Abstract
It still remains undetermined whether endovascular stent-graft placement (ESGP) is the optimal initial treatment for elective cases of thoracic aortic disease because of unknown long-term results. However, it is also recognized that ESGP contributes to better outcome as an initial treatment for aortic emergency, such as rupture, aortic injury, and complicated acute type B aortic dissection. Despite the fact that most patients are elderly, early mortality rates of ESGP are reportedly around 10% in cases of ruptured degenerative thoracic aortic aneurysm. Postoperative morbidity is also superior in ESGP compared with conventional open repair. Postoperative paraplegia has rarely occurred with ESGP. In cases of blunt aortic injury (BAI), other complications may also be present because of other serious injuries. ESGP has changed the surgical strategy for BAI and partially resolved some of the clinical dilemmas. Early mortality rate is almost zero when a stent graft can be placed before re-rupture. While BAI is a very good indication for ESGP, young patients need careful management and attention because of the unknown long-term outcome. In cases of complicated acute type B aortic dissection, the two main determinants of death, shock from rupture and visceral ischemia, could be managed by ESGP with or without conventional endovascular interventions. Recent reports disclosed less than 10% early mortality with ESGP for complicated acute aortic dissection. Even if the possibility of endotension remains, ESPG seems to be beneficial for these critical patients as the preferable initial treatment. The importance of close follow-up should be stressed to avoid some devastating late complications following ESGP.
Introduction
Recent advances in surgical management of thoracic aortic diseases has provided better outcomes and has led to an expansion of surgical indication for high-risk patients with comorbidities (CitationKouchoukos and Dougenis 1997; CitationSvensson et al 1997). However, surgical management of these diseases cannot avoid thoracotomy, extracorporeal bypass, and aortic clamping, which, given the invasiveness of these treatment methods, mean that there are still limitations for critically ill patients. For this reason, the emersion of endovascular stent-graft placement (ESGP), a completely different approach, had a substantial and positive impact on management of aortic disease (CitationParodi et al 1991; CitationDake et al 1994). Many studies have investigated the effectiveness of ESGP for abdominal aortic aneurysm (AAA) prior to its application for thoracic aortic disease (CitationMoore and Rutherford 1996; CitationButh and Laheij 2000; CitationHarris et al 2000; CitationButh, Harris, et al 2002). Although the long-term outcome of ESGP for AAA was not as satisfactory as initially expected (CitationBequemin et al 1999; CitationButh, van Marrewijk, et al 2002; CitationAlric et al 2003; CitationJacobs et al 2003; Sampram et al 2003), the number of ESGPs for AAA has not diminished as an initial treatment, even following the disappointing reports (CitationButh, Jajibi, et al 2002). We believe that the reason for this is that ESGP is less invasive.
As is the case with AAA, the early outcome of ESGP for thoracic aortic disease seems satisfactory compared with conventional open surgery. Recently, the middle-term outcome (a few years postoperative) of ESGP for thoracic aortic disease has been reported to be acceptable (CitationDoss et al 2004; CitationMilnitchouk et al 2004; CitationMakaroun et al 2005). Although there are only a few reports assessing the long-term outcome with follow-ups of five years or more (CitationOrend et al 2003; CitationDemers et al 2004), considerable stent-graft related events have been confirmed. It is easy to understand that localized aortic diseases, such as penetrating aortic ulcer, anastomotic pseudoaneurysm, and aortic isthmus injury, can be treated well by ESGP, but further follow-up assessments are still necessary to determine the appropriate treatment for elective cases of thoracic aortic disease.
On the other hand, ESGP has also been performed for emergency cases of acute thoracic aortic disease, in view of the high mortality of open surgical treatment (CitationMiller et al 1984; CitationCowley et al 1990; CitationCrawford et al 1991; CitationJohansson et al 1995; Citationvon Segesser et al 1996; CitationMiller and Calhoon 1997; CitationNienaber et al 1999; CitationLemaire et al 2002). It is rational that ESGP is applied for debilitated patients with aortic emergency if ESGP is less invasive. Based on the reported satisfactory early outcome of ESGP for thoracic aortic disease (CitationMitchell et al 1999; CitationBortone et al 2004), ESGP seems to contribute to better outcome as an initial treatment for aortic emergency.
