have been shown to induce TIMP-1 and PAI-1, resulting in reduced MMP and plasmin activity. This paradigm best applies to diabetic renal disease.
Endothelin-1
Endothelin-1 (ET-1) is a potent vasoconstrictor peptide, which can also exert pro-inflammatory, mitogenic and pro-fibrotic effects. Up-regulated by glucose, angiotensin II, TGF-β, ROS and PDGF in various renal cells (CitationZoja et al 1991; CitationKohno et al 1992; CitationKohno et al 1993; CitationHughes et al 1996; CitationHua et al 2001), retinal cells (CitationChakravarthy et al 1997; CitationPark et al 2000; CitationYokota et al 2003) and cardiomyocytes, ET-1 has been linked with matrix accumulation in diabetic kidney (CitationRuiz-Ortega et al 1994; CitationHargrove et al 2000) and cardiomyocyte hypertrophy (CitationChen et al 2007), and with hemodynamic and histopathological abnormalities in diabetic retina (CitationBursell et al 1995; CitationTakagi et al 1996; CitationChakrabarti et al 1998). Neutralizing ET-antibodies and anti-sense oligonucleotides, as well as ET-receptor antagonist treatment reduced proteinuria and the production of ECM proteins in the kidney (CitationLi et al 1999; CitationHocher et al 2001; CitationSugimoto et al 2002) and prevented myocardial and coronary dysfunction (CitationDing et al 2006; CitationWolkart et al 2006) in experimental diabetes.
Markers of fibrosis in biological fluids in diabetes
In order to assess accurately the morphological changes in the target organs and the extent to which the fibrotic changes affect their function, invasive studies (tissue biopsy) is commonly required. However, biopsy is not always feasible in human tissues and is associated with obvious risks. Therefore it is highly desirable to potentially estimate the severity of organ fibrosis by measuring ECM factors in biological fluids (peripheral blood or urine). Such markers, if proven they mirror the changes in specific organs structure and function, will allow a better monitoring of the disease. Moreover, if these markers levels show changes with treatment, they will be a useful tool to evaluate the therapeutic interventions. Thus the potential clinical value of the circulating or urinary levels of several ECM components and its regulators have been tested in diabetes and its complications.
ECM formation and degradation markers
Serum aminoterminal propeptide of type III (PIIINP) or type I (PINP) procollagen and carboxyterminal propeptide of type I procollagen (PICP) are released in a stoichiometric manner with collagen type III or I molecules during collagen biosynthesis, and therefore they are considered markers of the synthesis and deposition of type III and I collagen (CitationJensen et al 1990; CitationRisteli et al 1995; CitationWeber 1997). However, there is evidence that some of the PIIINP is also released during collagen degradation, because these propeptides are not completely cleaved during collagen synthesis (CitationFleischmajer et al 1985). Therefore, PIIINP could reflect both synthesis and degradation of collagen.
Circulating levels of collagen have been suggested as indicators of diabetic complications. Elevated serum levels of both PIIINP and laminin were associated with the development of diabetic microangiopathy (CitationOkazaki et al 1988). Another study (CitationMigdalis et al 1994) found elevated serum PIIINP levels in type 2 diabetic subjects with peripheral vascular disease and proposed that this reflected an increase in collagen deposition in the large arteries that accompanies the development of macroangiopathy. Serum PIIINP was also increased in type 2 diabetic patients with nephropathy (CitationIshimura et al 1996).
PICP concentration did not differ between uncomplicated type 2 diabetic patients and controls, but it was increased in complicated diabetes and related to the progression of nephropathy (CitationInukai et al 2000). In contrast, other investigators found decreased levels of serum PICP but increased PIIINP concentrations in subjects with proliferative retinopathy compared with those with no retinopathy (CitationArkkila et al 2001). Similarly, hypertensive type 2 diabetic patients had higher mean levels of PIIINP than controls, and lower mean levels of PINP and PICP (CitationAlla et al 2006). No association between collagen markers and neuropathy was found (CitationArkkila et al 2001).
ECM regulators as systemic fibrosis markers
In patients with type 1 diabetes plasma and urinary TGF-β1 levels were significantly higher than in controls. The effect of metabolic control on plasma TGF-β level is controversial (CitationAzar et al 2000; CitationFlores et al 2004; CitationThrailkill et al 2007). Increased TGF-β levels in both plama and urine were found especially in relation to diabetic nephropathy (CitationPfeiffer et al 1996; CitationHoulihan et al 2002). Urinary TGF-beta significantly decreased with ACEI, ARB or thiazolinediones treatment, in parallel with a decrease in albuminuria (CitationMatos et al 2005; CitationKatavetin et al 2006; CitationSong et al 2006; CitationWoo et al 2006).
While it seems correlated with glycemic control (CitationKakizawa et al 2004), plasma VEGF concentration was not strongly correlated with risk factor status or microvascular disease in type 1 diabetes, nor was affected by ACE inhibition (CitationChaturvedi et al 2001). Urinary VEGF concentrations were significantly higher in the diabetic groups, even at the normoalbuminuric stage, with further increase as diabetic nephropathy advanced (CitationKim et al 2004).
Diabetes status is associated with dysregulation in the circulating MMP/TIMP system even in the absence of complications. Thus, serum MMP-9 and TIMP-1 levels are increased in both type 1 (CitationMaxwell et al 2001) and type 2 diabetes (CitationTayebjee et al 2004), with no changes in MMP-2 concentration. In contrast, in another study urine and plasma MMP-2 levels and plasma MMP-2 activity were all significantly elevated in type 1 diabetic patients, with urine MMP-2 correlated with higher HbA1c, longer duration of diabetes, evidence of renal hyperfiltration and the presence of microalbuminuria (CitationThrailkill et al 2007). Increased serum MMP-2 and TIMP-1 concentrations, but no elevation of MMP-9 levels, were also found in a cohort of diabetic patients with mild or no complications (CitationLee et al 2005).
Some therapies have been proven efficient in reduction the plasma/serum MMPs and TIMP-1 levels in diabetic patients. Thiazolidinediones, which act via the PPARγ receptor, reduced the increase in circulating levels of MMP-9 but had no effect on circulating MMP-2. Furthermore, reductions in MMP-9 levels were associated with decrease in other inflammatory markers, such as C-reactive protein, PAI-1, IL-6, TNF-α and serum amyloid A (CitationHaffner et al 2002; CitationMarx et al 2003; CitationHanefeld et al 2007).
Circulating MMP-9 and TIMP-1 could also be reduced by lipid reduction therapy (plasma LDL apheresis) in diabetic patients with end-stage renal disease and arteriosclerosis obliterans (CitationNakamura et al 2003). Multifactorial cardiovascular risk reduction therapy with intensive glucose control and statin therapy caused significant reductions in circulating TIMP-1 levels (CitationTayebjee et al 2004). This effect was probably independent of blood pressure lowering, as the latter was relatively well controlled from the outset and did not fall significantly.
Current evidence for clinical utility of markers of fibrosis in diabetes
Diabetic renal disease
Type IV collagen in the circulation or urine has been identified as a possible indicator of renal injuries, especially in early stages of diabetic nephropathy, in numerous, albeit relatively small studies (CitationWatanabe et al 1991; CitationKotajima et al 2000; CitationXu et al 2002; CitationTashiro et al 2004). Both serum and urinary type IV collagen increased in accordance with the clinical stage of the renal disease (CitationWatanabe et al 1991, Citation2000; CitationXu et al 2002; CitationTashiro et al 2004). Serum carboxy-terminal propeptide type I procollagen (P1CP) levels may also reflect the progression of diabetic nephropathy in patients with type 2 diabetes (CitationInukai et al 2000). In type 1 diabetes, measurement of syndecan-1 in serum has shown significant increase even at the microalbuminuric stage compared to normoalbuminuric patients (CitationSvennevig et al 2006). TGF-β levels in serum were increased in patients with diabetic nephropathy (CitationSharma et al 1999) and decreased with ACEI (CitationEllis et al 1998; CitationSharma et al 1999).
Urinary levels of collagen IV positively correlated with uPA, and that of fibronectin negatively correlated with PAI-1 in the diabetic patients with microalbuminuria (CitationWoo et al 2006). Urinary TGF-β was significantly increased in type 2 diabetic patients with micro- or macroalbuminuria. ACEI, ARB, and TZD significantly reduced urinary excretion of TGF-β in these patients (CitationHoulihan et al 2002; CitationPraga et al 2003; CitationMatos et al 2005; CitationKatavetin et al 2006; CitationSong et al 2006; CitationWoo et al 2006).
