Abstract
The antithrombin binding sequence of heparin, a pentasaccharide, has been synthesized as fondaparinux, an indirect, selective, and reversible factor Xa inhibitor. It can be administered subcutaneously, is well absorbed, and has a half-life of c. 17 hours permitting once-daily injection. It has been evaluated in an extensive study program in major orthopedic surgery, including hip fracture, and in major abdominal surgery with a large proportion of surgery for cancer. The effect is at least as effective as for low-molecular-weight heparins and it has also been shown effective for extended prophylaxis in hip fracture patients. Several thousands of patients have been studied and the substance is safe, although a slightly higher frequency of bleedings is found than in patients on low-molecular-weight heparins. There is no specific antidote but if necessary, recombinant activated factor VII can be used. Other side-effects are rare. Fondaparinux is cost saving and sometimes cost neutral when compared with enoxaparin.
Introduction
Low-molecular-weight heparins are effective and safe to motivate their use for prevention of post-operative venous thromboembolism (VTE), and they are probably the dominating pharmacological principle in this respect (CitationGeerts et al 2002). In the very beginning of the low-molecular-weight heparin era it was considered important to have a high factor Xa inhibitor activity which ideally should be higher than the IIa inhibitor activity, but over the years the discussion came to focus on the optimal balance between inhibiting factor Xa and factor IIa. It was therefore of great theoretical interest when selective Xa inhibitors were identified, because it was then possible to answer the question: is it possible to obtain a good prophylactic effect by focusing on just one of the factors in the coagulation system? The discussion alluded to above on the role of Xa inhibition in the infancy of low-molecular-weight heparins could thereby be further argumued. The aim of this paper is to summarize present-day knowledge on the prophylactic effect of fondaparinux, a purely synthetic selective indirect inhibitor of factor Xa, the first representative of a new class of antithrombotic substances (CitationCheng 2002; CitationHoppensteadt et al 2003; CitationNijkeuter and Huisman 2004).
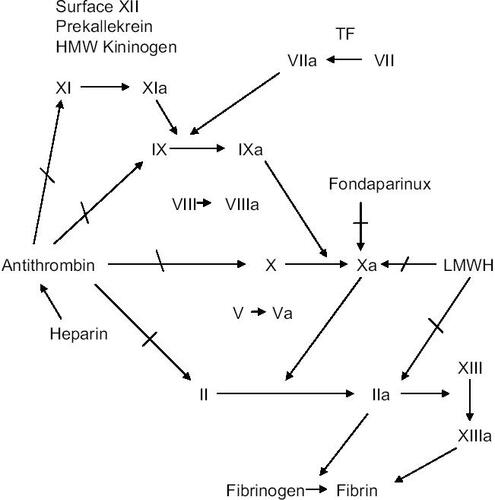
Inhibition of factor Xa ()
The mixture of polysulfated glycosaminoglycans called heparin binds to and activates antithrombin. The heparin–antithrombin complex inhibits the serine proteases in the coagulation system, including factor Xa. By fragmenting the heparin molecule to low-molecular-weight heparins, a relatively greater inhibitory effect on factor Xa is obtained. The minimum molecular sequence of heparin, which is necessary to bind antithrombin, was shown to be a pentasaccharide described by CitationLindahl et al (1979) and synthesized by CitationChoay et al (1983) as the pharmacological substance fondaparinux (molecular weight 1728 Da). It is an indirect, selective factor Xa inhibitor without interaction with factor II or platelets. TFPI (tissue factor pathway inhibitor) is not released by fondaparinux in contrast to heparins. The binding between fondaparinux and antithrombin is non-covalent and reversible. Also, when antithrombin in plasma has been saturated, circulating free fondaparinux has no anticoagulant effect by itself and is renally excreted. When the conformationally altered antithrombin binds to factor Xa, fondaparinux is released and can continue the process, acting as a catalyst. Fondaparinux is approved in Europe and by the FDA (Food and Drug Administration) at a dosage of 2.5 mg/day s.c. to be used for prevention of venous thromboembolism in major orthopedic surgery (specified as total hip and knee arthroplasty and hip fracture surgery). The bioavailability is 100% and maximal plasma concentration is seen at 2 hours with an elimination half-life of around 17 hours, which permits once-daily dosage (CitationBauer et al 2002). The excretion is in unmetabolized form through the kidneys, which motivates caution and dose modification in patients with impaired kidney function. In patients with a creatinine clearance of less than 30 mL/min fondaparinux is contraindicated. Because of the relatively long half life and its prolongation in patients with renal insufficiency, there has been a concern regarding reversibility. This is a potential problem should severe bleeding occur or emergent surgery be indicated. From a practical point of view but based on experiments in healthy volunteers, and with the duration between 2 and 6 hours, recombinant factor VIIa may be used intravenously (CitationBijsterveld et al 2002). Case reports are coming on the clinical value of recombinant factor VIIa to reverse bleeding complications after fondaprinux (CitationHuvers et al 2005).
