Abstract
Angiotensin-converting enzyme (ACE) inhibitors effectively interfere with the renin–angiotensin system and exert various beneficial actions on vascular structure and function beyond their blood pressure-lowering effects. Zofenopril, a potent sulphydryl ACE inhibitor, is characterized by high lipophilicity, sustained cardiac ACE inhibition, and antioxidant and tissue protective activities. Its ancillary properties, such as antioxidant activity and cardiovascular (CV) protection, make this drug potentially suitable for the treatment and prevention of certain CV diseases. The Survival of Myocardial Infarction Long term Evaluation trials have demonstrated that the early administration of zofenopril to patients with acute myocardial infarction is associated with a significant reduction in the 6-week occurrence of major CV events in high-risk patients with anterior non-thrombolyzed myocardial infarction. The fixed combination of zofenopril–hydrochlorothiazide (HCTZ) 30/12.5mg/day is approved for the management of mild-to-moderate hypertension in different European countries. In clinical trials comparing zofenopril–HCTZ with each agent administered as monotherapy, combination therapy was clearly more effective in normalizing blood pressure (BP). In addition, combination therapy provided sustained and consistent BP control over the entire 24 hour dosing interval. The efficacy and safety profile of zofenopril–HCTZ highlights that this combination is a potentially useful addition to currently available therapy for patients with BP inadequately controlled by monotherapy, as well as for patients who require more rapid and intensive BP control.
Introduction
Hypertension is the most commonly occurring independent risk factor for cardiovascular (CV) disease in both developed and developing countries (CitationEzzati et al 2002). The life-long prevalence of developing hypertension after the sixth decade of life is reportedly 90% (CitationFranklin et al 2001). On the other hand, effective treatment of hypertension is associated with a reduction in adverse CV events (CitationCollins and MacMahon 1994; CitationHansson et al 1998). A meta-analysis of data sampled in more than 47 000 patients showed that a sustained reduction of 5 mmHg to 6 mmHg in diastolic blood pressure (DBP) resulted in a risk reduction of more than 50% for heart failure, up to 40% for stroke and 20% to 25% for coronary heart disease (CitationVasan et al 2001). Data from the Hypertension Optimal Treatment (HOT) trial, evaluating approximately 19 000 patients reported that the lowest incidence of major cardiovascular events occurred at a mean achieved DBP of 82.6 mmHg. In addition, in a subgroup analysis, a 50% reduction in major CV events was observed in patients with hypertension and diabetes randomized to a target DBP of ≤80 mmHg (CitationHansson et al 1998; CitationWHO–ISH 2003).
Current guidelines for the management of hypertension recommend treating all hypertensive patients to target systolic blood pressure (SBP)/DBP values of ≤140/90 mmHg, and for patients with hypertension and comorbidities such as diabetes or renal disease to a target of <130/80 mmHg (CitationESH–ESC 2003; JNC-7 2003).
Beyond these recommendations, according to recent US data (CitationHajjar and Kotchen 2003), a large proportion of the hypertensive population (about 50%) are unaware of their high BP values and, consequently, are not currently treated for hypertension. Among those who are aware and treated for hypertension, the actual degree of adequate BP control is far from satisfactory, and does not attain the 30% level of treated patients across almost all Western countries (CitationWang and Vasan 2005).
Similar results have been provided by an extensive review of the extent of blood pressure management in European countries (CitationEUROASPIRE II 2001). Poor BP control is responsible for a significant increase in the economic burden of treating hypertension, since it increases the proportion of patients who do not achieve any clinical benefit from treatment, despite their involvement in a costly treatment program. Several different reasons have been identified to explain the poor extent of BP control, including: 1) inadequate compliance to treatment; 2) insufficient use of drug combinations; and 3) the high proportion of patients who withdraw from treatment because of incomplete BP control (CitationAmbrosioni et al 2000).
The main ways to improve the patient compliance to antihypertensive treatment appear to be the use of first-line therapy that are: 1) well tolerated; 2) mono-administered; 3) efficacious (JNC 7 2003).
In this context, angiotensin-converting enzyme (ACE) inhibitors are one of the most safe and efficacious class of antihypertensive drugs (CitationKhalil et al 2001).
Rationale for combination therapy
It is now recognized that the majority of patients will require at least two antihypertensive drugs to achieve optimal BP control (CitationHannson et al 1998; CitationKearney et al 2005). The European Society of Hypertension – European Society of Cardiology (ESH–ESC) guidelines recommend the use of either low-dose monotherapy or low-dose combination therapy with two agents (eg, a beta-blocker and a diuretic or an ACE inhibitor and a diuretic) (CitationESH–ESC 2003). The Joint National Committee 7 (JNC-7) report states that the addition of a second agent from a different class should be initiated when the use of monotherapy fails to achieve adequate control of BP (CitationJNC-7 2003).