Increasing evidence has emerged on circulating and urinary CTGF as indicators of renal disease in diabetes. Elevated plasma CTGF concentrations have been found in diabetic nephropathy. CTGF levels in the circulation correlated with urinary albumin excretion, creatinine clearance, glycemic control and duration of diabetes (CitationRoestenberg et al 2004). Of note, there was a wide overlap in plasma CTGF between normoalbuminuric patients and those with diabetic nephropathy. Urinary concentrations of CTGF (U-CTGF) has been more extensively investigated than circulating CTGF, in diabetic renal disease (CitationGilbert et al 2003; CitationRiser et al 2003; CitationNguyen et al 2006). U-CTGF was correlated with clinical markers of renal disease severity (urinary albumin excretion rate and glomerular filtration rate). Furthermore, the association of U-CTGF with diabetic nephropathy was comparable with that of the established risk factors: HbA1c and systemic blood pressure. Significantly higher in patients with microalbuminuria or overt nephropathy, urinary CTGF excretion varied largely across the studies, from 1.6-fold (CitationNguyen et al 2006) to 6-fold (CitationRiser et al 2003) and were 10–100-fold higher (CitationGilbert et al 2003) in diabetic nephropathy versus normoalbuminuric subjects. Large overlaps in U-CTGF were also noted between patients and controls (CitationNguyen et al 2006). In patients with DN, U-CTGF correlated positively with urinary albumin excretion and negatively with GFR (CitationNguyen et al 2006). It is unclear to what extent plasma CTGF levels contribute to U-CTGF. CTGF and its fragments are predicted to be cleared from plasma by glomerular filtration.
Animal studies have shown increased CTGF mRNA in renal cortex in a very early phase of nephropathy, which paralleled an increase in plasma and U-CTGF (CitationRoestenberg et al 2006). Local production of CTGF in the kidney, renal filtration of (elevated) plasma CTGF, together with tubular dysfunction and/or saturation of tubular reabsorption capacity in proteinuric patients may all be involved in increased U-CTGF (CitationNguyen et al 2006). Interventional studies have shown that in type 1 diabetes with nephropathy, RAS blockade with ARB significantly decreased U-CTGF concentration, and this reduction was associated with a slower rate of decline in GFR in a cohort of hypertensive type 1 diabetic patients with diabetic kidney disease (CitationAndersen et al 2005). However, plasma CTGF remained unchanged throughout the study, suggesting that circulating CTGF is at least partly independent of U-CTGF and renal dysfunction.
In diabetic patients, plasma VEGF levels were found to be positively correlated with plasma urea and urinary ACR, and urinary VEGF was positively correlated with urinary ACR and creatinine. Urinary VEGF and serum creatinine were independently correlated with urinary ACR (CitationKim et al 2004). Urinary excretion of VEGF increased during the earlier stage of diabetic nephropathy and was significantly correlated with urinary albumin excretion. This suggests that urinary VEGF might be used as a sensitive marker of diabetic nephropathy and for predicting disease progression (CitationKim et al 2004). In addition, another study implicated the potential of plasma VEGF as a DN marker (CitationBaba et al 2004).
Dysregulations in circulating and urinary MMP/TIMP systems have been found in diabetic renal disease. Thus, increased levels of plasma MMP-9 have been shown in DN patients (CitationNakamura et al 2000; CitationZaoui et al 2000), and were significantly reduced by ACEI treatment (CitationNakamura et al 2000). Moreover, it seems that elevation in plasma MMP-9 may precede the onset of microalbuminuria in type 2 diabetic patients (CitationEbihara et al 1998). Higher levels of MMP-9 were also found in the urine of type 2 diabetic patients with macroalbuminuria (CitationTashiro et al 2004). Interestingly, urinary MMP-9 levels were elevated not only in patients with diabetic renal disease but also in their first-degree relatives when compared with healthy controls (CitationZaoui et al 2000). It has been suggested that increased urinary MMP-2 and MMP-9 activities, but not serum MMP levels, may be sensitive markers of the extent of renal disease in type 1 diabetic patients (CitationTashiro et al 2004). Urine MMP-2 concentrations correlated with higher HbA1c levels, longer duration of disease, evidence of renal hyperfiltration, and the presence of microalbuminuria (CitationThrailkill et al 2007).
Another study has shown a significant increase in urinary TIMP-1 in association with urinary albumin and the progress of glomerular diffuse lesions, while no correlation between serum TIMP-1 and urinary TIMP-1 was found (CitationKanauchi et al 1996). TIMP-1 was also increased in the urine in a group of nondiabetic patients with chronic renal disease and was correlated with progressive reduction in renal function, but not with proteinuria (CitationHorstrup et al 2002). In an animal model urinary protein excretion showed a significant positive correlation with glomerular and tubular TIMP-2 protein, and a negative correlation with MMP-2 expression (CitationHan et al 2006).
Diabetic retinopathy
Increased synthesis of type III collagen (serum PIIINP), reflecting deposition of matrix and BM connective tissue, has been reported in patients with DPR (CitationArkkila et al 2001). While some investigators have found no significant differences of serum carboxy-terminal propeptide of human type I procollagen (PICP) across the differing severity of diabetic retinopathy in type 2 diabetic patients (CitationInukai et al 2000), others have actually observed progressively decreased levels of serum PICP, which can result in weakened vascular integrity in subjects with retinopathy (CitationArkkila et al 2001).
Diabetic retinopathy is associated with increased concentrations of type IV collagen in serum (CitationYano et al 1998; CitationKotajima et al 2001). Although in the vitreous the NH2-terminal CTGF fragment content was increased in patients with active PDR, suggesting that it may play a pathogenic role or may represent a surrogate marker of CTGF activity in DR (CitationHinton et al 2004), the elevated level of plasma CTGF found in the circulation in patients with DR compared with patients without DR seems likely to be due to associated nephropathy rather than to the retinopathy itself (CitationRoestenberg et al 2004). In addition, no changes in urinary CTGF were noted in DR (CitationNguyen et al 2006).
Increased plasma levels of VEGF and Ang II were found in diabetic patients, with the highest VEGF and Ang II levels being seen among patients with pre-proliferative and proliferative retinopathy (CitationLip et al 2004). The clinical utility of plasma VEGF levels after photocoagulation has yielded contradictory results (CitationLip et al 2000; CitationLip et al 2004).
Diabetic patients with retinopathy also displayed elevated systemic values of MMP-9 and MMP-9/TIMP-1 ratio when compared with patients without retinopathy. Logistic regression analysis identified diabetes duration firstly, and MMP-9 serum levels secondly as significant and independent variables associated with the existence of retinopathy in type 1 diabetic patients who were free of other overt vascular complications (CitationJacqueminet et al 2006).
Diabetic cardiomyopathy, heart failure
A strong correlation has been reported between myocardial collagen content and serum concentration of PICP in systemic hypertension (CitationQuerejeta et al 2000). Moreover, serum PICP has been found to be secreted by the heart via the coronary sinus in patients with hypertensive heart disease (CitationQuerejeta et al 2004). Thus, even if these markers could be released from various other tissues in diabetes or hypertension, the measurement of serum collagen degradation products may offer a reasonable evaluation of myocardial ECM changes in diabetes. In a highly selected group of uncomplicated type 2 diabetic patients, parameters of myocardial function were positively correlated with glutathione peroxidase and serum PICP, but not with levels of angiotensin II, aldosterone or endothelin-1 (CitationGonzalez-Vilchez et al 2005). Serum propeptide of procollagen type I (PIP) appears an independent predictor of new heart failure episodes, readmission and death and a single serum measurement of PIP may have prognostic value in patients presenting with decompensated heart failure (CitationRuiz-Ruiz et al 2007).
In hypertensive subjects, plasma TIMP-1 levels were increased and associated with LVH and LV diastolic impairment in some (CitationLaviades et al 1998; CitationLindsay et al 2002; CitationTimms et al 2002) but not all studies (CitationLi-Saw-Hee et al 2000). Treatment of hypertension with ACEI has been shown to decrease TIMP-1 levels (CitationLaviades et al 1998). Previous studies of small samples of patients with heart failure or LV dilatation have yielded inconsistent results, with both increased and decreased levels of myocardial or serum TIMP-1 being reported. (CitationLi et al 1998; CitationSchwartzkopff et al 2002). The Framingham Heart Study (CitationSundstrom et al 2004) has shown that plasma total TIMP-1 is directly related to major CVD risk factors and to echocardiographic indices of LVH, and inversely to systolic dysfunction.
It has also been suggested that elevated TIMP-1 might be a useful noninvasive marker of left ventricular diastolic dysfunction and fibrosis (CitationLindsay et al 2002). Serum TIMP-1 concentrations over 500 ng/ml showed good specificity and positive predictive value for detecting diastolic dysfunction among untreated patients with hypertension (CitationLindsay et al 2002). In an asymptomatic population with either diabetes or hypertension, but with no evidence of LV hypertrophy, plasma TIMP-1 negatively correlated with e’ (early diastolic velocity at the annulus). This suggests that the higher circulating levels of TIMP-1 may reflect structural changes within the heart that result in diastolic dysfunction. The correlation was however stronger among the hypertensive patients when compared with the diabetic group, suggesting again that systemic hypertension may mainly mediate the TIMP-1 and diastolic dysfunction link (CitationTayebjee et al 2005a).
Increased serum levels of AGEs were associated with heart stiffness in patients with type 1 diabetes, possibly mediated by the cross-linking properties of AGEs (CitationBerg et al 1999).
Atherosclerosis and arteriosclerosis
Plasma plasminogen activator inhibitor (PAI)-1, a potent inhibitor of fibrinolysis, was elevated in a number of clinical situations that are associated with high incidence of cardiovascular disease (CVD) (obesity, hypertension, type 2 diabetes) (CitationHoekstra et al 2004).
