Abstract
Survivors of myocardial infarction (MI) are at high risk of disability and death. This is due to infarct-related complications such as heart failure, cardiac remodeling with progressive ventricular dilation, dysfunction, and hypertrophy, and arrhythmias including ventricular and atrial fibrillation. Angiotensin (Ang) II, the major effector molecule of the renin–angiotensin–aldosterone system (RAAS) is a major contributor to these complications. RAAS inhibition, with angiotensin-converting enzyme (ACE) inhibitors were first shown to reduce mortality and morbidity after MI. Subsequently, angiotensin receptor blockers (ARBs), that produce more complete blockade of the effects of Ang II at the Ang II type 1 (AT1) receptor, were introduced and the ARB valsartan was shown to be as effective as an ACE inhibitor in reducing mortality and morbidity in high-risk post-MI suvivors with left ventricular (LV) systolic dysfunction and and/or heart failure and in heart failure patients, respectively, in two major trials (VALIANT and Val-HeFT). Both these trials used an ACE inhibitor as comparator on top of background therapy. Evidence favoring the use of valsartan for secondary prevention in post-MI survivors is reviewed.
Introduction
This article reviews the rationale and evidence for inhibition of the renin–angiotensin–aldosterone system (RAAS) by the angiotensin (Ang) II type 1 (AT1) receptor blocker (ARB) valsartan in survivors of myocardial infarction (MI) with left ventricular (LV) systolic dysfunction and/or heart failure, either on top of background therapy including angiotensin-converting enzyme (ACE) inhibitors or instead of ACE inhibitors in patients who are intolerant to them. The results of Valsartan in Acute MI trial (VALIANT) in high-risk survivors of MI and Valsartan Heart Failure Trial (Val-HeFT) in heart failure patients and their substudies, and the evidence favoring the use of valsartan for secondary prevention in survivors of MI are also reviewed.
RAAS inhibition: ACE inhibitors and ARBs
The role of the RAAS in cardiovascular (CV) disease was first recognized nearly five decades ago. The initial focus was on hypertension and the neurohumoral paradigm. Over the last two decades, ACE inhibitors have become established for the treatment of hypertension, heart failure, and MI as a result of several large-scale, multicenter randomized clinical trials (RCTs). The rationale for using ACE inhibitors was to inhibit ACE () and thereby decrease the formation of Ang II, the primary effector molecule of the RAAS that was linked to the pathophysiology of CV disease (). Several major ACE inhibitor trials () have established its use for improving the survival of patients with heart failure and acute MI. This was a major advance in CV medicine during the latter half of the 20th century.
Figure 1 Angiotensin II formation and degradation pathways. Updated from Jugdutt BI. 1998. Angiotensin receptor blockers. In: Crawford MH (ed). Cardiology Clinics Annual of Drug Therapy. Philadelphia: WB Saunders Pub, Vol 2, pp 1–17. Copyright © 1998. Reprinted with permission from Elsevier, with data from Ferrario CM, Trask AJ, Jessup JA. 2005. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol, 289:H2281-90. Copyright © 2005.
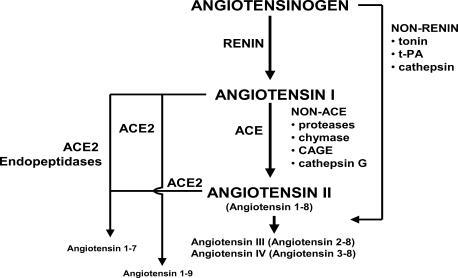
Figure 2 Major cardiovascular effects of angiotensin II. Updated from Jugdutt BI. 1998. Angiotensin receptor blockers. In: Crawford MH (ed). Cardiology Clinics Annual of Drug Therapy. Philadelphia: WB Saunders Pub, Vol 2, pp 1–17. Copyright © 1998. Reprinted with permission from Elsevier.
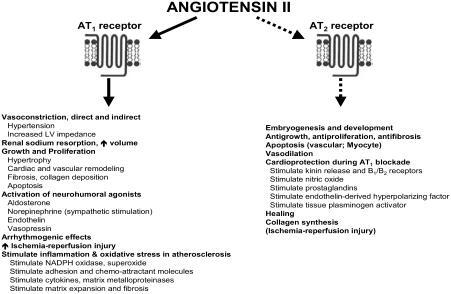
Table 1 Major trials of ACE inhibitors in heart failure and myocardial infarction
Over the last one and a half decades, several RCTs have investigated the benefits of using ARBs in patients with heart failure and MI (). The rationale for using ARBs was to achieve specific and selective blockade of the effects of Ang II via the AT1 receptor (CitationTimmermans et al 1991). Several other reasons were later proposed as justification for using ARBs on top of or instead of the already established ACE inhibitors. First, compared with ACE inhibitors, ARBs might provide more complete inhibition of Ang II derived from all sources, including non-ACE and non-renin pathways, especially as the latter is increased during ACE inhibition (CitationUrata et al 1990; Citationde Gasparo and Levens 1998). However, ARBs were later found to increase renin, Ang I and Ang II levels as well as Ang 1-7 levels (CitationFerrario, Jessup, et al 2005; CitationFerrario, Trask, et al 2005). Second, ARBs do not inhibit kininase II or increase, via this mechanism, systemic peptides of the inflammatory response such as bradykinin, substance P, and other tachykinins known to produce cough and angioedema: side effects associated with ACE inhibitors (CitationBenz et al 1997; CitationHowes and Tran 2002). Third, ARBs might produce unopposed stimulation of the Ang II type 2 (AT2) receptor resulting in added benefits (), including long-term CV structural changes over that seen with ACE inhibitors (Citationde Gasparo and Levens 1998). The discovery of ARBs may therefore be considered as another major breakthrough in CV medicine towards the end of the 20th century.
Figure 3 Pathways of ACE-inhibitor and ARB-induced cardiovascular protection. Updated from Jugdutt BI. 1998. Angiotensin receptor blockers. In: Crawford MH (ed). Cardiology Clinics Annual of Drug Therapy. Philadelphia: WB Saunders Pub, Vol 2, pp 1–17. Copyright © 1998. Reprinted with permission from Elsevier.
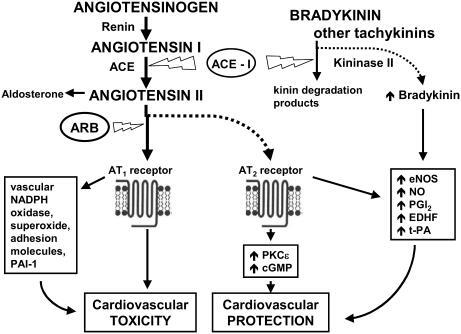
Table 2 Major trials of ARBs in heart failure and myocardial infarction
However, since the benefits of ACE inhibitors in hypertension, heart failure, and MI were already established when ARBs were introduced, it became necessary to demonstrate that ARBs were superior to ACE inhibitors or equally effective in patients intolerant to them and receiving other background therapies in RCTs, rather than relative to a true placebo group. Two RCTs have studied the effects of valsartan in post-MI LV systolic dysfunction and/or heart failure (CitationPfeffer, McMurray, et al 2003) and chronic heart failure (CitationCohn et al 2001), respectively.