We review reports on the use of ESGP for thoracic aortic emergency, divided into three categories; ruptured thoracic aortic aneurysm, blunt aortic injury, and complicated aortic dissection.
Ruptured degenerative thoracic aortic aneurysm
Generally, the affected population is of an older age with a variety of comorbidities. Despite advancements in open surgical techniques, early mortality rate is still high (CitationCrawford et al 1991; CitationMastroroberto and Chello 1999) and 50% in elderly patients (CitationHuynh et al 2002). Several important points must be addressed to perform ESGP successfully, including identification of an access artery and accurate configuration of the ruptured aortic aneurysm. One popular reason for unsuccessful ESGP was reported to be access route troubles (CitationMakaroun et al 2005). The latter investigators have reported that conduits to deliver the stent-graft system were required in approximately 15% of patients with thoracic aortic aneurysm. In order to choose ESGP for an emergency case, it is necessary that the left subclavian artery (LSA) does not originate from the ruptured aortic aneurysm (). Although the usual indications for elective ESGP have reported that an aortic diameter of proximal and distal landing zone should be less than 40 mm, depending on available endoprosthesis, and a length for both landing zones of more than 20 mm (at least 10 mm) (CitationGreenberg et al 2005; CitationMakaroun et al 2005), emergency ESGP had to be often performed in even unfavorable anatomical states. It is recognized that imaging modalities, such as computed tomography (CT) angiography and magnetic resonance (MR) angiography, are preferable to evaluate suitability of patients for ESGP if the patients' clinical condition allows further examinations. According to previously published reports, it seems to be a relatively safe technique to occlude the LSA intentionally by stent graft in order to make a proximal neck (CitationCzermak et al 2002; CitationGörich et al 2002; CitationDestrieux-Garnier et al 2004; CitationDagenais et al 2005). On the other hand, carotid left subclavian bypass has commonly been performed before placing stent garft proximal to the LSA in the US (CitationMakaroun et al 2005). Theoretically, cerebellar or brain stem ischemia is possible following occlusion of the LSA (Citationvan Herzeele et al 2003) and we experienced that LSA occlusion test revealed the patient in whom the LSA was crucial for brain circulation (CitationKurimoto et al 2005), but this infrequent adverse event might be an acceptable risk in such emergency situations.
Figure 1 92-year-old man with distal aortic arch aneurysm ruptured into mediastinum. The left subclavian artery is covered by the stent graft, but full patency of the left carotid artery is achieved by the fenestrated stent graft (white arrow).
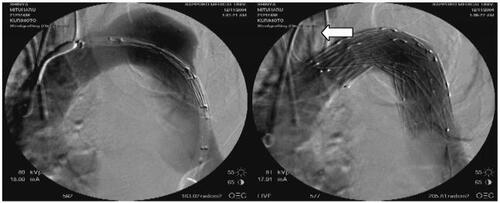
Currently, there are only a few reports which consist of more than 10 cases of ruptured degenerative thoracic aortic aneurysm (CitationDoss et al 2004; CitationScheinert et al 2004), and early mortality rates are reportedly around 10%, although there are some reports with a small number of cases in which early mortality rate is almost 0% (CitationSemba et al 1997; Citationvan Herzeele et al 2003; CitationBortone et al 2004; CitationIanneli et al 2004; CitationMilnitchouk et al 2004; CitationMorishita et al 2004). Despite the fact that there are no reports presenting the results of ruptured degenerative thoracic aortic aneurysm in exclusion from other acute aortic diseases, there is a possibility that ESGP provides a better early mortality rate than conventional open surgery. In addition, postoperative morbidity is also superior in ESGP, as exemplified by shorter periods of mechanical ventilator support and lower rates of postoperative renal failure. In particular, postoperative paraplegia, a devastating complication which sometimes occurs following descending thoracic aorta operation, has rarely happened with ESGP, even in emergency operations. Most studies have reported a 0% postoperative paraplegia rate (CitationSemba et al 1997; Citationvan Herzeele et al 2003; CitationBortone et al 2004; CitationDestrieux-Garnier et al 2004; CitationIanneli et al 2004; CitationMorishita et al 2004; CitationScheinert 2004) and a few disclose a 4% rate of paraparesis (CitationDake et al 1994; CitationDagenais et al 2005). It is likely that post-stent-grafting paraplegia is very rare even in emergency cases of thoracic aortic diseases, as well as in elective cases. In addition, most cases with post-stent-grafting spinal ischemic complication seem to be able to recover to some degree following some rehabilitation.
