Abstract
Cardiovascular (CV) disease remains the number 1 cause of death in the USA. Nonetheless, there has been a decline in the age-adjusted death rate for coronary heart disease (CHD) which may be due to more aggressive treatment guidelines for treating CV risk factors, such as hypertension, diabetes, and dyslipidemia. The recent update to the National Cholesterol Education Program (NCEP) guidelines have recommended lower low-density lipoprotein cholesterol (LDL-C) goals in high-risk patients. Based on the new targets for LDL-C, clinicians will need more efficacious lipid-lowering therapies. One of these newer therapies is the combination of ezetimibe and simvastatin. This article reviews the implications of the updated guidelines and discusses the efficacy and safety of ezetimibe/simvastatin for lowering LDL-C.
Introduction
Coronary heart disease (CHD) remains the number 1 cause of death in the USA. Over the past 2 decades, the age-adjusted death rate for cardiovascular (CV) disease has declined by over 50% (CitationMorbidity and Mortality 2002). This decline is due in part to an increased awareness and implementation of evidence-based guidelines for treating multiple CV risk factors, in particular, hypertension (CitationChobanian et al 2003), diabetes (CitationACE 2002), and dyslipidemia (CitationNCEP 2001). Along with these guidelines, therapeutic options for treating hypertension, diabetes, and dyslipidemia have improved, making it easier to reach target goals for blood pressure control, diabetes management, and cholesterol lowering.
While some of the therapeutic advances have entered the market as new drug classes, many new therapies have been created by combining established drugs to produce greater efficacy. These drug combinations offer clinicians the ability to achieve target goals at lower dosages of each drug. For instance, when treating hypertension, the combination of a low dose of an angiotensin-converting enzyme (ACE)-inhibitor, plus a low dose of a diuretic, lowers blood pressure more effectively than a higher dose of either one of these agents alone. Lower doses of two agents in a single drug also have the potential to reduce the side-effect profile, the cost of therapy, and to improve patient compliance by reducing the total pill burden.
The concept of combination therapy, which already provides better control of blood pressure and more recently, diabetes, has now entered the arena for treating dyslipidemia. The first combination therapy for treating dyslipidemia was the lovastatin/niacin extended-release (ER) combination (Advicor®). This drug produced enhanced lowering of both low-density lipoprotein cholesterol (LDL-C) and tri-glycerides, as well as significant increases in high-density lipoprotein cholesterol (HDL-C) (CitationKashyap et al 2002). Several clinical trials have also suggested niacin can improve CV endpoints when added to statin therapy (CitationBrown et al 1998, Citation2001). Despite its efficacy, this therapy has not been widely embraced by clinicians, largely due to intolerance with the niacin component.
Recently a new combination therapy for treating dyslipidemia was approved by the US Food and Drug Administration agency. This lipid-lowering therapy (LLT) combines ezetimibe (Zetia®), a cholesterol absorption inhibitor, with simvastatin (Zocor®), one of the most clinically studied statins for CV risk reduction. This combination (Vytorin®) has been shown to significantly enhance LDL-C lowering over statin monotherapy, and improve attainment of evidence-based LDL-C goals (CitationBallantyne et al 2004; CitationGoldberg et al 2004). This review will discuss the rationale for the more intensive LDL-C goals from the National Cholesterol Education Program (NCEP), and the clinical data supporting the efficacy and safety of the ezetimibe/simvastatin combination.
The challenge ahead
While the risk of age-adjusted CHD death is declining, the total number of CHD-related deaths remains high. There are more than 1 million deaths due to CHD annually and there were approximately 1.7 million acute coronary syndromes (ACS) (CitationAHA 2002). Several epidemiologic trends have continued to fuel the high rate of CHD. From 1991 to 2001, the prevalence of multiple CHD risk factors have significantly increased. The prevalence of hypertension increased by 11.9%, diabetes by 46.5%, obesity by 64.7%, and dyslipidemia by 14.2% (CitationCDCP 2004).