From a prognostic point of view, what appears to be more relevant in the first choice of an antihypertensive agent is its efficacy in normalizing BP: the HOT trial demonstrated that greater BP reductions and higher response rates are achieved with the use of combination therapy (CitationHannson et al 1997, Citation1998). In this trial, a total of 85% of patients achieved a DBP of ≤90 mmHg, but only approximately one-third of patients remained on monotherapy. Interestingly, 27% of patients had well controlled BP when treated according to step 2 treatment, consisting of a low-dose combination of two agents.
A lack of patient compliance is not sufficient to completely account for the poor treatment rates and low target attainment observed in patients with hypertension (CitationWetzels et al 2004). As demonstrated by the HOT trial, monotherapy simply does not result in BP lowering to or below target levels in the majority of patients.
Fixed-dose combination therapy is likely to be more effective in achieving BP goals and result in greater patient compliance than titrating individual doses of two single agents (CitationMoser 2003). Furthermore, fixed dose combination therapy offers beneficial BP lowering effects while minimizing the adverse effects that are potentially associated with the titration of each agent administered singly (CitationMancia and Grassi 1999; CitationZanchetti 1999).
An effective fixed-dose two-drug combination should have: complementary mechanisms of action; an antihypertensive effect that is at least as, if not more, effective than either agent administered singly or as sequential combination therapy; enhanced ability to offer end-organ protection, and minimal adverse effects (including, hemodynamic and metabolic effects) (CitationMancia and Grassi 1999; CitationZanchetti 1999).
The investigation of the renin–angiotensin system began with the discovery of renin in 1898 by Tiegerstedt and Bergman and was followed by the observation in 1940 that renin acted on a plasma protein substrate to catalyze the formation of a pressor peptide, angiotensin (CitationVane 1999). This plasma precursor is angiotensinogen and two forms of angiotensin, angiotensin I and II, were recognized in the mid 1950s. The conversion of inactive angiotensin I to the active angiotensin II occurs via ACE (CitationSoubrier et al 1988). Since these initial discoveries, efforts to manipulate the renin–angiotensin system have resulted in the synthesis of the first orally effective ACE inhibitor, captopril. A series of other ACE inhibitors have now been synthesized (CitationCushman et al 1977).
Thiazides are effective and inexpensive antihypertensive agents, but their use at full doses is associated with various side effects and low patient compliance (CitationJNC-7 2003).
There are three distinct advantages associated with the combination of an ACE inhibitor and a diuretic (CitationMancia et al 1997; CitationZanchetti 1999). Firstly, this combination, utilizing a low-dose diuretic, reduces the probability of adverse metabolic effects often associated with the use of high-dose diuretic therapy (CitationJNC-7 2003). ACE inhibitors are also known to effectively counteract the tendency of thiazide diuretics to lower serum potassium levels. In addition, HCTZ may increase oxidant activity via an activation of the renin–angiotensin system, and zofenopril has a direct antioxidant effect in humans (CitationNapoli et al 2004).
Secondly, the use of a fixed combination reduces the number of tablets taken daily, thus improving patient compliance.
Thirdly, combination therapy may be, at least, as equally effective as monotherapy in the prevention of organ damage associated with hypertension. ACE inhibitor therapy in combination with a diuretic has been shown to be effective in reducing left ventricular hypertrophy (LVH) (CitationMancia et al 1997). In clinical trials in patients with hypertension and diabetes, an ACE inhibitor in combination with a diuretic plays a role in retarding the progression of renal failure in diabetic and other types of nephropathy (CitationLewis et al 1993; CitationGiatras et al 1997; CitationZanchetti and Ruilope 2002).
Clinical efficacy of Zofenopril–HCTZ
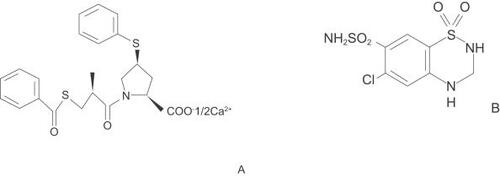
Zofenopril, a new potent sulphydryl ACE inhibitor, is characterized by high lipophilicity, sustained cardiac ACE inhibition, antioxidant and tissue protective activities (CitationBorghi and Ambrosioni 2000; CitationMatarrese et al 2004) ().
