Abstract
The pathogenesis of ST-elevation myocardial infarction (STEMI) involves plaque disruption, platelet aggregation and intracoronary artery thrombus formation. Aspirin is the cornerstone of antiplatelet therapy in patients with STEMI, reducing the risk of recurrent myocardial infarction or death during the acute phase and long term by about one-quarter. Recent large randomized trials have demonstrated that the addition of clopidogrel to aspirin reduces the risk of major ischemic events by up to a further one-third in patients with STEMI treated with fibrinolytic therapy and undergoing percutaneous coronary intervention, with no significant increase in bleeding. Thus, dual antiplatelet therapy with the combination of clopidogrel and aspirin is becoming the new standard of care for the management of patients with STEMI.
Introduction
In the USA, approximately 865000 people will suffer an acute ST-segment elevation myocardial infarction (STEMI) per year (CitationAmerica Heart Association 2006). Most cases of STEMI are a result of ruptured atherothrombotic plaque leading to thrombotic occlusion of a coronary artery. Timely restoration of coronary blood flow with fibrinolytic therapy (CitationRentrop et al 1979) or percutaneous coronary intervention salvages myocardium, reduces infarct size, and prolongs survival (CitationDeWood et al 1980; CitationKeeley et al 2003).
Platelets play a pivotal role in the pathogenesis of acute coronary artery thrombosis. Spontaneous atherosclerotic plaque rupture or mechanical plaque disruption during catheterization of an atheromatous coronary artery exposes subendothelial proteins to the circulating blood which leads to platelet adhesion, activation, and aggregation. Release of tissue factor activates the coagulation system and leads to thrombin generation. Thrombin converts fibrinogen to fibrin and further activates platelet, leading to the recruitment of additional platelets to the site of vascular injury. Activated platelets provide a phospholipid surface for the assembly of pro-coagulant clotting factors. The resulting intracoronary thrombus can obstruct blood flow, causing myocardial ischemia and the clinical manifestations of STEMI.
The benefits of antiplatelet drugs in patients with STEMI were first conclusively demonstrated in the Second International Study of Infarct Survival (ISIS-2) trial which randomized 17187 patients presenting within 24 hours of onset of suspected STEMI to intravenous streptokinase, aspirin 162.5 mg/day for 30 days, both, or neither (CitationISIS-2 1988). At the end of 5 weeks, aspirin reduced the risk of vascular death by 23% and non-fatal myocardial infarction or stroke by 49% and 46%, respectively, with no increase in major or intracranial bleeding. Despite the use of aspirin and thrombolysis; however, 20% of STEMI patients did not obtain reperfusion of the infarct-related artery, and an additional 4%–6% of patients developed re-occlusion of the infarct-related artery during the index hospitalization (CitationDalen et al 1988, CitationFibrinolytic Therapy Trialists’ [FTT] Collaborative Group 1994). Furthermore, as many as 10% of patients with acute coronary syndromes (ACS) who are treated with long-term aspirin experience myocardial infarction, stroke, or death during the next 2–3 years (CitationAntithrombotic Trialists’ Collaboration 2002) These data suggest that more intensive antiplatelet therapy may be required.
Clopidogrel (Plavix®, Sanofi-Aventis, Paris France) is an oral antiplatelet drug of thienopyridine class. An active metabolite of clopidogrel irreversibly inhibits platelet activation and aggregation by blocking the P2Y12 adenosine diphosphate (ADP) receptor (CitationQuinn and Fitzgerald 1999; CitationPatrono et al 2001). In patients with non-ST elevation ACS and those undergoing percutaneous coronary intervention (PCI), the combination of clopidogrel and aspirin reduces the risk of recurrent myocardial infarction (MI) or death by 20%–30% (CitationMehta et al 2001; CitationYusuf et al 2001; CitationSteinhubl et al 2002). Dual antiplatelet therapy with the combination of clopidogrel and aspirin has recently also been evaluated in patients with STEMI. This review will discuss the role of clopidogrel in STEMI patients treated medically (no PCI), undergoing PCI, and undergoing coronary artery bypass surgery.
STEMI patients treated medically (no PCI)
Given the complementary antiplatelet effects of aspirin and clopidogrel among patients with non ST-elevation myocardial infarction (CitationYusuf et al 2001) it was hypothesized that clopidogrel on a background of standard care (including aspirin and fibrinolytic therapy) would prevent re-occlusion, enhance infarct-related artery patency, and improve clinical outcomes in patients with acute STEMI. Two large randomized trials have compared the combination of clopidogrel and aspirin with aspirin alone as an adjunct to standard therapy in patients with STEMI (CitationChen et al 2005; CitationSabatine et al 2005a).
