Abstract
Leptin, a peptide discovered more than 10 years ago, decreases food intake and increases sympathetic nerve activity to both thermogenic and nonthermogenic tissue. Leptin was initially believed to be an anti-obesity hormone, owing to its metabolic effects. However, obese individuals, for unknown reasons, become resistant to the satiety and weight-reducing effect of the hormone, but preserve leptin-mediated sympathetic activation to nonthermogenic tissue such as kidney, heart, and adrenal glands. Leptin has been shown to influence nitric oxide production and natriuresis, and along with chronic sympathetic activation, especially to the kidney, it may lead to sodium retention, systemic vasoconstriction, and blood pressure elevation. Consequently, leptin is currently considered to play an important role in the development of hypertension in obesity.
Introduction
Obesity is considered a world health problem especially in western industrialized countries, where its incidence and prevalence are rising steadily, along with several comorbities associated with it, such as hypertension, diabetes, dyslipidemia, atherosclerosis, and chronic renal failure. Among these, hypertension – a very important cardiovascular risk factor – has been observed in roughly 50% of obese individuals, which has led researchers to consider obesity as one of the most common causes of hypertension. Hence, hypertension in obesity has become a topic of extensive ongoing research.
Now, several mechanisms have been implicated in the association between obesity and hypertension, including activation of sympathetic nervous system, abnormal renal sodium handling, insulin resistance, and physical compression of the kidney (CitationHaynes et al 1998).
In this respect, sympathetic activation appears to mediate at least part of the obesity-induced hypertension, and leptin, the adipocyte-derived hormone, has recently been postulated as one of the possible causes of this sympathetic activation in obesity.
Leptin is a 167 amino acid hormone discovered in 1994 that is almost exclusively produced by adipose tissue and possibly secreted by a constitutive mechanism. The effects of this peptide are mediated by receptors (Ob-R), most of them located in the hypothalamus, belonging to the class l cytokine receptor family. As of yet, 6 leptin receptor isoforms are known (CitationAhima and Flier 2000).
Leptin is considered a homeostatic hormone regulating food intake and body weight. Acting on the hypothalamic nuclei, leptin decreases appetite, and increases energy expenditure through sympathetic activation, which consequently decreases adipose tissue mass and body weight. The hormone levels are decreased during fasting and increased after several days of overfeeding as an effort to help regulate energy balance in humans. Due to latter homeostatic control mechanism, leptin is an anti-obesity hormone, based on the hypothetical fact that high leptin levels would prevent the occurrence of obesity. Unfortunately, this is not the case, and so the strong correlation between serum leptin levels and body fat mass found in obese individuals now suggests the existence of an endogenous leptin-resistant mechanism in obesity (CitationConsidine et al 1996).
In addition to regulating food intake and adipose tissue mass, leptin has been found to be involved in cardiovascular physiological processes like sympathetic nerve system activation, renal hemodynamics, blood vessel tone, and blood pressure. This review will focus on the leptin-mediated sympathetic activation and its relevance to the mechanism of obesity-related hypertension.