Hydrochlorothiazide is the 3,4-dihydro-derivative of chlorothiazide (). It affects the renal tubular mechanisms of electrolyte resorption, directly increasing excretion of sodium. Indirectly, the diuretic action of HCTZ reduces plasma volume with consequent increases in plasma renin activity, aldosterone secretion and urinary potassium loss, and decreases in serum potassium (CitationReyes 2002).
The fixed-dose combination of zofenopril–HCTZ 30/12.5 mg/day is approved for the management of mild-to-moderate hypertension. In clinical studies this combination was more effective in maintaining BP reduction than either agent administered as monotherapy (CitationMalacco and Omboni 2005; CitationParati et al 2005). This result was particularly evident in patients who were non-responsive to zofenopril monotherapy (CitationMalacco and Omboni 2005).
Three pivotal studies have investigated the clinical efficacy of this combination (CitationMalacco and Omboni 2005; CitationParati et al 2005). In all studies a stable baseline elevated BP was confirmed by repeated measurement of seated or standing SBP/DBP during a single-blind, placebo run-in period of 2 to 4 weeks duration. Inclusion criteria for these studies included a seated DBP of: 95 mmHg to 110 mmHg for the dose-response study (CitationParati et al 2005); 95 mmHg to 115 mmHg for the parallel-group comparative study (Study 1), and 90 mmHg to 110mmHg for the non-responder group study (Study 2) (CitationMalacco and Omboni 2005). Patients eligible for randomization to treatment were required to meet the criteria for seated DBP at inclusion and also for intra-and inter-visit variability (<10 mmHg). Randomized, double-blind treatment was given as a once daily regimen at the same time each day. BP was measured approximately 24 h after the previous dose by cuff sphygmomanometer and in the dose-response study by ambulatory BP monitoring (ABPM).
Dose-response study
The results of a 12-week multi-center dose-response study in 353 patients with essential hypertension demonstrated that combination therapy with zofenopril–HCTZ (30/12.5 mg/day or 60/12.5 mg/day) was more effective in maintaining continuous 24 h BP control than either agent administered alone (CitationParati et al 2005).
Patients aged 18 to 75 years were randomized to double-blind treatment with zofenopril 15 mg, 30 mg or 60 mg, HCTZ 12.5 mg or 25 mg or their combination for 12 weeks. The primary efficacy endpoint, proportion of patients achieving office BP normalization (seated DBP <90 mmHg), was greater for the combination of zofenopril–HCTZ than either agent administered as monotherapy and reached significance at the 30/12.5 mg/day, 60/12.5 mg/day and 30/25 mg/day doses (p<0.05).
The combination of zofenopril–HCTZ 30/12.5 mg normalized BP in 57% of patients compared with a normalization rate of 33% for HCTZ 12.5 mg/day (p<0.05). A total of 76% of patients administered the 30/12.5 mg combination exhibited normalized BP or a DBP reduction ≥10 mmHg (responders) in contrast with 42% on HCTZ 12.5 mg/day (p<0.01). Further reductions were observed with zofenopril–HCTZ 60/12.5 mg/day but not when HCTZ was increased to 25 mg/day. Similar results were observed with regard to SBP also, even if this was not a primary goal of the study.
Both 24 h and hourly changes in BP were greater with zofenopril–HCTZ 30/12.5 mg/day, 60/12.5 mg/day and 30/25 mg/day combination treatment than with either agent administered singly.
Furthermore, higher smoothness indices, evaluated by ABPM, indicated that combination therapy, in particular 30/12.5 mg/day and 30/25 mg/day, provided superior BP control over the dosing interval compared with monotherapy (CitationZanchetti et al 2006).
Comparative studies
Two international multi-center, randomized, double-blind, parallel-group studies have evaluated the combination of zofenopril–HCTZ 30/12.5 mg/day compared with monotherapy with zofenopril 30 mg/day (CitationMalacco and Omboni 2005).
A 36-week comparison of zofenopril–HCTZ and zofenopril monotherapy demonstrated the superior efficacy of combination therapy in lowering BP in 463 patients aged 18 to 75 years with mild-to-moderate hypertension. Following a 4-week washout period, the 12-week efficacy endpoint of reduction in DBP and SBP, and the proportion of responders were significantly greater with combination therapy than zofenopril monotherapy (p<0.001).