The CLopidogrel as Adjunctive ReperfusIon TherapY (CLARITY) – Thrombosis In Myocardial Infarction (TIMI) 28 Trial was a randomized, double-blind, trial comparing clopidogrel with placebo in 3491 patients aged 18–75 years who presented within 12 hours of onset of symptoms of STEMI (CitationSabatine et al 2005a). Patients were randomized to receive a 300-mg loading dose of clopidogrel followed by 75 mg once-daily or placebo. More than 99% of patients also received fibrinolytic therapy. Study treatment was continued until the time of angiography (2–8 days after randomization) or hospital discharge (maximum of 8 days) if angiography was not performed. Open-label clopidogrel therapy was continued post-angiography at the discretion of the treating physician. All patients also received aspirin, heparin (either unfractionated heparin [UFH] or low-molecular-weight heparin [LMWH]), and a fibrinolytic agent.
The incidence of the primary outcome, a composite of occlusion of infarct-related artery on angiography, or death or MI prior to angiography, was significantly reduced among patients receiving clopidogrel compared with placebo (15.0% clopidogrel vs 21.7% placebo, odds reduction 36%; 95% CI: 24%-47%; p<0.001), and a consistent treatment effect was evident across all subgroups of patients () (CitationSabatine et al 2005a). Clopidogrel had the greatest effect on reducing the rate of an occluded infarct-related artery (18.4% placebo vs 11.7% clopidogrel; 41% odds reduction; 95% CI: 0.28–0.52; p<0.001) and there was a consistent reduction in recurrent MI (3.6% placebo vs 2.5% clopidogrel; 30% odds reduction; p=0.08) but no reduction in death (2.2 placebo vs 2.6% clopidogrel; p=0.49).
Figure 1 Death, myocardial infarction or occlusion of infarct-related artery on angiography in the CLARITY randomized trial. Reproduced from CitationSabatine MS, Cannon CP, Gibson CM, et al. 2005a. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med, 352:1179–89.
Copyright © 2005 Massachusetts Medical Society.
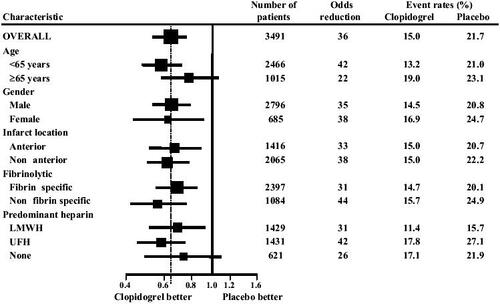
At 30 days, clopidogrel significantly reduced the odds of the composite outcome of cardiovascular death, recurrent MI, or recurrent ischemia leading to urgent revascularization by 20%: 0.80 (95% CI: 0.65–0.97; p=0.03). There also was a statistically significant 31% reduction in the odds of recurrent MI in the clopidogrel group compared with placebo but there was no difference in the rate of cardiovascular death, and a non-significant 24% reduction in the odds of recurrent myocardial ischemia leading to need for urgent revascularization (4.5% placebo vs 3.5% clopidogrel; p=0.11)
The incidence of TIMI major and minor bleeding was similar in the clopidogrel and placebo groups (major bleeding: 1.3% clopidogrel vs 1.1% placebo; p=0.64; and minor bleeding: 1.0% clopidogrel vs 0.5% placebo; p=0.17). There was no significant difference in the incidence of intracranial hemorrhage between the two randomized treatment groups (0.5% clopidogrel vs 0.7% placebo; p=0.38).
The Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT) randomized 45852 patients presenting within 24 hours of onset of symptoms of suspected MI to receive clopidogrel versus placebo and metoprolol versus placebo using a 2 × 2 factorial design (CitationChen et al 2005). Patients received aspirin 162 mg daily and were randomized to clopidogrel 75 mg daily (no loading dose) or placebo. 55% of patients received fibrinolytic therapy, which was administered prior to randomization in the majority of cases. Study treatments were continued until hospital discharge or for 28 days and the co-primary outcomes were death and the composite of death, recurrent MI, or stroke.
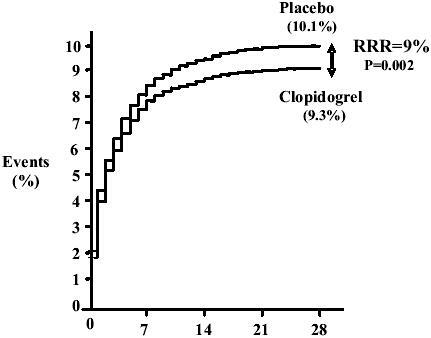
Among the broad range of acute STEMI patients randomized in the COMMIT study (ECG changes: ST-segment elevation or left bundle block branch) clopidogrel reduced the relative risk of death by 7% compared with placebo (7.5% clopidogrel vs 8.1% placebo; 95% CI: 0.01–0.13; p=0.03) () and reduced the relative risk of the composite outcome of death, recurrent MI or stroke by 9% (9.2% clopidogrel vs 10.1% placebo; 95% CI: 0.03–0.14; p=0.002) (). The results were consistent in patients treated with fibrinolytic therapy compared with those who did not receive fibrinolytic therapy. Clopidogrel also reduced the risk of non-fatal MI (1.2% clopidogrel vs 1.4% placebo; p=0.01), but did not reduce the risk of non-fatal stroke (0.6% clopidogrel vs 0.6% placebo; p=0.33). There was no difference in the combined incidence of ischemic and hemorrhagic strokes among patients treated with clopidogrel compared with placebo (0.9% clopidogrel vs 1.1% placebo; p=0.11).