Concept of selective leptin resistance
As stated above, obesity is associated with high leptin levels, reflecting the increased amount of adipose tissue in obese individuals. As a consequence, it has been postulated that the apparent loss of the anorexic and weight-reducing effects of leptin in obesity, is a result of a leptin-resistance mechanism. Nevertheless, obesity is associated with increased sympathetic nerve activity, and leptin has been proven to participate in autonomic nervous system control, in part, by increasing renal sympathetic nerve activity (RSNA). Therefore, it is contradictory that, in the presence of a leptin resistance state, the hormone can contribute to the sympathetic activation seen in obesity. This has led to the novel concept of selective leptin resistance, in which resistance appears to be primarily limited to the metabolic (satiety and weight-reducing) actions of leptin, sparing the renal sympathetic activation effects. This concept has emerged from observations made first in agouti yellow obese (Ay) mice (CitationCorreia et al 2002; CitationRahmouni et al 2002), and corroborated recently in a diet-induced obesity model (CitationRahmouni et al 2005). In these studies, leptin administered peripherally produced increase in RSNA in both obese and lean mice, but failed to decrease food intake and body weight in the obese mice to a similar degree as it did in their lean littermates. Interestingly, this same effect was observed when leptin was injected into the lateral ventricle of the brain (CitationRahmouni et al 2002, Citation2005). Here, leptin increased RSNA again, without changing significantly food intake and body weight in Ay and diet-induced obese mice, as compared with their respective lean littermates. These latter studies also served to demonstrate that is unlikely that leptin resistance is due to an impaired leptin transport mechanism across the blood–brain barrier as intracerebroventricular administration of the hormone also failed to enhance food intake and weight loss. Moreover, the increased RSNA achieved by central leptin administration was not accompanied by changes in plasma leptin levels, suggesting that leptin-induced RSNA was centrally-mediated.
In agreement with these observations, CitationMunzberg et al (2004) recently described that leptin resistance is not global within the hypothalamus, and found that the arcuate nuclei, which contains leptin-mediated anorexigenic neurons, becomes resistant to leptin in very early stages of diet-induced obesity in mice. On the contrary, other hypothalamic and extrahypothalamic sites appear to continue relatively sensitive to the actions of leptin, including the ventromedial hypothalamic nuclei, which has been previously shown to mediate leptin-induced sympathetic nerve activation (CitationSatoh et al 1999).
This hypothesis, however, must be proved in human studies as well, and if true, it would help explain how leptin could contribute to sympathetic activation and hypertension despite the fact that there is resistance to the satiety and weight-reducing effects of leptin in obesity.
Renal effects of leptin: the role of the sympathetic nervous system and nitric oxide
In the kidney, leptin may affect blood pressure mainly by two opposing ways: the first is through renal sympathetic activation (CitationHaynes, Sivitz, et al 1997), and the second is related to nitric oxide (NO) synthesis (CitationVecchione et al 2002). The renal effect of leptin also depends on the exposure time to the hormone. As it has been observed, acutely administered leptin usually does not increase blood pressure in animal or human studies, but when given for longer periods of time, leptin does elevate blood pressure in animal models.
Acute effects
Traditionally, obesity-induced renal sympathetic activation has been thought to contribute to the development of hypertension by enhancing sodium retention, since renal denervation attenuates the antinatriuretic and hypertensive effect of obesity in dogs (CitationKassab et al 1995). Although systemic administration of leptin stimulates sympathetic nerve activity to the kidney (CitationHaynes, Morgan, et al 1997), if infused for short period of time, leptin produces increased sodium excretion and urine output with no changes in blood pressure in lean animals (CitationJackson and Li 1997; CitationVillarreal et al 1998; CitationBeltowski, Wojcicka, Gorny, et al 2002; CitationBeltowski, Jochem, et al 2004). The increased natriuresis and fractional sodium excretion are not accompanied by substantial changes in glomerular filtration rate or the renin–aldosterone axis suggesting a decreased sodium tubular transport. CitationBeltowski, Wojcicka, et al (2002) described a decreased sodium–potassium exchanger (Na/K-ATPase) activity in the renal medulla following acute leptin administration. New evidence suggests that leptin stimulates systemic NO release which opposes the pressor and antinatriuretic effect of leptin-induced sympathetic activation (CitationBeltowski, Wojcicka, Borkowska, et al 2002; CitationBeltowski, Jochem, et al 2004). In this regard, CitationVecchione et al (2002) demonstrated that leptin stimulates NO production in endothelial cells and blood vessels. In other studies, leptin evoked a hypotensive effect when the sympathetic nervous system output was blocked pharmacologically, implying a likely leptin-mediated vasorelaxant effect (CitationFruhbeck 1999; CitationLembo et al 2000).
