Abstract
The role of glycoprotein (Gp) IIb/IIIa receptor antagonists remains controversial and these agents are infrequently utilized during non-ST-segment elevation acute coronary syndromes (NSTE-ACS) despite American Heart Association/American College of Cardiology guidelines. Despite recommendations, the NRMI-4 (National Registry of Myocardial Infarction 4) and CRUSADE (Can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA guidelines?) registries observed that only 25%–32% of eligible patients received early Gp IIb/IIIa therapy, despite a 6.3% absolute mortality reduction in NRMI-4 and a 2% absolute mortality reduction in CRUSADE. A pooled analysis of Gp IIb/IIIa data from these registries suggest a major reduction in mortality (Odds Ratio = 0.43, 95% Confidence Index 0.25–0.74, p = 0.002) with early Gp IIb/IIIa therapy, yet clinicians fail to utilize this option in NSTE-ACS. The evidence-based approach to NSTE-ACS involves aspirin, clopidogrel, low-molecular weight heparins, or unfractionated heparin in concert with Gp IIb/IIIa receptor antagonists, however, newer percutaneous coronary intervention (PCI)-based trials challenge current recommendations. Novel strategies emerging in NSTE-ACS include omitting Gp IIb/IIIa inhibitors altogether or using Gp IIb/IIIa inhibitors with higher doses of clopidogrel in selected patients. The ISAR-REACT (Intracoronary stenting and antithrombotic regimen–Rapid early action for coronary treatment) and ISAR-SWEET (ISAR–Is abciximab a superior way to eliminate elevated thrombotic risk in diabetics) trials question the value of abciximab when 600 mg of clopidogrel concurrently administered during PCI. The CLEAR-PLATELETS (Clopidogrel loading with eptifibatide to arrest the reactivity of platelets) and PEACE (Platelet activity extinction in non-Q-wave MI with ASA, clopidogrel, and eptifibatide) trials suggest more durable platelet inhibition when Gp IIb/IIIa inhibitors are used with higher doses clopidogrel. The ISAR-COOL (ISAR: Cooling off strategy) trial found no difference in ischemic outcomes when Gp IIb/IIIa inhibitors were excluded and ARMYDA-2 (Antiplatelet therapy for reduction of myocardial damage during angioplasty) suggested higher doses of clopidogrel are more appropriate during PCI when Gp IIb/IIIa inhibitors are not utilized. This constellation of new trials forces reconsideration of current recommendations in regards to patient risk stratification, choice of antithrombotic therapy, doses, and timing. These new data will impact emerging guidelines and updates are currently in progress.
Introduction
The typical pharmacotherapeutic strategy for patients experiencing non-ST-segment elevation acute coronary syndromes (NSTE-ACS) has been an intensive combination of aspirin (ASA), clopidogrel, glycoprotein (Gp) IIb/IIIa inhibitor (abciximab, tirofiban, eptifibatide) along with an antithrombin (unfractionated heparin [UFH] or low-molecular weight heparin [LMWH]). Emerging clinical trials are challenging the role of Gp IIb/IIIa-based strategies and suggesting new options that differ by omitting Gp IIb/IIIa-based antiplatelet therapeutics. A consequence of this new data has been a variety of pharmacotherapeutic regimens that differ from American Heart Association/American College of Cardiology (ACC/AHA) guidelines, altering the established timing and dosing of clopidogrel and challenging the utility of Gp IIb/IIIa inhibitors by omitting them from the antiplatelet regimen. This paper reviews the available evidence to reinforce the best evidenced-based practices with GP IIb/IIIa inhibitors and clopidogrel for patients experiencing NSTE-ACS.
Pathophysiology
Platelet activation and the evolution of arterial thrombosis are pivotal pathophysiologic events in ACS leading to myocardial infarction (MI), urgent revascularization, stroke, thrombotic embolization, and cardiovascular death. Early, aggressive, antiplatelet therapy with Gp IIb/IIIa inhibitors during ACS is endorsed by ACC/AHA and established by extensive evidence from multiple prospective, randomized, controlled trials. (CitationCAPTURE 1997; CitationEPILOG 1997; CitationIMPACT-II 1997; CitationRESTORE 1997; CitationPRISM 1998; CitationPRISM-PLUS 1998; CitationPURSUIT 1998; CitationESPRIT 2000; CitationSteinhubl et al 2001; CitationBraunwald et al 2002; CitationMahoney et al 2002).
Platelet activation during NSTE-ACS is driven by the pathobiology of vascular injury, inflammation, and amplification of the coagulation cascade. Vascular inflammation and impaired endothelium-dependent vasodilatation are regulated by a variety of cellular adhesion molecules (CAMs) (CitationMaksimowicz-McKinnon et al 2004). Vascular cellular adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1) stimulates NAPDH oxidase, which generates reactive oxygen species that alter endothelial cell structure and facilitate leukocyte migration into intracellular spaces (CitationIiyama et al 1999; CitationCook-Mills 2002; CitationMaksimowicz-McKinnon et al 2004). P-selectin mediates monocyte rolling and infiltration and platelet-neutrophil interactions (CitationBhatt 2003).