Figure 2 Effect of clopidogrel on death before first discharge from hospital in COMMIT. Reproduced from CitationChen ZM, Jiang LX, Chen YP, et al. 2005. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction:randomised placebo-controlled trial. Lancet, 366:1607–21. Copyright © 2005, with permission from Elsevier.
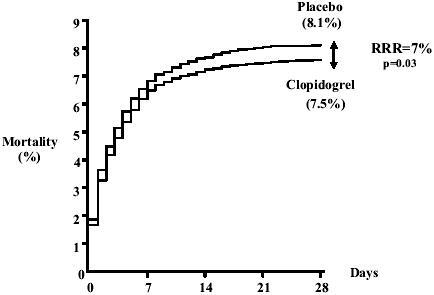
Figure 3 Effects of clopidogrel on primary end point, a composite of death, reinfarction, or stroke before first discharge from hospital in COMMIT Reproduced from CitationChen ZM, Jiang LX, Chen YP, et al. 2005. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction:randomised placebo-controlled trial. Lancet, 366:1607–21. Copyright © 2005, with permission from Elsevier.
Clopidogrel compared with placebo did not increase the risk of major non-cerebral bleeding (fatal and transfused) and cerebral bleeding (0.58% clopidogrel vs 0.55% placebo; p=0.59) and did not increase fatal cerebral bleeding (0.17% vs 0.18%; p=NS) or non-fatal cerebral bleeding (0.07% clopidogrel vs 0.07% placebo).
Comparing CLARITY and COMMIT trials
The major differences between the CLARITY and COMMIT trials are summarized in . The CLARITY trial (CitationSabatine et al 2005a) did not include patients older than 75 years or patients presenting more than 12 hours after symptom onset. All patients received a 300-mg loading dose of clopidogrel and the duration of exposure to study drug was 2–3 days (median 4 doses). Almost all patients received fibrinolysis (slightly more than one-half of the patients subsequently underwent PCI), and patency of the infarct-related artery was included as a component of the primary efficacy outcome. By contrast, the COMMIT trial (CitationChen et al 2005) did not have an upper age limit (26% were aged greater than 70 years), also included patients between 12 and 24 hours after onset of symptoms, and did not administer a loading dose of clopidogrel. Study drug was continued for 2–3 weeks (mean 14.9 days), one-half of patients did not receive fibrinolysis, and only 3% of patients subsequently underwent PCI.
Table 1 Comparison of the CLARITY and COMMIT randomized clinical trials (CitationChen et al 2005; CitationSabatine et al 2005a)
The results of the CLARITY and COMMIT studies are complementary. The CLARITY study demonstrated that the addition of clopidogrel to aspirin in patients with STEMI improved coronary perfusion and the COMMIT study demonstrated improvements in clinical outcomes, with a reduction in both death and recurrent ischemic events among patients randomized to clopidogrel. Clopidogrel did not increase major bleeding or intracranial hemorrhage, despite the use of a loading dose in CLARITY and the inclusion of patients aged greater than 75 years in COMMIT.
Based on the results of COMMIT and CLARITY, for every one thousand patients with STEMI treated in hospital for about 2–3 weeks, the addition of clopidogrel to standard care, including aspirin, saves 50 lives and prevents another 50 major cardiovascular events.