It has been demonstrated that endogenously-produced NO inhibits renal sodium reabsorption in most of the tubule segments apparently through a decrease in the activity of both apical transporters and basolateral Na/K-ATPases (CitationOrtiz and Garvin 2002). Likewise, CitationVillarreal et al (2004) found that chronic inhibition of NO synthesis impairs the acute leptin-mediated natriuretic effect in normotensive lean rats. The kidney has been shown to express high amounts of leptin receptors (CitationSerradeil-Le Gal et al 1997), yet it is not clear whether leptin evokes a direct effect on leptin receptors or indirectly via induction of NO release in renal tubules.
Interestingly, the short-term leptin-induced natriuretic effect seen in lean animals was attenuated in obese rats and was even blunted in spontaneously hypertensive rats (SHR) (CitationVillarreal et al 1998; CitationBeltowski, Wojcicka, Gorny, et al 2002). This suggests a certain level of peripheral leptin resistance, probably as a result of several factors including renal receptor down-regulation induced by long-standing hyperleptinemia (CitationCoatmellec-Taglioni et al 2003) and/or the presence of chronic antinatriuretic mechanisms, typical of these states, such as an enhanced RSNA and renin–angiotensin system (CitationVillarreal et al 1998; CitationBeltowski, Wojcicka, Gorny, et al 2002), which may have overcome leptin-mediated natriuresis. In agreement with this statement, CitationVillarreal et al (2000) found that SHR after renal denervation recovered the sodium excretory capacity in response to acute leptin administration.
Chronic effects
Conversely, long-term leptin administration increases both RSNA and blood pressure. Recent data indicate that chronic hyperleptinemia decreases natriuresis and urinary excretion of NO metabolites (NOx) (CitationBeltowski, Jamroz-Wisniewska, et al 2004). In humans, a negative correlation between leptin and NOx was observed recently in obese hypertensive individuals (CitationGolan et al 2002). Apparently, long-term hyperleptinemic states like obesity increase the level of systemic and intrarenal oxidative stress, leading to NO deficiency (CitationBeltowski, Wojcicka, et al 2004). Leptin, chronically, would reduce natriuresis by up-regulation of the Na/K-ATPase pump, especially in the renal medulla, thereby promoting sodium retention (CitationBeltowski, Jamroz-Wisniewska, et al 2004). This antinatriuretic response to chronic hyperleptinemia, could be either secondary to renal sympathetic activation (CitationEchtenkamp and Dandridge 1989; CitationKirchheim et al 1989; CitationBeach 1992) and/or deficiency of systemic and intrarenal NO production (CitationBeltowski, Wojcicka, et al 2004). Chronic inhibition of NO synthesis in the renal medulla may reduce medullary blood flow by 30%, which is associated with sodium retention and high blood pressure (CitationMattson et al 1994).
Supporting these observations, CitationBickel et al (2001) reported an increased number of different sodium tubular transporters in the kidney of obese rats. These animals exhibited a decreased natriuretic effect after a saline load when compared with their lean age mates. In addition, thiazide-sensitive Na/Cl cotransporters' abundance has been shown to be associated with inhibition of NO synthesis.