A second study of 369 patients, 18 to 70 years of age, confirmed the efficacy of combination therapy in treating patients with mild-to-moderate hypertension who were not responsive to zofenopril monotherapy (CitationMalacco and Omboni 2005). Following a 4-week placebo run-in phase, eligible patients were administered 4-weeks’ treatment with zofenopril 30 mg/day in a single-blind fashion. Non-responders (SBP ≥130 mmHg and DBP ≥85 mmHg and/or SBP reduction <20 mmHg and/or DBP reduction <10 mmHg) were then randomized to double-blind treatment with zofenopril–HCTZ 30/12.5 mg/day or zofenopril 30 mg/day for 8 weeks. Significantly greater and more consistent reductions in BP and higher response rates were reported with combination therapy than with zofenopril monotherapy. In those patients receiving zofenopril monotherapy BP reached a plateau at week 8 but continued to decrease in patients receiving combination therapy.
Finally, while these results are encouraging, further clinical data are needed to assess the long-term efficacy of the zofenopril–HCTZ combination on specific clinical outcomes
Zofenopril–HCTZ and end-organ protection
In addition to hypertension and other CV risk factors (ie, hyperlipidemia, diabetes) it has been recognized that activation of the renin–angiotensin–aldosterone system (RAAS) plays a role in the risk of CV disease-related morbidity and mortality (CitationToto et al 2004; CitationDzau 2005). Angiotensin II has been shown to have a direct effect on various tissues including endothelial, vascular and renal tissues (CitationToto et al 2004).
Pharmacological agents such as ACE inhibitors, that actively prevent the formation of angiotensin II, may therefore have a beneficial effect in terms of end-organ protection beyond their activity on BP. Data from clinical trials with an ACE inhibitor, such as the Heart Outcomes Prevention Evaluation (HOPE) study, confirm the efficacy of these agents in reducing the risk of CV death, myocardial infarction and stroke (CitationYusuf et al 2000).
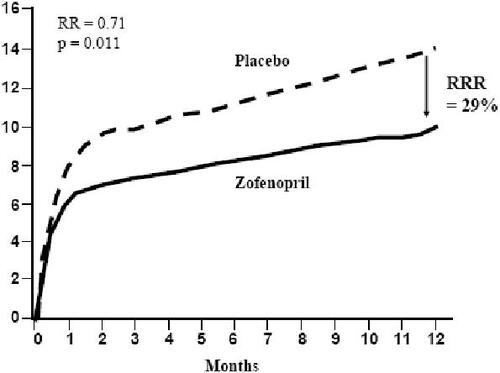
No large randomized clinical trial has yet demonstrated the long-term efficacy of the zofenopril–HCTZ combination on CV morbidity and mortality. The main data on zofenopril efficacy come from the Survival of Myocardial Infarction Long-Term Evaluation (SMILE) and SMILE-2, where patients received this agent in addition to standard therapy. In SMILE, 1556 patients with no history of congestive heart failure and presenting <24 after onset of symptoms were randomized to zofenopril 7.5–30 mg twice daily (bid). and placebo. Incidence of death or severe congestive heart failure at 6 weeks was significantly reduced by zofenopril compared with placebo (7.1% vs 10.6%; p<0.05); the cumulative risk reduction was 34% (p=0.018). The risk reduction with zofenopril was 46% (p<0.018) for severe congestive heart failure and 25% (p=0.19) for death (). After 1 year, the mortality rate was significantly lower in the zofenopril group than in the placebo group (10% vs 14.1%) with a risk reduction of 29% (p=0.011) () (CitationAmbrosioni et al 1995). Similar results have been observed in a subgroup of patients affected by type 2 diabetes (CitationBorghi et al 2003).
Figure 2 Early efficacy of zofenopril treatment in patients with no history of congestive heart failure and presenting <24 h after onset of myocardial infarction symptoms: results from the Survival of Myocardial Infarction Long-term Evaluation (SMILE) study.
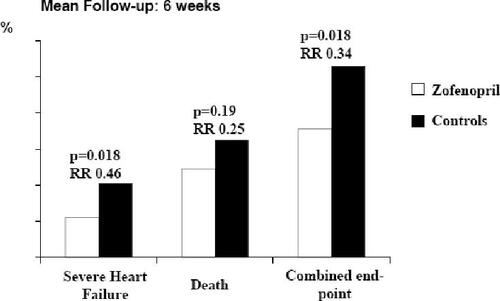
Figure 3 Relative risk reduction of overall mortality related to one year treatment with Zofenopril compared with placebo in the Survival of Myocardial Infarction Long-term Evaluation (SMILE) study.