Clinical study program
After having established the mechanism of action it is important to choose a reasonably correct dose and administration routine to be used with the most relevant patient populations. For some time it has been considered appropriate to start with major orthopedic surgery with rather standardized surgical procedures and high risk to develop venous thromboembolism. This study model was used for fondaparinux, where the initial program evaluated several thousand patients undergoing elective hip and knee arthroplasty or hip fracture surgery, the comparator being enoxaparin. This documentation has lead to approval in several countries as prophylaxis for these indications. Later on, studies on high-risk abdominal surgery have been performed. Before starting the large-scale clinical evaluation program a dose finding study was performed in 993 patients undergoing total hip replacement, where the optimal dose from a risk–benefit point of view was defined as 2.5 mg once daily with start 6±2 hours post-operatively (CitationTurpie et al 2001). The phase III program of today has included more than 15 000 patients.
Prophylactic effect of fondaparinux ()
Studies on post-operative prophylaxis have included more than 10 000 patients. The four key phase III studies on 7344 orthopedic patients (CitationBauer et al 2001; CitationEriksson et al 2001; CitationLassen et al 2002; CitationTurpie et al 2002) (trial acronyms EPHESUS (elective hip), PENTAMAKS (elective knee), PENTHIFRA (hip fracture), PENTAHLON (elective hip)) have been summarized in a meta-analysis (CitationTurpie et al 2002). The ability to reduce venographically detected deep vein thrombosis and symptomatic venous thromboembolism is clear and significantly better than the comparator which has been the low-molecular-weight heparin enoxaparin, in two studies with the European regimen (40 mg daily with pre-operative start) and in two with the American (30 mg twice daily with post-operative start). The overall result was a 55% common odds reduction in favor of fondaparinux (95% CI 46%–63%) in risk of VTE (13.7 vs 6.8%; p<0.001). The effect is true also when proximal deep vein thrombosis is considered (2.9 vs 1.3%) but less clear or non-existent when the focus of analysis is put on clinically established venous thromboembolism or mortality (no difference), a fact which has been used as an argument against fondaparinux (see for instance (CitationVormfelde 2002; CitationLowe et al 2003)). Fatal pulmonary embolism was seen in two fondaparinux and three enoxaparin patients. Subgroup analyses showed that the beneficial effect of fondaparinux was found regardless of gender, age, body mass index, duration of surgery, use of cement, and type of anesthesia (CitationTurpie et al 2002). After the publication of the fondaparinux studies the ACCP (American College of Chest Physicians) conference on antithrombotic therapy (CitationGeerts et al 2004) and the European Committee for Proprietary Medicinal Products (CitationCPMP 2000) have proposed not taking asymptomatic distal deep vein thrombosis into consideration. This view has also been taken in a letter to the Lancet by CitationLowe et al (2003). The fondaparinux results were therefore re-analyzed (CitationTurpie et al 2004) and using the ACCP definition the results were 1.7% and 3.3% respectively (reduction of 49.6%, p<0.001) and with CPMP 2.1 and 3.9% respectively (reduction of 48% p<0.01).