Clinical relevance
Chronic hyperleptinemia, as mentioned, augments the blood pressure by means of different mechanisms in animal models. In humans, there have been several studies attempting to link leptin to hypertension (). Serum leptin levels are elevated in obesity due to increase amount of adipose tissue which is the main source of the hormone and possibly secondary to some degree of central resistance to its action. A number of studies have found leptin to be positively correlated with systolic and diastolic blood pressure in both obese (CitationKunz et al 2000; CitationGolan et al 2002; CitationItoh et al 2002; CitationAl-Hazimi and Syiamic 2004; CitationCanatan et al 2004a; CitationSchutte et al 2005) and non-obese individuals (CitationUckaya et al 1999; CitationAdamczak et al 2000; CitationTakizawa et al 2001; CitationBarba et al 2003; CitationCanatan et al 2004b). CitationAl-Hazimi and Syiamic (2004), for example, found that serum leptin and angiotensin II levels were strong predictors of elevated blood pressure in obese women. Likewise, other investigators (CitationKunz et al 2000; CitationGolan et al 2002; CitationCanatan et al 2004b) reported higher leptin levels in obese hypertensives compared with obese normotensive individuals, even after controlling for confounders such as age and body mass index (BMI). In another study leptin correlated stronger with systolic and diastolic blood pressure than did with BMI (or waist circumference) in untreated male adults (CitationBarba et al 2003). Moreover, one study found that after a 3-month weight reduction program, leptin continued to correlate positively with mean arterial blood pressure in the hypertensive, but not in the normotensive obese group (CitationItoh et al 2002). Interestingly, leptin has been found to be elevated in several hypertensive states of pregnancy as well (CitationAnato et al 2000; CitationVitoratos et al 2001). For instance, CitationVitoratos et al (2001) reported increased leptin levels in pre-eclamptics compared with normotensive pregnant women of the same gestational age. Of note is also the observation that a positive correlation between leptin and platelet count levels exist in pre-eclamptic women (CitationAnato et al 2000).
Figure 1 Renal and blood pressure effect of leptin. Adapted from CitationHall et al 2001. Copyright © 2001. Reproduced with permission from Hall JE, Hildebrandt DA, Kuo J. 2001. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertension, 14(6 Pt 2):103S-115S.
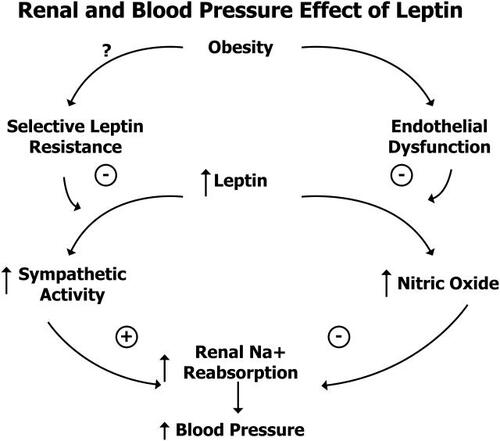
In disagreement to some of these data, one study found that leptin levels were higher in hypertensives than in normotensive African Americans, but once these individuals were adjusted for obesity, no significant relationship was further observed between leptin and blood pressure (CitationEl-Gharbawy et al 2002).
In summary, it seems evident that serum leptin levels are significantly elevated in obese hypertensive individuals when compared with obese normotensives. However this relationship between leptin and blood pressure may not always be as evident after controlling for BMI in some subjects, making the assumption of leptin as a one of the potential causes of hypertension in obesity, less consistent.
Possible treatment considerations
The elevated leptin concentrations seen in obese hypertensive humans are mainly due to abdominal adipose tissue secretion. Current studies have elucidated that some antihypertensives might be more relevant than others in terms of reducing leptin levels in obesity. For example, angiotensin II has recently been found to stimulate leptin production in human adipose tissue (CitationSkurk et al 2005), possibly by means of activation of the angiotensin II type 1 receptor subtype. This effect was completely abolished when the angiotensin receptor blocker (ARB) candesartan was employed prior to angiotensin II administration in human fat cells. In agreement with this observation, valsartan, another ARB, aside from lowering blood pressure, decreased leptin levels and BMI in obese individuals, when compared with the calcium channel blocker (CCB) felodipine (CitationFogari et al 2005). Similarly, the angiotensin-converting enzyme (ACE) inhibitor, enalapril, in combination with a weight reduction program, evidenced the greatest benefits in terms of weigh loss and diminution of plasma norepinephrine, insulin, and leptin levels in comparison with control groups treated with weight reduction program alone or combined with the CCB amlodipine (CitationMasuo et al 2001). In another study, the beta-blocker (BB) pindolol showed a marked suppressive effect on serum leptin levels, not seen in hypertensive individuals on perindopril, or felodipine (CitationFicek et al 2002).