In SMILE-2, 1024 thrombolyzed patients with acute MI were randomized to receive oral zofenopril (30–60 mg/day) or lisinopril (5–10 mg/day), starting within 12 hours of completion of thrombolytic therapy and continuing for 42 days. The overall incidence of severe hypotension was 10.9% with zofenopril and 11.7% with lisinopril (p=0.38). The incidence of drug-related severe hypotension was slightly but significantly lower with zofenopril than with lisinopril (6.7 vs 9.8%, p=0.048). The 6-week mortality rate was 3.2% in the zofenopril group and 4.0% in the lisinopril group (p=0.38), and no significant differences were observed in the incidence of major cardiovascular complications or any safety variables between the 2 ACE inhibitors (CitationBorghi and Ambrosioni 2003).
Moreover, the ischemic heart protective effect of zofenopril has been clearly demonstrated in different animal models (CitationFrascarelli et al 2004; CitationEvangelista and Manzini 2005; CitationLeva et al 2006): the main proposed mechanisms of action are interference with bradykinin metabolism, preservation of protein sulfhydryl groups, and antioxidant activity.
It has also been suggested (but yet not demonstrated in humans) that the zofenopril–HCTZ combination may be used to treat congestive heart failure. Preclinical data show that, as with captopril, zofenopril significantly increases the myocardial expression of collagen type III and normalized the collagen type I/III ratio, thus preventing myocardial hypertrophy in spontaneous hypertensive rats. The hypertension related organ damage was more completely prevented by zofenopril–HCTZ than by the enalapril–HCTZ combination and it was related to an activation of endothelial nitric oxide (NO) synthase expression and to a normalization of the oxidative stress components due to angiotensin II inhibition (CitationGagnon et al 2004).
Dietary sodium restriction (similar to that achievable with HTCZ) increases the zofenopril-related attenuation of left ventricular dysfunction induced by myocardial infarction (CitationWestendorp et al 2004).
Preclinical data on combination therapy with zofenopril–HCTZ indicated that, as well as preventing the development of arterial hypertension, it provided more complete organ protection than the combination of enalapril–HCTZ (CitationGarcía-Estan et al 2006). This was demonstrated in a 8 week study in hypertensive rats by a reduction in mortality, and normalization of renal morphological and functional alterations including improvements in excretory parameters.
Other preclinical data suggest that HCTZ could also contribute to the cardiac and renal protective effect of zofenopril by increasing the concentration of its metabolite, zofenoprilat, in these target organs (CitationWestendorp et al 2005).
Interestingly, part of the organ-damage protection offered by zofenopril may also be mediated by its antioxidant effect and this has been clearly shown in both laboratory models and in humans (CitationNapoli et al 2004). For example, one clinical study demonstrated that zofenopril, as with other sulfhidryl compounds, was able to improve the oxidative balance in hypertensive patients. This effect was accompanied by a parallel increase in NO synthesis, counteracting the usual inactivation of NO by O2 radical production and was not observed with enalapril (CitationScribner et al 2003). Furthermore, the negative effect of thiazides, of increasing free radical levels through induction of the renin–angiotensin system, could be counterbalanced by zofenopril's antioxidant effect in the combination.
Tolerability of Zofenopril–HCTZ
In controlled clinical trials involving approximately 600 patients, adverse events observed with the combination of zofenopril–HCTZ were in line with those previously reported with zofenopril or HCTZ monotherapy (CitationBorghi et al 2004). The most commonly reported adverse events were dizziness, headache, and cough, as would be expected with ACE inhibitor therapy. These adverse events were generally mild-to-moderate in severity and were not correlated with age or gender. Theoretically, the presence of the sulphydryl group may be associated with an increased prevalence of dysgeusia, but available clinical data do not support the hypothesis that this side effect is more frequent with zofenopril than with other drugs of the same class (CitationZanchetti et al 2006).
In the dose-response study, a total of 9.9% of patients reported an adverse event; 64.3% of these events were of mild intensity (CitationParati et al 2005). In these patients 61.9% of adverse events were determined to be treatment-related but the majority of adverse events disappeared upon discontinuation of treatment. The occurrence of treatment-related adverse events was comparable among the treatment groups, and the most common adverse events were cough and polyuria. Treatment withdrawal occurred in only 1.7% of patients. There were no increases in low-density lipoprotein cholesterol levels or triglycerides, blood glucose or uric acid levels with combination therapy.
Importantly, in two studies zofenopril–HCTZ 30/12.5 mg/day was at least as well tolerated as zofenopril 30 mg/day monotherapy with the combination having therapy no detrimental effect on heart rate (CitationMalacco and Omboni 2005).
Finally, even though the available safety data are encouraging, further clinical studies are needed to fully confirm the long-term safety of the zofenopril–HCTZ combination.