It is estimated that approximately 50 million Americans have hypertension, 10–12 million have diabetes, and more than 100 million have dyslipidemia (CitationAHA 2004). Diabetes is now considered a CHD risk equivalent (CitationNCEP 2001). Moreover, diabetes significantly increases the risk of CV events 3–4 fold when combined with any other risk factor (CitationBeckman et al 2002). Because of this increased prevalence of CHD risk factors, more intensive treatment of at-risk patients is needed to prevent more CV events.
The metabolic syndrome represents a clustering of CV risk factors that may be the result of insulin resistance, although the exact cause is not known. The diagnostic criteria for the metabolic syndrome are abdominal obesity (increased waist circumference), low HDL-C, and high triglycerides, as well as hypertension and impaired fasting glucose. According to the Adult Treatment Panel III (ATP III), patients with the metabolic syndrome have a high risk for CV disease, and may be considered for an optional LDL-C goal of <100 mg/dL (CitationNCEP 2001).
The clinical evidence supporting LLT with statins is overwhelming. Statins have been shown to effectively reduce LDL-C, to have a highly favorable risk–benefit ratio, and to lower CV events by 25%–40%. It is estimated that if statin therapy were implemented for all high-risk patients in the USA, approximately 67 000 coronary deaths, 78 000 strokes, 117 000 nonfatal myocardial infarctions, and 146 000 revascularization procedures would be prevented per year (CitationBallantyne, O'Keefe, et al 2005). This means that for every physician managing 1000 high-risk patients, statin use could prevent one CHD death, myocardial infarction, or stroke every 12 days.
National Cholesterol Education Program guidelines (ATPIII): new clinical data supports intensive LDL-C treatment
The ATP III guidelines were released in May 2001 (CitationNCEP 2001). While ATP III reemphasized the same CHD risk factors as ATP II, the major shift was to focus on the global risk of the patient. Several distinctions were made with regards to optimal LDL-C goals and patient risk categories. An LDL-C level of <100 mg/dL was established as the optimal LDL-C level and patients with the diagnosis of CHD, or CHD risk equivalents, were candidates for this goal. ATP III defined three patient groups as CHD risk equivalents:
diabetes mellitus (removed as a risk factor in ATP II);
noncoronary atherosclerosis (such as peripheral artery disease, abdominal aortic aneurysms, or symptomatic carotid artery disease); and
patients with multiple risk factors that confer a 10-year CHD risk of greater than 20%. ().
Table 1 ATPIII Guidelines 2001: CHD and CHD risk equivalents – target LDL-C < 100 mg/dL
Since 2001, several new statin clinical trials have been completed, which evaluated their impact on CV endpoints in more than 60 000 patients and formed the basis for an update to ATP III in 2004 (CitationGrundy et al 2004). The five major outcomes trials considered in the ATP III update are:
The Heart Protection Study (HPS) (CitationHPS 2002);
Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) (CitationShepherd et al 2002);
Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) (CitationALLHAT 2000);
Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA) (CitationSever et al 2003); and
Pravastatin on Atorvastatin Evaluation and Infection (PROVE-IT) (CitationCannon et al 2004).
The Collaborative Atorvastatin Diabetes Study (CARDS) (CitationColhoun et al 2004) was published soon after the update; but will be discussed here. Most of these subjects were highrisk primary prevention, with the exception of the PROVEIT and a subgroup of the HPS. All were comparisons of a statin vs placebo, except for PROVE-IT, which compared two statins.
Heart Protection Study
The HPS was a randomized, double-blind, placebocontrolled 5-year trial of 20 536 high-risk adults, comparing simvastatin 40 mg to placebo. Simvastatin significantly reduced all-cause mortality by 13% (p = 0.0003) and major coronary events (nonfatal myocardial infarction and coronary death) by 24% (p < 0.0001). More importantly, HPS was able to demonstrate a benefit with statin therapy regardless of baseline LDL-C level, particularly in those subjects with LDL-C < 100 mg/dL.
Prospective Study of Pravastatin in the Elderly at Risk trial
The PROSPER trial was a randomized, placebo-controlled trial of 5804 older high-risk patients, with or without known CHD, between the ages of 70–82 years old who were assigned to either pravastatin 40 mg or placebo. Over 3.2 years, there was a 15% (p = 0.014) reduction in combined clinical endpoints and a 24% (p = 0.043) reduction in CHD mortality.
Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial – Lipid Lowering Trial
The ALLHAT-LLT randomized 10 355 hypertensive patients, age 55 and older with total cholesterol <250 mg/dL to pravastatin 40 mg or usual care for 5 years. The LDL-C reduction in the pravastatin group was 28% compared with 11% in the usual care group and there was a nonsignificant difference in major cardiac events. ALLHAT-LLT was the first major statin clinical trial to not show a statistically significant benefit in reduction of CV events. The investigators attributed this result to the small difference in the LDL-C levels between the treatment group (LDL-C 104 mg/dL) and the usual care group (LDL-C 122 mg/dL). While there was no benefit in the overall population, the African American subgroup did have a significant 24% reduction in CV events.
Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm trial
The ASCOT-LLA randomized more than 10 000 patients with hypertension and 3 or more risk factors, and a total cholesterol ≥250 mg/dL to atorvastatin 10 mg or placebo. While the trial was planned for 5 years, it was terminated at 3.3 years because of a 27% reduction (p = 0.024) favoring atorvastatin in the primary endpoint. The LDL-C reduction with atorvastatin was 34% (mean LDL-C 90 mg/dL) vs 4% with placebo (mean LDL-C 126 mg/dL). As in HPS, there was evidence of clinical benefit across the baseline cholesterol levels, including those with total cholesterol <194 mg/dL.
Pravastatin on Atorvastatin Evaluation and Infection Trial
The PROVE IT trial randomized 4162 patients with ACS to receive either intense therapy with atorvastatin 80 mg vs moderate therapy with pravastatin 40 mg. The intensive therapy group achieved a LDL-C of 62 mg/dL at 2 years while the moderate therapy group achieved a LDL-C of 95 mg/dL. The intensive therapy group had a 16% reduction (p < 0.005) in the composite end point. This study demonstrated that more intensive LDL-C lowering initiated at the time of ACS reduced CV events better than less intensive therapy, with good tolerance.
Cardiovascular Atorvastatin Diabetes Study trial
The CARDS study was a planned 6-year, randomized, double-blind, placebo-controlled trial of 2383 patients with a history of Type II diabetes and average LDL-C level (baseline 119 mg/dL in the treatment group and 118 mg/dL in the placebo). Patients were randomized to atorvastatin 10 mg or placebo. The trial was stopped at 3.9 years due to a 37% reduction (p = 0.001) in CV events. The mean on-treatment LDL-C was 82 mg/dL, and patients who were above or below the baseline mean had similar clinical benefit from active treatment.
Treat to New Targets trial
The Treat to New Targets (TNT) trial compared atorvastatin 10 mg with atorvastatin 80 mg in 10 001 patients with CHD for 5 years (CitationLaRosa et al 2005). Patients on atorvastatin 10 mg achieved a LDL-C of 101 mg/dL while those on atorvastatin 80 mg achieved a LDL-C of 77 mg/dL. There was an overall 22% reduction in major CV events with more intensive compared with less intensive therapy. These clinical trial data, along with HPS and PROVE-IT, provide strong evidence that lower LDL-C is better in high-risk patients.
ATP III addendum – the new optional LDL-C goal
The results of these trials in high-risk primary and secondary prevention patients confirm that clinical benefit occurs with more intensive lowering of LDL-C levels. The ATP III addendum has therefore recommended an optional LDL-C goal of less than 70 mg/dL for very high-risk patients, and an optional goal of less than 100 mg/dL for moderate to high-risk (10% to 20% 10-year CHD risk) patients (CitationGrundy et al 2004). The very high-risk patient category is defined as established CHD plus one of the following: (1) diabetes mellitus; (2) severe or poorly controlled risk factors (ie, cigarette smoking, hypertension, or hyperlipidemia); (3) multiple risk factors associated with the metabolic syndrome; or (4) acute coronary syndrome (). Consistent with this recommendation and the results of the randomized clinical trials, the ATP III Update has stated that statin therapy is first line treatment and that the dose of the statin should lower LDL-C by at least 30%–40%. lists the therapeutic options available with statin monotherapy to achieve 30%–40% LDL-C lowering.