In contrast to these findings, CitationSonmez et al (2001) in an earlier study, found no clear effect of the ARB Losartan on leptin levels in young hypertensive individuals despite its hypotensive action. Likewise, both enalapril and clonidine reduced heart sympathetic activity and blood pressure in another clinical trial, but failed to decrease serum leptin levels in normotensive obese and non-obese subjects after 7 days of treatment (CitationAmador et al 2004).
It is worth mentioning that obesity is associated with sodium retention and increased extracellular volume inducing hypervolemia and increased cardiac output, leading to the activation of the renin–angiotensin and sympathetic nervous system; all of these factors contributing ultimately to the development of hypertension and eccentric-concentric left ventricular hypertrophy: changes that may induce congestive heart failure, arrhythmia, and sudden death () (CitationZhang and Reisin 2000).
Figure 2 Potential pathways in which obesity can cause cardiovascular dysfunction. Effects of obesity-hypertension on the heart. Adapted from CitationZhang et al 2000. Copyright © 2004. Reproduced with permission from Zhang R, Reisin E. 2000. Obesity-hypertension: the effects on cardiovascular and renal systems. Am J Hypertens, 13:1308-14.
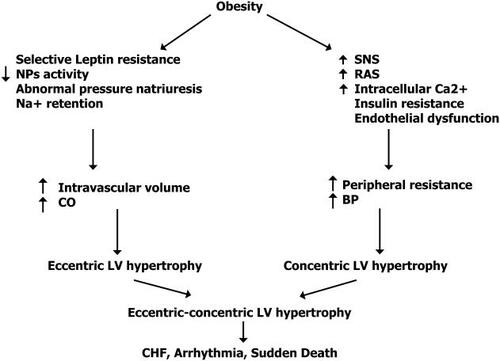
Although the studies are not yet consistent, it seems that pharmacologic suppression of the sympathetic nervous system, the renin–agiotensin system and, lately hyperleptinemia with ACE inhibitors, ARB and BB, may resemble the blood pressure reduction effect induced by weight loss. Diuretics, on the other hand, by eliminating urinary sodium and extracellular fluid, might also play a significant role in the treatment of hypertension in obesity, as these individuals are known for being sodium retainers and hypervolemics. Nevertheless, BBs and diuretics may affect insulin sensitivity and cause hyperglycemia and so their use should be carried out with close monitoring of glycemia in this patient population.
Summary
In summary, leptin the adipose tissue-derived hormone by way of distinct neurochemical pathways stimulates sympathetic nerve activity in thermogenic and nonthermogenic tissue, affecting the metabolic and cardiovascular system respectively. Leptin, acutely, could have a dual influence on blood pressure control, in which the net effect would depend on the balance between the pressor action through activation of the sympathetic nervous system and a possible natriuretic and peripheral vasorelaxant effect of the hormone on the renal tubules and endothelium. In contrast, chronic hyperleptinemia may lead to abnormal renal sodium retention and vasoconstriction associated with renal sympathetic activation and NO deficiency, both contributing to pressure elevation in obese individuals, who may develop resistance to the satiety effect of leptin with preservation of the cardiovascular effect. Treatment of these individuals might be focused in overcoming the hemodynamic alterations seen in obesity, such as antinatriuresis and overactivity of the sympathetic and renin–angiotensin systems.