Serum 7S-collagen levels in diabetic patients with essential hypertension were significantly higher than in normal subjects, and significantly correlated with systolic blood pressure. Thus it has been suggested that the metabolic alteration of basement membrane occurring in patients with diabetes mellitus may worsen in the presence of high systolic blood pressure (CitationYano et al 1997). In hypertensive type 2 diabetic patients there were also noted an imbalance between serum MMP-1 which was decreased, and its tissue inhibitor, TIMP-1 which was not significantly changed compared to controls (CitationAlla et al 2006).
Diabetic patients with acute coronary syndromes showed increased plasma levels of MMP-9, TIMP-1, and TIMP-2 (CitationDerosa et al 2007). A high percent of patients with coronary artery disease (CAD) or acute coronary syndromes (ACS) had elevated urine values of MMP-9 and TIMP-1 suggesting that these variables might be a useful marker of atherosclerotic disease (CitationFitzsimmons et al 2006). Plasma levels of MMP-9, TIMP-2, but not TIMP-1 were high in patients with stable CAD compared with healthy controls. However, no correlation with severity of CAD or collateralization was found (CitationTayebjee et al 2005b).
In patients with premature coronary atherosclerosis, the levels of plasma MMP-9 and TIMP-1 were significantly higher, and the levels of MMP-2, MMP-3, and TIMP-2 were significantly lower than those of controls, with significant positive correlation between plasma MMP-9 levels and low-density lipoprotein (LDL)-cholesterol levels, and significant negative correlation between plasma MMP-9 levels and high-density lipoprotein (HDL)-cholesterol levels. TIMP-2 levels were negatively correlated with total cholesterol and LDL-cholesterol levels (CitationNoji et al 2001).
In patients with CAD the low TGF-β group had a significantly poor prognosis in terms of survival without cardiovascular events and survival without coronary interventions as compared with the high TGF-β group, while other prognoses were comparable between the two groups. These results suggest that lower plasma concentrations of TGF-β may have an adverse prognostic significance in patients with CAD (CitationTashiro et al 2002). In another study, plasma TGF-β levels were significantly lower in patients with ischemic heart disease than they were in controls (CitationTashiro et al 1997).
Liver fibrosis
The serum glyceraldehyde-derived AGEs level may be a useful biomarker for discriminating NASH from simple steatosis. Moreover, it correlated with adiponectin (CitationHyogo et al 2007). Serum hyaluronic acid could identify NAFLD in patients with severe fibrosis in some (CitationSakugawa et al 2005; CitationKaneda et al 2006) , but not all studies (CitationYoneda et al 2007). Type IV collagen 7 s domain and type IV collagen 7s domain concentrations in the circulation have been identified as potential markers in differentiating between NASH and NAFLD (CitationSakugawa et al 2005), and between advanced and mild liver fibrosis (CitationYoneda et al 2007). Serum levels of endothelin-1 (ET-1), an inflammatory and marker of increased endothelial tone, also showed a significant positive correlation with liver fibrosis severity in patients with NASH (CitationDegertekin et al 2007).
TIMP-1 and MMP-1 levels in serum or peripheral blood mononuclear cells (PBMCs) seem to have some value in assessing liver fibrosis. The combination of serum PDGF-BB, TIMP-1 mRNA and TIMP-1mRNA/MMP-1mRNA ratio in PBMCs was suggested as an efficient test in screening for the presence of liver fibrosis (CitationZhang et al 2003).
Research required in circulating and urinary markers of fibrosis in diabetes
Important issues appear when interpreting levels of the ECM markers and their regulators in blood or urine from diabetic patients. First, it is not yet clear whether the assessment of these markers in biological fluids provide clinically valuable information of the fibrosis process in the affected organs and whether tissue levels are reflected accurately in blood or urine. Factors such as the relative contribution of a tissue to a particular circulating marker and also the rate of clearance of the marker from the circulation and its dependence on renal function can have significant effects on its level. Nevertheless, the fact that different therapies impact upon their levels indicates that they may be involved in the pathological process.
A second issue is the presence of conditions that commonly co-exist with diabetes, such as hypertension and/or dyslipidemia, which could also influence the levels of circulating/urinary markers of fibrosis. The differentiation between the contribution of diabetes and other co-morbidities to the total level of ECM markers in blood/urine could therefore be difficult to assess.
summarizes the main findings of circulating and urinary markers and their regulators and their potential value in diabetes complications. At this time, Type IV collagen and CTGF in plasma and urine hold promise in diabetic renal disease, MMP-9 has promise in acute coronary syndromes as does TIMP-1 and also in myocardial dysfunction. Longitudinal and large studies in appropriate populations of people with diabetes, will be needed to further investigate the possible clinical value of these and other ECM components and ECM regulators as makers for incipient or progressive diabetes complications. Ideally, such markers in their natural history and after therapy, would show predictive value independent of other common clinical variables such as hypertension, degree of glycemic control, and microalbuminuria. It is envisaged that clinically verified algorithms will be generated where automated plasma or urine measures will aid in the calculation of risk of tissue fibrosis, and related organ dysfunction. It may be that other, tissue specific measures such as BNP for the heart or liver function tests for liver fibrosis, will be useful in combination with such ECM markers. Urinary measures do appear to often be independent of circulating levels, although whether such measures have advantage over albuminuria and estimated GFR levels, remains to be determined.
References
- Abdel-WahabNWestonBSRobertsTConnective tissue growth factor and regulation of the mesangial cell cycle: role in cellular hypertrophyJ Am Soc Nephrol20021324374512239232
- AbreuJGKetpuraNIReversadeBConnective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-betaNat Cell Biol2002459960412134160
- Abu El-AsrarAMVan den SteenPEAl-AmroSAExpression of angiogenic and fibrogenic factors in proliferative vitreoretinal disordersInt Ophthalmol200727112217375263
- AdlerSGKangSWFeldSGlomerular mRNAs in human type 1 diabetes: biochemical evidence for microalbuminuria as a manifestation of diabetic nephropathyKidney Int2001602330611737607
- AlessiMCJuhan-VagueIContribution of PAI-1 in cardiovascular pathologyArch Mal Coeur Vaiss200497673815283042
- AllaFKearney-SchwartzARadauceanuAEarly changes in serum markers of cardiac extra-cellular matrix turnover in patients with uncomplicated hypertension and type II diabetesEur J Heart Fail200681475316198628
- AndersenSvan NieuwenhovenFATarnowLReduction of urinary connective tissue growth factor by Losartan in type 1 patients with diabetic nephropathyKidney Int2005672325915882275
- AnguloPNonalcoholic fatty liver diseaseN Engl J Med200234612213111961152
- ArkkilaPERonnemaaTKoskinenPJBiochemical markers of type III and I collagen: association with retinopathy and neuropathy in type 1 diabetic subjectsDiabet Med2001188162111678972
- AzarSTSaltiIZantoutMSAlterations in plasma transforming growth factor beta in normoalbuminuric type 1 and type 2 diabetic patientsJ Clin Endocrinol Metab2000854680211134127
- BabaTOsterbyRNeugebauer-BabaSNo nephropathy in Type 2 diabetic patient with POEMS syndrome with an elevated plasma VEGFDiabet Med200421292415008843
- BaeldeHJEikmansMLappinDWReduction of VEGF-A and CTGF expression in diabetic nephropathy is associated with podocyte lossKidney Int2007716374517264876
- BatallerRSancho-BruPGinesPActivated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin IIGastroenterology20031251172512851877
- BergTJSnorgaardOFaberJSerum levels of advanced glycation end products are associated with left ventricular diastolic function in patients with type 1 diabetesDiabetes Care19992211869010388987
- BishopJELaurentGJCollagen turnover and its regulation in the normal and hypertrophying heartEur Heart J199516Suppl C38447556271
- BlomIEvan DijkAJWietenLIn vitro evidence for differential involvement of CTGF, TGFbeta, and PDGF-BB in mesangial response to injuryNephrol Dial Transplant20011611394811390712
- BorderWANobleNAInteractions of transforming growth factor-beta and angiotensin II in renal fibrosisHypertension19983118189453300
- BrigstockDRRegulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61)Angiogenesis200251536512831056
- BrillaCGScheerCRuppHRenin-angiotensin system and myocardial collagen matrix: modulation of cardiac fibroblast function by angiotensin II type 1 receptor antagonismJ Hypertens Suppl199715S1399493122
- BrownleeMBiochemistry and molecular cell biology of diabetic complicationsNature20014148132011742414