STEMI patients undergoing PCI
Efficacy of clopidogrel in PCI
Early trials demonstrated the efficacy of ticlopidine plus aspirin compared with aspirin plus anticoagulation to prevent stent thrombosis in patients undergoing PCI (CitationBerger et al 1998; CitationBertrand et al 1998; CitationLeon et al 1998; CitationUrban et al 1998; CitationSmith et al 2001). The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS) suggested that clopidogrel was as effective as ticlopidine to prevent ischemic complications in patients undergoing PCI (CitationBertrand et al 2000) and clopidogrel subsequently replaced ticlopidine for this indication because, unlike ticlopidine, it does not cause potentially fatal neutropenia. The PCI substudy of the Clopidogrel in Unstable Angina to prevent Recurrent Ischemic Events (CURE) trial demonstrated that pre-treatment with clopidogrel (median duration of pre-treatment of 10 days) compared with placebo was associated with a 30% reduction in myocardial infarction, stroke, or cardiovascular death at 30 days (RR 0.70; 95% CI: 0.50–0.97, p=0.03) and a continuing benefit out to 1 year (RR 0.72; 95% CI: 0.52–0.96) (CitationMehta et al 2001). The Clopidogrel for the Reduction of Events During Observation (CREDO) trial provided additional supporting evidence of a benefit of clopidogrel pre-treatment in patients undergoing PCI (CitationSteinhubl et al 2002). Analysis by duration of pre-treatment demonstrated that patients who received clopidogrel at least 6 hours before PCI had a 38.6% reduction in the risk events which was borderline statistically significant (p=0.051).
STEMI patients undergoing primary PCI
There are no randomized trials that have specifically evaluated the use of combined clopidogrel and aspirin in patients with STEMI undergoing primary PCI.
STEMI patients undergoing PCI after initial medical therapy
The PCI-CLARITY study was a prospectively planned analysis of the 1863 patients undergoing PCI after mandated angiography in the CLARITY trial (CitationSabatine et al 2005b). Pre-treatment with clopidogrel for an average of 3 days prior to PCI significantly reduced the incidence of cardiovascular death, MI, or stroke (3.6% clopidogrel vs 6.2% placebo; adjusted OR 0.54, 95% CI: 0.35–0.85; p=0.008). Pretreatment with clopidogrel also reduced the incidence of MI or stroke prior to PCI (4.0% clopidogrel vs 6.2% placebo; OR, 0.62, 95% CI: 0.40–0.95; p=0.03). Overall, pretreatment with clopidogrel resulted in a highly significant reduction in cardiovascular death, MI, or stroke from randomization through 30 days (7.5% clopidogrel vs 12.0% placebo; adjusted OR, 0.59, 95% CI: 0.43–0.81; p=0.001; number needed to treat = 23). There was no significant excess in the rates of TIMI major or minor bleeding (2.0% clopidogrel vs 1.9% placebo; p=0.99). These data add further support to the early use of clopidogrel in STEMI and the strategy of routine clopidogrel pre-treatment in patients undergoing PCI.
A pooled analysis of the results of PCI-CLARITY, PCI-CURE, and CREDO trials by Sabatine and colleagues demonstrated a significant reduction in the risk of cardiovascular events before and after PCI suggesting that clopidogrel pre-treatment should be started as soon as possible in patients undergoing PCI, including patients presenting with STEMI (CitationSabatine et al 2005b).
STEMI patients undergoing coronary artery bypass graft (CABG) surgery
There are limited randomized data regarding the role of clopidogrel in patients undergoing CABG surgery and the majority of the available data relate to non-STEMI patients. In the CURE study, 2072 of 12 562 patients with non-ST-elevation ACS underwent surgical revascularization during the trial (CitationFox et al 2004). The median time from randomization to CABG in the overall trial was 26 days and among patients undergoing CABG during initial hospitalization was 12 days. Compared with aspirin alone, clopidogrel combined with aspirin was associated with a 19% reduction in the risk of cardiovascular death, MI, or stroke among patients undergoing CABG surgery during the initial hospitalization (13.4% clopidogrel vs 16.4% placebo; RR 0.81, 95% CI: 0.59–1.12), and an 11% relative risk reduction among patients who underwent CABG at any time during the 9-month treatment period (14.5% clopidogrel vs 16.2% placebo; RR 0.89, 95% CI: 0.71–1.11). The benefits of clopidogrel were seen mainly in the preoperative period (RR 0.82; 95% CI: 0.58–1.16) and less so in the post-operative period (RR 0.97; 95% CI: 0.74–1.26). However, the post-operative data need to be interpreted with caution because clopidogrel was withheld for a median of 10 days after surgery, and was restarted in only 75% of those who stopped the drug before surgery.
Observational studies suggest that pre-operative exposure to clopidogrel and aspirin in patients undergoing CABG surgery increases the risk of post-operative bleeding. In a prospective observational study involving 312 consecutive patients requiring urgent or emergent CABG surgery, Chu and colleagues reported that clopidogrel use within 4 days of surgery was an independent risk factor for increased transfusion requirements (OR 4.22, 95% CI: 20.7–9.34; p=0.001) and was associated with increased blood loss and reoperation due to bleeding (CitationChu et al 2004). Yende and colleagues reported an increase in transfusion requirements (72.6% clopidogrel vs 51.6% no clopidogrel, p=0.007) and re-operation rates (9.8% vs 1.6%, p=0.01) among 247 patients who had received clopidogrel and aspirin within 5 days of surgical revascularization compared with those who did not (CitationYende and Wunderink 2001). Hongo and colleagues reported a statistically significant increase in 24 hour chest tube blood loss and transfusion requirements and a tenfold increase in re-operation for bleeding (0.6% no clopidogrel vs 6.8% clopidogrel, p=0.018) among 224 patients who had received clopidogrel within 7 days of surgery compared with those who did not (CitationHongo et al 2002).