References
- AdamczakMKokotFWiecekAWRelationship between plasma renin profile and leptinaemia in patients with essential hypertensionJ Hum Hypertens200014503910962518
- AhimaRSFlierJSLeptinAnn Rev Physiol2000624133710845097
- Al-HazimiAMSyiamicAYRelationship between plasma angiotensinII, leptin and arterial blood pressureSaudi Med J2004251193815448764
- AmadorNPerez-LuqueEMalacaraJMLeptin and heart sympathetic activity in normotensive obese and non-obese subjectsItal Heart J20045293515080578
- AnatoVGarmendiaJVBiancoNESerum leptin levels in different types of hypertension during pregnancyRes Commun Mol Pathol Pharmacol20001081475311913707
- BarbaGRussoOSianiAPlasma leptin and blood pressure in men: graded association independent of body mass and fat patternObes Res200311160612529499
- BeachRERenal nerve-mediated proximal tubule solute reabsorption contributes to hypertension in spontaneously hypertensive ratsClin Exp Hypertens A199214685971628413
- BeltowskiJWojcickaGGornyDHuman leptin administered intraperitoneally stimulates natriuresis and decreases renal medullary Na+, K+-ATPase activity in the rat–impaired effect in dietary-induced obesityMed Sci Monit20028BR221912070427
- BeltowskiJJamroz-WisniewskaABorkowskaEUp-regulation of renal Na(+), K(+)-ATPase: the possible novel mechanism of leptin-induced hypertensionPol J Pharmacol2004562132215156072
- BeltowskiJJochemJWojcickaGInfluence of intravenously administered leptin on nitric oxide production, renal hemodynamics and renal function in the ratRegul Pept2004120596715177921
- BeltowskiJWojcickaGBorkowskaEHuman leptin stimulates systemic nitric oxide production in the ratObes Res2002109394612226143
- BeltowskiJWojcickaGMarciniakAOxidative stress, nitric oxide production, and renal sodium handling in leptin-induced hypertensionLife Sci2004742987300015051422
- BickelCAVerbalisJGKnepperMAIncreased renal Na-K-ATPase, NCC, and beta-ENaC abundance in obese Zucker ratsAm J Physiol Renal Physiol2001281F6394811553510
- CanatanHBakanIAkbulutMComparative analysis of plasma leptin levels in both genders of patients with essential hypertension and healthy subjectsEndocr Res2004a309510515098923
- CanatanHBakanIAkbulutMRelationship among levels of leptin and zinc, copper, and zinc/copper ratio in plasma of patients with essential hypertension and healthy normotensive subjectsBiol Trace Elem Res2004b1001172315326361
- Coatmellec-TaglioniGDausseJPGiudicelliYSexual dimorphism in cafeteria diet-induced hypertension is associated with gender-related difference in renal leptin receptor down-regulationJ Pharmacol Exp Ther2003305362712649390
- ConsidineRVSinhaMKHeimanMLSerum immunoreactive-leptin concentrations in normal-weight and obese humansN Engl J Med199633429258532024
- CorreiaMLHaynesWGRahmouniKThe concept of selective leptin resistance: evidence from agouti yellow obese miceDiabetes2002514394211812752
- EchtenkampSFDandridgePFInfluence of renal sympathetic nerve stimulation on renal function in the primateAm J Physiol1989257F20492669527
- El-GharbawyAHKotchenJMGrimCEGender-specific correlates of leptin with hypertension-related phenotypes in African AmericansAm J Hypertens2002159899312441220
- FicekJKokotFChudekJInfluence of antihypertensive treatment with perindopril, pindolol or felodipinon plasma leptin concentration in patients with essential hypertensionHorm Metab Res200234703812660886
- FogariRDerosaGZoppiAComparison of the effects of valsartan and felodipine on plasma leptin and insulin sensitivity in hypertensive obese patientsHypertens Res2005282091416097363
- FruhbeckGPivotal role of nitric oxide in the control of blood pressure after leptin administrationDiabetes199948903810102710
- GolanETalBDrorYReduction in resting metabolic rate and ratio of plasma leptin to urinary nitric oxide: influence on obesity-related hypertensionIsr Med Assoc J200244263012073415
- HallJEHildebrandtDAKuoJObesity hypertension: role of leptin and sympathetic nervous systemAm J Hypertension2001146 Pt 2103S115S