In contrast to the satisfactory early outcome (CitationMitchell et al 1999; CitationBortone et al 2004; CitationGrabenwoger et al 2004), there seems to be problems in the follow-up periods after ESGP (CitationButh and Laheij 2000). In the first few years after ESGP, there are rarely serious stent-graft related events. However, re-rupture secondary to endoleak or stent-graft migration has been reported in the middle-term periods, especially 5 or more years after ESGP (CitationButh and Laheij 2000), in cases who were not closely checked by CT examination after ESGP and for whom appropriate treatments, such as re-ESGP, were not performed.
Nevertheless, for elderly patients with significant comorbidity, ESGP is still a preferable initial treatment of choice for ruptured degenerative thoracic aortic aneurysm if the configuration of aneurysm allows it. In near future, the use of branched grafts might further expand the indication for stent-grafting for ruptured distal arch aneurysm (CitationSchneider et al 2003).
Blunt aortic injury
Since an anecdotal report by CitationParmley et al (1958), immediate open surgical treatment has been recommended to avoid re-rupture of pseudoaneurysm at the aortic isthmus. However, most patients with blunt aortic injury (BAI) are complicated with other serious injuries, such as head injury, which do not always allow immediate open repair of the injured aorta. Despite recent advancements in surgical techniques, extracorporeal bypass and aortic clamping are still potentially harmful for these patients. In this context, the timing of open repair of injured aorta has been controversially discussed for a decade because conventional open repair is possibly too invasive for multi-trauma patients (CitationWalker and Pate 1990; CitationHolmes et al 2002; CitationRousseau et al 2005).
However, the emergence of ESGP has changed surgical strategy for BAI and partially resolved some clinical dilemmas regarding thoracotomy, unilateral lung ventilation, aortic clamping, and heparinization for extracorporeal bypass. In the period when there were only the two choices of open repair or conservative treatment, initial conservative treatment and delayed open repair may well have been a reasonable strategy (CitationHolmes et al 2002; CitationRousseau et al 2005), considering the high mortality associated with immediate open repair (CitationWalker and Pate 1990). Nowadays, we know that ESGP is less invasive and very effective for BAI (CitationMarty-Ane et al 2003; CitationDunham et al 2004; CitationNeuhauser et al 2004; CitationWellons et al 2004; CitationRousseau et al 2005). Considering the fact that 10% of BAIs have the possibility to re-rupture in the acute phase despite medical treatment (CitationFabian et al 1997; CitationHolmes et al 2002), ESGP should be performed as early as possible if the patients can be transferred to an operating room or angiography suite.
Although there is no report that describes which type of BAI is indicated for initial conservative treatment, traumatic type B aortic dissection and pseudoaneurysm without mediastinal hematoma are probably candidates (CitationFrykberg et al 1991). Since ascending aorta injury is fatal, commonly combining with cardiac injury or cardiac tamponade (CitationParmley et al 1958; CitationFishbone et al 1973), the incidence of BAIs affecting the aortic isthmus is high at more than 80% in patients who can be clinically treated for BAI (CitationDuhaylongsod et al 1992; CitationFabian et al 1997). With the exception of young patients with acutely angled distal arch, ESGP seems to be very well suited to BAI because of the localized affect of the lesion, small diameter of aorta for landing neck and easy access to the injured aorta ().
Figure 2 74-year-old woman with blunt aortic injury associated with intracranial hemorrhage and pelvic fracture. Emergency stent-grafting was performed 2 hours after arrival. Typical aortic isthmus injury (white arrow) is well excluded by the stent graft.