Table 2 NCEP ATP III addendum: optional LDL-C goal <70 mg/dL for the very high-risk patient
Table 3 Therapeutic options for achieving a 30%–40% reduction in LDL-C
Since the prevalence of CV risk is increasing and new clinical trials confirm reducing LDL-C to lower targets improves outcomes, more efficacious therapies are needed to successfully achieve these goals. New statins with greater LDL-C efficacy (CitationJones et al 2003) and new combination therapies improve the ability to reach new LDL-C targets. The most recent update from the National Health and Nutrition Examination Survey III (NHANES III) suggests that only 16% of CHD patients reach an LDL-C level of <100 mg/dL and that more than 60% of CHD patients are not currently on LLT (CitationNHANES 1999). Furthermore, NHANES III notes that the average LDL-C level for a CHD patient is 133 mg/dL. Therefore, for the average CHD patient to achieve a LDL of <100 mg/dL, a statin dose that lowers LDL-C by 25%–30% is needed. For those at very high-risk with an optional LDL-C goal of <70 mg/dL, a statin dose that reduces LDL-C by 45%–50% is required.
To reach 50% LDL-C reduction efficacy, clinicians must prescribe moderate to high doses of the one of three most efficacious statins, which are atorvastatin, simvastatin, and rosuvastatin. Atorvastatin can reduce LDL-C by 45%–50% at the 40 mg to 80 mg dose, while simvastatin at the 80 mg dose, and rosuvastatin at 10 mg and 20 mg doses, have similar efficacy (CitationJones et al 2003). For many clinicians, however, there are safety and tolerability concerns about using the highest doses of a statin. Except for these three statin monotherapies doses, combination therapy is necessary to reduce LDL-C by 45%–50% or greater.
A unique perspective for cholesterol lowering: the ezetimibe and simvastatin combination
Ezetimibe offers a unique mechanism of action not available in any other LLT (). It blocks cholesterol transport at the level of the proximal small bowel (CitationVan Heek et al 2000). Recent work by CitationAltmann et al (2004) has revealed that the Nieman-Pick C1 Like 1 protein is critical for the update of cholesterol across the plasma membrane of the intestinal enterocyte. Ezetimibe inhibits approximately 54% of intestinal cholesterol, which is predominately derived from biliary excretion, before it can be incorporated into chylomicrons. This effect decreases the cholesterol ester (CE) content in chylomicrons as they enter into the plasma, which results in reduced delivery of cholesterol to the liver, causing an upregulation of LDL-C receptors and increased clearance of LDL-C from the plasma. Ezetimibe is absorbed, undergoes glucuconidation in the liver, and is excreted in bile, where it returns to the intestinal lumen and continues to inhibit cholesterol absorption. There is minimal systemic exposure. In clinical trials evaluating ezetimibe efficacy, monotherapy at 10 mg was found to lower LDL-C by 17% (p < 0.01 compared with placebo) (CitationKnopp et al 2003). The safety and tolerability was found to be comparable with placebo, specifically with respect to hepatic transaminases and creatine kinase (CK) levels.
Figure 1 A schematic for sites of action of cholesterol-lowering agents, ezetimibe and simvastatin.
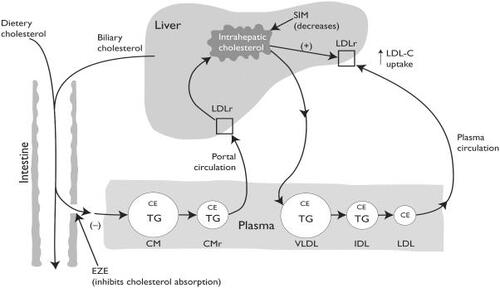
Statins inhibit the rate-limiting step of cholesterol synthesis by competitively inhibiting HMG CoA reductase in the liver. The inhibition reduces the hepatic cholesterol pool, which causes an upregulation of LDL-C receptors on the hepatic surface and enhanced removal of LDL-C particles from the plasma. Since ezetimibe also reduces the hepatic cholesterol pool by decreasing the return of intestinal cholesterol, it would appear that this action should be complimentary to that of statins.