Pharmacology of the RAAS
The pertinent aspects of RAAS inhibition have been reviewed (CitationJugdutt 1998). Ang II has several important physiological actions, including vasoconstriction, aldosterone and catecholamine release, drinking, secretion of prolactin and adrenocorticotrophic hormone, and glycogenolysis (CitationJugdutt 1998). Ang II is also a pleiotropic cytokine that plays a critical role in the pathophysiology of several CV diseases. Thus, Ang II induces vasoconstriction and stimulates growth, contributes to LV dysfunction and progression of heart failure, mediates adverse structural cardiac and vascular remodeling (CitationDzau 1993), and causes deleterious activation of other neurohumoral agonists such as norepinephrine, aldosterone, and endothelin ().
Collective evidence indicates that Ang II is produced in the circulation and tissues and acts on the AT1 and AT2 receptors (CitationJugdutt 1998; CitationDzau 2001), but most of the effects of Ang II are mediated through the AT1 receptor. However, in CV diseases such as myocardial hypertrophy, vascular injury, MI, heart failure, and wound healing, the AT2 receptor is upregulated and may mediate some CV effects of Ang II. For example, in heart failure, there is a decrease in AT1 and an increase in AT2 receptors. It has been proposed that the antiproliferative and vasodilatory effects of AT2 balance the growth-stimulating and vasoconstricting effects of AT1 receptors. In that concept, an ARB would completely block effects of Ang II via AT1 and result in unopposed AT2 receptor stimulation that might augment its beneficial effects (Citationde Gasparo and Levens 1998). However, the role of AT2 in humans remains controversial (CitationOpie and Sack 2001).
Collective evidence also suggests that the CV protective effects of ACE inhibitors are related not only to inhibition of Ang II formation via ACE, but also to inhibition of the breakdown of bradykinin and other tachykinins due to ACE's kininase II activity (). Thus during ACE inhibition, Ang II presented to both AT1 and AT2 receptors is decreased, at least initially, so that decreased but balanced AT1 and AT2 effects would be expected. However, increased bradykinin stimulates nitric oxide (NO), prostaglandins such as prostacyclin (PGI2,), endothelial-derived hyperpolarizing factor (EDHF) and tissue-thromboplastin activator (t-PA), thereby contributing to the vasodilation, CV protection and other favorable vascular effects associated with ACE inhibitors (CitationDrexler 1994). Of note, increased bradykinin may contribute to hypotensive effect of ACE inhibitors.
In contrast, the CV protective effect of ARBs is mediated largely by AT1 blockade and partly via AT2 receptor activation and via release of kinins and stimulation of kinin B1 or B2 receptors (CitationSeyedi et al 1995; CitationLiu et al 1997) and/or direct AT2 mediated signaling via protein kinase C (PKCɛ), nitric oxide (NO), and cyclic guanosine monophosphate (cGMP) (CitationJugdutt et al 2000; CitationXu et al 2000; CitationJugdutt and Balghith 2001) ().
The discovery that ACE inhibitors do not block the formation of all Ang II, such as that from Ang I via chymase and other non-ACE enzymes, and/or that from angiotensinogen via non-renin pathways, and Ang II levels persist during long-term ACE inhibitor therapy (CitationKawamura et al 1992; CitationJorde et al 2000), fueled the concept that the combination of ACE inhibition and AT1 receptor blockade may produce more complete blockade of the deleterious effects of Ang II and produce greater benefits. Support for this concept came from experimental (CitationSpinale et al 1997) and clinical (CitationHamroff et al 1999) studies in heart failure. In the rat model of post-MI heart failure, valsartan combined with the ACE inhibitor fosinopril suppressed histopathologic changes associated with remodeling, and normalized collagen I, macrophages and myofibroblasts (CitationYu et al 2001). Extending that concept, valsartan combined with the endothelin blocker bosentan was shown to produce additive beneficial effects on loading, neurohumoral activity, and LV performance in the atrial pacing-induced heart failure in pigs (CitationNew et al 2000).
Recent advances have modified some traditional concepts about the RAAS (). Several studies have underscored the importance of Ang II degradation by ACE2, a regulator of cardiac function, to Ang-(1-7), a vasodilator, antitrophic and antifibrotic heptapeptide that functions as an endogenous inhibitor of Ang II (CitationFerrario, Trask, et al 2005; CitationIwata et al 2005). Both ACE2 and Ang-(1-7) have been demonstrated in rat and human cardiomyocytes. Experimentally in rats, ACE inhibition was shown to decrease Ang II formation and increase Ang-(1-7), and ATM1 blockade to increase Ang II and Ang-(1-7) (CitationFerrario, Jessup, et al 2005). The increase in Ang-(1-7) with ACE inhibition was attributed to increased Ang I and inhibition of Ang-(1-7) metabolism, and that with AT1 blockade to formation from increased Ang I. After MI in rats, AT1 blockade was shown to upregulate ACE2 (CitationIshiyama et al 2004), which may contribute to its cardioprotective effect via Ang-(1-7) formation, as verified by Ang-(1-7) infusion (CitationLoot et al 2002). As Ang-(1-7) is a substrate for inactivation by ACE, it competes with Ang I and bradykinin for degradation, thereby inhibiting Ang II formation and augmenting bradykinin activity and its vasodilatory effects (CitationTom et al 2001). Increased Ang-(1-7) with ACE inhibition may further augment bradykinin activity. Recently, AT1 blockade was shown to increase bradykinin levels in hypertensive humans, probably due to decreased metabolism by ACE and neutral endopeptidase (CitationCampbell et al 2005). The authors suggested that the increased bradykinin with ARBs may augment therapeutic actions, but also lead to angioedema. Collectively, the findings indicate that both ACE inhibitors and ARBs increase Ang-(1-7) and bradykinin.
It should be noted that ACE inhibitors and ARBs are used on top of background therapy which often includes beta-blockers, especially in patients with LV systolic dysfunction and heart failure. Since beta-blockers also reduce renin (CitationBuhler et al 1972) and Ang II (CitationCampbell et al 2001), they produce effects that are additive to those of ACE inhibitors (CitationSharpe 1999).