Monocyte chemoattractant protein-1 (MCP-1) regulates monocyte and macrophage migration and infiltration to sites of active inflammation. MCP-1 further upregulates CAM production and induces pro-inflammatory cytokines, chemokines, and matrix metalloproteinases (MMPs) (CitationJiang et al 1992; CitationYamamoto et al 2000). MCP-1 induces proinflammatory interleukin-6 (IL-6) and promotes vascular smooth muscle cell proliferation at inflamed plaques (CitationViedt et al 2002). Activated T-cells and prothrombotic, proinflammatory CD40–CD40L interactions trigger Gp IIb/IIIa receptor expression on platelet surfaces. The concentration of MMP-9 becomes elevated, which further destabilizes vulnerable necrotic plaque by stimulating reactive oxygen species, enhancing lipid peroxidation, and destroying cellular membranes (CitationViedt et al 2002; CitationEgashira 2003).
Proinflammatory, prothrombotic tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) modulates a variety of functions including CAM expression, vascular smooth muscle differentiation, MMP activation and further amplifies the coagulation cascade (CitationRidker et al 2000; CitationSack 2002). This progression facilitates atherosclerotic plaque rupture exposing collagen and von Willebrand factor on the damaged endothelium to create an extensive surface for platelet adhesion. Adherent platelets subsequently aggregate, following a release of a variety of platelet intracellular signaling molecules including thromboxane A2(TxA2), adenosine diphoshphate (ADP), serotonin (5-HT), and epinephrine in addition to systemically circulating thrombin. Additional platelets are recruited to the site of injury and adhesion where conformational changes in platelet cytoskeletal proteins result in upregulation and expression of Gp IIb/IIIa receptors on platelet surfaces. Gp IIb/IIIa receptor to fibrinogen-binding causes extensive cross-linking, which facilitates thrombus formation. Considering that multiple mechanisms are responsible for activating the final common pathway of platelet activation, multiple aggressive antithrombotic strategies are warranted to prevent ischemic complications during percutaneous coronary intervention (PCI). The Gp IIb/IIIa receptor target has been the subject of many clinical trials, spirited debate, and extensive controversy.
Gp IIb/IIIa receptor antagonists (abciximab, tirofiban, eptifibatide) inhibit fibrinogen from cross-linking with platelets which is the scaffold for thrombus growth and formation. This “final common pathway” of Gp IIb/IIIa receptor activation has been evaluated in several clinical trials. (CitationPRISM-PLUS 1998; CitationPURSUIT 1998; CitationESPRIT 2000) Small molecule Gp IIb/IIIa inhibitors (eptifibatide, tirofiban) and chimeric antibody-based abciximab have fundamentally different pharmacokinetics and pharmacodynamics which only partially explain the variability observed during invitro-platelet aggregometry and in-vivo outcomes data (CitationBatchelor et al 2002). Patients with diabetes have overtly accelerated platelet pathobiology, making the findings from existing trials difficult to interpret and apply clinically. (CitationSilva and Gandhi 2004) Considering this challenge, guideline endorsement of Gp IIb/IIIa inhibitors during ACS has varied according to clinical features and ACS presentation. We review the data to reinforce the best evidence-based strategies for antiplatelet therapy during NSTE-ACS.
ACC/AHA recommendations for small-molecule Gp IIb/IIIa inhibitors in NSTE-ACS
NSTE-ACS
ACC/AHA recommendations for eptifibatide or tirofiban during unstable angina (UA)/NSTE-ACS ACS are supported by extensive clinical evidence (CitationRESTORE 1997; CitationPRISMPLUS 1998; CitationPURSUIT 1998; CitationESPRIT 2000). A Gp IIb/IIIa antagonist should be considered for patients with UA/NSTE-ACS receiving ASA and UFH, scheduled for catheterization and PCI. (Class Ia, grade A evidence) (CitationSteinhubl et al 2001; CitationBraunwald et al 2002). Eptifibatide, in addition to ASA, UFH or LMWH is also recommended for patients with ongoing ischemia, elevated troponin, who are not candidates for an invasive strategy. (Class IIa, grade A evidence) (CitationBraunwald et al 2002). Eptifibatide or tirofiban, in the presence of clopidogrel, ASA and UFH in patients scheduled for PCI has also been endorsed by the UA/NSTE-ACS guidelines. (Class IIa, grade B evidence) (CitationBraunwald et al 2002).