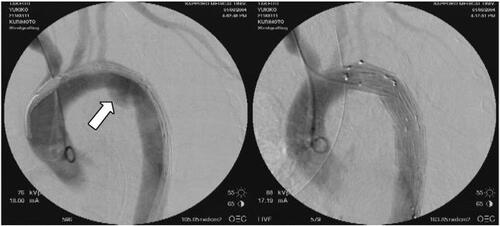
Early mortality rate of ESGP for BAI is almost zero in many reports (CitationMarty-Ane et al 2003; CitationDunham et al 2004; CitationNeuhauser et al 2004; CitationWellons et al 2004; CitationRousseau et al 2005). The cause of early death after ESGP was reportedly due to other concomitant critical injuries (CitationDunham et al 2004). The postoperative paraplegia rate following open repair for BAI is approximately 5% even when using distal aortic perfusion, which has been a concern because the patients are relatively young compared with those with degenerative aortic aneurysm (CitationFabian et al 1997; CitationRousseau et al 2005). Certainly, the introduction of ESGP has made this devastating complication very rare (CitationMarty-Ane et al 2003; CitationDunham et al 2004; CitationNeuhauser et al 2004; CitationWellons et al 2004; CitationRousseau et al 2005) but a small number of cases have needed re-ESGP due to secondary endoleak (CitationNeuhauser et al 2004). Critical events, such as rupture, are very rare in the middle term after ESGP. However, considering the small diameter of the aorta in young patients, migration of the deployed stent graft of small size is highly possible in some decades after ESGP (CitationDuhaylongsod et al 1992). For the time being, indication of ESGP for young patients should be limited to those complicated with severe brain injury.
Complicated aortic dissection
Except for Stanford type A retrograde aortic dissection (CitationDoenst et al 2000), Stanford type B aortic dissection is a possible candidate for ESGP. Generally, type B aortic dissection has been managed by conservative treatment in acute phase if no dissection-related complications were present (CitationUmana et al 2002; CitationSuzuki et al 2003; CitationKusagawa et al 2005). The in-hospital mortality rate of acute type B was an average of 13% and was highest for patients who required surgery, at 32% (CitationSuzuki et al 2003). Seventy percent of patients died from rupture with visceral ischemia being the next most frequent cause at 19% (CitationSuzuki et al 2003). Emergency surgery has been performed for ruptured descending thoracic aorta and organ ischemia secondary to aortic branch malperfusion. Mortality rates for type B dissection with hypotension due to rupture or with organ ischemia were as high as 61.6% and 45.5%, respectively (CitationSuzuki et al 2003).
Aortic side branch occlusion associated with spontaneous acute aortic dissection was common, with an incident rate of 40% (CitationCambria et al 1988; CitationLauterbach et al 2001). Surgical procedure to restore the flow into an ischemic organ depends on whether the mechanism of aortic branch occlusion is dynamic or static (CitationLauterbach et al 2001; CitationBeregi et al 2003; CitationVedantham et al 2003). Open surgical fenestration has been performed in cases of dynamic mechanism in which a true lumen of aorta is critically compressed by an expanded false lumen (CitationCambria et al 1988; CitationDeeb et al 1997; CitationOderich and Panneton 2002). CitationLauterbach et al (2001) reported a recent improvement of in-hospital mortality rate to 23% in the group with end-organ ischemia (CitationCambria et al 1988). It was reported that after aortic rupture or tamponade is ruled out, mesenteric and renal revascularization precedes a proximal aortic operation, most often by open aortic fenestration (CitationLauterbach et al 2001). CitationDeeb et al (1997) also recommended management of end-organ malperfusion prior to proximal aortic replacement even in cases of acute type A dissection (CitationOderich and Panneton 2002). Contrarily, CitationFann et al (1990) reported a 92% resolution of upper and lower limb ischemia following proximal aortic repair and insisted that proximal aortic operation should be performed first for cases of acute aortic dissection complicated with peripheral vascular malperfusion, including mesenteric ischemia (CitationElefteriades et al 1990). Although endovascular treatments, such as balloon fenestration and stent placement, for end-organ ischemia caused by acute aortic dissection have been reported for selected patients (CitationFann et al 1990; CitationLauterbach et al 2001; CitationBeregi et al 2003), the emergence of ESGP has changed surgical strategy for cases with complicated acute aortic dissection including rupture at the descending thoracic aorta (CitationSlonim et al 1999; CitationBortone et al 2002; CitationHerold et al 2002; CitationHutschala et al 2002; CitationDuebener et al 2004).