- HaynesWGMorganDAWalshSAReceptor-mediated regional sympathetic nerve activation by leptinJ Clin Invest199710027089218503
- HaynesWGMorganDAWalshSACardiovascular consequences of obesity: role of leptinClin Exp Pharmacol Physiol1998256599493562
- HaynesWGSivitzWIMorganDASympathetic and cardiorenal actions of leptinHypertension199730619239322991
- ItohKImaiKMasudaTRelationship between changes in serum leptin levels and blood pressure after weight lossHypertens Res200225881612484512
- JacksonEKLiPHuman leptin has natriuretic activity in the ratAm J Physiol1997272F33389087676
- KassabSKatoTWilkinsFCRenal denervation attenuates the sodium retention and hypertension associated with obesityHypertension19952589377721450
- KirchheimHEhmkeHPerssonPSympathetic modulation of renal hemodynamics, renin release and sodium excretionKlin Wochenschr198967858642681964
- KunzISchorrUKlausSResting metabolic rate and substrate use in obesity hypertensionHypertension200036263210904008
- LemboGVecchioneCFrattaLLeptin induces direct vasodilation through distinct endothelial mechanismsDiabetes200049293710868946
- MasuoKMikamiHOgiharaTWeight reduction and pharmacologic treatment in obese hypertensivesAm J Hypertens200114530811411732
- MattsonDLLuSNakanishiKEffect of chronic renal medullary nitric oxide inhibition on blood pressureAm J Physiol1994266H1918268203591
- MunzbergHFlierJSBjorbaekCRegion-specific leptin resistance within the hypothalamus of diet-induced obese miceEndocrinology20041454880915271881
- OrtizPAGarvinJLRole of nitric oxide in the regulation of nephron transportAm J Physiol Renal Physiol2002282F7778411934686
- RahmouniKHaynesWGMorganDASelective resistance to central neural administration of leptin in agouti obese miceHypertension2002394869011882595
- RahmouniKMorganDAMorganGMRole of selective leptin resistance in diet-induced obesity hypertensionDiabetes20055420121815983201
- SatohNOgawaYKatsuuraGSympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretionDiabetes19994817879310480609
- SchutteRHuismanHWSchutteAELeptin is independently associated with systolic blood pressure, pulse pressure and arterial compliance in hypertensive African women with increased adiposity: the POWIRS studyJ Hum Hypertens2005195354115759020
- Serradeil-Le GalCRaufasteDBrossardGCharacterization and localization of leptin receptors in the rat kidneyFEBS Lett1997404185919119061
- SkurkTvan HarmelenVBlumWFAngiotensin II promotes leptin production in cultured human fat cells by an ERK1/2-dependent pathwayObes Res2005139697315976138
- SonmezAKisaUUckayaGEffects of losartan treatment on T-cell activities and plasma leptin concentrations in primary hypertensionJ Renin Angiotensin Aldosterone Syst200121121611881109
- TakizawaHUraNSaitohSGender difference in the relationships among hyperleptinemia, hyperinsulinemia, and hypertensionClin Exp Hypertens2001233576811349826
- UckayaGOzataMSonmezAPlasma leptin levels strongly correlate with plasma renin activity in patients with essential hypertensionHorm Metab Res199931435810450836
- VecchioneCMaffeiAColellaSLeptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathwayDiabetes2002511687311756337
- VillarrealDReamsGFreemanRHEffects of renal denervation on the sodium excretory actions of leptin in hypertensive ratsKidney Int2000589899410972663
- VillarrealDReamsGFreemanRHRenal effects of leptin in normotensive, hypertensive, and obese ratsAm J Physiol1998275R2056609843897
- VillarrealDReamsGSamarHEffects of chronic nitric oxide inhibition on the renal excretory response to leptinObes Res20041210061015229341
- VitoratosNChrystodoulacosGKouskouniEAlterations of maternal and fetal leptin concentrations in hypertensive disorders of pregnancyEur J Obstet Gynecol Reprod Biol200196596211311762
- ZhangRReisinEObesity-hypertension: the effects on cardiovascular and renal systemsAm J Hypertens20001313081411130776