US registry data
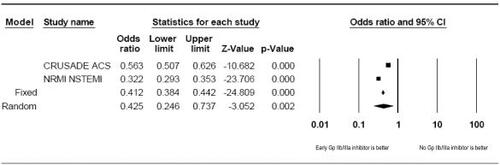
In light of strong recommendations by the ACC/AHA supported by extensive evidence from clinical trials, registry data from CRUSADE and NRMI suggest that US practice with Gp IIb/IIIa inhibitors is suboptimal. (CitationPeterson et al 2003; CitationHoekstra et al 2005) The CRUSADE registry consisted of 65 424 patients with NSTE-ACS across the US. Early Gp IIb/IIIa inhibitor therapy was initiated in 35.5% of the entire cohort. Among patients receiving a Gp IIb/IIIa inhibitor, only 32% received early upstream Gp IIb/IIIa therapy in the emergency department; 33% received therapy in the coronary care unit; 32% received therapy in the catheterization laboratory; and 3% elsewhere (CitationHoekstra et al 2005). Comparing patients who received early Gp IIb/IIIa with patients not receiving early therapy suggested that patient features are predictive of the choice and timing of therapy. Several patient features were associated with failure to receive a Gp IIb/IIIa inhibitor including: females (p < 0.0001), elevated heart rate (p < 0.0001), heart failure (p < 0.0001), prior MI (p < 0.0001), prior hypertension (p < 0.0001), prior stroke (p < 0.0001), renal insufficiency (p < 0.0001), and Medicare/Medicaid coverage (p < 0.0001). Males with hypercholesterolemia receiving care from cardiologists were most likely to receive early Gp IIb/IIIa therapy (p < 0.0001) (CitationHoekstra et al 2005). Patients in the CRUSADE registry who received early Gp IIb/IIIa inhibitors had 2% absolute reduction in overall mortality and a number needed to treat (NNT) = 50. The NRMI-4 registry consisted of 60 770 patients experiencing NSTE-ACS across the US (CitationPeterson et al 2003). Only 25% received early Gp IIb/IIIa within 24 hours of presentation. A consistent pattern of similar characteristics predicted failure to use Gp IIb/IIIa inhibitors in the NRMI-4 registry: females (p < 0.0001), increasing age (p < 0.0001), prior stroke (p < 0.0001), prior peripheral vascular disease (p = 0.004), and nonwhite status (p < 0.0001) (CitationPeterson et al 2003). Patients in the NRMI-4 receiving Gp IIb/IIIa inhibitor experienced a 6.3% absolute reduction of in-hospital mortality with a NNT = 16. Taken together, a pooled analysis of the CRUSADE and NRMI-4 data suggests that early Gp IIb/IIIa inhibitor therapy is associated with a reduction in crude rate of mortality (Odds ratio [OR] = 0.43; 95% Confidence interval [CI] 0.25–0.74; p = 0.002) (). The complicated and evolving presentation of NTSE-ACS is difficult to evaluate and may explain the failure to use early Gp IIb/IIIa therapy. Appropriate selection of pharmacotherapy requires a careful evaluation of risks, clinical features, and patient characteristics most associated with benefits. Possible options to assist with this evaluation are the use of TIMI (Thrombolysis in MI)-risk scores or NRMI–NSTE-ACS risk scores as guides for Gp IIb/IIIa selection.
Established evidence – the foundation of guideline recommendations
Several trials have demonstrated increased inhibition of platelet aggregation with the cocktail of antiplatelet–antithrombin combinations used in the ESPRIT, CLEAR-PLATELETS, and PEACE (Platelet activity extinction in non-Q-wave MI with ASA, clopidogrel, and eptifibatide) trials. Pharmacodynamic evaluations of eptifibatide in the doses used in the IMPACT II and PURSUIT trials suggested <80% platelet inhibition; a dose which is less than optimal for protection against occlusive thrombus formation. These pharmacodynamic findings are still considered unfavorable despite a 2.5% absolute reduction (p = 0.035) in 30-day death, MI, or urgent revascularization in IMPACT II and 1.5% absolute reduction (p = 0.04) in death or MI in PURSUIT. The ESPRIT trial used a high dose, double-bolus/infusion regimen of 180/2/180 (180 μg bolus of eptifibatide followed by a 2 μg/kg/min infusion for 18–24 hours with second 180 μg bolus within 10 minutes of the first bolus) or placebo along with ASA, clopidogrel, and weight-adjusted UFH. The trial was designed to uniformly achieve >80% platelet inhibition and reduce ischemic complications. The primary endpoint was 48-hour death, MI, revascularization, or thrombotic bailout. ESPRIT was powered to achieve a 33% reduction in 30-day death, MI, or revascularization (1-β = 0.86; two-tailed-α = 0.05). Patients receiving eptifibatide had a 3.9% absolute reduction (NNT = 26; p = 0.015) of death, MI, revascularization, or thrombotic bailout at 48-hours and a 3.7% absolute reduction (NNT = 29; p = 0.0034) in 30-day death, MI, or revascularization. The majority of ischemic events (88%) occurred within 48 hours of PCI suggesting that antiplatelet therapy should be maximized early within the first 48 hours after PCI. Reductions in these short-term composite endpoints are largely driven by the reduction of small, non-Q-wave MI. Large MI, death, and urgent revascularization were not different between eptifibatide or placebo in ESPRIT. Among patients receiving the eptifibatide double-bolus/infusion, 90% achieved 90% platelet inhibition using standard platelet aggregometry. A long-term follow up in the ESPRIT cohort revealed a 4.4% absolute reduction in death or MI at 12 months (NNT = 23) and a 3.6% absolute reduction in death, MI, or revascularization at 12 months (NNT = 28) (CitationO'Shea et al 2002). The mechanism by which a short-acting, small molecule glycoprotein IIb/IIIa inhibitor administered at the time of PCI protects against death, MI, and revascularization at 12-months is unknown, yet the benefits are documented. Thus, eptifibatide 180/2/180 double bolus reduced both short-term and long ischemic outcomes and produced durable, predictable platelet inhibition in a contemporary study design where clopidogrel bolus and maintenance doses were used with a variety of different stents.
Pooling all data from the PRISM, PRISM-PLUS, PURSUIT, GUSTO IV (Effect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularization: the GUSTO IV-ACS randomized trial), PARAGON (platelet IIb/IIIa antagonism for the reduction of acute coronary syndrome events in a global organization network) A, and PARAGON B trials in patients with NSTE-ACS receiving medical management suggested that Gp IIb/IIIa inhibition is associated with early reduction in mortality at 30 days (absolute risk reduction [ARR] = 1.6%; NNT = 63) among patients with diabetes, but no corresponding reduction in mortality was observed among patients without diabetes. (CitationRoffi et al 2001) Further analysis of the GUSTO IV trial alone suggested that medical management of NSTE-ACS with abciximab is not beneficial at 30 days, thus benefit seen in previous meta-analysis were driven by small-molecule Gp IIb/IIIa inhibitors rather than abciximab (CitationSimoons et al 2001).