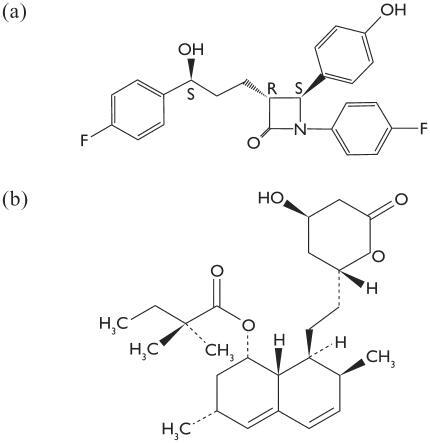
The chemical name of ezetimibe is 1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone (). The empiric formula is C24H2F2NO3. Simvastatin is butanoic acid, 2,2-dimethyl-,1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-hexadryro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxyl-6-oxo-2H-pyran-2-yl)-ethyl]-1-naphthalenyl ester. The empiric formula of simvastatin is C25H38O5 ().
As expected, several clinical trials have documented the enhanced LDL-C efficacy of the ezetimibe/simvastatin combination over statin monotherapy (). The Ezetimibe Study Group reported that coadministration of ezetimibe/simvastatin was more effective than simvastatin alone (p < 0.001) in reducing LDL-C levels (CitationGoldberg et al 2004). CitationBallantyne et al (2004) compared the safety and efficacy of coadministration with ezetimibe and simvastatin vs atorvastatin, and found that there was significantly better LDL-C lowering with ezetimibe/simvastatin 10/10 mg and 10/20 mg (46.1–50.3%) when compared with atorvastatin 10 mg (37.2%) (p ≥ 0.001). Each group reported that the safety and tolerability of the ezetimibe/simvastatin combination was similar to statin monotherapy. Importantly, pharmacokinetic data demonstrate no effect on concentration maximum (C max) or area under the curve (AUC) of either drug on the other when coadministered.
Table 4 Ezetimibe plus simvastatin versus atorvastatin
In the Vytorin Versus Atorvastatin (VYVA) Study, 1902 patients were randomized to either ezetimibe/simvastatin or atorvastatin monotherapy (CitationBallantyne, Abase, et al 2005). This multicenter, double-blind, 6-week parallel-group study compared ezetimibe/simvastatin 10/10 mg to 10/80 mg with atorvastatin 10–80 mg to determine the ability of each dose to achieve ATP III target LDL-C levels. The ezetimibe/simvastatin combination achieved greater LDL-C reduction across the dose ranges (47%–59%) compared with atorvastatin (36%–53%). Moreover, significantly more patients at the starting dosage of ezetimibe/simvastatin 10/20 mg achieved their LDL-C goals than did patients at the starting dose of atorvastatin 10 mg (86.1% vs 69.4%, p < 0.001). In addition, more CHD and CHD-risk equivalent patients achieved an LDL-C goal of <100 mg/dL, as well as the optional LDL-C of <70 mg/dL, when treated with the ezetimibe/simvastatin combination (). Finally, the ezetimibe/simvastatin combination provided significantly greater raising of HDL-C than atorvastatin (7.9% vs 4.3%, p < 0.001) while both treatments lowered triglyceride (TG) values similarly (27.4% vs 25.5%, p-NS). Recently the EASE (Ezetimibe Add-on to Statin for Effectiveness) trial determined the benefit of ezetimibe therapy added to statins vs placebo, which lowered LDL-C levels and attained ATP III goals (CitationPearson et al 2005). In a study of more than 3000 patients, ezetimibe added to statin achieved an additional 25.8% reduction in LDL-C compared with a 2.7% reduction with placebo (p < 0.001). Attainment of ATP III LDL-C goals occurred in 71% of patients who received add-on ezetimibe compared with 20.6% of patients who received placebo (p < 0.001).
Table 5 Percent attainment of LDL-C goal <100 mg/dL with ezetimibe/simvastatin vs atorvastatin
While the majority of the data regarding the ezetimibe/simvastatin combination therapy has focused on lipid efficacy, recent trials have demonstrated a reduction in highsensitivity C-reactive protein (hsCRP). While ezetimibe alone has a modest effect on CRP, Sager et al showed that the combination of ezetimibe/simvastatin was superior to simvastatin alone in reducing hsCRP (CitationSager et al 2005).