Information regarding the bleeding risk of clopidogrel in CABG surgery is also available from randomized trials. In a subgroup analysis of 912 patients who had stopped clopidogrel less than 5 days before CABG surgery in the CURE trial (CitationYusuf et al 2001) there was increased risk of minor bleeding (5.1% clopidogrel vs 2.4% placebo, p<0.001) and a trend towards an increased risk of major bleeding (9.6% clopidogrel vs 6.3% placebo, p=0.06) among patients in the clopidogrel group compared with placebo. In contrast, there was no excess of major bleeding after CABG surgery among patients who discontinued clopidogrel at least 5 days prior to surgery (4.4% clopidogrel vs 5.3% placebo; p=0.53).
In the CREDO trial (CitationLeon et al 1998), three-quarters of all major bleeds occurred in patients undergoing CABG, although there was no difference in bleeding risk between treatment groups (68% clopidogrel vs 78% placebo).
In the CLARITY trial, pre-treatment with clopidogrel did not lead to a significant increase in major bleeding at 30 days among 136 patients who underwent CABG during the initial hospitalization (7.5% clopidogrel vs 7.2% placebo; p=1.00), including those who had CABG within 5 days of cessation of clopidogrel (9.1% clopidogrel vs 7.9% placebo; p=1.00) (CitationUrban et al 1998).
Taken together, these data suggest that caution should be exercised when prescribing clopidogrel to those in whom CABG surgery is likely to be performed within 5–7 days, balancing the risk of recurrent cardiac ischemia and the risk of post-operative bleeding and its consequences (CitationLevine et al 2003).
Cannon and colleagues recently reviewed the literature evaluating the benefits and risks of oral antiplatelet therapy among patients undergoing CABG surgery and proposed the following approach: For patients undergoing elective CABG, clopidogrel should be withdrawn 5 days prior to surgery. Among high-risk patients requiring urgent CABG, if safe to do so, surgery should be scheduled in 3–5 days while withholding clopidogrel. If not, proceed to surgery on a background of antiplatelet therapy with the aim of reducing the pre-operative risk of MI, and intervene with early platelet transfusion and aprotinin and tranexamic acid if bleeding occurs (CitationCannon et al 2005).
Other unresolved issues associated with clopidogrel in STEMI
Need for a loading dose of clopidogrel and possible effect of loading dose on bleeding
The CLARITY and COMMIT studies do not clarify whether a loading dose of clopidogrel (300 mg) compared with no loading dose confers additional benefit or if it increases the risk of bleeding in elderly patients. An initial loading dose of clopidogrel achieves more rapid and complete platelet inhibition but, even without its use, there was already a separation of the time-to-event curves during the first 12 hours in the COMMIT study, and there was a significant reduction in death by the end of day one (11%, 99% CI: 0%–20%; p=0.01). The benefits of clopidogrel administered without a loading dose are achieved without an excess of major bleeding, even in the those aged greater than 70 years (n=11934). In the younger STEMI patient population (mean age 57 years) in the CLARITY trial, the use of a loading dose of clopidogrel of 300mg followed by 75 mg daily for 2–3 days did not increase bleeding. These data indicate that a loading dose of clopidogrel can be safely administered in STEMI patients aged less than 75 years and that clopidogrel is effective in those aged greater than 70 years even without a loading dose.
Optimal duration of combined antiplatelet therapy in patients with STEMI
There are no randomized trials assessing the long-term (>28 days) effect of clopidogrel in patients with STEMI. A benefit of extended duration treatment with clopidogrel has, however, been suggested in patients with unstable coronary artery disease without STEMI and in patients undergoing PCI. In the CURE study involving 12 562 aspirin-treated patients with non-ST elevation acute coronary syndrome, the combination of clopidogrel administered as a 300-mg loading dose followed by 75 mg/day for 3–12 months was superior to placebo in preventing the primary composite outcome of stroke, MI, and vascular death (RR 0.80; 95% CI: 0.72–0.90, p<0.001) (CitationYusuf et al 2001). The benefits of clopidogrel emerged within the first 24 hours of commencing treatment and continued to accrue throughout the duration of the study.