Emerging trials
Current and emerging trials introduce new strategies to intervene against early thrombosis and ischemic outcomes in NSTE-ACS, however, the results are confusing since the heterogeneity of patients, methods, and endpoints creates difficulty in extrapolating outcomes data to patients presenting to the catheterization laboratory of most institutions. Confusion extends into questions regarding patient selection, urgent or elective PCI, and choice of pharmacotherapy. ACC/AHA guideline-directed interventions in the case of urgent or elective PCI include the early use of ASA to antagonize TxA2 mediated platelet activation, 300 mg clopidogrel loading dose prior to PCI followed by 75 mg maintenance dosing afterward, targeting ADP-mediated P2Y12 platelet surface receptors, LMWH-, or UFH-based antithrombin strategies and the use of either eptifibatide or abciximab to block platelet Gp IIb/IIIa receptors on the platelet surface (CitationBraunwald et al 2002). Radical trial designs in progress are evaluating the extent of platelet inhibition and ischemic endpoints in the absence of Gp IIb/IIIa-based strategies opting for larger doses of clopidogrel or the use of bivalrudin to achieve platelet inhibition. These strategies may cause confusion in drug selection and undermine previous research that demonstrated robust and durable platelet inhibition when combinations of ASA, clopidogrel, LMWH, or UFH and a Gp IIb/IIIa inhibitor are selected.
Clopidogrel alone or in combination with Gp IIb/IIIa inhibitors
The ISAR (Intracoronary stenting and antithrombotic regimen) trials examined the necessity of Gp IIb/IIIa inhibitors in coronary artery disease in varying patient populations, settings and pharmacotherapeutic regimens (CitationKandzari et al 2004; CitationMehilli et al 2004). ISAR-REACT (ISAR-Rapid early action for coronary treatment) determined the benefit of abciximab in low-risk patients undergoing elective PCI after pretreatment with clopidogrel (CitationKandzari et al 2004). Patients were excluded if they experienced a MI within 14 days, unstable angina with electrocardiographic changes, positive biomarkers, thrombus percentage, lesions in bypass grafts, left ventricular ejection fraction of less than 30%, insulin-dependent diabetes mellitus, hemodynamic instability, stroke in the past 3 months, and pericarditis. In a double-blind-fashion, patients were randomized to abciximab (n = 1079) or to placebo (n = 1080). All patients were pretreated at least two hours before PCI with 325 mg–500 mg of ASA and 600 mg of clopidogrel. Larger doses of UFH were administered to the placebo group (140 U/kg) than to the abciximab group (70 U/kg). Post-PCI patients received 100 mg–325 mg of ASA, clopidogrel 75 mg twice daily for three days or until discharge and then once daily thereafter, and any other cardiac medications as prescribed. The primary endpoint was the cumulative incidence of all-cause mortality, MI, or urgent revascularization at 30 days. The event rate was lower than initially predicted and subsequently, the sample size of the trial was doubled in an attempt to demonstrate a 40% reduction in the occurrence of the primary endpoint. The frequency of the primary endpoint at 30 days was 4% in both groups (Relative risk [RR] associated with abciximab: 1.05; 95% CI, 0.69–1.59; p = 0.82) (CitationKandzari et al 2004). There was no significant difference between abciximab and placebo in regards to major or minor bleeding events (p = 0.37 and p = 0.38, respectively). There was a greater occurrence of thrombocytopenia and blood transfusions in the abciximab group (p = 0.002 and p = 0.007, respectively). The authors concluded no clinical benefit was observed with abciximab within 30 days of PCI. This trial raises the question of omitting Gp IIb/IIIa receptor blockade, at least with abciximab in selected patients, since there was no difference in all-cause mortality, MI, or 30-day revascularization, nor were there differences in bleeding. There are several limitations to consider. The patient population studied in the ISAR-REACT is generally not the population that receives Gp IIb/IIIa inhibitors. Previous studies demonstrated that intermediate-to-high-risk patients requiring PCI receive the most benefit from these agents (CitationEPIC 1994; CitationPRISM-PLUS 1998; CitationPURSUIT 1998; CitationESPRIT 2000; CitationSteinhubl et al 2001; Topol et al 2001; CitationBraunwald et al 2002). In essence, ISAR-REACT is an underpowered study that suggests low-to-moderate risk patients undergoing elective PCI after pretreatment with 600 mg of clopidogrel do not receive additional benefit from abciximiab and would further imply that Gp IIb/IIIa inhibitors are best reserved for moderate-to-high risk patients requiring PCI. This suggestion reinforces the need for careful risk stratification when selecting antithrombotic regimens.