The two main determinants of death due to acute type B dissection, shock from rupture () and visceral ischemia () (CitationUmana et al 2002; CitationSuzuki et al 2003), could be managed less invasively by ESGP with or without conventional endovascular interventions (CitationFann et al 1990; CitationSlonim et al 1999; CitationDoenst et al 2000; CitationLauterbach et al 2001; CitationHerold et al 2002; CitationHutschala et al 2002; CitationBeregi et al 2003; CitationDuebener et al 2004). Considering reports of insufficient fenestrated re-entry size to rescue malperfusion in the acute phase and rupture in the late phase following percutaneous fenestration, primary entry closure using stent grafts seems to be preferable in cases with malperfusion by the dynamic mechanism (CitationCambria et al 1988; CitationBortone et al 2002). Initially, an early mortality rate of 16% was reported in patients with complicated acute type B dissection treated by ESGP (CitationWilliams et al 1990). Recent reports disclosed less than 10% early mortality with ESGP for complicated acute aortic dissection (CitationDoenst et al 2000; CitationHerold et al 2002; CitationHutschala et al 2002; CitationDuebener et al 2004). Early outcomes of ESGP were satisfactory. However, ESGP cannot close all entries or re-entries through the aorta, which means there is a possibility that endotension of a false lumen continues and causes critical late complications, such as re-rupture and aorto-esophageal fistula (CitationDake et al 1999; CitationDuebener et al 2004). Even if the possibility of endotension remains, the value of ESPG is not reduced because patients undergo a second intervention, including open surgery, with acceptable results due to their stabilized condition. There are still some unclear problems in ESGP for aortic dissection including intramural hematoma which is obviously one possible lethal disease. Thus, there is a need to stress the importance of close follow-up to avoid devastating late complications.
Figure 3 57-year-old man with acute type IIIa aortic dissection ruptured into the left pleural cavity. In addition to complete entry closure, full patency of the left carotid artery and the left subclavian artery is achieved by the fenestrated stent graft (white arrow).
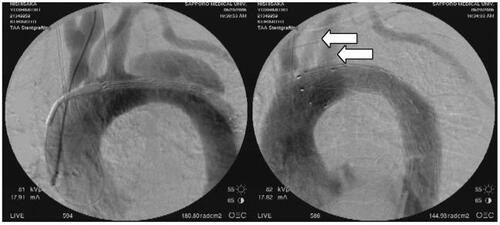
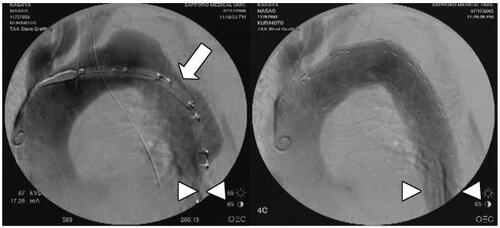
Figure 4 81-year-old man with acute type IIIa aortic dissection complicated with visceral and leg ischemia. The entry site (white arrow) was revealed by intravascular ultrasound (IVUS) before deployment of the stent graft. Critically compressed true lumen of the distal descending thoracic aorta is sufficiently expanded by the entry closure using the stent graft (white arrowheads).