Place of Zofenopril–HCTZ in the management of hypertension
Hypertension is the most commonly occurring independent CV disease risk factor in the world (CitationKearney et al 2004). One of the primary challenges for physicians in the management of patients with hypertension is achieving target BP levels in clinical practice. Physicians are now more aware than ever before that optimal antihypertensive treatment should be effective, ensure timely reductions in BP levels, and be well tolerated to enhance patient compliance.
The use of combination therapy in the treatment of patients with hypertension is recommended by the ESH–ESC, JNC-7 and the World Health Organization–International Society of Hypertension (WHO-ISH) 2003 guidelines (CitationESH–ESC 2003; CitationJNC-7 2003; CitationWHO–ISH 2003). ESH–ESC and JNC-7 guidelines acknowledge that the majority of patients will require two or more antihypertensive agents to achieve target BP goals (CitationESH–ESC 2003; CitationJNC-7 2003). Therefore, in patients with uncontrolled BP, physicians should consider initiating combination therapy either with two separate agents or as a fixed-dose combination. Combination therapy is also justified in certain patient populations with hypertension and co-morbidities (ie, diabetes, chronic kidney disease) for whom rapid reduction of BP is required (CitationESH–ESC 2003; CitationJNC-7 2003).
The use of combination therapy as first-line treatment is increasing as BP goals for antihypertensive therapy are becoming more ambitious for all patients and, in particular, for patients at a higher risk of CV complications. While most guidelines (CitationESH–ESC 2003; CitationJNC-7 2003) advocate the combination of two agents from different classes as an alternative to monotherapy, many physicians feel that this does not allow the freedom to vary the dose of the individual components according to patient response. It should be emphasized that for fixed-dose combinations the individual doses of each component have been selected on the basis of careful investigation to determine the dose that provides the greatest BP reductions with the lowest incidence of adverse events in the largest proportion of patients (CitationZanchetti 1999).
The use of fixed-dose combination therapy with an ACE inhibitor and a diuretic provides physicians with the means to achieve BP control without the need for prolonged and laborious dose-titrations necessary with initial monotherapy (CitationGavras and Rosenthal 2004).
The results of a dose-response study comparing zofenopril–HCTZ with each agent administered as monotherapy clearly demonstrate that a greater antihypertensive effect, in terms of the rate of patients with normalized DBP, is achieved with combination therapy. In addition, combined therapy provided sustained and consistent BP control over the entire 24 h dosing interval.
These results suggest that the combination of zofenopril–HCTZ may be indicated in patients who do not achieve target BP on zofenopril 30 mg/day or up to 25 mg/day of HCTZ. A well-designed study confirmed the greater BP lowering efficacy of zofenopril–HCTZ 30/12.5 mg/day versus zofenopril 30 mg/day monotherapy (CitationBorghi et al 2004). The advantage of zofenopril–HCTZ combination therapy in normalizing BP was particularly evident in patients who were non-responsive to monotherapy (CitationMalacco and Omboni 2005).
The overall tolerability profile of zofenopril–HCTZ is consistent with the profile of each individual agent. A direct comparison of zofenopril–HCTZ with each agent administered as monotherapy did not show any significant differences in the nature, severity or incidence of treatment-related adverse events (CitationMalacco and Omboni 2005; CitationParati et al 2005). Headache, dizziness, cough and polyuria were the most frequently reported treatment-related adverse events. Neither patient gender nor age had any effect on the tolerability of the zofenopril–HCTZ combination. Notably, fewer patients discontinued treatment with combination therapy due to adverse events (CitationZanchetti et al 2006).
The fixed combination of zofenopril–HCTZ is expected to demonstrate potential additive cardiovascular protective properties as suggested by the efficacy of zofenopril monotherapy in reducing the incidence of death or severe congestive heart failure post myocardial infarction in the SMILE study (CitationAmbrosioni et al 1995). Preclinical data support its efficacy in providing cardiovascular and renal end-organ protection (CitationBorghi et al 2004).
Nevertheless, as for all fixed-combination treatment there are also some potential disadvantages: firstly, the difficulty in modifying the dosage of any of the individual drug components according to BP response; secondly, the difficulty in distinguishing the causative agent in any cases of atypical side effects; and finally, the administration of two drugs may also increase the risk of pharmacological interaction with other agents given concomitantly.
Conclusion
In conclusion, data from clinical trials with zofenopril–HCTZ indicate that this combination provides optimal BP control in a larger proportion of patients compared with monotherapy, whilst maintaining the tolerability profile observed with each individual component and thereby enhancing patient compliance. The efficacy and safety profile of zofenopril–HCTZ highlights that this combination is a useful addition to currently available therapy for patients with BP inadequately controlled by monotherapy, as well as for patients who require more rapid and intensive BP control. Further long-term large randomized clinical trials are needed to establish that the zofenopril–HCTZ combination has the same cardiovascular and renal protective effects demonstrated by other ACE inhibitors.