The CREDO study examined the effect of long-term (12 months) treatment with clopidogrel in patients undergoing elective PCI (CitationSteinhubl et al 2002). All patients received clopidogrel for the first 28 days after PCI but thereafter patients who had received pre-treatment with clopidogrel continued treatment with clopidogrel 75 mg/day while those who received pre-treatment placebo continued treatment with placebo. At 1 year, clopidogrel compared with placebo was associated with a 26.9% (95% CI: 3.9%–44.4%; p=0.02) reduction in the primary composite outcome of MI, stroke, and cardiovascular death. A subgroup analysis demonstrated that continued treatment with clopidogrel beyond 28 days was associated with a further 37.4% relative risk reduction in the composite outcome of MI, stroke, and cardiovascular death (95% CI: 1.8%–60.1%, p=0.04). There was a no significant increase in the risk of bleeding at 1 year (8.8% clopidogrel and aspirin vs 6.7% aspirin alone, p=0.07) (CitationSteinhubl et al 2002).
Stent thrombosis following PCI is a serious complication, associated with a 30-day mortality rate of approximately 10% (CitationCutlip et al 2001). Refinement in stent deployment techniques in combination with dual antiplatelet therapy (aspirin and thienopyridine) have reduced the incidence of stent thrombosis to approximately 1% (CitationLeon et al 1998; CitationBertrand et al 2000; CitationMuller et al 2000; CitationCutlip et al 2001). The combination of clopidogrel 75 mg/day with or without a loading dose of 300 mg started post procedure and continued for 4 weeks among patients receiving bare-metal (non-drug-eluting) stents is highly effective in reducing the rate of thrombotic stent occlusion with no significant increase the rate of bleeding (CitationBertrand et al 2000; CitationMuller et al 2000).
Drug-eluting stents containing sirolimus (Cordis, Miami Lakes, FL, USA) or paclitaxel (Boston Scientific, Natick, MA, USA) markedly reduce neo-intimal hyperplasia and subsequent restenosis (CitationSousa et al 2001; CitationMorice et al 2002; CitationPark et al 2003) especially among patients with risk factors for restenosis, such as diabetes mellitus, small-vessel disease, and long-lesion lengths. Because of the concern that late stent thrombosis may occur in such patients, most randomized clinical trials comparing the efficacy and safety of drug-eluting and bare-metal stents extended combined clopidogrel and aspirin treatment to 3–6 months in those who received a drug-eluting stent (CitationMoses et al 2003; CitationPark et al 2003; CitationHolmes et al 2004; CitationStone et al 2004a, Citation2004b). However, the occurrence of stent thrombosis after discontinuation of clopidogrel at 6 months in 1% of patients who have received a drug-eluting stent has prompted some clinicians to recommend long-term or indefinite clopidogrel in these patients.
Taken together, the data indicate that clopidogrel should be continued for up to 1 month in patients with STEMI, for at least 1 month after PCI involving a bare metal stent, and for at least 3–6 months after PCI involving a drug-eluting stent. There is no direct evidence for continuing clopidogrel beyond 1 month in patients with STEMI or beyond 3–6 months in those undergoing PCI with a drug-eluting stent but indirect evidence from trials of patients with unstable coronary artery disease suggests that clopidogrel may be beneficial in these patients if continued long term.
Suboptimal response to clopidogrel (clopidogrel “resistance”)
Recent demonstration of variability in the antiplatelet response of clopidogrel has evoked the concept of clopidogrel resistance. However, a standardized definition and laboratory approach to the measurement of the antiplatelet effect of clopidogrel has not been established, and there is uncertainty concerning the prognostic importance of suboptimal antiplatelet response to clopidogrel in STEMI patients. Matetzky and colleagues divided 60 patients with STEMI undergoing primary PCI in quartiles based on the percent inhibition of ADP-induced platelet aggregation at day 6 compared with baseline (CitationMatetzky et al 2004). All patients received a loading dose of clopidogrel of 300 mg followed by a maintenance dose of 75 mg/day. Six of the 15 patients in the first (lower) quartile of percent inhibition of ADP-induced platelet aggregation developed a recurrent vascular event (STEMI, subacute stent thrombosis, acute coronary syndrome, or peripheral arterial occlusion) during 6 months of follow up compared with only 1 of 15 patients in the second quartile and none in the third and fourth (upper) quartiles. Before any firm conclusions can be made about the clinical relevance of suboptimal antiplatelet response to clopidogrel, the results need to be reproduced in larger prospective studies.