References
- AlricPHinchliffeRJWenhamPWLessons learned from the long-term follow-up of a first-generation aortic stent graftJ Vasc Surg2003373677312563208
- BequeminJPLapieVFavreJPMid-term results of a second generation bifurcated endovascular graft for abdominal aortic aneurysm repair: The French Vanguard trialJ Vasc Surg1999302091810436440
- BeregiJPHaulonSOtalPEndovascular treatment of acute complications associated with aortic dissection: Midterm results from a multicenter studyJ Endovasc Ther2003104869312932159
- BortoneASSchenaSD'AgostinoDImmediate versus delayed endovascular treatment of post-traumatic aortic pseudoaneurysms and type B dissections: Retrospective analysis and premises to the upcoming European trialCirculation2002106Suppl II-2344012354739
- BortoneASCillisEDD'AgostinoDEndovascular treatment of thoracic aortic disease: four years of experienceCirculation2004110Suppl IIII-262715364873
- ButhJLaheijRJFEarly complications and endoleaks after endovascular abdominal aortic aneurysm repair: Report of a multicenter studyJ Vasc Surg2000311344610642716
- ButhJHarrisPLvan MarrewijikCCauses and outcomes of open conversion and aneurysm rupture after endovascular abdominal aortic aneurysm repair: Can type II endoleaks be dangerous?J Am Coll Surg2002194Suppl 1S9810211800362
- ButhJvan MarrewijkCJHarrisPLOutcome of endovascular abdominal aortic aneurysm repair in patients with conditions considered unfit for an open procedure: A report on the EUROSTAR experienceJ Vasc Surg2002352112111854717
- ButhRLJajibiSLinPHConservatism and new technology: The impact on abdominal aortic aneurysm repairAm Surg200268576012467319
- CambriaRPBrewsterDCGertlerJVascular complications associated with spontaneous aortic dissectionJ Vasc Surg198871992093276932
- CowleyRATurneySZHankinsJRRupture of thoracic aorta caused by blunt trauma. A fifteen-year experienceJ Thorac Cardiovasc Surg1990100652612232829
- CrawfordESHessKRCohenESRuptured analysis according to size and treatmentAnn Surg1991213417262025061
- CzermakBVWaldenbergerPPerkmannRPlacement of endovascular stent-grafts for emergency treatment of acute disease of the descending thoracic aortaAJR20021793374512130430
- DagenaisFNormandJPTurcotteRChanging trends in management of thoracic aortic disease: Where do we stand with thoracic endovascular stent grafts?Can J Cardiol200521173815729417
- DakeMDMillerDCSembaCPTransluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysmsN Engl J Med19943311729347984192
- DakeMDKatoNMitchellRSEndovascular stent-graft placement for the treatment of acute aortic dissectionN Engl J Med199934015465210332016
- DeebGMWilliamsDMBollingSFSurgical delay for acute type A dissection with malperfusionAnn Thorac Surg1997641669779436553
- DemersPMillerDCMitchellRSMidterm results of endovascular repair of descending thoracic aortic aneurysms with first-generation stent graftsJ Thorac Cardiovasc Surg20041276647315001894
- Destrieux-GarnierLHaulonSWilloteauxSMidterm results of endoluminal stent grafting of the thoracic aortaVascular2004121798515586526
- DoenstTSchlensakCBeyersdorfFLimitations to the therapeutic potential of endoluminal stent placement in the thoracic aortaCirculation2000101e96
- DossMWoodJPBalzerJEmergency endovascular interventions for acute thoracic aortic rupture: Four-year follow-upJ Thorac Cardiovasc Surg20041296455115746750
- DuebenerLFLorenzenPRichardtGEmergency endovascular stent-grafting for life-threatening acute type B aortic dissectionsAnn Thorac Surg2004781261715464482
- DuhaylongsodFGGlowerDDWolfeWGAcute traumatic aortic aneurysm: the Duke experience from 1970 to 1990J Vasc Surg199215331431735894
- DunhamMBZygunDPetrasekPEndovascular stent grafts for acute blunt aortic injuryJ Trauma2004561173815211121
- EggebrechtHBaumgartDRadeckeKAortoesophageal fistula secondary to stent-graft repair of the thoracic aortaJ Endovasc Ther200411161715056021