- BrownleeMSpiroRGBiochemistry of the basement membrane in diabetes mellitusAdv Exp Med Biol197912414156388998
- BrownleeMCeramiAVlassaraHAdvanced glycosylation end products in tissue and the biochemical basis of diabetic complicationsN Engl J Med19883181315213283558
- BugianesiELeoneNVanniEExpanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinomaGastroenterology20021231344012105842
- BursellSEClermontACOrenBThe in vivo effect of endothelins on retinal circulation in nondiabetic and diabetic ratsInvest Ophthalmol Vis Sci1995365966077890491
- CandidoRForbesJMThomasMCA breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changesCirc Res2003927859212623881
- ChaDRKangYSHanSYRole of aldosterone in diabetic nephropathyNephrology (Carlton)200510SupplS37916174286
- ChakrabartiSGanXTMerryAAugmented retinal endothelin-1, endothelin-3, endothelin A and endothelin B gene expression in chronic diabetesCurr Eye Res19981730179543639
- ChakravarthyUHayesRGStittAWEndothelin expression in ocular tissues of diabetic and insulin-treated ratsInvest Ophthalmol Vis Sci1997382144519331278
- ChaqourBYangRShaQMechanical Stretch Modulates the Promoter Activity of the Profibrotic Factor CCN2 through Increased Actin Polymerization and NF-{kappa}B ActivationJ Biol Chem2006281206082216707502
- CharltonMNonalcoholic fatty liver disease: a review of current understanding and future impactClin Gastroenterol Hepatol2004210485815625647
- ChaturvediNFullerJHPokrasFCirculating plasma vascular endothelial growth factor and microvascular complications of type 1 diabetes mellitus: the influence of ACE inhibitionDiabet Med2001182889411437859
- ChaturvediNSjolieAKStephensonJMEffect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes MellitusLancet199835128319433426
- ChenSKhanZAKarmazynMRole of endothelin-1, sodium hydrogen exchanger-1 and mitogen activated protein kinase (MAPK) activation in glucose-induced cardiomyocyte hypertrophyDiabetes Metab Res Rev2007233566717024690
- CichaIYilmazAKleinMConnective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitroArterioscler Thromb Vasc Biol20052510081315761189
- CohenMPShearmanCWLautenslagerGTSerum type IV collagen in diabetic patients at risk for nephropathyDiabetes Care2001241324711473064
- CookerLAPetersonDRambowJTNF-{alpha}, but not IFN-{gamma}, regulates CCN2 (CTGF), collagen type I, and proliferation in mesangial cells: possible roles in the progression of renal fibrosisAm J Physiol Renal Physiol2007293F1576517376761
- CreanJKFinlayDMurphyMThe role of p42/44 MAPK and protein kinase B in connective tissue growth factor induced extracellular matrix protein production, cell migration, and actin cytoskeletal rearrangement in human mesangial cellsJ Biol Chem2002277441879412218048
- D’ArmientoJMatrix metalloproteinase disruption of the extracellular matrix and cardiac dysfunctionTrends Cardiovasc Med2002129710112007733
- DasAKDasJPChandrasekarSSpecific heart muscle disease in diabetes mellitus – a functional structural correlationInt J Cardiol1987172993023679609
- de VrieseASTiltonRGElgerMAntibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetesJ Am Soc Nephrol200112993100011316858
- DeathAKFisherEJMcGrathKCHigh glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact on atherosclerosis in diabetesAtherosclerosis2003168263912801609
- DegertekinBOzenirlerSElbegSThe Serum Endothelin-1 Level in Steatosis and NASH, and Its Relation with Severity of Liver FibrosisDig Dis Sci2007411
- DerosaGCiceroAFScaliseFMetalloproteinase-2 and -9 in diabetic and nondiabetic subjects during acute coronary syndromesEndothelium200714455117364896
- DerubertisFRCravenPAActivation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathyDiabetes199443188262306
- DescampsFJMartensEKangaveDThe activated form of gelatinase B/matrix metalloproteinase-9 is associated with diabetic vitreous hemorrhageExp Eye Res200683401716643893
- Di PaoloSGesualdoLRanieriEHigh glucose concentration induces the overexpression of transforming growth factor-beta through the activation of a platelet-derived growth factor loop in human mesangial cellsAm J Pathol199614920951068952542
- DingYZouRJuddRLEndothelin-1 receptor blockade prevented the electrophysiological dysfunction in cardiac myocytes of streptozotocin-induced diabetic ratsEndocrine200630121717185800
- DresowBDelbruckAThe isolation and activity of growth-stimulating factors from human plateletsJ Clin Chem Clin Biochem198422527336238121
- DuncanMRFrazierKSAbramsonSConnective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMPFaseb J19991317748610506580
- EbiharaINakamuraTShimadaNIncreased plasma metalloproteinase-9 concentrations precede development of microalbuminuria in noninsulin-dependent diabetes mellitusAm J Kidney Dis199832544509774113
- EdwardsIJWagnerJDVogl-WillisCAArterial heparan sulfate is negatively associated with hyperglycemia and atherosclerosis in diabetic monkeysCardiovasc Diabetol20043615117408
- EfratiSBermanSGoldfingerNEnhanced angiotensin II production by renal mesangium is responsible for apoptosis/proliferation of endothelial and epithelial cells in a model of malignant hypertensionJ Hypertens20072510415217414669
- EllisDForrestKYErbeyJUrinary measurement of transforming growth factor-beta and type IV collagen as new markers of renal injury: application in diabetic nephropathyClin Chem19984495069590367
- EngeliSGorzelniakKKreutzRCo-expression of renin-angiotensin system genes in human adipose tissueJ Hypertens1999175556010404958
- FactorSMOkunEMMinaseTCapillary microaneurysms in the human diabetic heartN Engl J Med198030238487351930
- FarquharAMacDonaldMKIrelandJTThe role of fibrin deposition in diabetic glomerulosclerosis: a light, electron and immunofluorescence microscopy studyJ Clin Pathol197225657674561948
- FinckenbergPInkinenKAhonenJAngiotensin II induces connective tissue growth factor gene expression via calcineurin-dependent pathwaysAm J Pathol20031633556612819040
- FiorettoPMauerMHistopathology of diabetic nephropathySemin Nephrol20072719520717418688
- FisherEJMcLennanSVYueDKHigh glucose reduces generation of plasmin activity by mesangial cellsMicrovasc Res19975317389143550
- FitzsimmonsPJForoughRLawrenceMEUrinary levels of matrix metalloproteinase 9 and 2 and tissue inhibitor of matrix metalloproteinase in patients with coronary artery diseaseAtherosclerosis2006828
- FleischmajerRPerlishJSTimplRCollagen fibrillogenesis in human skinAnn N Y Acad Sci1985460246573914229
- FloresLNafSHernaezRTransforming growth factor beta at clinical onset of Type 1 diabetes mellitus. A pilot studyDiabet Med2004218182215270783
- ForbesJMCooperMEThallasVReduction of the accumulation of advanced glycation end products by ACE inhibition in experimental diabetic nephropathyDiabetes20025132748212401719
- FrazierKWilliamsSKothapalliDStimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factorJ Invest Dermatol1996107404118751978
- FukudaGKhanZABarbinYPEndothelin-mediated remodeling in aortas of diabetic ratsDiabetes Metab Res Rev2005213677515580650
- FukuiMNakamuraTEbiharaIECM gene expression and its modulation by insulin in diabetic ratsDiabetes199241152071280237
- GeigerMBinderBRPlasminogen activation in diabetes mellitus. Kinetics of plasmin formation with tissue plasminogen activator and plasminogen from individual diabetic donors and with in vitro glucosylated plasminogenEnzyme198840149572971532
- GendaAMizunoSNunodaSClinical studies on diabetic myocardial disease using exercise testing with myocardial scintigraphy and endomyocardial biopsyClin Cardiol19869375823731563
- GeorgeJTsutsumiMsiRNA-mediated knockdown of connective tissue growth factor prevents N-nitrosodimethylamine-induced hepatic fibrosis in ratsGene Ther20071479080317344905
- GilbertREAkdenizAWeitzSUrinary connective tissue growth factor excretion in patients with type 1 diabetes and nephropathyDiabetes Care2003262632612941731
- GilbertRECoxAWuLLExpression of transforming growth factor-beta1 and type IV collagen in the renal tubulointerstitium in experimental diabetes: effects of ACE inhibitionDiabetes199847414229519748
- GlickADJacobsonHRHaralsonMAMesangial deposition of type I collagen in human glomerulosclerosisHum Pathol199223137391468774
- GoldinABeckmanJASchmidtAMAdvanced glycation end products: sparking the development of diabetic vascular injuryCirculation200611459760516894049
- GomezDEAlonsoDFYoshijiHTissue inhibitors of metalloproteinases: structure, regulation and biological functionsEur J Cell Biol199774111229352216
- Gomez-GarreDRuiz-OrtegaMOrtegoMEffects and interactions of endothelin-1 and angiotensin II on matrix protein expression and synthesis and mesangial cell growthHypertension199627885928613264
- Gonzalez-VilchezFAyuelaJAresMOxidative stress and fibrosis in incipient myocardial dysfunction in type 2 diabetic patientsInt J Cardiol200510153815860383
- Gore-HyerEShegogueDMarkiewiczMTGF-beta and CTGF have overlapping and distinct fibrogenic effects on human renal cellsAm J Physiol Renal Physiol2002283F7071612217862
- GreeneDALattimerSASimaAASorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complicationsN Engl J Med19873165996063027558