Table 1 Studies on the prophylactic effect of fondaparinux on post-operative venous thromboembolism
After these four key studies the evaluation program continued to study the indication prolonged prophylaxis in hip fracture surgery (CitationEriksson and Lassen 2003), an approach that was further motivated by the finding in a logistic regression analysis of the four first studies that a longer duration of fondaparinux was associated with a lower incidence of venous thromboembolism (CitationAndersen 2004). Extending the prophylaxis from around 7 days to around 30 days reduced venographic deep vein thrombosis from 35% to 1.4% (p<0.001). This is obviously an exceptionally good prophylactic result. A similar reduction was found for proximal deep vein thrombosis (15.8%–0.9%) and symptomatic venous thromboembolism (2.7%–0.3%, p<0.02).
In the orthopedic studies fondaparinux has been instituted post-operatively. In a post-hoc analysis it was shown that delaying the start to between 6 and 9 hours post-operatively reduced the risk of bleeding complications significantly, without diminishing the effectivity (CitationTurpie et al 2002). In an open randomized study on 2000 elective hip patients with blinded adjudication of clinically documented venous thromboembolism, it was further shown that delaying the first dose of fondaparinux from 6 to 8 hours post-operatively to the first post-operative morning did not influence the outcome (1.9% VTE vs 1.8%) (CitationDavidsson et al 2005). Thus fondaparinux can be started post-operatively and therefore does not increase surgical bleeding.
Another high risk group of importance to study is found within the various indications for abdominal surgery. In a recently published trial (PEGASUS) in 2927 high risk abdominal surgery 2.5 mg fondaparinux started 6 hours after surgery was compared with dalteparin started 2 hours before the operation (the first two doses of 2500 units and thereafter 5000 units daily) (CitationAgnelli et al 2005). The study was designed as a non-inferiority trial and the hypothesis was verified. The frequency of venographically detected deep vein thrombosis on day 10 in the efficacy analysis of 2048 patients was 4.6% in the fondaparinux group and 6.1 % in the dalteparin group, a relative risk reduction of 25% (95% CI – 9.0%–47.9%). In the subgroup of 1408 patients (69%) operated on for cancer, the corresponding frequencies of thrombosis were 4.7% and 7.7% respectively (p<0.01). The frequency of symptomatic-adjudicated venous thromboembolic events was 0.4% in both groups.
In the APOLLO study with 1070 patients (CitationTurpie et al 2005) once daily fondaparinux combined with intermittent pneumatic compression was significantly more effective than intermittent pneumatic compression alone to prevent venous thromboembolism after major abdominal surgery. Of 842 patients included in the efficacy analysis (cancer surgery 39%), the frequency of venous thromboembolism was reduced from 5.3% to 1.7% (p<0.004).
In a recently published multicenter placebo-controlled trial it was shown that fondaparinux also has a significant effect in preventing VTE in acutely ill medical patients (CitationCohen et al 2006).
Safety, side-effects
The main concern is whether fondaparinux may induce bleeding complications as, by its mechanism of action, it influences the hemostatic system. Generally, fondaparinux has been well tolerated. Fondaparinux does not influence platelet function. In the early dose finding study the incidence of major bleeding was significantly dose-related (CitationTurpie et al 2001). In the studies on hip surgery (both arthroplasty and hip fracture) the frequency of major hemorrhagic events did not differ between the fondaparinux and enoxaparin groups. In the study on major knee surgery (CitationBauer et al 2001) the frequency of major bleeding complications was 2.1% in the fondaparinux group vs 0.2% in the enoxaparin group, a difference which was highly significant (p<0.006). In a meta-analysis (CitationTurpie et al 2002) of all four short-time orthopedic studies the frequency of adjudicated major bleeding events in the fondaparinux group was significantly higher than in the enoxaparin group (2.9% vs 1.7%; p<0.008). The difference was mainly driven by a difference in the so called bleeding index (≥2). There was no difference in fatal bleeding, or bleeding leading to re-operation or in a critical organ (CitationNijkeuter and Huisman 2004). One important finding in a post hoc analysis was the significant frequency of major bleeding whether the first fondaparinux injection was given <6 hours after wound closure or ≥6 hours after (3.2% vs 2.1% p=0.045 (CitationTurpie et al 2002).