Pharmacology of valsartan
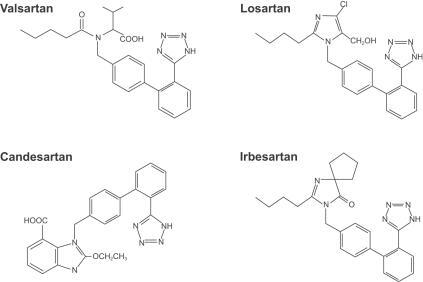
The pharmacology of valsartan has been reviewed (CitationCriscione et al 1993; CitationMarkham and Goa 1997; CitationChiolero and Bernier 1998; CitationChung et al 1999; CitationWellington and Faulds 2002). The chemical structure has similarities and differences compared with other ARBs such as losartan, candesartan, and irbesartan (). Valsartan displays non-competitive antagonism at the AT1 receptor (CitationChung et al 1999). It also demonstrates partial insurmountable antagonism in vitro, as do some other ARBs including irbesartan and EXP3174. This feature of valsartan antagonism may reflect slow dissociation from the AT1 receptor (half-life = 17 minutes) and may explain its prolonged blood pressure (BP) lowering effect in clinical studies (CitationVerheijen et al 2000). Valsartan is quickly absorbed after oral dosing, reaching peak plasma concentration in 2 hours, and has 20% bioavailability. Binding to protein, mainly albumin, is 94% to 97%. Valsartan is mainly eliminated unchanged in bile and <10% in urine. The elimination half-life is 5–7 hours in patients. Metabolism appears to be independent of the cytochrome P450 isoenzyme system and 20% of the dose is recovered as metabolites. Accumulation after daily dosage is minimal. The kinetics is not affected by renal dysfunction. However, the dosage has to be reduced in patients with liver dysfunction.
Figure 4 Chemical structures of valsartan and some other AT1 receptor blockers.
In heart failure it is advisable to start at a dose of 40 mg twice daily (BID) and titrate to 160 mg BID. In heart failure patients, valsartan produces significant decreases in pulmonary capillary wedge pressure (p = 0.013), systolic BP (p = 0.003), and plasma norepinephrine (p = 0.013) by 28 days (CitationBaruch et al 1999). In LV dysfunction and/or heart failure post MI (CitationPfeffer, McMurray, et al 2003), valsartan was begun at 20 mg and escalated in steps to 40 mg, 80 mg, and 160 mg BID depending on the dose of captopril (target 50 mg three times daily [TID]).
Double jeopardy of MI survivors and secondary prevention after MI
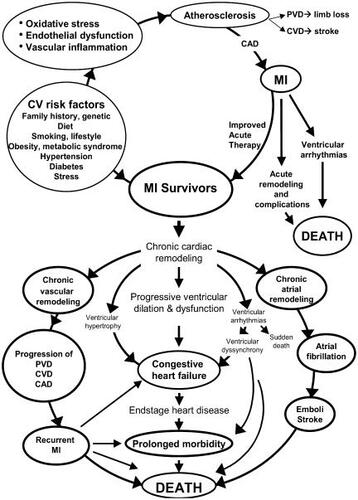
Survivors of MI represent a special group of patients at double jeopardy for increased CV events, morbidity, and mortality (). First, they are at high risk for infarct-related complications (such as progressive cardiac and vascular remodeling, LV dysfunction and heart failure, arrhythmias, and death). Second, they are exposed to risk factors that antedated the MI and contribute to atherosclerosis progression, myocardial ischemia, recurrent MI, restenosis after revascularization/reperfusion procedures, metabolic syndrome and type II diabetes mellitus, peripheral vascular disease (PVD), arrhythmogenic syndromes, ventricular dyssynchrony, stroke and other cerebrovascular disease (CVD), and renal complications. Importantly, MI survivors are increasing as a result of improved therapies that have reduced mortality. Comprehensive secondary prevention is therefore an important aspect of therapy in the MI survivors.
Prevention of LV remodeling after MI and RAAS inhibition
The pathophysiology and prevention of LV structural remodeling after MI has been extensively reviewed (CitationPfeffer and Braunwald 1990; CitationJugdutt 1993, Citation1995, Citation1996, Citation2003b). A continuum, from post-MI LV remodeling to heart failure and death as well as other cycles leading to disability and death in survivors of MI, has been emphasized (). The discovery that LV remodeling post MI, with progressive LV dilation was a major determinant of morbidity and mortality, and that this process could be limited by RAAS inhibition, has led to a major paradigm shift in CV medicine and has had a tremendous positive impact on post-MI survival and outcome.
Recent advances in knowledge of the biology of post-MI remodeling underscore its complexity and the participation of various molecules including cytokines, growth factors, and hormones as well as cellular responses and signaling pathways (CitationJugdutt 2003a, Citation2003b). Newer therapies attempt to modify and modulate these processes in efforts to optimize outcome. Irrespective of the therapeutic approach that is selected, outcome depends critically on the timing and duration of therapy, and attention to the pathological processes (CitationJugdutt 1993, Citation2003b). A caveat with early unloading therapy after MI emphasizes the avoidance of hypotension, the paradoxical J-curve effect and hypoperfusion (CitationJugdutt 1983, Citation1991; CitationSwedberg et al 1992). Thus, low-dose intravenous nitroglycerin for 48 hours given to high-risk acute MI patients while avoiding hypotension resulted in anti-remodeling effects and a survival benefit in anterior MI (CitationJugdutt and Warnica 1988, Citation1989).
At least two mechanisms explain the inhibition of LV structural remodeling in MI survivors by ACE inhibition and ARBs: (i) a hemodynamic mechanism involving decreased BP, preload and afterload, and wall stress; and (ii) a cellular mechanism involving inhibition of Ang II-induced growth, hypertrophy, and apoptosis (CitationDzau 1993; CitationLeri et al 2000).
The rationale for RAAS inhibition using ACE inhibitors in chronic MI was first provided by experimental studies showing that chronic captopril therapy reduced LV dysfunction, LV remodeling and mortality in rats (CitationPfeffer et al 1985). Subsequent multicenter RCTs involving over 100 000 patients established that ACE inhibitors improved survival in patients with acute (CitationACE 1998) and chronic (CitationFlather et al 2000) MI. The greatest benefits were found in high-risk patients with LV dysfunction (CitationPfeffer 1998). Three trials, namely the Survival and Ventricular Enlargement (SAVE) (CitationPfeffer et al 1992), CitationAcute Infarction Ramipril Efficacy (AIRE) (1993) and Trandolapril Cardiac Evaluation (TRACE) (CitationKober et al 1995), provided strong evidence for the reduction of mortality and morbidity in MI survivors, the odds ratio reduction being 0.74% (95% confidence interval [CI], 0.66%–0.83%) for all-cause mortality, 0.73% (95% CI, 0.63%–0.85%) for heart failure hospitalization and 0.80% (95% CI, 0.69%–0.94%) for recurrent MI.
The prevention of progressive LV remodeling, dilation, and LV dysfunction after MI with ACE inhibitors was established in the SAVE and Healing and Early Afterload Reducing Therapy (HEART) trials (CitationSt John Sutton et al 1994; CitationPfeffer et al 1997; CitationAikawa et al 2001). This anti-remodeling effect was associated with limitation of heart failure and improved survival in SAVE (CitationPfeffer et al 1992). In the Randomized Evaluation of Strategies for LV Dysfunction (RESOLVD) trial, the combination of candesartan and enalapril more effectively prevented LV remodeling than either alone (CitationMcKelvie et al 1999). In the Veterans Administration Cooperative Vasodilator-Heart Failure Trial (V-HeFT), increase in LV ejection fraction and decreased volumes were suggested as markers of regression of adverse LV remodeling induced by ACE inhibition (CitationWong et al 1993). In Val-HeFT, valsartan limited adverse LV remodeling in heart failure and patients with the most LV dilation (LV internal dimension in diastole ≥7.5 cm) and worse ejection fraction (EF < 22%) gained most from its anti-remodeling effect (CitationCohn et al, 2001; CitationWong et al 2004). In VALIANT, valsartan limited adverse LV remodeling and improved LV function after MI to a similar degree as captopril and the combination of valsartan and captopril (CitationSolomon et al 2005). Importantly, the anti-remodeling effect of valsartan in both Val-HeFT and VALIANT was associated with survival benefits (CitationCohn et al 2001; CitationPfeffer, McMurray, et al 2003).