- GressnerOALahmeBDemirciIDifferential effects of TGF-beta on connective tissue growth factor (CTGF/CCN2) expression in hepatic stellate cells and hepatocytesJ Hepatol2007626
- GrotendorstGROkochiHHayashiNA novel transforming growth factor beta response element controls the expression of the connective tissue growth factor geneCell Growth Differ19967469809052988
- GuhaMXuZGTungDSpecific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetesFASEB J20072133556817554073
- HaffnerSMGreenbergASWestonWMEffect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitusCirculation20021066798412163427
- HagiwaraHKaizuKUriuKExpression of type-1 plasminogen activator inhibitor in the kidney of diabetic rat modelsThromb Res2003111301914693179
- HanSYJeeYHHanKHAn imbalance between matrix metalloproteinase-2 and tissue inhibitor of matrix metalloproteinase-2 contributes to the development of early diabetic nephropathyNephrol Dial Transplant20062124061616728425
- HanefeldMMarxNPfutznerAAnti-inflammatory effects of pioglitazone and/or simvastatin in high cardiovascular risk patients with elevated high sensitivity C-reactive protein: the PIOSTAT StudyJ Am Coll Cardiol200749290717239709
- HannekenAde JuanEJrLuttyGAAltered distribution of basic fibroblast growth factor in diabetic retinopathyArch Ophthalmol19911091005112064554
- HargroveGMDufresneJWhitesideCDiabetes mellitus increases endothelin-1 gene transcription in rat kidneyKidney Int20005815344511012888
- HashimotoGInokiIFujiiYMatrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165J Biol Chem2002277362889512114504
- HaydenMRSowersJRTyagiSCThe central role of vascular extracellular matrix and basement membrane remodeling in metabolic syndrome and type 2 diabetes: the matrix preloadedCardiovasc Diabetol20054915985157
- Heart Outcomes Prevention Evaluation Study InvestigatorsEffects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudyLancet2000355253910675071
- HintonDRSpeeCHeSAccumulation of NH2-terminal fragment of connective tissue growth factor in the vitreous of patients with proliferative diabetic retinopathyDiabetes Care2004277586414988298
- HishikawaKOemarBSTannerFCOverexpression of connective tissue growth factor gene induces apoptosis in human aortic smooth muscle cellsCirculation199910021081210562268
- HocherBSchwarzAReinbacherDEffects of endothelin receptor antagonists on the progression of diabetic nephropathyNephron200187161911244312
- HoekstraTGeleijnseJMSchoutenEGPlasminogen activator inhibitor-type 1: its plasma determinants and relation with cardiovascular riskThromb Haemost2004918617215116245
- HorstrupJHGehrmannMSchneiderBElevation of serum and urine levels of TIMP-1 and tenascin in patients with renal diseaseNephrol Dial Transplant20021710051312032189
- HosodaYOkadaMMatsumuraMEpiretinal membrane of proliferative diabetic retinopathy: an immunohistochemical studyOphthalmic Res199325289948259261
- HoulihanCAAkdenizATsalamandrisCUrinary transforming growth factor-beta excretion in patients with hypertension, type 2 diabetes, and elevated albumin excretion rate: effects of angiotensin receptor blockade and sodium restrictionDiabetes Care2002251072712032117
- HuaHGoldbergHJFantusIGHigh glucose-enhanced mesangial cell extracellular signal-regulated protein kinase activation and alpha1(IV) collagen expression in response to endothelin-1: role of specific protein kinase C isozymesDiabetes20015023768311574422
- HueberAWiedemannPEsserPBasic fibroblast growth factor mRNA, bFGF peptide and FGF receptor in epiretinal membranes of intraocular proliferative disorders (PVR and PDR)Int Ophthalmol199620345509237137
- HughesJMKuiperEJKlaassenIAdvanced glycation end products cause increased CCN family and extracellular matrix gene expression in the diabetic rodent retinaDiabetologia20075010899817333105
- HughesAKStricklettPKPadillaEEffect of reactive oxygen species on endothelin-1 production by human mesangial cellsKidney Int19964918198770966
- HuntJVSmithCCWolffSPAutoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucoseDiabetes199039142042227114
- HyogoHYamagishiSIwamotoKElevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitisJ Gastroenterol Hepatol2007221112917559366
- IgarashiAOkochiHBradhamDMRegulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repairMol Biol Cell19934637458374172
- IkedaSMakinoHHaramotoTChanges in glomerular extracellular matrices components in diabetic nephropathyJ Diabet Complications1991518681770041
- InukaiTFujiwaraYTayamaKSerum levels of carboxy-terminal propeptide of human type I procollagen are an indicator for the progression of diabetic nephropathy in patients with type 2 diabetes mellitusDiabetes Res Clin Pract20004823810704696
- IoachimEStefaniotouMGorezisSImmunohistochemical study of extracellular matrix components in epiretinal membranes of vitreoproliferative retinopathy and proliferative diabetic retinopathyEur J Ophthalmol2005153849115945009
- IshimuraENishizawaYShojiSSerum type III, IV collagens and TIMP in patients with type II diabetes mellitusLife Sci199658133178614290
- JacqueminetSBen AbdesselamOChapmanMJElevated circulating levels of matrix metalloproteinase-9 in type 1 diabetic patients with and without retinopathyClin Chim Acta2006367103716426593
- JensenLTHorslev-PetersenKToftPSerum aminoterminal type III procollagen peptide reflects repair after acute myocardial infarctionCirculation1990815272297848
- JerdanJAGlaserBMRetinal microvessel extracellular matrix: an immunofluorescent studyInvest Ophthalmol Vis Sci1986271942033510998
- KagamiSBorderWAMillerDEAngiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cellsJ Clin Invest199493243178200978
- KakizawaHItohMItohYThe relationship between glycemic control and plasma vascular endothelial growth factor and endothelin-1 concentration in diabetic patientsMetabolism200453550515131756
- KanauchiMNishiokaHNakashimaYRole of tissue inhibitors of metalloproteinase in diabetic nephropathyNippon Jinzo Gakkai Shi19963812488721333
- KanedaHHashimotoEYatsujiSHyaluronic acid levels can predict severe fibrosis and platelet counts can predict cirrhosis in patients with nonalcoholic fatty liver diseaseJ Gastroenterol Hepatol20062114596516911693
- KatavetinPEiam-OngSSuwanwalaikornSPioglitazone reduces urinary protein and urinary transforming growth factor-beta excretion in patients with type 2 diabetes and overt nephropathyJ Med Assoc Thai200689170716579002
- KawasakiDKosugiKWakiHRole of activated renin-angiotensin system in myocardial fibrosis and left ventricular diastolic dysfunction in diabetic patients – reversal by chronic angiotensin II type 1A receptor blockadeCirc J200771524917384453
- KhanZAChakrabartiSEndothelins in chronic diabetic complicationsCan J Physiol Pharmacol2003816223412839273
- KimNHKimKBKimDLPlasma and urinary vascular endothelial growth factor and diabetic nephropathy in Type 2 diabetes mellitusDiabet Med2004215455115154937
- KimYKleppelMMButkowskiRDifferential expression of basement membrane collagen chains in diabetic nephropathyAm J Pathol1991138413201992766
- KiryuKMoritaHFujitaYPhenotypic expressions of type I, III, IV, V, and VI collagens in patients with diabetic nephropathy: immunohistochemical comparison between HD and non-HD patientsNippon Jinzo Gakkai Shi199436365738022109
- KobayashiTInoueTOkadaHConnective tissue growth factor mediates the profibrotic effects of transforming growth factor-beta produced by tubular epithelial cells in response to high glucoseClin Exp Nephrol200591142115980944
- KohnoMHorioTIkedaMAngiotensin II stimulates endothelin-1 secretion in cultured rat mesangial cellsKidney Int19924286061333547
- KohnoMIkedaMJohchiMInteraction of PDGF and natriuretic peptides on mesangial cell proliferation and endothelin secretionAm J Physiol1993265E67398238492
- KoitabashiNAraiMKogureSIncreased connective tissue growth factor relative to brain natriuretic peptide as a determinant of myocardial fibrosisHypertension2007491120717372041
- KolmVSauerUOlgemoollerBHigh glucose-induced TGF-beta 1 regulates mesangial production of heparan sulfate proteoglycanAm J Physiol1996270F812218928843
- KotajimaNKandaTYuukiNType IV collagen serum and vitreous fluid levels in patients with diabetic retinopathyJ Int Med Res200129292611675902
- KotajimaNKimuraTKandaTType IV collagen as an early marker for diabetic nephropathy in non-insulin-dependent diabetes mellitusJ Diabetes Complications20001413710925061
- KuiperEJWitmerANKlaassenIDifferential expression of connective tissue growth factor in microglia and pericytes in the human diabetic retinaBr J Ophthalmol2004881082715258030
- LamSvan der GeestRNVerhagenNASecretion of collagen type IV by human renal fibroblasts is increased by high glucose via a TGF-beta-independent pathwayNephrol Dial Transplant200419169470115150349
- LamSvan der GeestRNVerhagenNAConnective tissue growth factor and igf-I are produced by human renal fibroblasts and cooperate in the induction of collagen production by high glucoseDiabetes20035229758314633859
- LaviadesCVaroNFernandezJAbnormalities of the extracellular degradation of collagen type I in essential hypertensionCirculation199898535409714110
- LeeHBHaHPlasminogen activator inhibitor-1 and diabetic nephropathyNephrology (Carlton)200510SupplS11316174279