- ElefteriadesJAHammondGLGusbergRJFenestration revisitedArch Surg1990125786902346378
- FabianTCRichardsonJDCroceMAProspective study of blunt aortic injury: Multicenter trial of the American Association for the Surgery of TraumaJ Trauma199742374809095103
- FannJISarrisGEMitchellRSTreatment of patients with aortic dissection presenting with peripheral vascular complicationsAnn Surg1990212705132256762
- FattoriRNapoliGLovatoLIndication for, timing of, and results of catheter-based treatment of traumatic injury to the aortaAJR2002179603912185027
- FishboneGRobbinsDIOsbornDJTrauma to the thoracic aorta and great vesselsRadiol Clin North Am197311543534760301
- FrykbergERCrumpJMDennisJWNon-operative observation of clinically occult arterial injuries: a prospective evaluationSurgery199110985961984640
- GörichJAsquanYSeifarthHInitial experience with intentional stent-graft coverage of the subclavian artery during endovascular thoracic aortic repairsJ Endovasc Ther20029Suppl IIII39II4312166840
- GrabenwogerMFleckTEhrlichMSecondary surgical interventions after endovascular stent-grafting of the thoracic aortaEur J Cardio-thorac Surg20042660813
- GreenbergRKO'NeillSWalkerEEndovascular repair of thoracic aortic lesions with the Zenith TX1 and TX2 thoracic grafts: Intermediate-term resultsJ Vasc Surg2005415899615874921
- HarrisPLVallabhaneniSRDesgrangesPIncidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: The EUROSTAR experienceJ Vasc Surg2000327394911013038
- HeroldUPiotrowskiJBaumgartDEndoluminal stent graft repair for acute and chronic type B aortic dissection and atherosclerotic aneurysm of the thoracic aorta: an interdisciplinary taskEur J Cardio Thorac Surg2002228917
- HolmesJHBlochRDHallRANatural history of traumatic rupture of the thoracic aorta managed nonoperatively: A longitudinal analysisAnn Thorac Surg20027311495411998813
- HutschalaDFleckTCzernyMEndoluminal stent-graft placement in patients with acute aortic dissection type BEur J Cardio Thorac Surg2002219649
- HuynhTTTMillerCCEstreraALThoracoabdominal and descending thoracic aortic aneurysm surgery in patients aged 79 years or olderJ Vasc Surg2002364697512218969
- IanneliGPiscioneFDi TommasoLThoracic aortic emergencies: impact of endovascular surgeryAnn Thorac Surg200477591614759443
- JacobsTSWonJGravereauxECMechanical failure of prosthetic human implants: A 10-year experience with aortic stent graft devicesJ Vasc Surg200337162612514573
- JohanssonGMarkstromUSwedenborgJRuptured thoracic aortic aneurysms: a study of incidence and mortality ratesJ Vasc Surg19952198587776479
- KouchoukosNTDougenisDSurgery of the thoracic aortaN Engl J Med19973361876889197217
- KurimotoYAsaiYMaedaTHow to deal with the left subclavian artery in endovascular stent-grafting for distal aortic arch aneurysmCirculation2005112Suppl IIII712
- KusagawaHShimonoTIshidaMChanges in false lumen after transluminal stent-graft placement in aortic dissections. Six years' experienceCirculation20051112951715927978
- LauterbachSRCambriaRPBrewsterDCContemporary management of aortic branch compromise resulting from acute aortic dissectionJ Vasc Surg20013311859211389416
- LemaireSARiceDCSchmittingZCEmergency surgery for thoracoabdominal aortic aneurysms with acute presentationJ Vasc Surg2002351171812042727
- MakarounMSDillavouEDKeeSTEndovascular treatment of thoracic aortic aneurysms: Results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesisJ Vasc Surg2005411915696036
- Marty-AneCHBerthetJPBranchereauPEndovascular repair for acute traumatic rupture of the thoracic aortaAnn Thorac Surg2003751803712822619
- MastrorobertoPChelloMEmergency thoracoabdominal aortic aneurysm repair: clinical outcomeJ Thorac Cardiovasc Surg19991184778210469962
- MillerDCMitchellRSOyerPEIndependent determinants of operative mortality for patients with aortic dissectionCirculation198470Suppl. II-153646235061