Disclosure
Five years ago Professor Borghi received funding to support research on Zofenopril efficacy and safety in clinical practice.
References
- AmbrosioniEBorghiCMagnaniBfor the Survival of Myocardial Infarction Long-Term Evaluation (SMILE) Study InvestigatorsThe effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarctionN Engl J Med19953338057990904
- AmbrosioniELeonettiGPessinaACPatterns of hypertension management in Italy: results of a pharmacoepidemiological survey on antihypertensive therapy. Scientific Committee of the Italian Pharmacoepidemiological Survey on Antihypertensive TherapyJ Hypertens2000181691911081785
- BorghiCAmbrosioniEZofenopril: A review of the evidence of its benefits in hypertension and acute myocardial infarctionClin Drug Invest20002037184
- BorghiCAmbrosioniESurvival of Myocardial Infarction Long-term Evaluation-2 Working PartyDouble-blind comparison between zofenopril and lisinopril in patients with acute myocardial infarction: results of the Survival of Myocardial Infarction Long-term Evaluation-2 (SMILE-2) studyAm Heart J200314580712514658
- BorghiCBacchelliSDegli EspostiDA review of the angiotensin-converting enzyme inhibitor, zofenopril, in the treatment of cardiovascular diseasesExpert Opin Pharmacother2004519657715330734
- BorghiCBacchelliSEspostiDDSMILE StudyEffects of the early ACE inhibition in diabetic non-thrombolyzed patients with anterior acute myocardial infarctionDiabetes Care2003261862812766124
- CollinsRMacMahonSBlood pressure, antihypertensive drug treatment and the risks of stroke and of coronary heart diseaseBr Med Bull199450272988205459
- CushmanDWCheungHSSaboEFDesign of potent competitive inhibition of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acidsBiochemistry197716548491200262
- DzauVThe cardiovascular continuum and renin-angiotensin-aldosterone system blockadeJ Hypertens200523Suppl 1S9S17
- [ESH–ESC] European Society of Hypertension – European Society of Cardiology Guidelines Committee2003 European Society of Hypertension – European Society of Cardiology guidelines for the management of arterial hypertensionJ Hypertens20032110115312777938
- [EUROASPIRE II] EUROASPIRE II Euro Heart Survey ProgrammeLifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries. Principal results from EUROASPIRE II Euro Heart Survey ProgrammeEur Heart J2001225547211259143
- EvangelistaSManziniSAntioxidant and cardioprotective properties of the sulphydryl angiotensin-converting enzyme inhibitor zofenoprilJ Int Med Res200533425415651714
- EzzatiMLopezADRodgersASelected major risk factors and global and regional burden of diseaseLancet200236013476012423980
- FranklinSLarsonMGKhanSADoes the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart StudyCirculation20011031245911238268
- FrascarelliSGhelardoniSRonca-TestoniSCardioprotective effect of zofenopril in perfused rat heart subjected to ischemia and reperfusionJ Cardiovasc Pharmacol200443294914716220
- GagnonCLegaultFGeraldesPDiverse effects of Ace inhibitors and angiotensin II receptor antagonists on prevention of cardiac hypertrophy and collagen distribution in spontaneously hypertensive ratsInt J Cardiol2004973738115561321
- García-EstanJOrtizCO’ValleFEffects of angiotensin converting enzyme inhibitors in combination with diuretics on blood pressure and renal injury in nitric oxide deficiency induced hypertension in ratsClin Sci20061102273316197366
- GavrasIRosenthalTCombination therapy as first-line treatment for hypertensionCurr Hypertens Rep200462677215257860
- GiatrasILauJLeveyASEffect of angiotensin-converting-enzyme inhibitors on the progression of nondiabetic renal disease: A meta-analysis of randomized trialsAnn Intern Med1997127337459273824
- HajjarIKotchenTATrends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000J Am Med Assoc2003290199206
- HanssonLZanchettiAfor the HOT Study GroupThe Hypertension Optimal Treatment (HOT) study: 24-month data on blood pressure and tolerabilityBlood Pressure19976313179360003
- HanssonLZanchettiACarruthersSGEffects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomized trialLancet19983511755629635947
- [JNC-7] Joint National Committee VII Committee, National Heart, Lung, and Blood InstituteThe seventh report of the Joint National Committee on prevention, detection, evaluation and treatment of high blood pressure2003 NIH Publication No. 03-5233; May 2003