In the study in patients undergoing abdominal surgery (CitationAgnelli et al 2005) the frequency of major bleedings was 3.4% vs 2.4% in the dalteparin group (p<0.122). There were two fatal bleedings in each group (0.1%). The effect of timing of the first fondaparinux injection was similar to the orthopedic studies with 6 hours post-operatively as the divider (3.4% vs 2.8%, NS). In the APOLLO study major bleeding was significantly more frequent in the fondaparinux group compared with the placebo group, 1.6% vs 0.2% (p<0.006) (CitationTurpie et al 2005).
Thrombocytopenia is seen at a similar frequency after enoxaparin and dalteparin and there are no reports of serious HIT (heparin induced thrombocytopenia). In the study on extended prophylaxis after hip fracture there was no increase in liver enzymes (CitationLassen et al 2003). Because of the long half-life there is a concern about the risk of spinal hematoma in patients with epidural anesthesia but hitherto no cases have been reported with the 2.5 mg dose. Special concern should be given to patients with impaired renal function.
Up till now there are no reports on drug interactions (CitationReynolds et al 2004). Specific studies have been made with warfarin, aspirin, and piroxicam, no interaction being found (CitationKeam and Goa 2002).
There have been no differences reported in mortality between the fondaparinux and comparators in the various studies. As indicated above, one important concern when discussing fondaparinux is the current lack of an antidote.
Aspects on health economy
In addition to studies showing new substances to be effective and safe, in a reality with restrained resources it is also important to put a health economic perspective to the new methodology. One problem performing such studies is the differences between countries; another is to draw conclusions from well defined trial data to everyday health care. To overcome at least some of the difficulties, models are used where various assumptions are made and included in sensitivity analyses to establish the robustness of the outcome data. There are now several pharmacoeconomic studies showing that fondaparinux compares favorably with enoxaparin in major orthopedic surgery (CitationLobo 2003; CitationReynolds et al 2004). In some studies, especially Spanish and UK trials, enoxaparin was cost saving at discharge, but in the long run fondaparinux became cost neutral or even cost saving. Health economic studies on long-term consequences such as post-thrombotic syndrome have not been undertaken.
Conclusion
Fondaparinux, a new selective indirect inhibitor of activated factor X, has been shown to be as effective as or even better than low-molecular-weight heparins to prevent postoperative venous thromboembolic complications, both after major orthopedic and major abdominal surgery. The bleeding risk is small.
Disclosures
The author belonged to the steering committee of the PEGASUS trial (fondaparinux in high risk abdominal surgery).