Valsartan and outcome post MI: VALIANT
The VALIANT trial was designed to assess the superiority of valsartan over captopril as comparator on top of conventional therapy, and compared the efficacy and safety of long-term treatment with valsartan, captopril, and their combination in high-risk patients with MI and LV systolic dysfunction and/or heart failure (CitationPfeffer, McMurray, et al 2003). The study enrolled 14 703 patients, similar to those in SAVE, AIRE, and TRACE, randomized them at 0.5 to 10 days after acute MI, and followed them for a median of 24.7 months. The patients received valsartan 160 mg BID (n = 4909), captopril 50 mg TID (n = 4909) or valsartan 50 mg BID plus captopril 50 mg TID (n = 4885). There was no difference in the primary end-point of all-cause mortality in the 3 treatment groups. Comparing valsartan with captopril, the upper limit of one-sided 97.5% CI was within the pre-specified margin for non-inferiority for mortality (p=0.004) and the composite end-point of fatal and nonfatal events (p<0.001). However, adverse events were most frequent with the combination of valsartan plus captopril. Valsartan monotherapy was associated with more hypotension and renal dysfunction. Captopril monotherapy was associated with cough, rash, and taste disturbance. The authors statistically compared the VALIANT results with previous results of SAVE, AIRE, and TRACE trials using an imputed placebo, and found that the 25% risk reduction in all-cause mortality in VALIANT was comparable with those in the ACE-inhibitor trials (CitationPfeffer, McMurray, et al 2003). This established conclusively that valsartan is as effective as an ACE inhibitor in reducing mortality in high-risk post-MI survivors.
Expanding the story: RAAS inhibition in heart failure
Heart failure is the end-point of several chronic CV diseases and is a growing medical and economic burden (CitationO'Connell 2000). Adverse LV remodeling post-MI leads to heart failure. In heart failure RCTs, >50% of patients were survivors of MI ().
Table 3 Cause of heart failure in some trials of ACE inhibitors and ARBs
Valsartan in heart failure: Val-HeFT
In Val-HeFT, 5010 patients with systolic heart failure were randomized to valsartan 160 mg or placebo BID on top of standard therapy consisting of different ACE inhibitors in 93%, digoxin in 67%, different beta-blockers in 35%, and the aldosterone blocker spironolactone in 5% (CitationCohn et al 2001). The patients were followed for an average of 23 months. There was no difference in the primary end-point of all-cause mortality. However, valsartan reduced the composite end-point of mortality and morbidity by 13.2% (relative rate of 0.87; 97.5% CI, 0.77–0.97; p = 0.009). Valsartan also improved clinical signs and symptoms of heart failure (p<0.01). Heart failure hospitalizations decreased by 24% with valsartan. Post-hoc analysis of the combined end-point revealed that valsartan had a favorable effect in patients receiving neither ACE inhibitors nor beta-blockers, but an adverse effect in the 30% of patients receiving the combination of valsartan, ACE inhibitor, and beta-blocker (CitationCohn et al 2001). Since only 5% of the patients were on spironolactone, this combination was not analyzed. Overall, the target dose of valsartan was well tolerated and valsartan was most beneficial in patients not taking ACE inhibitors.
There are several other noteworthy features of Val-HeFT. First, patients already taking ARBs were excluded. Second, valsartan produced sustained reduction of aldosterone in all subgroups, despite different clinical outcomes (CitationCohn et al 2003). Third, the V-HeFT group (CitationBaruch et al 1999) previously demonstrated physiologically active levels of Ang II in chronic heart failure patients receiving standard long-term ACE inhibitor therapy while 4 weeks of valsartan decreased plasma aldosterone and norepinephrine. Although Ang II levels while taking valsartan without ACE inhibitors were not measured, Ang II did not rise with coadministration of valsartan and an ACE inhibitor.
Tolerability and safety of valsartan post-MI and in heart failure
Overall, ARBs including valsartan are well tolerated. Tolerability in Optimal Therapy in MI with the Ang II Antagonist Losartan (OPTIMAAL) (CitationDickstein et al 2002) and Evaluation of Losartan in the Elderly (ELITE) (CitationPitt et al 1997) favored losartan over captopril after MI and in heart failure, respectively. Tolerability to candesartan in heart failure patients intolerant to ACE inhibitors was demonstrated in the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) trial (CitationGranger et al 2003; CitationMcMurray et al 2003; CitationPfeffer, Swedberg, et al 2003; CitationYusuf et al 2003). The CHARM data provides a useful guide since the patients who developed cough with the ACE inhibitor had a 0.3% chance of developing it with candesartan, while patients who developed angioedema, hypotension, and renal impairment with the ACE inhibitor had a 2.6%, 9.1%, 23.1% probability, respectively, of developing them with candesartan.
Tolerability of valsartan in heart failure was confirmed in Val-HeFT (CitationCohn et al 2001). Thus, the adverse event rate for valsartan was similar to placebo although serum creatinine increased slightly with valsartan. The finding of a potentially adverse effect of the combination of valsartan with an ACE inhibitor and a beta-blocker (triple therapy) suggests the need for caution with combination therapy. However, this finding should not detract from the other important benefits in Val-HeFT. Thus, in patients not taking ACE inhibitors, valsartan decreased mortality by 33% (p = 0.017) and the combined mortality and morbidity end-point by 44% (p < 0.001) (CitationMaggioni et al 2002; CitationCarson et al 2003). The overall findings of Val-HeFT support the use of valsartan as an alternative in heart failure patients intolerant to ACE inhibitors, but not as an add-on to ACE inhibitor therapy. Valsartan also improved other secondary end-points, reducing the incidence of atrial fibrillation by 37% (CitationMaggioni et al 2005), improving LV internal diastolic diameter and ejection fraction in all groups except in those taking valsartan with an ACE inhibitor and a beta-blocker (CitationWong et al 2004), and reducing brain natriuretic peptide (BNP) and plasma norepinephrine (CitationLatini et al 2002).
In the post-MI patients in VALIANT (CitationPfeffer, McMurray, et al 2003), the finding that valsartan on top of captopril increased adverse events without improving survival or the secondary outcomes, despite more BP lowering and increased rate of intolerance, did not support the concept of incremental benefits with combination therapy. More patients were not taking the study drug at one year in the valsartan plus captopril than in captopril (19.0 vs 16.8%, p = 0.007) or valsartan (15.3%) groups. Hypotension was common, and more frequent (p<0.05) with the combination (18.2%) than with valsartan (15.1%) or captopril (11.9%) monotherapy, underscoring the need for BP monitoring after acute MI. Cough and rash were more common with captopril and renal impairment with valsartan (4.9%) or valsartan plus captopril (4.8%).