- KearneyPMWheltonMReynoldsKGlobal burden of hypertension: analysis of worldwide dataLancet200536521223
- KearneyPMWheltonMReynoldsKWorldwide prevalence of hypertension: a systematic reviewJ Hypertens200422111915106785
- KhalilMEBasherAWBrownEJJRA remarkable medical story: benefits of angiotensin-converting enzyme inhibitors in cardiac patientsJ Am Coll Cardiol20013717576411401108
- LevaCMariscalcoGFerrareseSThe role of zofenopril in myocardial protection during cardioplegia arrest: an isolated rat heart modelJ Card Surg20062144916426347
- LewisEJHunsickerLGBainRPThe effect of angiotensin-converting-enzyme therapy on diabetic nephropathyN Eng J Med1993329145662
- MalaccoEOmboniSAntihypertensive efficacy of zofenopril plus hydrochlorothiazide fixed combination is superior to that of single componentsEur Heart J200526401
- ManciaGGrassiGRationale for the use of a fixed dose combination in the treatment of hypertensionEur Heart J Supplements19991Suppl. LL1419
- ManciaGZanchettiAAgabiti-RoseiEAmbulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophyCirculation1997951464709118514
- MatarreseMSalimbeniATurollaEA11C-Radiosynthesis and preliminary human evaluation of the disposition of the ACE inhibitor [11C]zofenoprilatBioorg Med Chem2004126031114738971
- MoserMRationale for combination therapy in the management of hypertensionJ Clin Hypertens200356 Suppl.41725
- NapoliCSicaVde NigrisFSulfhydryl angiotensin-converting enzyme inhibition induces sustained reduction of systemic oxidative stress and improves the nitric oxide pathway in patients with essential hypertensionAm Heart J2004148e515215814
- ParatiGOmboniSMalaccoEon behalf of the Study GroupAntihypertensive efficacy of zofenopril and hydrochlorothiazide and their different combinations assessed by 24h ambulatory blood pressure monitoringJ Hypertens200523Suppl 2S309
- ReyesAJDiuretics in the therapy of hypertensionJ Hum Hypertens200216Suppl 1S788311986901
- ScribnerAWLoscalzoJNapoliCThe effect of angiotensin converting enzyme inhibition on endothelial function and oxidant stressEur J Pharmacol200348295914660009
- SoubrierFAlhenc-GelasFHubertCTwo putative active centers in human angiotensin I-converting enzyme revealed by molecular cloningProc Natl Acad Sci U S A1988859386902849100
- TotoRDRinnerSRamCVSACE inhibitors and target organ protection. An expanded role for these antihypertensive agents?Postgrad Med2004116111615323151
- VaneJRThe history of inhibitors of angiotensin converting enzymeJ Physiol Pharmacol1999504899810639000
- VasanRSLarsonMGLeipEPImpact of high-normal blood pressure on the risk of cardiovascular diseaseN Eng J Med200134512917
- WangTJVasanRSEpidemiology of uncontrolled hypertension in the United StatesCirculation200511216516216157784
- WestendorpBSchoemakerRGBuikemaHDietary sodium restriction specifically potentiates left ventricular ACE inhibition by zofenopril, and is associated with attenuated hypertrophic response in rats with myocardial infarctionJ Renin Angiotensin Aldosterone Syst20045273215136971
- WestendorpBSchoemakerRGvan GilstWHHydrochlorothiazide increases plasma or tissue angiotensin-converting enzyme-inhibitor drug levels in rats with myocardial infarction: differential effects on lisinopril and zofenoprilEur J Pharmacol2005527141916310764
- WetzelsGECNelemansPSchoutenJSFacts and fiction of poor compliance as a cause of inadequate blood pressure: a systematic reviewJ Hypertens20042218495515361751
- [WHO–ISH] World Health Organization – International Society of Hypertension Writing Group2003. World Health Organization (WHO)–International Society of Hypertension (ISH) statement on management of hypertensionJ Hypertens20032119839214597836
- YusufSSleightPPogueJEffects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. The Heart Outcomes Prevention Evaluation (HOPE) Study InvestigatorsN Engl J Med20003421455310639539
- ZanchettiAContribution of fixed low-dose combinations to initial therapy in hypertensionEur Heart J Supplements19991Suppl. LL5L9
- ZanchettiAParatiGMalaccoEZofenopril plus hydroclorothiazide. Combination therapy for the treatment of mild to moderate hypertensionDrugs20066611071516789795
- ZanchettiARuilopeLMAntihypertensive treatment in patients with type-2 diabetes mellitus: what guidance from well controlled randomized trials?J Hypertens200220209511012359990