- LeeSWSongKEShinDSAlterations in peripheral blood levels of TIMP-1, MMP-2, and MMP-9 in patients with type-2 diabetesDiabetes Res Clin Pract200569175916005367
- LeeTSSaltsmanKAOhashiHActivation of protein kinase C by elevation of glucose concentration: proposal for a mechanism in the development of diabetic vascular complicationsProc Natl Acad Sci, USA198986514152740348
- LembachKJInduction of human fibroblast proliferation by epidermal growth factor (EGF): enhancement by an EGF-binding arginine esterase and by ascorbateProc Natl Acad Sci, USA19767318371061114
- LiMChenYZhangZInhibition of mesangial cell proliferation by antisense oligodeoxynucleotide targeting preproendothelin-1 mRNA in vitroChin Med J (Engl)1999112790311717946
- LiYYFeldmanAMSunYDifferential expression of tissue inhibitors of metalloproteinases in the failing human heartCirculation1998981728349788826
- LindsayMMMaxwellPDunnFGTIMP-1: a marker of left ventricular diastolic dysfunction and fibrosis in hypertensionHypertension2002401364112154103
- LipPLBelgoreFBlannADPlasma VEGF and soluble VEGF receptor FLT-1 in proliferative retinopathy: relationship to endothelial dysfunction and laser treatmentInvest Ophthalmol Vis Sci2000412115910892852
- LipPLChatterjeeSCaineGJPlasma vascular endothelial growth factor, angiopoietin-2, and soluble angiopoietin receptor tie-2 in diabetic retinopathy: effects of laser photocoagulation and angiotensin receptor blockadeBr J Ophthalmol2004881543615548809
- Li-Saw-HeeFLEdmundsEBlannADMatrix metalloproteinase-9 and tissue inhibitor metalloproteinase-1 levels in essential hypertension. Relationship to left ventricular mass and anti-hypertensive therapyInt J Cardiol20007543711054505
- LiuXLuoFPanKHigh glucose upregulates connective tissue growth factor expression in human vascular smooth muscle cellsBMC Cell Biol20078117224075
- LiuYWangZYinWSevere insulin resistance and moderate glomerulosclerosis in a minipig model induced by high-fat/ high-sucrose/ high-cholesterol dietExp Anim200756112017283886
- LjubimovAVBurgesonREButkowskiRJBasement membrane abnormalities in human eyes with diabetic retinopathyJ Histochem Cytochem1996441469798985139
- LorenziMGerhardingerCEarly cellular and molecular changes induced by diabetes in the retinaDiabetologia20014479180411508263
- LuCHLuJXHuaGPEffects of antisense RNA of connective tissue growth factor expressing plasmid on rat liver fibrosisZhonghua Gan Zang Bing Za Zhi2007151182117362637
- MakinoHKashiharaNSugiyamaHPhenotypic changes of the mesangium in diabetic nephropathyJ Diabetes Complications1995928248573747
- MakinoHShikataKWieslanderJLocalization of fibril/microfibril and basement membrane collagens in diabetic glomerulosclerosis in type 2 diabetesDiabet Med199411304118033531
- MakinoHYamasakiYHaramotoTUltrastructural changes of extracellular matrices in diabetic nephropathy revealed by high resolution scanning and immunoelectron microscopyLab Invest19936845558423676
- MarxNFroehlichJSiamLAntidiabetic PPAR gamma-activator rosiglitazone reduces MMP-9 serum levels in type 2 diabetic patients with coronary artery diseaseArterioscler Thromb Vasc Biol200323283812588772
- MasonRMWahabNAExtracellular matrix metabolism in diabetic nephropathyJ Am Soc Nephrol20031413587312707406
- MathewsMKMergesCMcLeodDSVascular endothelial growth factor and vascular permeability changes in human diabetic retinopathyInvest Ophthalmol Vis Sci1997382729419418725
- MatosJPde Lourdes RodriguesMIsmerimVLEffects of dual blockade of the renin angiotensin system in hypertensive type 2 diabetic patients with nephropathyClin Nephrol200564180916175942
- MaxwellPRTimmsPMChandranSPeripheral blood level alterations of TIMP-1, MMP-2 and MMP-9 in patients with type 1 diabetesDiabet Med2001187778011678966
- McClainDAPatersonAJRoosMDGlucose and glucosamine regulate growth factor gene expression in vascular smooth muscle cellsProc Natl Acad Sci, USA199289815041518840
- McLennanSVFisherEMartellSYEffects of glucose on matrix metalloproteinase and plasmin activities in mesangial cells: possible role in diabetic nephropathyKidney Int Suppl200077S81710997695
- McLennanSVWangXYMorenoVConnective tissue growth factor mediates high glucose effects on matrix degradation through tissue inhibitor of matrix metalloproteinase type 1: implications for diabetic nephropathyEndocrinology200414556465515345671
- McLennanSVYueDKTurtleJREffect of glucose on matrix metalloproteinase activity in mesangial cellsNephron19987929389678429
- MigdalisINKalogeropoulouKZachariadisDSerum levels of type III procollagen peptide and peripheral vascular disease in diabetic patientsDiabetes Res Clin Pract199423179827924878
- MorganKWhartonJWebbJCCo-expression of renin-angiotensin system component genes in human atrial tissueJ Hypertens Suppl199412S1197965269
- MoriyaTGroppoliTJKimYQuantitative immunoelectron microscopy of type VI collagen in glomeruli in type I diabetic patientsKidney Int2001593172311135085
- MurphyMGodsonCCannonSSuppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor and other genes in human mesangial cellsJ Biol Chem19992745830410026205
- NaftilanAJPrattREDzauVJInduction of platelet-derived growth factor A-chain and c-myc gene expressions by angiotensin II in cultured rat vascular smooth muscle cellsJ Clin Invest1989831419242649516
- NahmanNSJrLeonhartKLCosioFGEffects of high glucose on cellular proliferation and fibronectin production by cultured human mesangial cellsKidney Int1992413964021552712
- NakamuraTFukuiMEbiharaIAbnormal gene expression of matrix metalloproteinases and their inhibitor in glomeruli from diabetic ratsRen Physiol Biochem199417316257533311
- NakamuraTMatsudaTSuzukiYEffects of low-density lipoprotein apheresis on plasma matrix metalloproteinase-9 and serum tissue inhibitor of metalloproteinase-1 levels in diabetic hemodialysis patients with arteriosclerosis obliteransAsaio J200349430412918586
- NakamuraTUshiyamaCSuzukiSUrinary excretion of podocytes in patients with diabetic nephropathyNephrol Dial Transplant20001513798310978394
- NakashimaYFujiiHSumiyoshiSEarly human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltrationArterioscler Thromb Vasc Biol20072711596517303781
- NerlichASchleicherEImmunohistochemical localization of extracellular matrix components in human diabetic glomerular lesionsAm J Pathol1991139889991928305
- NguyenTQTarnowLAndersenSUrinary connective tissue growth factor excretion correlates with clinical markers of renal disease in a large population of type 1 diabetic patients with diabetic nephropathyDiabetes Care20062983816373901
- NieuwdorpMHollemanFde GrootEPerturbation of hyaluronan metabolism predisposes patients with type 1 diabetes mellitus to atherosclerosisDiabetologia20075012889317415544
- NojiYKajinamiKKawashiriMACirculating matrix metalloproteinases and their inhibitors in premature coronary atherosclerosisClin Chem Lab Med200139380411434385
- NunodaSGendaASugiharaNQuantitative approach to the histopathology of the biopsied right ventricular myocardium in patients with diabetes mellitusHeart Vessels198514374093355
- OberhammerFAPavelkaMSharmaSInduction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1Proc Natl Acad Sci, USA1992895408121608949
- OkazakiRMatsuokaKHoriuchiAAssays of serum laminin and type III procollagen peptide for monitoring the clinical course of diabetic microangiopathyDiabetes Res Clin Pract19885163703219988
- OlgemollerBSchleicherEAlterations of glomerular matrix proteins in the pathogenesis of diabetic nephropathyClin Investig199371S139
- OtaniATakagiHOhHAngiotensin II-stimulated vascular endothelial growth factor expression in bovine retinal pericytesInvest Ophthalmol Vis Sci2000411192910752960
- PandolfiACetrulloDPolishuckRPlasminogen activator inhibitor type 1 is increased in the arterial wall of type II diabetic subjectsArterioscler Thromb Vasc Biol20012113788211498469
- ParadisVPerlemuterGBonvoustFHigh glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitisHepatology2001347384411584370
- ParkISKiyomotoHAbboudSLExpression of transforming growth factor-beta and type IV collagen in early streptozotocin-induced diabetesDiabetes199746473809032105
- ParkJYTakaharaNGabrieleAInduction of endothelin-1 expression by glucose: an effect of protein kinase C activationDiabetes20004912394810909984
- PaueksakonPReveloMPMaLJMicroangiopathic injury and augmented PAI-1 in human diabetic nephropathyKidney Int2002612142812028454
- PaulMWagnerJDzauVJGene expression of the renin-angiotensin system in human tissues. Quantitative analysis by the polymerase chain reactionJ Clin Invest1993912058648387539
- PfeifferAMiddelberg-BispingKDrewesCElevated plasma levels of transforming growth factor-beta 1 in NIDDMDiabetes Care199619111378886558
- PragaMAndradeCFLunoJAntiproteinuric efficacy of losartan in comparison with amlodipine in non-diabetic proteinuric renal diseases: a double-blind, randomized clinical trialNephrol Dial Transplant20031818061312937228
- QiWTwiggSChenXIntegrated actions of transforming growth factor-beta1 and connective tissue growth factor in renal fibrosisAm J Physiol Renal Physiol2005288F800915536170
- QuerejetaRLopezBGonzalezAIncreased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosisCirculation20041101263815313958