In contrast, the ISAR-SWEET (ISAR: Is abciximab a superior way to eliminate elevated thrombotic risk in diabetics) trial determined if abciximab provides benefit in diabetic patients after pretreatment with 600 mg of clopidogrel and 500 mg of ASA at least two hours before elective PCI (CitationMehili et al 2004). Patients were excluded by similar criteria as in ISAR-REACT trial with the exception of diabetes mellitus. In a randomized double-blind fashion, patients received abciximab (n = 351) or placebo (n = 350) along with UFH, ASA, and clopidogrel as in ISAR-REACT (CitationKandzari et al 2004). No statistical difference was observed between groups in the primary composite endpoint of allcause mortality and MI at one year (8.3% in the abciximab group vs 8.6% in the placebo group; p = 0.91) (CitationMehili et al 2004). Unlike the ISAR-REACT trial, there were no significant differences relative to major (p = 0.99) or minor (p = 0.09) bleeding events, blood transfusions (p = 0.11), or thrombocytopenia (p = 0.12). These results should be interpreted cautiously. Although the patient population included patients with diabetes, these patients were not highrisk because they had acceptable glycemic control as reflected by an estimated mean hemoglobin A1c of 7.1%, but they were excluded if they had ACS, and the mean ejection fraction was 55%. Furthermore, the management practice patterns are in stark contrast to practice in the US. This is exemplified by the dose of UFH and clopidogrel, and that only 10% of the patients received drug-eluting stent. Most importantly, there was an 11% loss in statistical power (reduction in power from 80% to 69%) which was attributed to unexpectedly lower event rates in the placebo group (expected event rate of 14% vs the actual event rate of 8%). The ISAR trials evaluated low-risk patients undergoing elective PCI and this is not representative of the ACC/AHA recommendations or US registry data. A clinical trial observed that a Gp IIb/IIIa based therapy was not associated with fewer instances of all-cause mortality and MI at 30 days with equivocal bleeding risk. Some early interpretations suggest omitting Gp IIb/IIIa antiplatelet agents in select patients undergoing PCI. Considering early data with abciximab–clopidogrel combinations, there has been some equivocal evidence in regards to ischemic outcomes in small trials. It is not valid to assume the same equivalence when considering eptifibatide or tirofiban since these combinations have not been evaluated under similar protocols and abciximab has never been shown to be superior to eptifibatide or tirofiban in head-to-head trials based on outcomes data.
The CLEAR-PLATELETS study was the largest pharmacodynamic comparison to date evaluating platelet aggregation in response to 300 mg or 600 mg of clopidogrel with and without eptifibatide in the setting of PCI. Investigators evaluated the extent of antiplatelet activity of 300 mg or 600 mg clopidogrel alone and in the presence of eptifibatide dosed according to the ESPRIT 180/2/180 protocol (CitationESPRIT 2000; CitationGurbel et al 2005). Patients received ASA 81 mg, UFH 60 U/kg bolus prior to PCI. Clopidogrel-based strategies in the absence of eptifibatide may not be sufficient to provide adequate antiplatelet protection alone. Previous pharmacodynamic studies have indicated that a clopidogrel 300 mg dose achieves 40% platelet inhibition within 8–18 hours of the loading dose. Eptifibatide dosing according to the ESPRIT protocol routinely achieves ≥80% platelet inhibition (CitationGurbel et al 2002). Patients receiving clopidogrel and eptifibatide had consistently greater platelet inhibition at 18 and 24 hours compared with those receiving clopidogrel alone (p = 0.001) (CitationGurbel et al 2005). Clopidogrel in the absence of eptifibatide does not produce adequate platelet inhibition at either 300 mg or 600 mg doses. Those receiving 300 mg of clopidogrel alone achieved approximately 20% platelet inhibition at 18–24 hours in response to physiologic concentrations of 20 μM ADP. A 600 mg dose alone results in <35% platelet inhibition at 18–24 hours in response to 20 μM ADP. Only patients receiving eptifibatide in addition to clopidogrel were able to consistently achieve platelet inhibition of ≥80% at 18–24 hours. Patients receiving 600 mg of clopidogrel and eptifibatide had greater platelet inhibition compared with those receiving 300 mg of clopidogrel and eptifibatide (p = 0.05) Thus, patients receiving higher doses of clopidogrel achieve greater platelet inhibition with and without eptifibatide. Patients receiving clopidogrel and eptifibatide achieve approximately ≥2-fold greater platelet inhibition compared with clopidogrel alone. Patients receiving clopidogrel 600 mg or 300 mg in conjunction with eptifibatide had less Gp IIb/IIIa receptor expression at 18–24 hours after stent placement (p ≥ 0.05). Patients receiving clopidogrel and eptifibatide had less troponin-I (p = 0.04), myoglobin (p = 0.02), and CKMB (p = 0.05) release. A higher dose of clopidogrel (600 mg) plus eptifibatide offers better platelet inhibition, less Gp IIb/IIIa expression, and limits the extent of myocardial necrosis better than a 300 mg loading-dose of clopidogrel with and without eptifibatide. Thus, in the setting of elective PCI the combination of clopidogrel–eptifibatide may provide more protection against myocardial necrosis and ischemia compared with clopidogrel alone without a Gp IIb/IIIa inhibitor. The limitation to CLEAR PLATELETS was its design as a pharmacodynamic investigation using platelet aggregometry and markers of myocardial necrosis rather than true ischemic outcomes. These pharmacodynamic findings suggest that a combination of clopidogrel–eptifibatide may offer greater protection against ischemic outcomes.
The PEACE trial further supports the necessity of multidrug antiplatelet therapy during NSTE-ACS (CitationDalby et al 2004). PEACE evaluated the differences in Gp IIb/IIIa receptor expression and fibrinogen binding between patients receiving eptifibatide–clopidogrel or clopidogrel monotherapy among 32 people experiencing NSTE-ACS. All patients received ASA 500 mg intravenous followed by 75 mg daily, enoxaparin 1 mg/kg subcutaneous twice daily, and other cardiac medications as deemed appropriate. Platelet activation was assessed at baseline (ASA and enoxaparin only), 3 hours after a 300 mg loading-dose of clopidogrel, and then 12 hours after the initiation of the eptifibatide infusion (180 μg/kg bolus, followed by 2 μg/kg/minute infusion) which was immediately administered before cardiac catheterization. Greater reductions in Gp IIb/IIIa receptor expression were observed in the eptifibatide/clopidogrel group when ADP (48% vs 80%; p < 0.0001) or thrombin receptor-activating peptide (43% vs 78%, p < 0.001) was used to activate platelets (CitationDalby et al 2004). Using the same platelet agonists, fibrinogen binding was significantly reduced with eptifibatide and clopidogrel, then with clopidogrel monotherapy (p < 0.001). These data suggest that the combination of eptifibatide–clopidogrel provides greater antiplatelet activity than clopidogrel alone.