To further establish the efficacy and safety of ezetimibe/simvastatin therapy, as well as determine the effect on CV outcomes, several randomized clinical trials are underway. The Study of Heart and Renal Protection (SHARP) study will evaluate the effects of ezetimibe/simvastatin versus placebo in 9000 patients with chronic kidney disease. The Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) study is a placebo-controlled trial to evaluate outcomes in patients with of aortic stenosis. The Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerotic Regression (ENHANCE) study will compare ezetimibe/simvastatin 10/80 mg with simvastatin 80 mg on carotid artery disease by measuring intimal-medial thickness. The study will recruit 725 men and women with heterozygous familial hypercholesterolemia. And finally the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE IT) will evaluate ezetimibe/simvastatin 10/40 mg vs simvastatin 40 mg in reducing death and major coronary events in approximately 10 000 patients with ACS.
Conclusion
Recent evidence-based data suggest that more intensive lowering of LDL-C, particularly in the high-risk population, continues to reduce CV risk. Updated guidelines direct clinicians to achieve lower LDL-C goal levels for their highest risk patients, which requires the use of more efficacious statins or combination therapies. Lowering LDL-C by more than 45%–50% may be required to achieve the optional LDL-C target of <70 mg/dL in very high-risk patients. The ezetimibe/simvastatin combination offers an effective therapy with a greater likelihood of achieving LDL goals, and with similar safety, when compared with equivalent statin monotherapy doses.
Despite the absence of significant evidence-based data that ezetimibe therapy lowers CV risk, it is important to point out that other nonstatin clinical trials have shown significant CV risk reduction in association with lowering cholesterol. The final report of the Lyon Diet Heart Study (Citationde Lorgeril et al 1999) found significant CV risk reduction in patients randomized to the Mediterranean-type diet over the western-type diet. The Programs on Surgical Control of the Hyperlipidemias (POSCH) trial (CitationBuchwald et al 1990) revealed that ileal-bypass surgery lowered LDL-C and was associated with lower CV event rates. In addition, the nonstatin pharmacotherapy trial with cholestyramine, the Lipid Research Clinics – Coronary Primary Prevention Trial (LRC-CPPT) (CitationLRC 1984) found that patients treated with cholestyramine significantly reduced their risk of CV events.
While simvastatin has significant outcomes data for CV risk reduction, there is no significant outcomes data for ezetimibe. Nonetheless, it would be anticipated that the enhanced LDL-C lowering capacity of this combination could result in CHD risk reduction, and endpoint trials in progress will help to determine this hypothesis.
Acknowledgements
The authors would like to thank Terri Glim for her enduring effort and commitment in helping to prepare this manuscript.
References
- [ACE] American College of EndocrinologyConsensus statement on guidelines for glycemic controlEndocr Pract20028Suppl611
- [AHA] American Heart AssociationHeart disease and stroke statistical update2002Dallas, TXAHA 2001
- [AHA] American Heart AssociationHeart disease and stroke statistics – 2004 update2004Dallas, TXAHA11 2003
- [ALLHAT] ALLHAT Officers and Coordinators for the ALLHAT Collaborative GroupThe Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)JAMA200028319677510789664
- AltmannSWDavisHRZhuLNeimann-Pick C1 like 1 protein is critical for intestinal cholesterol absorptionScience20043031201414976318
- BallantyneCMAbaseNYuanZDose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: The Vytorin Versus Atorvastatin (VYVA) StudyAm Heart J20051494647315864235
- BallantyneCMBlazingMAKingTREfficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemiaAm J Cardiol20049314879415194018
- BallantyneCMO'KeefeJHGottoGMOverview of dyslipidemia. dyslipidemia essentials20052ng EdPhysician Pr18
- BeckmanJACreagerMALibbyPDiabetes and atherosclerosis epidemiology, pathophysiology, and managementJAMA200228725708112020339