There is evolving evidence that higher doses of clopidogrel improve laboratory response. Among patients undergoing elective PCI, several observational studies have reported that a loading dose of clopidogrel of 600 mg is associated with greater platelet function inhibition compared with a 300-mg loading dose (CitationAngiolillo et al 2004; CitationGurbel et al 2005). The Antiplatelet therapy for Reduction of Myocardial Damage during Angioplasty (ARMYDA-2) Study reported that compared with a loading dose of clopidogrel of 300 mg, pretreatment with a 600-mg loading dose of clopidogrel 4–8 hours before elective PCI significantly reduces the primary outcome at 30 days, a composite of death, MI, or target vessel revascularization (12% vs 4%; p=0.041) and 50% risk reduction of MI (OR 0.48; 95% CI: 0.15–0.97; p=0.044) (CitationPatti et al 2005). The size of the treatment effect of the 600-mg loading dose of clopidogrel observed in ARMYDA-2 is, however, implausibly large and requires confirmation. There is no evidence that a higher dose of clopidogrel improves clinical outcome among STEMI patients.
Guidelines for antiplatelet therapy in ST-segment elevation myocardial infarction
The American College of Cardiology/American Heart Association (ACC/AHA) Guidelines for the Management of Patients with STEMI 2004 recommend aspirin 160–325 mg/day given on day 1 of acute myocardial infarction and continued indefinitely thereafter in all patients using a dose of 75–162 mg/day (CitationAntman et al 2004). In light of the results of the COMMIT (no loading dose of clopidogrel given) and CLARITY (loading dose of clopidogrel in STEMI patients <75 years) trials, it seems reasonable to add clopidogrel to aspirin in patients presenting with STEMI as an adjunct to fibrinolytic therapy (CitationChen et al 2005; CitationSabatine et al 2005a). The safety of using a loading dose of clopidogrel of 300 mg in STEMI patients aged over 70 years is unknown, and should be balanced against the risk of bleeding. The US Food and Drug Administration recently approved clopidogrel for use in patients with STEMI not undergoing PCI based on the results of the COMMIT and CLARITY studies (CitationFDA 2006).
For STEMI patients undergoing primary PCI, clopidogrel should be given as a loading dose of clopidogrel of 300 mg followed by 75 mg daily. Treatment should be continued for at least 1 month after bare metal stent implantation and for at least 3–6 months after drug-eluting stent implantation (3 months for sirolimus, 6 months for paclitaxel). In patients who are not at high risk for bleeding, it is reasonable to continue clopidogrel for 12 months or more to prevent late stent thrombosis (CitationAntman et al 2004; CitationSmith et al 2006). For STEMI patients taking clopidogrel in whom CABG is planned, the drug should be withheld for at least 5 days and preferably for 7 days unless the urgency for revascularization outweighs the risks of bleeding.
Acknowledgements
Dr Tran is the recipient of the Schering/Haematology Society of Australia & New Zealand Young Investigator Scholarship for 2006. Dr Mehta holds a New Investigator Award from the Canadian Institutes of Health Research. Dr Eikelboom holds a Tier II Canada Research Chair in Cardiovascular Medicine from the Canadian Institutes of Health Research.
Disclosures
Dr John W Eikelboom has received honoraria and/or research grants from Bayer, GSK, McNeil Pharmaceuticals, Pfizer, Sanofi-Aventis, Thrombovision, and Ventracor. Dr Shamir R Mehta has received research grant support via the Population Health Research Institute, Hamilton Health Sciences and McMaster University from Sanofi-Aventis, GSK and Bristol Myers Squibb. He has also received honoraria from Sanofi-Aventis, Bristol Myers Squibb, McNeil Johnson and Johnson, GSK, Eli Lilly, and AstraZeneca
References
- America Heart AssociationCardiovascular disease statistics [online]2006 Accessed 29 Sept 2006. URL: http://www.americanheart.org/downloadable/heart/1140534985281Statsupdate06book.pdf
- AngiolilloDJFernandez-OrtizABernardoEHigh clopidogrel loading dose during coronary stenting:effects on drug response and interindividual variabilityEur Heart J20042519031015522469
- Antithrombotic Trialists’ CollaborationCollaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patientsBMJ2002324718611786451
- AntmanEMAnbeDTArmstrongPWACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary:a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction)Circulation200411058863615289388
- BergerPBBellMRGrillDEFrequency of adverse clinical events in the 12 months following successful intracoronary stent placement in patients treated with aspirin and ticlopidine (without warfarin)Am J Cardiol19988171389527080
- BertrandMELegrandVBolandJRandomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The full anticoagulation versus aspirin and ticlopidine (fantastic) studyCirculation19989815976039778323
- BertrandMERupprechtHJUrbanPDouble-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS)Circulation2000102624910931801
- CannonCPMehtaSRArankiSFBalancing the benefit and risk of oral antiplatelet agents in coronary artery bypass surgeryAnn Thorac Surg2005807687916039260
- ChenZMJiangLXChenYPAddition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction:randomised placebo-controlled trialLancet200536616072116271642