A recent editorial cautioned against ARBs increasing MI (CitationVerma and Strauss 2004), based on data from the Valsartan Antihypertensive Long-term Use Evaluation Trial (VALUE) (19% increase) and CHARM (36% increase) compared with ACE inhibitors (>20% decrease). However, a post-hoc analysis of VALIANT showed a downward trend in the numbers of patients developing recurrent MI: 840 for captopril, 820 for valsartan and 775 for the combination (CitationMcMurray 2005). Furthermore, a meta-analysis of RCTs using ARBs showed that ARBs were not associated with an excess risk of MI and the frequency of MI was similar for ARBs versus placebo or ACE inhibitors (CitationVerdecchia et al 2005).
A meta-analysis of ARBs in chronic heart failure and high-risk acute MI patients showed that ARBs similarly reduced all-cause mortality and heart failure deaths as ACE inhibitors and should be considered as suitable alternatives to ACE inhibitors (CitationLee et al 2004). The authors found that ARBs produced a statistically significant reduction in all-cause mortality relative to placebo, contrary to a previous meta-analysis (CitationJong et al 2002).
Expanding the RAAS-inhibition paradigm in post-MI survivors
Six points are pertinent. First, over the last decade, two RCTs have investigated the benefits of using aldosterone antagonists in patients with heart failure and MI. The rationale was that Ang II stimulates the release of aldosterone (, ) thereby activating the mineralocorticoid receptor and activation of this receptor persists despite the use of ACE inhibitors, ARBs and beta-blockers. Aldosterone blockade was shown to limit LV remodeling, fibrosis, matrix metalloproteinase (MMP) activation and angiogenesis in the coronary embolization model of heart failure in dogs (CitationSuzuki et al 2002), and limit collagen synthesis and LV remodelling in post-MI patients (CitationModena et al 2001). In the Randomized Aldactone Evaluation Study (RALES) (CitationPitt et al 1999), 1663 patients with chronic heart failure (LV ejection fraction ≥35%) received the aldosterone blocker spironolactone or placebo on top of background therapy with an ACE inhibitor, diuretic, digoxin, and beta-blocker. RALES was prematurely terminated due to the early finding of a 30% reduction in all-cause mortality (p < 0.001). In the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) (CitationPitt et al 2003), 6642 patients with acute MI, LV ejection fraction ≥40%, and heart failure were randomized to receive the selective aldosterone blocker, eplerenone or placebo on top of optimal background therapy. Eplerenone reduced all-cause mortality by 15% (p = 0.008) and cardiovascular mortality by 17% (p = 0.005). In a substudy of RALES (CitationZannad et al 2000), spironolactone was associated with increased levels of markers of collagen synthesis, suggesting that limitation of excessive extracellular matrix (ECM) turnover may have contributed to the benefits. In a substudy of EPHESUS (CitationPitt et al 2005), eplerenone was shown to reduce the 30-day all-cause mortality after acute MI, supporting early initiation of therapy.
Second, polypharmacy is becoming common in post-MI survivors and heart failure. Although experimental studies suggest that combination therapy is more beneficial than monotherapy, this can result in unsuspected interactions among known effects as well as previously not so well known pleotropic effects. Although one potential concern with use of ACE-inhibitors, ARBs, and aldosterone antagonists in combination is that each drug can effectively decrease infarct collagen as well non-infarct interstitial fibrosis (CitationJugdutt 2003b), the evidence of benefits in the RCTs and demonstrated anti-remodeling collectively serve to allay this concern somewhat. While the effect may improve LV diastolic function, the possibility remains that reduction in collagen matrix may increase LV distensibility on the long-term and contribute to deterioration, especially in patients with large transmural MI. This area needs further study.
Another concern unmasked by Val-HeFT was a possible interaction of ARBs with beta-blockers. In addition, both VALIANT and Val-HeFT drew attention to potential adverse effects with the combination of ARBs and ACE inhibitors.
Third, although most patients with acute MI receive reperfusion therapy that may be associated with significant reperfusion injury and stunning (CitationSolomon et al 2001), there is little data on whether ARBs might improve functional recovery after reperfused MI. Recently in the dog model of reperfusion after prolonged ischemia, valsartan was shown to improve LV function, limit acute infarct remodeling and infarct size, and normalize the balance between MMP-9 and the tissue inhibitor of MMP (TIMP-3), suggesting improved ECM remodeling (CitationSawicki et al 2004). In the same model, valsartan reversed the changes in metabolic, functional and structural proteins induced by post-ischemic reperfusion (CitationJugdutt and Sawicki 2004; CitationSawicki and Jugdutt 2004). In both dog and rat models of post-ischemic reperfusion, valsartan induced cardioprotection, which was associated with enhanced AT2 receptor expression (CitationJugdutt and Menon 2004a, Citation2004b). Collectively, the findings suggest that valsartan limits myocardial reperfusion injury after reperfused MI and this effect may involve improved ECM remodeling and AT2 stimulation.
Fourth, experimental evidence suggests that the combination of valsartan and enalapril produces added benefits relative to monotherapy with respect to endothelial function (Citationde Gasparo et al 2002) and combination therapy is currently being evaluated in patients with vascular disease (CitationYusuf 2002). Although AT2 receptor stimulation may explain the vasculoprotective effects of ARBs and ACE inhibitors, recent experimental evidence suggests that AT2 receptor stimulation may promote cardiac hypertrophy and vascular fibrosis, and reduce neovascularization in ischemic tissue (CitationLevy 2004). These negative effects of AT2 stimulation may explain why ARBs are clinically not found to be superior to ACE inhibitors with respect to some end-points.
Fifth, should ARBs such as valsartan be considered for all survivors of MI? Can ARBs prevent both infarct and non-infarct related complications in MI survivors? The evidence from VALIANT and Val-HeFT supports the use of valsartan as an alternative to ACE inhibitors in high-risk patients with LV systolic dysfunction and/or heart failure as well as in chronic heart failure, including that after MI. In view of the evidence gap, it might be prudent not to extrapolate the existing data and extend the use to include all post-MI patients.
Sixth, several RCTs in hypertension have shown that RAAS inhibition reverses adverse cardiac remodeling and improves prognosis beyond BP control, and valsartan showed similar efficacy relative to ACE inhibitors in that respect (CitationCorea et al 1996; CitationLangtry and McClellen 1999). Collectively, the RCTs in hypertension suggest that, besides BP control and CV protection, reduction of stroke and new-onset diabetes are important long-term benefits with ACE-inhibitors and ARBs. Results of RCTs and substudies in heart failure and MI favor these agents, including valsartan, for prevention of adverse atrial remodeling, atrial fibrillation and stroke, and recent onset diabetes in MI survivors.