- MillerOLCalhoonJHKaiserLRAcute traumatic aortic transsectionMastery of cardiothoracic surgery1997Philadelphia, PA, USALippincott-Raven4789
- MilnitchoukSPfammatterTKadnerAEmergency stent-graft placement for hemorrhage control in acute thoracic ruptureEur J Cardio Thorac Surg20042510328
- MitchellRSMillerDCDakeMDThoracic aortic aneurysm repair with an endovascular stent graft: The “first generation”Ann Thrac Surg19996719714
- MooreWSRutherfordRBTransfemoral endovascular repair of abdominal aortic aneurysm: results of the North American EVT phase I trialJ Vasc Surg199623543538627888
- MorishitaKKurimotoYKawaharadaNDescending thoracic aortic rupture: role of endovascular stent-graftingAnn Thorac Surg2004781630415511446
- NeuhauserBCzermakBJaschkeWStent-graft repair for acute traumatic thoracic aortic ruptureAm Surgeon20047010394415663041
- NienaberCAFattoriRLundGNonsurgical reconstruction of thoracic aortic dissection by stent-graft placementN Engl J Med199934015394510332015
- OderichGSPannetonJMAcute aortic dissection with side branch vessel occlusion: Open surgical optionsSemin Vasc Surg200215899612060898
- OrendKHScharrer-PalmerRKapferXEndovascular treatment in diseases of the descending thoracic aorta: 6-year results of a single centerJ Vasc Surg20033791912514583
- ParmleyLFMattinglyTWManionWCNonpenetrating traumatic injury of the aortaCirculation1958XVII108610113547374
- ParodiJCPalmazJCBaroneHDTransfemoral intraluminal graft implantation for abdominal aortic aneurysmsAnn Vasc Surg1991549191837729
- RousseauHDambrinCMarcheixBAcute traumatic aortic rupture: A comparison of surgical and stent-graft repairJ Thorac Cardiovasc Surg20051291050515867779
- SampramESKKarafaMTMaschaEJNature, frequency and predictors of secondary procedures after endovascular repair of abdominal aortic aneurysmJ Vasc Surg200337930712756335
- ScheinertDKrankenbergHSchmidtAEndoluminal stentgraft placement for acute rupture of the descending thoracic aortaEur Heart J20042569470015084375
- SchneiderDBCurryTKReillyLMBranched endovascular repair of aortic arch aneurysm with a modular stent-graft systemJ Vasc Surg20033885514560244
- SembaCPKatoNKeeSTAcute rupture of the descending thoracic aorta: repair with use of endovascular stent-graftsJVIR19978337429152904
- SlonimSMMillerDCMitchellRSPercutaneous balloon fenestration and stenting for life-threatening ischemic complications in patients with acute aortic dissectionJ Thorac Cardiovasc Surg199911711182710343260
- SuzukiTMehtaRHInceHClinical profiles and outcomes of acute type B aortic dissection in the current era: Lessons from the international registry of aortic dissection (IRAD)Circulation2003108Suppl IIII-312II-31712970252
- SvenssonLGNatural history of aneurysms of the descending and thoracoabdominal aortaJ Card Surg199712279849271757
- UmanaJPMillerDCMichellRSWhat is the best treatment for patients with acute type B aortic dissections-Medical, surgical, or endovascular stent-grafting?Ann Thorac Surg200274S1840312440677
- van HerzeeleIVermassenFDurieuxCEndovascular repair of aortic ruptureEur J Endovasc Surg20032631116
- VedanthamSPicusDSanchezLAPercutaneous management of ischemic complications in patients with type-B aortic dissectionJ Vasc Interv Radiol2003141819312582186
- von SegesserLKGenoniMKunzliASurgery for ruptured thoracic and thoraco-abdominal aortic aneurysmsEur J Cardiothoracic Surg1996109961001
- WalkerWAPateJWMedical management of acute traumatic rupture of the thoracic aortaAnn Thorac Surg19905096572241388
- WellonsEDMilnerRSolisMStent-graft repair of traumatic thoracic aortic disruptionsJ Vasc Surg200440109510015622361
- WilliamsDMBrothersTEMessinaLMRelief of mesenteric ischemia in type III aortic dissection with percutaneous fenestration of the aortic septumRadiology199017445022136956