- QuerejetaRVaroNLopezBSerum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart diseaseCirculation200010117293510758057
- RachfalAWBrigstockDRConnective tissue growth factor (CTGF/CCN2) in hepatic fibrosisHepatol Res2003261912787797
- RatziuVBonyhayLDi MartinoVSurvival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosisHepatology20023514859312029634
- RatziuVGiralPCharlotteFLiver fibrosis in overweight patientsGastroenterology200011811172310833486
- RazzaqueMSKojiTHaradaTIdentification of type VI collagen synthesizing cells in human diabetic glomerulosclerosis using renal biopsy sectionsAnal Cell Pathol199715175819497854
- RazzaqueMSKojiTTaguchiTIn situ localization of type III and type IV collagen-expressing cells in human diabetic nephropathyJ Pathol199417413187965408
- ReganTJLyonsMMAhmedSSEvidence for cardiomyopathy in familial diabetes mellitusJ Clin Invest19776088499893679
- RiserBLCortesPDeNichiloMUrinary CCN2 (CTGF) as a possible predictor of diabetic nephropathy: preliminary reportKidney Int200364451812846740
- RiserBLCortesPYeeJMechanical strain- and high glucose-induced alterations in mesangial cell collagen metabolism: role of TGF-betaJ Am Soc Nephrol19989827369596080
- RiserBLDenichiloMCortesPRegulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosisJ Am Soc Nephrol200011253810616837
- RisteliJRisteliLAnalysing connective tissue metabolites in human serum. Biochemical, physiological and methodological aspectsJ Hepatol19952277817665854
- RobertsABSpornMBAssoianRKTransforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitroProc Natl Acad Sci, USA1986834167712424019
- RoestenbergPvan NieuwenhovenFAJolesJATemporal expression profile and distribution pattern indicate a role of connective tissue growth factor (CTGF/CCN-2) in diabetic nephropathy in miceAm J Physiol Renal Physiol2006290F13445416380465
- RoestenbergPvan NieuwenhovenFAWietenLConnective tissue growth factor is increased in plasma of type 1 diabetic patients with nephropathyDiabetes Care20042711647015111539
- RothSMichelKGressnerAM(Latent) transforming growth factor beta in liver parenchymal cells, its injury-dependent release, and paracrine effects on rat hepatic stellate cellsHepatology1998271003129537440
- RoySMaielloMLorenziMIncreased expression of basement membrane collagen in human diabetic retinopathyJ Clin Invest199493438428282817
- RoySSalaRCaglieroEOverexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memoryProc Natl Acad Sci, USA19908740482296596
- RoySSatoTParyaniGDownregulation of fibronectin overexpression reduces basement membrane thickening and vascular lesions in retinas of galactose-fed ratsDiabetes20035212293412716757
- Ruiz-OrtegaMBustosCHernandez-PresaMAAngiotensin II participates in mononuclear cell recruitment in experimental immune complex nephritis through nuclear factor-kappa B activation and monocyte chemoattractant protein-1 synthesisJ Immunol199816143099647253
- Ruiz-OrtegaMGomez-GarreDAlcazarRInvolvement of angiotensin II and endothelin in matrix protein production and renal sclerosisJ Hypertens Suppl199412S5187965275
- Ruiz-OrtegaMGonzalezSSeronDACE inhibition reduces proteinuria, glomerular lesions and extracellular matrix production in a normotensive rat model of immune complex nephritisKidney Int1995481778918587237
- Ruiz-RuizFJRuiz-LaiglesiaFJSamperiz-LegarrePPropeptide of procollagen type I (PIP) and outcomes in decompensated heart failureEur J Intern Med2007181293417338965
- RuperezMLorenzoOBlanco-ColioLMConnective tissue growth factor is a mediator of angiotensin II-induced fibrosisCirculation2003a108149950512952842
- RuperezMRuiz-OrtegaMEstebanVAngiotensin II increases connective tissue growth factor in the kidneyAm J Pathol2003b16319374714578193
- RutschowSLiJSchultheissHPMyocardial proteases and matrix remodeling in inflammatory heart diseaseCardiovasc Res2006696465616417902
- SakugawaHNakayoshiTKobashigawaKClinical usefulness of biochemical markers of liver fibrosis in patients with nonalcoholic fatty liver diseaseWorld J Gastroenterol200511255915633226
- SanyalAJAGA technical review on nonalcoholic fatty liver diseaseGastroenterology200212317052512404245
- SatoAHayashiKNaruseMEffectiveness of aldosterone blockade in patients with diabetic nephropathyHypertension20034164812511531
- SchaeferLRaslikIGroneHJSmall proteoglycans in human diabetic nephropathy: discrepancy between glomerular expression and protein accumulation of decorin, biglycan, lumican, and fibromodulinFaseb J2001155596111259366
- ScheinmanJIFishAJMichaelAFThe immunohistopathology of glomerular antigens. The glomerular basement membrane, collagen, and actomyosin antigens in normal and diseased kidneysJ Clin Invest1974541144544138529
- SchjoedtKJRossingKJuhlTRBeneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathyKidney Int2006705364216775595
- SchneebergerSAHjelmelandLMTuckerRPVascular endothelial growth factor and fibroblast growth factor 5 are colocalized in vascular and avascular epiretinal membranesAm J Ophthalmol1997124447549323936
- SchwartzkopffBFassbachMPelzerBElevated serum markers of collagen degradation in patients with mild to moderate dilated cardiomyopathyEur J Heart Fail20024439412167381
- ShanklandSJLyHThaiKGlomerular expression of tissue inhibitor of metalloproteinase (TIMP-1) in normal and diabetic ratsJ Am Soc Nephrol19967971048808115
- SharmaKEltayebBOMcGowanTACaptopril-induced reduction of serum levels of transforming growth factor-beta1 correlates with long-term renoprotection in insulin-dependent diabetic patientsAm J Kidney Dis1999348182310561136
- SharmaKJinYGuoJNeutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic miceDiabetes199645522308603776
- SharmaKZiyadehFNHyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediatorDiabetes1995441139467556948
- SharmaKZiyadehFNAlzahabiBIncreased renal production of transforming growth factor-beta1 in patients with type II diabetesDiabetes19974685499133555
- SheetzMJKingGLMolecular understanding of hyperglycemia’s adverse effects for diabetic complicationsJama200228825798812444865
- ShimizuMUmedaKSugiharaNCollagen remodelling in myocardia of patients with diabetesJ Clin Pathol1993463267679418
- ShirkRAParthasarathyNSan AntonioJDAltered dermatan sulfate structure and reduced heparin cofactor II-stimulating activity of biglycan and decorin from human atherosclerotic plaqueJ Biol Chem2000275180859210749870
- SinghRAlaviNSinghAKRole of angiotensin II in glucose-induced inhibition of mesangial matrix degradationDiabetes19994820667310512375
- SipersteinMDUngerRHMadisonLLStudies of muscle capillary basement membranes in normal subjects diabetic, and prediabetic patientsJ Clin Invest1968471973995675423
- SongJHChaSHLeeHJEffect of low-dose dual blockade of renin-angiotensin system on urinary TGF-beta in type 2 diabetic patients with advanced kidney diseaseNephrol Dial Transplant200621683916330466
- SongWErgulAType-2 diabetes-induced changes in vascular extracellular matrix gene expression: relation to vessel sizeCardiovasc Diabetol20065316503991
- SpirinKSSaghizadehMLewinSLBasement membrane and growth factor gene expression in normal and diabetic human retinasCurr Eye Res199918490910435836
- StokesMBHollerSCuiYExpression of decorin, biglycan, and collagen type I in human renal fibrosing diseaseKidney Int2000574879810652025
- SugimotoKTsuruokaSFujimuraARenal protective effect of YM598, a selective endothelin ET(A) receptor antagonist, against diabetic nephropathy in OLETF ratsEur J Pharmacol2002450183912206857
- SugimotoREnjojiMKohjimaMHigh glucose stimulates hepatic stellate cells to proliferate and to produce collagen through free radical production and activation of mitogen-activated protein kinaseLiver Int20052510182616162162
- SundstromJEvansJCBenjaminEJRelations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham heart studyEur Heart J20042515091615342170
- SungSHZiyadehFNWangABlockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in miceJ Am Soc Nephrol200617309310416988063
- SvennevigKKolsetSOBangstadHJIncreased syndecan-1 in serum is related to early nephropathy in type 1 diabetes mellitus patientsDiabetologia2006492214616832664
- TakagiCBursellSELinYWRegulation of retinal hemodynamics in diabetic rats by increased expression and action of endothelin-1Invest Ophthalmol Vis Sci1996372504188933767
- TakahashiMIncreased urinary fibronectin excretion in type II diabetic patients with microalbuminuriaNippon Jinzo Gakkai Shi199537336427666599
- TashiroHShimokawaHSadamatuKPrognostic significance of plasma concentrations of transforming growth factor-beta in patients with coronary artery diseaseCoron Artery Dis2002131394312131016
- TashiroHShimokawaHYamamotoKAltered plasma levels of cytokines in patients with ischemic heart diseaseCoron Artery Dis1997814379237023
- TashiroKKoyanagiIOharaILevels of urinary matrix metalloproteinase-9 (MMP-9) and renal injuries in patients with type 2 diabetic nephropathyJ Clin Lab Anal2004182061015103687
- TayebjeeMHLimHSMacFadyenRJMatrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 and -2 in type 2 diabetes: effect of 1 year’s cardiovascular risk reduction therapyDiabetes Care20042720495115277439