With respect to stroke, cumulative evidence indicates that atrial fibrillation leads to embolism and stroke and is associated with adverse outcome in MI and heart failure, and RAAS inhibition can decrease the incidence of atrial fibrillation. In a substudy of VALIANT (CitationVelazquez et al 2004), heart failure and/or LV systolic dysfunction preceded 80.3% of all in-hospital deaths, and that group had higher rates of atrial fibrillation (16%) and stroke (2.2%). In another substudy (CitationSzummer et al 2005), in-hospital stroke was found in 1.5% of the post-MI heart failure patients, in-hospital mortality was greater in patients with stroke (27.2 vs 6.5%, p<0.001), heart failure on admission increased the risk of stroke, and atrial fibrillation was more frequent in stroke victims. In a substudy of Val-HeFT, atrial fibrillation was associated with worse outcome in chronic heart failure patients and the addition of valsartan reduced atrial fibrillation by 37% (CitationMaggioni et al 2005), indicating that chronic therapy with valsartan on top of ACE inhibitors and beta-blockers reduces atrial fibrillation.
With respect to diabetes, VALUE (CitationJulius et al 2004) noted a decrease in new-onset diabetes with valsartan in hypertensive patients. A substudy of VALIANT confirmed that diabetes, whether new-onset or known, predicts poor long-term outcomes in high-risk MI patients (CitationAguilar et al 2004). In CHARM (CitationPfeffer, Swedberg, et al 2003), candesartan was suggested to ‘prevent’ diabetes. This effect of ARBs may have contributed to CV protection in RCTs.
Conclusions and future directions
The totality of the evidence from RCTs to date supports the use of ACE inhibitors as first line therapy in post MI survivors and ARBs as proven life saving alternatives. For patients intolerant to ACE inhibitors, valsartan is a prime candidate. Valsartan is approved for the treatment of heart failure and hypertension, as is candesartan. Potential additional indications for ARBs include: (i) limiting ventricular and atrial remodeling in MI survivors; (ii) preventing atrial fibrillation and stroke, especially in MI survivors; (iii) improving BP control, cardioprotection, vasculoprotection, and CV outcomes in hypertensive patients with LV hypertrophy; (iv) reducing new-onset diabetes and insulin resistance; (v) preventing progression of atherosclerosis and its complications. Aggressive measures, including the use of RAAS inhibition with ARBs and/or ACE inhibitors and aldosterone antagonists on top of approved background therapies, are needed in MI survivors for closing the healthcare gap and reducing risk in high-risk CV patients who do not achieve treatment targets recommended by evidence-based guidelines.
Acknowledgements
Supported by an operating grant from the Heart and Stroke Foundation of Canada.
References
- [ACE] ACE Inhibitor Myocardial Infarction Collaborative GroupIndications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100 000 patients in randomized trialsCirculation1998972202129631869
- AguilarDSolomonSDKoberLNewly diagnosed and previously known diabetes mellitus and 1-year outcomes of acute myocardial infarction: the VALsartan In Acute myocardial iNfarcTion (VALIANT) trialCirculation20041101572815364810
- AikawaYRohdeLPlehnJRegional wall stress predicts ventricular remodeling after anteroseptal myocardial infarction in the Healing and Early Afterload Reducing Trial (HEART): an echocardiography-based structural analysisAm Heart J20011412344211174337
- [AIRE] The Acute Infarction Ramipril Efficacy (AIRE) Study InvestigatorsEffect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failureLancet199334282188104270
- AmbrosioniEBorghiCMagnaniBThe Survival of Myocardial Infarction Long-Term Evaluation (SMILE) Study InvestigatorsThe effect of the angiotensin-converting–enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarctionN Engl J Med19953328057990904
- BaruchLAnandICohenISAugmented short- and longterm hemodynamic and hormonal effects of an angiotensin receptor blocker added to angiotensin converting enzyme inhibitor therapy in patients with heart failure. Vasodilator Heart Failure Trial (V-HeFT) Study GroupCirculation19999926586410338459
- BenzJOshrainCHenryDValsartan, a new angiotensin II receptor antagonist: a double-blind study comparing the incidence of cough with lisinopril and hydrochlorothiazideJ Clin Pharmacol19973710179055135
- BuhlerFRLaraghJHBaerLPropranolol inhibition of renin secretion. A specific approach to diagnosis and treatment of renindependent hypertensive diseasesN Engl J Med19722871209145084985
- CampbellDJAggarwalAEslerMβ-blockers, angiotensin II, and ACE inhibitors in patients with heart failureLancet200135816091011716889
- CampbellDJKrumHEslerMDLosartan increases bradykinin levels in hypertensive humansCirculation20051113152015655136
- CarsonPTognoniGCohnJNEffect of Valsartan on hospitalization: results from Val-HeFTJ Card Fail200391647112815565
- ChioleroABurnierMPharmacology of valsartan, an angiotensin II receptor antagonistExpert Opin Investig Drugs19987191525
- ChungOCsikosTUngerTAngiotensin II receptor pharmacology and AT1-receptor blockersJ Hum Hypertens199913Suppl 1S112010076916
- CohnJNAnandISLatiniRValsartan Heart Failure Trial InvestigatorsSustained reduction of aldosterone in response to the angiotensin receptor blocker valsartan in patients with chronic heart failure: results from the Valsartan Heart Failure TrialCirculation20031081306912939207
- CohnJNTognoniGValsartan Heart Failure Trial InvestigatorsA randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failureN Engl J Med200134516677511759645
- [CONSENSUS] The CONSENSUS Trial Study GroupEffects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS)N Engl J Med19873161429352883575
- CoreaLCardoniOFogariRValsartan, a new angiotensin II antagonist for the treatment of essential hypertension: a comparative study of the efficacy and safety against amlodipineClin Pharmacol Ther19966034168841157
- CriscioneLde GasparoMBuhlmayerPPharmacological profile of valsartan: a potent, orally active, nonpeptide antagonist of the angiotensin II AT1-receptor subtypeBr J Pharmacol1993110761718242249
- de GasparoMHessPClozelMCombination of low-dose valsartan and enalapril improves endothelial dysfunction and coronary reserve in Nomega-nitro-L-arginine methyl ester-treated spontaneously hypertensive ratsJ Cardiovasc Pharmacol20024078980012409988
- de GasparoMLevensNDoes blockade of angiotensin II receptors offer clinical benefits over inhibition of angiotensin-converting enzyme?Pharmacol Toxicol199882257719677617
- DicksteinKKjekshusJOPTIMAAL Steering Committee of the OPTIMAAL Study GroupEffects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist LosartanLancet20023607526012241832
- DrexlerHEndothelial dysfunction in heart failure and potential for reversal by ACE inhibitionBr Heart J1994723 SupplS11147946797
- DzauVJTissue renin-angiotensin system in myocardial hypertrophy and failureArch Intern Med1993153937428386920
- DzauVJTheodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesisHypertension20013710475211304501
- FerrarioCMJessupJChappellMCEffect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2Circulation200511126051015897343
- FerrarioCMTraskAJJessupJAAdvances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular functionAm J Physiol2005289H228190