References
- AgnelliGBergqvistDCohenATRandomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgeryBr J Surg20059212122016175516
- AndersenJCAdvances in anticoagulation therapy: the role of selective inhibitors of factor Xa and thrombin in thromboprophylaxis after major orthopedic surgerySemin Thromb Hemost2004306091815630666
- BauerKAErikssonBILassenMRFondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgeryN Engl J Med200134513051011794149
- BauerKAHawkinsDWPetersPCFondaparinux, a synthetic pentasaccharide: the first in a new class of antithrombotic agents - the selective factor Xa inhibitorsCardiovasc Drug Rev200220375212070533
- BijsterveldNRMoonsAHBoekholdtSMAbility of recombinant factor VIIa to reverse the anticoagulant effect of the pentasaccharide fondaparinux in healthy volunteersCirculation20021062550412427650
- ChengJWFondaparinux: a new antithrombotic agentClin Ther200224175769 discussion 171912501872
- ChoayJPetitouMLormeauJStructure-activity relationship in heparin: a synthetic pentasaccharide with high affinity for antithrombin III and eliciting high antifactor Xa activityBiochem Biophys Res Commun198311649296651824
- CohenATDavidsonBLGallusASEfficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trialBMJ20063327537325916439370
- [CPMP] Committee for Proprietary Medicinal ProductsPoint to consider on clinical investigation of medicnal products for prophylaxis of iintra-and postoperative venous thromboembolic risk: The European Agency for the Evaluation of Medical Products, London, UK2000 200, CPMP/EWP/707/98
- DavidssonBTurpieAKwongLFLEXTRA: Early Vs delayed initiation of postoperative fondaparinux prophylaxis after joint replacement: A clinical outcome study [abstract]2005 Congress ISTH 2005
- ErikssonBIBauerKALassenMRFondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgeryN Engl J Med2001345129830411794148
- ErikssonBILassenMRDuration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind studyArch Intern Med200316313374212796070
- GeertsWHeitJClagettGPrevention of venous thromboembolismChest2002121207812065386
- GeertsWHPineoGFHeitJAPrevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic TherapyChest2004126Suppl 3S 338400
- HoppensteadtDWalengaJMFareedJHeparin, low-molecular-weight heparins, and heparin pentasaccharide: basic and clinical differentiationHematol Oncol Clin North Am2003173134112627673
- HuversFSlappendelRBenraadBTreatment of postoperative bleeding after fondaparinux with rFVIIa and tranexamic acidNeth J Med200563184615952489
- KeamSJGoaKLFondaparinux sodiumDrugs200262167385 discussion 1686-712109927
- LassenMRBauerKAErikssonBIPostoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: a randomised double-blind comparisonLancet2002359931917152012049858
- LassenMRBauerKAErikssonBAbscence of transaminase increase after 4-week administrationof fondaparinux (Arixtra), a new synthetic and selective inhibition of factor Xa, in the PENTHIFRA-plus studyJ Thormb Haemost2003ISupplP2052
- LindahlUBackstromGHookMStructure of the antithrombin-binding site in heparinProc Natl Acad Sci U S A1979763198202226960
- LoboBLPharmacoeconomic considerationsAm J Health Syst Pharm200360Suppl 7S11414650861
- LoweGSandercockPRosendaalFPrevention of venous thromboembolism after major orthopaedic surgery: Is fondaparinux an advance?Lancet2003362504512932379
- NijkeuterMHuismanMVPentasaccharides in the prophylaxis and treatment of venous thromboembolism: a systematic reviewCurr Opin Pulm Med2004103384415316429
- ReynoldsNAPerryCMScottLJFondaparinux sodium: a review of its use in the prevention of venous thromboembolism following major orthopaedic surgeryDrugs20046415759615233593
- TurpieABauerKCapriniJFondaparinux combined withintermittent pneumatic compression (IPC) versus IPC alone in the prevention of VTE after major abdominal surgery2005 Results of the APOLLO study [abstract]. XX Congress ISTH 2005
- TurpieAGBauerKAErikssonBIFondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studiesArch Intern Med200216218334012196081
- TurpieAGBauerKAErikssonBIPostoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trialLancet200235993191721612049860
- TurpieAGBauerKAErikssonBISuperiority of fondaparinux over enoxaparin in preventing venous thromboembolism in major orthopedic surgery using different efficacy end pointsChest2004126501815302737
- TurpieAGGallusASHoekJAA synthetic pentasaccharide for the prevention of deep-vein thrombosis after total hip replacementN Engl J Med20013446192511228275
- VormfeldeSEnoxaparin or fondaparinux for thrombosis prevention after orthopaedic surgeryLancet2002360170112457831