- TayebjeeMHLimHSNadarSTissue inhibitor of metalloproteinse-1 is a marker of diastolic dysfunction using tissue doppler in patients with type 2 diabetes and hypertensionEur J Clin Invest2005a3581215638813
- TayebjeeMHLipGYTanKTPlasma matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-2, and CD40 ligand levels in patients with stable coronary artery diseaseAm J Cardiol2005b963394516054454
- The Diabetes Control and Complications Trial Research GroupThe effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitusN Engl J Med1993329977868366922
- The EUCLID Study GroupRandomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuriaLancet19973491787929269212
- ThomsonSMcLennanSVKirwanPRenal connective tissue growth factor (CTGF) correlates with glomerular basement membrane thickness and prospective microalbuminuria in a non-human primate model of diabetes: Possible predictive marker for diabetic nephropathyJ Diab Complic2007in press
- ThrailkillKMBunnRCMoreauCSMMP-2 dysregulation in Type 1 Diabetes MellitusDiabetes Care2007302321617563344
- TikellisCCooperMETwiggSMConnective tissue growth factor is up-regulated in the diabetic retina: amelioration by angiotensin-converting enzyme inhibitionEndocrinology2004145860614592956
- TimmsPMWrightAMaxwellPPlasma tissue inhibitor of metalloproteinase-1 levels are elevated in essential hypertension and related to left ventricular hypertrophyAm J Hypertens2002152697211939619
- TranPKAgardhHETran-LundmarkKReduced perlecan expression and accumulation in human carotid atherosclerotic lesionsAtherosclerosis20071902647016620836
- TsengSCSavionNSternRFibroblast growth factor modulates synthesis of collagen in cultured vascular endothelial cellsEur J Biochem1982122355606460622
- TsilibaryECMicrovascular basement membranes in diabetes mellitusJ Pathol20032005374612845621
- TsutsuiHMatsushimaSKinugawaSAngiotensin II type 1 receptor blocker attenuates myocardial remodeling and preserves diastolic function in diabetic heartHypertens Res2007304394917587756
- TwiggSMCooperMEThe time has come to target connective tissue growth factor in diabetic complicationsDiabetologia200447965815168021
- TwiggSMCaoZSVMCRenal connective tissue growth factor induction in experimental diabetes is prevented by aminoguanidineEndocrinology2002b14349071512446618
- TwiggSMChenMMJolyAHAdvanced glycosylation end products up-regulate connective tissue growth factor (insulin-like growth factor-binding protein-related protein 2) in human fibroblasts: a potential mechanism for expansion of extracellular matrix in diabetes mellitusEndocrinology20011421760911316739
- TwiggSMJolyAHChenMMConnective tissue growth factor/IGF-binding protein-related protein-2 is a mediator in the induction of fibronectin by advanced glycosylation end-products in human dermal fibroblastsEndocrinology2002a1431260911897682
- TyagiSCKumarSGBanksJCo-expression of tissue inhibitor and matrix metalloproteinase in myocardiumJ Mol Cell Cardiol1995272177898576934
- UK Prospective Diabetes Study (UKPDS) GroupIntensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33)Lancet1998352837539742976
- UmezonoTToyodaMKatoMGlomerular expression of CTGF, TGF-beta 1 and type IV collagen in diabetic nephropathyJ Nephrol200619751717173248
- van den MeirackerAHBaggenRGPauliSSpironolactone in type 2 diabetic nephropathy: Effects on proteinuria, blood pressure and renal functionJ Hypertens20062422859217053552
- van HoevenKHFactorSMA comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart diseaseCirculation199082848552394006
- VaughanDEPAI-1 and atherothrombosisJ Thromb Haemost2005318798316102055
- VranesDCooperMEDilleyRJCellular mechanisms of diabetic vascular hypertrophyMicrovasc Res1999578189882558
- WagnerJJan DanserAHDerkxFHDemonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin systemBr J Ophthalmol199680159638814748
- WahabNAHarperKMasonRMExpression of extracellular matrix molecules in human mesangial cells in response to prolonged hyperglycaemiaBiochem J1996316Pt 3985928670179
- WahabNASchaeferLWestonBSGlomerular expression of thrombospondin-1, transforming growth factor beta and connective tissue growth factor at different stages of diabetic nephropathy and their interdependent roles in mesangial response to diabetic stimuliDiabetologia20054826506016270194
- WahabNAYevdokimovaNWestonBSRole of connective tissue growth factor in the pathogenesis of diabetic nephropathyBiochem J2001359778711563971
- WatanabeHSanadaHShigetomiSUrinary excretion of type IV collagen as a specific indicator of the progression of diabetic nephropathyNephron200086273510971150
- WatanabeTNegishiKKatayamaSSerum or urinary concentration of type IV collagen in diabeticsJ Diabet Complications1991519121770043
- WeberKTExtracellular matrix remodeling in heart failure: a role for de novo angiotensin II generationCirculation1997964065829403633
- WellsJAMurthyRChibberRLevels of vascular endothelial growth factor are elevated in the vitreous of patients with subretinal neovascularisationBr J Ophthalmol19968036368703891
- WestermannDRutschowSJagerSContributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonismDiabetes200756641617327431
- WestonBSWahabNAMasonRMCTGF mediates TGF-beta-induced fibronectin matrix deposition by upregulating active alpha5beta1 integrin in human mesangial cellsJ Am Soc Nephrol2003146011012595495
- WilliamsonJRChangKFrangosMHyperglycemic pseudohypoxia and diabetic complicationsDiabetes199342801138495803
- WolkartGStesselHSaadZCardioprotective effects of atrasentan, an endothelin-A receptor antagonist, but not of nitric oxide in diabetic mice with myocyte-specific overexpression of endothelial nitric oxide synthaseBr J Pharmacol20061486718116702986
- WooVNiLSHakDEffects of losartan on urinary secretion of extracellular matrix and their modulators in type 2 diabetes mellitus patients with microalbuminuriaClin Invest Med2006293657217330452
- WuLLCoxARoeCJTransforming growth factor beta 1 and renal injury following subtotal nephrectomy in the rat: role of the renin-angiotensin systemKidney Int1997511553679150473
- XuXWuZZhouQThe role of determining the levels of serum collagen type IV in diagnosing early diabetic nephropathyRen Fail2002247475312472197
- YagameMKimYZhuDDifferential distribution of type IV collagen chains in patients with diabetic nephropathy in non-insulin-dependent diabetes mellitusNephron1995704287617116
- YamamotoTNakamuraTNobleNAExpression of transforming growth factor beta is elevated in human and experimental diabetic nephropathyProc Natl Acad Sci, USA199390181487680480
- YangCPatelKHardingPRegulation of TGF-beta1/MAPK-mediated PAI-1 gene expression by the actin cytoskeleton in human mesangial cellsExp Cell Res200731312405017328891
- YanoYUraHGabazzaECCirculating levels of 7 S domain of type IV collagen and atrial natriuretic peptide in normotensive type 2 diabetic patients with or without retinopathyHorm Metab Res19983010379543694
- YanoYUraHSumidaYSerum 7S domain of type IV collagen levels in essential hypertension and hypertensive type 2 diabetic patientsDiabet Med199714466719212312
- YokotaTMaRCParkJYRole of protein kinase C on the expression of platelet-derived growth factor and endothelin-1 in the retina of diabetic rats and cultured retinal capillary pericytesDiabetes2003528384512606528
- YonedaMMawatariHFujitaKType IV collagen 7s domain is an independent clinical marker of the severity of fibrosis in patients with nonalcoholic steatohepatitis before the cirrhotic stageJ Gastroenterol2007423758117530362
- ZannadFAllaFDoussetBLimitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales InvestigatorsCirculation20001022700611094035
- ZaouiPCantinJFAlimardani-BessetteMRole of metalloproteases and inhibitors in the occurrence and progression of diabetic renal lesionsDiabetes Metab200026Suppl 425910922970
- ZhangBBCaiWMWengHLDiagnostic value of platelet derived growth factor-BB, transforming growth factor-beta1, matrix metalloproteinase-1, and tissue inhibitor of matrix metalloproteinase-1 in serum and peripheral blood mononuclear cells for hepatic fibrosisWorld J Gastroenterol200392490614606082
- ZhouGLiCCaiLAdvanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor beta-independent pathwayAm J Pathol200416520334315579446
- ZhuDKimYSteffesMWGlomerular distribution of type IV collagen in diabetes by high resolution quantitative immunochemistryKidney Int199445425338164429
- ZimmetPGlobalization, coca-colonization and the chronic disease epidemic: can the Doomsday scenario be averted?J Intern Med20002473011010762445
- ZiyadehFNHanDCCohenJAGlycated albumin stimulates fibronectin gene expression in glomerular mesangial cells: involvement of the transforming growth factor-beta systemKidney Int19985363189507208
- ZiyadehFNHoffmanBBHanDCLong-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic miceProc Natl Acad Sci, USA20009780152010859350
- ZiyadehFNSharmaKEricksenMStimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-betaJ Clin Invest199493536428113392
- ZojaCOrisioSPericoNConstitutive expression of endothelin gene in cultured human mesangial cells and its modulation by transforming growth factor-beta, thrombin, and a thromboxane A2 analogueLab Invest19916416201703585