- FlatherMDYusufSKoberLACE-Inhibitor Myocardial Infarction Collaborative GroupLong-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patientsLancet200035515758110821360
- [GISSI-3] Gruppo Italiano per lo Studio della Sopravvivenza nell'infarto MiocardicoGISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarctionLancet19943431115227910229
- [GISSI-3] Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto MiocardicoSix-month effects of early treatment with lisinopril and transdermal glyceryl trinitrate singly and together withdrawn six weeks after acute myocardial infarctionJ Am Coll Cardiol199627337448557903
- GrangerCBMcMurrayJJYusufSCHARM Investigators and CommitteesEffects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trialLancet2003362772613678870
- HamroffGKatzSDManciniDAddition of angiotensin II receptor blockade to maximal angiotensin-converting enzyme inhibition improves exercise capacity in patients with severe congestive heart failureCirculation199999990210051289
- HowesLGTranDCan angiotensin receptor antagonists be used safely in patients with previous ACE inhibitor-induced angioedema?Drug Saf20022573611888349
- IshiyamaYGallagherPEAverillDBUpregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptorsHypertension200443970615007027
- ISIS-4 (Fourth International Study of Infarct Survival) Collaborative GroupISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58 050 patients with suspected acute myocardial infarctionLancet1995345669857661937
- IwataMCowlingRTGurantzDAngiotensin-(1-7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effectsAm J Physiol2005289H235663
- JongPDemersCMcKelvieRSAngiotensin receptor blockers in heart failure: meta-analysis of randomized controlled trialsJ Am Coll Cardiol2002394637011823085
- JordeUPEnnezatPVLiskerJMaximally recommended doses of angiotensin-converting enzyme (ACE) inhibitors do not completely prevent ACE-mediated formation of angiotensin II in chronic heart failureCirculation2000101844610694521
- JugduttBIMyocardial salvage by intravenous nitroglycerin in conscious dogs: loss of beneficial effect with marked nitroglycerin-induced hypotensionCirculation198368673846409447
- JugduttBIIntravenous nitroglycerin unloading in acute myocardial infarctionAm J Cardiol19916852D63D
- JugduttBIPrevention of ventricular remodelling post myocardial infarction: timing and duration of therapyCan J Cardiol19939103148439824
- JugduttBIDhallaNSBeamishRENaganoMModification of left ventricular remodelling after myocardial infarctionThe failing heart1995New YorkRaven Pr23145
- JugduttBIPrevention of ventricular remodeling after myocardial infarction and in congestive heart failureHeart Failure Rev1996111529
- JugduttBICrawfordMHAngiotensin II receptor blockersCardiology Clinics Annual of Drug Therapy1998Vol 2PhiladelphiaWB Saunders Pub117
- JugduttBIRemodeling of the myocardium and potential targets in the collagen degradation and synthesis pathwaysCurr Drug Targets Cardiovasc Haematol Disord2003a313012769643
- JugduttBIVentricular remodeling after infarction and the extracellular collagen matrix: when is enough enough?Circulation2003b108139540312975244
- JugduttBIBalghithMEnhanced regional AT2-receptor and PKCɛ expression during cardioprotection induced by AT1-receptor blockade after reperfused myocardial infarctionJ Renin Angiotensin Aldosterone Syst200121344011881113
- JugduttBIMenonVAT1 receptor blockade limits myocardial injury and upregulates AT2 receptors during reperfused myocardial infarctionMol and Cell Biochem2004a2601111815228092
- JugduttBIMenonVValsartan-induced cardioprotection involves angiotensin II type 2 receptor upregulation in dog and rat in vivo models of reperfused myocardial infarctionJ Cardiac Failure2004b107482
- JugduttBISawickiGAT1 receptor blockade alters metabolic, functional and structural proteins after reperfused myocardial infarction. Detection using proteomicsMol and Cell Biochem20042631798815524179
- JugduttBIWarnicaJWIntravenous nitroglycerin therapy to limit myocardial infarct size, expansion and complications: effect of timing, dosage and infarct locationCirculation198878906193139326
- JugduttBIWarnicaJWTolerance with low dose intravenous nitroglycerin therapy in acute myocardial infarctionAm J Cardiol19896458172506751
- JugduttBIXuYBalghithMCardioprotection induced by AT1R blockade after reperfused myocardial infarction: association with regional increase in AT2R, IP3R and PKCɛ proteins and cGMPJ Cardiovasc Pharmacol Ther200053011111150400
- JuliusSKjeldsenSEWeberMfor the VALUE trial groupOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet200436320223115207952
- KawamuraMImanashiMMatsushimaYCirculating angiotensin II levels under repeated administration of lisinopril in normal subjectsClin Exp Pharmacol Physiol199219547531326422
- KoberLTorp-PedersenCCarlsenJEA clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study GroupN Engl J Med1995333167067477219
- LangtryHDMcClellanKJValsartan/hydrochlorothiazideDrugs199957751510353300
- LatiniRMassonSAnandIValsartan Heart Failure Trial InvestigatorsEffects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: the Valsartan Heart Failure Trial (Val-HeFT)Circulation20021062454812417542
- LeeVCRhewDCDylanMMeta-analysis: angiotensinreceptor blockers in chronic heart failure and high-risk acute myocardial infarctionAnn Intern Med200414169370415520426
- LeriALiuYLiBUp-regulation of AT1 and AT2 receptors in postinfarcted hypertrophied myocytes and stretch-mediated apoptotic cell deathAm J Pathol200015616637210793077
- LevyBICan angiotensin II type 2 receptors have deleterious effects in cardiovascular disease? Implications for therapeutic blockade of the renin-angiotensin systemCirculation200410981314707017
- LishengLLiuLSWangWChinese Cardiac Study Collaborative GroupOral captopril versus placebo among 13 634 patients with suspected acute myocardial infarctio: interim report from the Chinese Cardiac Study (CCS-1)Lancet199534568677885123
- LiuYHYangXPSharovVGEffects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptorsJ Clin Invest1997991926359109437
- LootAERoksAJHenningRHAngiotensin-(1-7) attenuates the development of heart failure after myocardial infarction in ratsCirculation200210515485011927520
- MaggioniAPAnandIGottliebSOVal-HeFT Investigators (Valsartan Heart Failure Trial)Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensinconverting enzyme inhibitorsJ Am Coll Cardiol20024014142112392830
- MaggioniAPLatiniRCarsonPEVal-HeFT InvestigatorsValsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT)Am Heart J20051495485715864246
- MarkhamAGoaKLValsartan. A review of its pharmacology and therapeutic use in essential hypertensionDrugs1997542993119257084
- McKelvieRSYusufSPericakDComparison of candesartan, enalapril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. The RESOLVD Pilot Study InvestigatorsCirculation199910010566410477530
- McMurrayJJOstergrenJSwedbergKCHARM Investigators and CommitteesEffects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trialLancet20033627677113678869
- McMurrayJAngiotensin receptor blockers and myocardial infarction: Analysis of evidence is incomplete and inaccurate. Rapid responseBMJ2005330126915920134
- ModenaMGAvetaPMenozziAAldosterone inhibition limits collagen synthesis and progressive left ventricular enlargement after anterior myocardial infarctionAm Heart J200114141611136485