Clopidogrel pretreatment or post-treatment?
In a randomized controlled trial, ISAR-COOL (ISAR: Cooling off strategy) evaluated the outcomes of prolonged antithrombotic pretreatment (3–5 days) to early intervention (less than six hours) prior to PCI in 410 patients with ACS. All patients received UFH 60 U/kg bolus followed by a continuous infusion adjusted to maintain partial thromboplastin time of 60–85 seconds, ASA 500 mg intravenous bolus followed by 100 mg twice daily oral dose, clopidogrel 600 mg loading dose followed by 75 mg twice daily, and tirofiban 10 μg/kg bolus followed by a continuous infusion of 0.10 μg/kg/minute. Eligibility criteria included: angina at rest, angina with minimal exertion, and either positive troponin or ST-segment changes. The primary endpoint was the 30-day composite of all–cause mortality and MI. A statistical difference was observed between groups at 30 days (RR 1.96; 95% CI (1.01–3.82), p = 0.05) (CitationKandzari et al 2004). Early intervention was superior to prolonged antithrombotic pretreatment. The tirofiban dose used in this study was found to be suboptimal as evidenced in TARGET (Do Tirofiban and ReoPro give similar efficacy trial) (CitationSteinhubl et al 2001). Caution is warranted when extrapolating conclusions that clopidogrel is more beneficial than Gp IIb/IIIa inhibitors in this context; considering that a major difference between treatment arms in the ISAR-COOL was time-to-catheterization in addition to different pharmacotherapeutic strategies. The ISAR trials propose this controversy, questioning whether clopidogrel, Gp IIb/IIIa inhibitor, or both, are warranted.
Variability in response to the antiplatelet activity of clopidogrel may result in a higher proportion of thrombotic events and recurrent ischemia in nonresponders; a pharmacodynamic problem likely to be overcome in this minority of patients with higher doses of clopidogrel (CitationMüller et al 2003; CitationLau et al 2004). Contemporary 300 mg doses of clopidogrel 6 hours prior to PCI when used in conjunction with ASA, UFH, or LMWH and eptifibatide, may still lead to early in-stent thrombosis and periprocedural ischemia (CitationPatti et al 2005). Periprocedural ischemia occurs within the first 8 hours of PCI, and more commonly among patients in the highest quartiles of postprocedural platelet reactivity (CitationSaucedo et al 2005). The differences in platelet reactivity, patient variability, and insufficient metabolite production may be overcome with increasing doses of clopidogrel. Loading-doses of 600 mg may provide improved antiplatelet activity over 300 mg doses alone or in the presence of Gp IIb/IIIa inhibitor-based therapy. A 600 mg loading dose of clopidogrel provides better early inhibition at 2 hours compared with 300 mg doses and peak inhibitory effects of 30%–50% platelet inhibition occurs at 8 hours with 600 mg doses compared with patients receiving 300 mg loading doses who have peak inhibitory effects between 8–18 hours (CitationGurbel et al 2005).
Clopidogrel loading-dose and insufficient metabolite production are related reasons for observed post-treatment platelet aggregation. Defining clopidogrel “responders” and “nonresponders” may help direct drug therapy. The relationship between clopidogrel loading-dose and responsiveness has been studied in a cohort of 190 patients undergoing elective coronary stenting randomized to either 300 mg or 600 mg of clopidogrel immediately after stent placement (CitationGurbel et al 2002). A 600 mg load was associated with greater changes in platelet aggregation inhibition in response to either 5 μM ADP (30% vs 20%, p < 0.001) or 20 μM ADP (29% vs 16%, p < 0.001). More patients responded to 600 mg of clopidogrel at 5 μM ADP (35% vs 20%, p = 0.03) and at 20 μM ADP (37% vs 21%, p = 0.02). Across increasing tertiles of post-treatment platelet aggregation, the 600 mg loading-dose was consistently associated with fewer nonresponders, even in the group with the highest post-procedural platelet aggregation (6% vs 17%), suggesting overall improved antiplatelet activity across all groups and a reduction in the proportion of patients not responding to clopidogrel. The limitations to this data set were that recent Gp IIb/IIIa inhibitor use was considered criteria for exclusion and ischemic outcomes data were not part of the study design.