- BrownBGBrockenbroughAZhaoXQVery intensive lipid therapy with lovastatin, niacin, and colestipol for prevention of death and myocardial infarction: a 10-year Familial Atherosclerosis Treatment Study (FATS) follow-upCirculation199898Suppl I16359665051
- BrownBGZhaoXQChaitASimvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary artery diseaseN Engl J Med200134515839211757504
- BuchwaldHVarcoRLMattsJPEffect of partial ileal bypass surgery on mortality and morbidity of coronary heart disease in patients with hyperlipidemia. Report of the Program on Surgical Control of the Hyperlipidemias (POSCH)N Engl J Med1990323946552205799
- CannonCPBraunwaldEMcCabeCMIntensive versus moderate lipid lowering statins after acute coronary syndromes. (PROVE-IT)N Engl J Med2004380149550415007110
- [CDCP] Centers for Disease Control and PreventionMorbidity Weekly ReportMMWR2004534714724558
- ChobanianAVBakinGLBlackHRThe seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 ReportJAMA200328925607212748199
- ColhounHMBetteridgeDJDurringtonPNPrimary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS); multicentre randomized placebo-controlled trialLancet20043646859615325833
- de LorgerilMSalenPMartinJLMediterranean diet, traditional risk factors, and the rate of cardiovascular complication after myocardial infarction: final report of the Lyon Diet Heart StudyCirculation199999779859989963
- GoldbergACSapreALinJEfficacy and safety of ezetimibe coadministration with simvastatin in patients with primary hypercholesterolemia: a randomized, double-blind, placebo-controlled trialMayo Clin Proc2004796203015132403
- GrundySMCleemanJIMerzBImplications of recent clinical for the national cholesterol education program Adult Treatment Panel III guidelinesCirculation2004931935
- [HPS] Heart Protection Study Collaborative StudyMRC/BHF Heart protection study of cholesterol lowering with simvastatin in 20536 high risk individuals: a randomized placebo-controlled trialLancet200236072212114036
- JonesPHDavidsonMHSteinEAComparison of the Efficacy and Safety of Rosuvastatin Versus Atorvastatin, Simvastatin, and Pravastatin Across Doses (STELLAR*) TrialAm J Cardiol2003921526012860216
- KashyapMLMcGovernMEBerraKLong-term safety and efficacy of a once-daily niacin/lovastatin formulation for patients with dyslipidemiaAm J Cardiol200289672811897208
- KnoppRHDujovneCALe BeautAEvaluation of the efficacy, safety, and tolerability of ezetimibe in primary hypercholesterolemia: a pooled analysis from two Phase III clinical studiesInt J Clin Proct2003573638
- LaRosaJCGrundySMWatersDDThe treating to new targets investigatorsN Engl J. Med200535214253515755765
- [LRC] The Lipid Research Clinics-Coronary Primary Prevention Trial InvestigatorsThe relationship of incidence of coronary heart disease to cholesterol loweringJAMA1984251351646361299
- Morbidity and MortalityChart book on cardiovascular, lung, and blood diseases2002NIH
- [NCEP] National Cholesterol Education ProgramExecutive summary of the third report of the National Cholesterol Education Program (NCEP): expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (ATP III)JAMA200128524869711368702
- [NHANES] National Health and Nutrition Examination SurveyThird National Health and Nutrition Examination (NHANES III 1988–1994) [online]1999 Accessed 12 May 2005. URL: http://www.cdc.gov/nchs/nhanes.htm
- PearsonTPDenkeMAMcBridePEA community-based, randomized trial of ezetimibe added to statin therapy to attain NCEP ATP III goals for LDL cholesterol in hypercholesterolemic patients: the Ezetimibe Add-on to Statin Effectiveness (EASE) trialMayo Clin Proc2005805879515887425
- SagerPTCapeceRLipkaLEffects of ezetimibe coadministered with simvastatin on C-reactive protein in a large cohort of hypercholesterolemic patientsAtherosclerosis2005179361715777554
- SeverPSDahlofBPoulterNRThe Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicenter randomized controlled trialLancet200336111495812686036
- ShepherdJBlounGJMurphyMBProspective Study of Pravastatin in the Elderly at Risk. (PROSPER)Lancet200236016233012457784
- Van HeekMFarleyCComptonDSComparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663Br J Pharmocol2000129174854