- NewRBSampsonACKingMKEffects of combined angiotensin II and endothelin receptor blockade with developing heart failure: effects on left ventricular performanceCirculation200010214475310993866
- O'ConnellJBThe economic burden of heart failureClin Cardiol200023Suppl III6103
- OpieLHSackMNEnhanced angiotensin II activity in heart failure: reevaluation of the counterregulatory hypothesis of receptor subtypesCirc Res200188654811304486
- PfefferJMPfefferMABraunwaldEInfluence of chronic captopril therapy on the infarcted left ventricle of the ratCirc Res19855784953891127
- PfefferMAACE inhibitors in acute myocardial infarction: patient selection and timingCirculation199897219249631866
- PfefferMABraunwaldEVentricular remodeling after myocardial infarction. Experimental observations and clinical implicationsCirculation1990811161722138525
- PfefferMABraunwaldEMoyéLASAVE InvestigatorsEffect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarctionN Engl J Med1992327669771386652
- PfefferMAGreavesSCArnoldJMEarly versus delayed angiotensin-converting enzyme inhibition therapy in acute myocardial infarction. The healing and early afterload reducing therapy trialCirculation1997952643519193433
- PfefferMAMcMurrayJJVelazquezEJValsartan in Acute Myocardial Infarction Trial InvestigatorsValsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or bothN Engl J Med2003349189390614610160
- PfefferMASwedbergKGrangerCBCHARM Investigators and CommitteesEffects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programmeLancet20033627596613678868
- PittBPoole-WilsonPASegalREffect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial—the Losartan Heart Failure Survival Study ELITE IILancet20003551582710821361
- PittBRemmeWZannadFEplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study InvestigatorsEplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarctionN Engl J Med200334813092112668699
- PittBSegalRMartinezFARandomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE)Lancet1997349747529074572
- PittBWhiteHNicolauJEPHESUS InvestigatorsEplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failureJ Am Coll Cardiol2005464253116053953
- PittBZannadFRemmeWJThe effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study InvestigatorsN Engl J Med19993417091710471456
- SawickiGJugduttBIDetection of changes in protein levels in the in vivo canine model of acute heart failure following ischemiareperfusion injury – Functional proteomics studiesProteomics20044219520215221779
- SawickiGMenonVJugduttBIImproved balance between TIMP-3 and MMP-9 after regional myocardial ischemia-reperfusion during AT1 receptor blockadeJ Cardiac Failure2004104429
- SeyediNXuXNasjlettiACoronary kinin generation mediates nitric oxide release after angiotensin receptor stimulationHypertension199526164707607720
- SharpeNBenefit of beta-blockers for heart failure: proven in 1999Lancet19993531988910376609
- SolomonSDGlynnRJGreavesSRecovery of ventricular function after myocardial infarction in the reperfusion era: the healing and early afterload reducing therapy studyAnn Intern Med2001134451811255520
- SolomonSDSkaliHAnavekarNSChanges in ventricular size and function in patients treated with valsartan, captopril, or both after myocardial infarctionCirculation200511134111915967846
- [SOLVD] The SOLVD InvestigatorsEffect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failureN Engl J Med19913252933022057034
- [SOLVD] The SOLVD InvestigatorsEffect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractionsN Engl J Med1992327685911463530
- SpinaleFGde GasparoMWhitebreadSModulation of the renin-angiotensin pathway through enzyme inhibition and specific receptor blockade in pacing-induced heart failure: I. Effects on left ventricular performance and neurohormonal systemsCirculation1997962385969337215
- St John SuttonMPfefferMAPlappertTSAVE InvestigatorsQuantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captoprilCirculation19948968758281697
- SuzukiGMoritaHMishimaTEffects of long-term monotherapy with eplerenone, a novel aldosterone blocker, on progression of left ventricular dysfunction and remodeling in dogs with heart failureCirculation200210629677212460880
- SwedbergKHeldPKjekshusJCONSENSUS Study GroupEffects of the early administration of enalapril on mortality in patients with acute myocardial infarction. Results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II)N Engl J Med1992327678841495520
- SzummerKESolomonSDVelazquezEJVALIANT RegistryHeart failure on admission and the risk of stroke following acute myocardial infarction: the VALIANT registryEur Heart J20052621141915972293
- TimmermansPBCariniDJChiuATThe discovery of a new class of highly specific nonpeptide angiotensin II receptor antagonistsAm J Hypertens19914275S81S1854452
- TomBde VriesRSaxenaPRBradykinin potentiation by angiotensin-(1-7) and ACE inhibitors correlates with ACE C- and Ndomain blockadeHypertension20013895911463767
- UrataHHealyBStewartRWAngiotensin II-forming pathways in normal and failing human heartsCirc Res199066883902156635
- VelazquezEJFrancisGSArmstrongPWVALIANT registryAn international perspective on heart failure and left ventricular systolic dysfunction complicating myocardial infarction: the VALIANT registryEur Heart J20042519111915522470
- VerdecchiaPAngeliFGattobigioRDo angiotensin II receptor blockers increase the risk of myocardial infarction?Eur Heart J2005262381616081468
- VerheijenIFierensFLDebackerJPInteraction between the partially insurmountable antagonist valsartan and human recombinant angiotensin II type 1 receptorsFundam Clin Pharmacol2000145778511206708
- VermaSStraussMAngiotensin receptor blockers and myocardial infarctionBMJ20043291248915564232
- WellingtonKFauldsDMValsartan/hydrochlorothiazide: a review of its pharmacology, therapeutic efficacy and place in the management of hypertensionDrugs2002621983200512215069
- WongMJohnsonGShabetaiREchocardiographic variables as prognostic indicators and therapeutic monitors in chronic congestive heart failure. Veterans Affairs cooperative studies V-HeFT I and II. V-HeFT VA Cooperative Studies GroupCirculation1993876 SupplVI-65708500242
- WongMStaszewskyLLatiniRSeverity of left ventricular remodeling defines outcomes and response to therapy in heart failure: Valsartan heart failure trial (Val-HeFT) echocardiographic dataJ Am Coll Cardiol2004432022715172407
- XuYMenonVJugduttBICardioprotection after angiotensin II type 1 blockade involves angiotensin II type 2 receptor expression and activation of protein kinase C-epsilon in acutely reperfused myocardial infarction in the dog. Effect of UP269-6 and losartan on AT1 and AT2-receptor expression and IP3 receptor and PKCe proteinsJ Renin Angiotensin Aldosterone Syst200011849511967812
- YuCMTipoeGLWing-Hon LaiKEffects of combination of angiotensin-converting enzyme inhibitor and angiotensin receptor antagonist on inflammatory cellular infiltration and myocardial interstitial fibrosis after acute myocardial infarctionJ Am Coll Cardiol20013812071511583905
- YusufSPfefferMASwedbergKCHARM Investigators and CommitteesEffects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved TrialLancet20033627778113678871
- YusufSFrom the HOPE to the ONTARGET and the TRANSCEND studies: challenges in improving prognosisAm J Cardiol2002892A18A25A11779516
- ZannadFAllaFDoussetBLimitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales InvestigatorsCirculation20001022700611094035