The ARMYDA-2 (Antiplatelet therapy for reduction of myocardial damage during angioplasty) trial randomized 255 patients with NSTE-ACS to 600 mg or 300 mg of clopidogrel within 4–8 hours of PCI and evaluated 30-day death, MI, or revascularization (CitationPatti et al 2005). Gp IIb/IIIa receptor use and choice of stent were left to the operator's discretion. Though ARMYDA-2 was a small clinical trial, an impressive 8% absolute reduction of 30-day death, MI, or revascularization (p = 0.041, NNT = 13) was observed in the group randomized to 600 mg of clopidogrel. Periprocedural myocardial ischemia was measured by evaluating postprocedural creatinine kinase MB isoenzyme, troponin I, and myoglobin release. The 600 mg dose of clopidogrel was associated with significant reductions in all postprocedural biomarkers and high-dose clopidogrel was independently predictive of decreased risk of periprocedural MI in a related multivariate analysis (OR 0.48, 95% CI 0.15–0.97; p = 0.044) (CitationPatti et al 2005). Considering that data exists suggesting benefit of 600 mg of clopidogrel prior to PCI and after PCI, the debate now extends to optimal timing of clopidogrel in patients experiencing NSTE-ACS. Early treatment with clopidogrel reduces early ischemic events, provides benefit among those ultimately receiving PCI, but increases risk of bleeding if coronary anatomy is unknown, and coronary artery bypass grafting (CABG) is a possibility (CitationMehta et al 2001; CitationYusuf et al 2003). Approximately 50%–60% of those presenting with ACS will receive PCI and 8%–20% would be considered for CABG (CitationCannon 2005). Delaying clopidogrel minimizes bleed risks for the minority of CABG patients and benefit or reducing early ischemia, and treatment benefits are lost for the majority proceeding to PCI. Selective, early identification of patients who may need urgent CABG using TIMI risk score tools may help identify those who should not receive early 600 mg clopidogrel loading-doses, minimizing bleeding risk of those proceeding to CABG, and preserving benefit for the majority requiring PCI (CitationCannon 2005).
Summary
Pharmacotherapeutic approaches to NSTE-ACS requiring PCI are complicated by a myriad of evolving antiplatelet strategies that have existed experimentally and outside our current evidence-driven guidelines. Optimal use of Gp IIb/IIIa receptor antagonists involves identifying the appropriate window for therapy, drug choice, appropriate dosing, and patient selection. Heterogeneity in clinical trials has borne a mix of data suggesting both benefit and equivalence making interpretation difficult for both clinical and interventional cardiologists. Higher-risk patients with NSTE-ACS requiring PCI are most likely to achieve benefit from Gp IIb/IIIa receptor antagonists when they have ongoing ischemia, dynamic EKG changes, and positive troponins due to unstable plaque with active inflammation. Gp IIb/IIIa receptor blockade limits ischemic complications of PCI across all indications, among various devices, throughout multiple anticoagulation approaches using a variety of agents. Future guidelines should provide more specific direction regarding risk stratification in an era where Gp IIb/IIIa receptor blockade and clopidogrel may be used in concert in NSTE-ACS and PCI.
Disclosure
Dr Matthew A Silva has no conflicts of interest to report. Dr Jennifer L Donovan has no conflicts of interest to report. Dr Pritesh J Gandhi was a former employee of Millenium Pharmaceuticals (eptifibatide) and currently owns Millenium Stock (just purchased by Schering). Dr Gregory A Volturo has no conflicts of interest to report.
References
- BatchelorWBTollesonTRHuangYRandomized COMparison of platelet inhibition with abciximab, tiRofiban and eptifibatide during percutaneous coronary intervention in acute coronary syndromes: the COMPARE trial. Comparison Of Measurements of Platelet aggregation with Aggrastat, Reopro, and EptifibatideCirculation20021061470612234950
- BhattDLScientific and therapeutic advances in antiplatelet therapyNat Rev Drug Discov20032152812509756
- BraunwaldEAntmanEMBeasleyJWACC/AHA guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction – 2002: summary article: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the management of patients with unstable angina)Circulation20021061893190012356647
- CannonCPWhat is the optimal timing of clopidogrel in acute coronary syndromes?Crit Pathways in Cardiol200544650
- [CAPTURE] CAPTURE InvestigatorsRandomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE studyLancet19973491429359164316
- Cook-MillsJVCAM-1 signals during lymphocyte migration: role of reactive oxygen speciesMol Immunol20023949950812431382
- DalbyMMontalescotGBal dit SollierCEptifibatide provides additional platelet inhibition in non-ST-elevation myocardial infarction patients already treated with aspirin and clopidogrel: Results of the platelet activity extinction in non-Q-Wave myocardial infarction with aspirin, clopidogrel, and eptifibatide (PEACE) studyJ Am Coll Cardiol200443162814736431
- EgashiraKMolecular mechanisms mediating inflammation in vascular disease: special reference to monocyte chemoattractant protein-1Hypertension2003418344112624005
- [EPIC] The EPIC InvestigatorsUse of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in highrisk coronary angioplasty. The EPIC InvestigationN Engl J Med1994330956618121459
- [EPILOG] EPILOG InvestigatorsPlatelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularizationN Engl J Med19973361689969182212
- [ESPRIT] ESPRIT Investigators; Enhanced Suppression of the Platelet IIb/IIIa Receptor with Integrilin TherapyNovel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trialLancet200035620374411145489
- GurbelPABlidenKPZamanKAClopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading with Eptifibatide to Arrest the Reactivity of Platelets (CLEAR-PLATELETS) studyCirculation20051111153915738352
- GurbelPAGalbutBBlidenKPEffect of eptifibatide for acute coronary syndromes: rapid versus late administration—therapeutic yield on platelets (The EARLY Platelet Substudy)J Thromb Thrombolysis2002142131912913401
- HoekstraJWRoeMTPetersonEDEarly glycoprotein IIb/IIIa inhibitor use for non-ST-segment elevation acute coronary syndrome: patient selection and associated treatment patternsAcad Emerg Med200512431815863399
- IiyamaKHajraLIiyamaMPatterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formationCirc Res19998519920710417402
- [IMPACT-II] IMPACT-II InvestigatorsRandomised placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. Integrilin to Minimise Platelet Aggregation and Coronary Thrombosis-IILancet19973491422889164315
- JiangYBellerDIFrendlGMonocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytesJ Immunol1992148242381348518
- KandzariDEBergerPBKastratiAISAR-REACT Study InvestigatorsInfluence of treatment duration with a 600-mg dose of clopidogrel before percutaneous coronary revascularizationJ Am Coll Cardiol2004442133615582309
- LauWCGurbelPAWatkinsPBContribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistanceCirculation20041091667114707025
- MahoneyEMJurkovitzCTChuHTACTICS-TIMI 18 Investigators. Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy-Thrombolysis in Myocardial InfarctionCost and cost-effectiveness of an early invasive vs conservative strategy for the treatment of unstable angina and non-ST-segment elevation myocardial infarctionJAMA20022881851812377083
- Maksimowicz-McKinnonKBhattDLCalabreseLHRecent advances in vascular inflammation: C-reactive protein and other inflammatory biomarkersCurr Opin Rheumatol200416182414673384
- MehilliJKastratiASchühlenHIntracoronary Stenting and Antithrombotic Regimen – Rapid Early Action for Coronary Treatment (ISAR-REACT) Study InvestigatorsRandomized clinical trial of abciximab in diabetic patients undergoing elective percutaneous coronary interventions after treatment with a high loading dose of clopidogrelCirculation200411036273515531766
- MehtaSRYusufSPetersRJClopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) InvestigatorsEffects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE studyLancet20013585273311520521
- MüllerIBestaFSchulzCPrevalence of clopidogrel nonresponders among patients with stable angina pectoris scheduled for elective coronary stent placementThromb Haemost200389783712719773
- O'SheaJCBullerCECantorWJESPRIT InvestigatorsLongterm efficacy of platelet glycoprotein IIb/IIIa integrin blockade with eptifibatide in coronary stent interventionJAMA20022876182111829701
- PattiGColonnaGPasceriVRandomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) studyCirculation2005111209910615750189
- PetersonEDPollackJCharlesVNational Registry of Myocardial Infarction (NRMI) 4 InvestigatorsEarly use of glycoprotein IIb/IIIa inhibitors in non-ST-elevation acute myocardial infarction: observations from the National Registry of Myocardial Infarction 4J Am Coll Cardiol200342455312849658
- RidkerPMRifaiNPfefferMElevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarctionCirculation200010121495310801754
- RoffiMChewDPMukherjeeDPlatelet glycoprotein IIb/IIIa inhibitors reduce mortality in diabetic patients with non-STsegment-elevation acute coronary syndromesCirculation200110427677111733392
- SackMTumor necrosis factor-alpha in cardiovascular biology and the potential role for anti-tumor necrosis factor-alpha therapy in heart diseasePharmacol Ther2002941233512191598
- SaucedoJFAudeWPachecoRInhibition of platelet aggregation with eptifibatide, bivalirudin, and heparin in patients undergoing percutaneous coronary intervention receiving clopidogrel pretreatment (The PharmacoDynamic Evaluation of Angiomax, Clopidogrel with or without INtegrilin [DEACON] study)Am J Cardiol2005951453615950569
- SilvaMGandhiPJSelection of glycoprotein IIb/IIIa inhibitors for upstream use in patients with diabetes experiencing unstable angina or non-ST segment elevation myocardial infarction. What have we learned in the last 10 years?J Clin Pharm Ther20042949751015584937
- SimoonsMLGUSTO IV-ACS InvestigatorsEffect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularisation: the GUSTO IV-ACS randomised trialLancet200135719152411425411
- SmithJSidneyCDoveJTACC/AHA guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines) – executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) endorsed by the Society for Cardiac Angiography and InterventionsJ Am Coll Cardiol20013722153811419905
- SonelAFGoodCBMulgundJCRUSADE InvestigatorsRacial variations in treatment and outcomes of black and white patients with high-risk non-ST-elevation acute coronary syndromes: insights from CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines?)Circulation200511112253215769762
- SteinhublSREllisSGWolskiKTiclopidine pretreatment before coronary stenting is associated with sustained decrease in adverse cardiac events: data from the Evaluation of Platelet IIb/IIIa Inhibitor for Stenting (EPISTENT) TrialCirculation20011031403911245644
- [PRISM] Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study InvestigatorsA comparison of aspirin plus tirofiban with aspirin plus heparin for unstable anginaN Engl J Med1998338149815059599104
- [PRISM-PLUS] Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) Study InvestigatorsInhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarctionN Engl J Med19983381488979599103
- [PURSUIT] PURSUIT Trial InvestigatorsInhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromesN Engl J Med1998339436439705684
- [RESTORE] RESTORE InvestigatorsEffects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplastyCirculation1997961445539315530
- TopolEJLincoffAMKereiakesDJMulti-year follow-up of abciximab therapy in three randomized, placebo-controlled trials of percutaneous coronary revascularizationAm J Med20021131612106616
- ViedtCVogelJAthanasiouTMonocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-kappaB and activator protein-1Arterioscler Thromb Vasc Biol2002229142012067898
- YamamotoTEckesBMauchCMonocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1 alpha loopJ Immunol20001646174910843667
- YusufSMehtaSRZhaoFClopidogrel in Unstable angina to prevent Recurrent Events Trial InvestigatorsEarly and late effects of clopidogrel in patients with acute coronary syndromesCirculation20031079667212600908
- YusufSZhaoFMehtaSRClopidogrel in Unstable Angina to Prevent Recurrent Events Trial InvestigatorsEffects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevationN Engl J Med200134549450211519503