- ChuMWWilsonSRNovickRJDoes clopidogrel increase blood loss following coronary artery bypass surgery?Ann Thorac Surg20047815364115511426
- CutlipDEBaimDSHoKKStent thrombosis in the modern era:a pooled analysis of multicenter coronary stent clinical trialsCirculation200110319677111306525
- DalenJEGoreJMBraunwaldESix- and twelve-month follow-up of the phase I Thrombolysis in Myocardial Infarction (TIMI) trialAm J Cardiol198862179853135737
- DeWoodMASporesJNotskeRPrevalence of total coronary occlusion during the early hours of transmural myocardial infarctionN Engl J Med19803038979027412821
- Fibrinolytic Therapy Trialists’ (FTT) Collaborative GroupIndications for fibrinolytic therapy in suspected acute myocardial infarction:collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patientsLancet1994343311227905143
- FoxKAMehtaSRPetersRBenefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome:the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) TrialCirculation20041101202815313956
- GurbelPABlidenKPZamanKAClopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) studyCirculation20051111153915738352
- HolmesDRJrLeonMBMosesJWAnalysis of 1-year clinical outcomes in the SIRIUS trial:a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosisCirculation20041096344014769686
- HongoRHLeyJDickSEThe effect of clopidogrel in combination with aspirin when given before coronary artery bypass graftingJ Am Coll Cardiol200240231712106925
- ISIS-2 (Second International Study of Infarct Survival) CollaborativeRandomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2Lancet19882349602899772
- KeeleyECBouraJAGrinesCLPrimary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction:a quantitative review of 23 randomised trialsLancet2003361132012517460
- LeonMBBaimDSPopmaJJA clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study InvestigatorsN Engl J Med19983391665719834303
- LevineGNKernMJBergerPBManagement of patients undergoing percutaneous coronary revascularizationAnn Intern Med20031391233612859162
- MatetzkySShenkmanBGuettaVClopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarctionCirculation20041093171515184279
- MehtaSRYusufSPetersRJEffects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention:the PCI-CURE studyLancet20013585273311520521
- MoriceMCSerruysPWSousaJEA randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularizationN Engl J Med200234617738012050336
- MosesJWLeonMBPopmaJJSirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary arteryN Engl J Med200334913152314523139
- MullerCButtnerHJPetersenJA randomized comparison of clopidogrel and aspirin versus ticlopidine and aspirin after the placement of coronary-artery stentsCirculation2000101590310673248
- ParkSJShimWHHoDSA paclitaxel-eluting stent for the prevention of coronary restenosisN Engl J Med200334815374512700373
- PatronoCCollerBDalenJEPlatelet-active drugs: the relationships among dose, effectiveness, and side effectsChest200111939S63S11157642
- PattiGColonnaGPasceriVRandomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention:results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) studyCirculation2005111209910615750189
- QuinnMJFitzgeraldDJTiclopidine and clopidogrelCirculation199910016677210517740
- RentropKPBlankeHKarschKRAcute myocardial infarction:intracoronary application of nitroglycerin and streptokinaseClin Cardiol1979235463121799
- SabatineMSCannonCPGibsonCMAddition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevationN Engl J Med2005a35211798915758000
- SabatineMSCannonCPGibsonCMEffect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics:the PCI-CLARITY studyJAMA2005b29412243216143698
- SmithSCJrDoveJTJacobsAKACC/AHA guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines)-executive summary:a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) endorsed by the Society for Cardiac Angiography and InterventionsCirculation200110330194111413094
- SmithSCJrFeldmanTEHirshfeldJWJrACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention—summary article:a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention)Circulation20061131567516391169
- SousaJECostaMAAbizaidALack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries:a quantitative coronary angiography and three-dimensional intravascular ultrasound studyCirculation2001103192511208675
- SteinhublSRBergerPBMannJT3rdEarly and sustained dual oral antiplatelet therapy following percutaneous coronary intervention:a randomized controlled trialJAMA200228824112012435254
- StoneGWEllisSGCoxDAOne-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent:the TAXUS-IV trialCirculation2004a1091942715078803
- StoneGWEllisSGCoxDAA polymer-based, paclitaxeleluting stent in patients with coronary artery diseaseN Engl J Med2004b3502213114724301
- [FDA] US Food and Drug AdministrationFDA Approves New Medical Use for Plavix - Drug benefits Patients with common form of Heart Attack [online]2006 Accessed 29 Sept 2006. URL: http://www.fda.gov/bbs/topics/NEWS/2006/NEW01431.html
- UrbanPMacayaCRupprechtHJRandomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients:the multicenter aspirin and ticlopidine trial after intracoronary stenting (MATTIS)Circulation1998982126329815866
- YendeSWunderinkRGEffect of clopidogrel on bleeding after coronary artery bypass surgeryCrit Care Med2001292271511801823
- YusufSZhaoFMehtaEffects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevationN Engl J Med200134549450211519503