Abstract
In most patients with hypertension, especially Stage 2 hypertension, adequate control of blood pressure (BP) is only achieved with combination drug therapy. When using combination therapy, antihypertensive agents with complementary mechanisms of action are recommended, for example, an angiotensin receptor blocker (ARB) in combination with hydrochlorothiazide (HCTZ), a β-blocker + HCTZ, an ACE inhibitor + HCTZ, or a calcium channel blocker + an ACE inhibitor. One such combination is olmesartan medoxomil + HCTZ, which is available as fixed-dose, single-tablet combinations for once-daily administration. In clinical trials, olmesartan medoxomil/HCTZ reduced systolic BP (SBP) and diastolic BP (DBP) to a greater extent than either component as monotherapy. A clinical study in patients with Stage 1 or 2 hypertension showed that olmesartan medoxomil/HCTZ achieved a similar mean reduction in DBP, but a significantly greater mean reduction in SBP and higher rate of BP control (<140/90 mmHg) than observed with losartan/HCTZ, at US/European-approved starting doses. In a non-inferiority trial, the antihypertensive efficacy of olmesartan medoxomil/HCTZ was comparable to that of atenolol/HCTZ. Furthermore, indirect comparisons have shown that olmesartan medoxomil/HCTZ compares favorably with other antihypertensive combination therapies, including other ARB/HCTZ combinations and amlodipine besylate/benazepril. Olmesartan medoxomil/HCTZ is generally well tolerated. In conclusion, olmesartan medoxomil/HCTZ is an effective and well-tolerated combination antihypertensive therapy that results in significant BP reductions and BP control in many patients.
Introduction
Hypertension is a highly prevalent cardiovascular risk factor, affecting an estimated 65 million people in the United States alone (CitationAmerican Heart Association 2006). The control of blood pressure (BP) is important for the prevention of cardiovascular morbidity and mortality; however, as many as two-thirds of patients do not have their BP adequately controlled (CitationChobanian et al 2003). Treatment goals recommended by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) and international guidelines are <140/90 mmHg, or <130/80 mmHg for patients with diabetes or chronic renal disease (CitationEuropean Society of Hypertension 2003; CitationChobanian 2003; CitationWhitworth 2003) In most patients, especially those with Stage 2 hypertension (systolic BP [SBP] ≥160 mmHg or diastolic [DBP] ≥100 mmHg), combination therapy is needed to achieve adequate control of BP, and it is generally recommended that drugs with complementary mechanisms of action should be used (CitationChobanian et al 2003).
Several fixed-dose combinations are now available, including β-blockers + hydrochlorothiazide (HCTZ), ACE inhibitors + HCTZ, angiotensin receptor blockers (ARBs) + HCTZ, and ACE inhibitors + calcium channel blockers. Many of these agents combine drugs with synergistic mechanisms of action allowing for substantially greater reductions in BP compared with component monotherapies. For example, patients are more responsive to the BP-lowering effects of ARBs and ACE inhibitors upon the addition of HCTZ. Although the mechanism(s) involved are not clearly understood, HCTZ may activate the renin-angiotensin system (RAS), making BP more dependent on angiotensin II (CitationKjeldsen et al 2005). These effects on counter-regulatory processes may help to explain why the combination of an ARB or an ACE inhibitor with HCTZ reduces BP more effectively than either agent alone (CitationBrown et al 1990; CitationChrysant 1994; CitationBenz et al 1998; CitationKochar et al 1999; CitationManolis et al 2000; CitationChrysant et al 2004).
Olmesartan medoxomil/HCTZ is the most recent fixed-dose ARB/HCTZ combination to be approved for the treatment of hypertension. This review will focus on the pharmacodynamics, antihypertensive efficacy, and tolerability of olmesartan medoxomil/HCTZ and how it compares with other currently available fixed-dose combinations.
Pharmacology of olmesartan medoxomil and HCTZ
The clinical effects of angiotensin II, including vasoconstriction, increasing intravascular volume, and hormone secretion are mediated by AT1 receptors (CitationBurnier 2001). Olmesartan medoxomil is an AT1 receptor antagonist, demonstrating specificity for receptors in vascular tissue (CitationMizuno et al 1995; CitationKoike et al 2001). In vitro, olmesartan medoxomil has been shown to be a competitive antagonist, displaying high affinity, slow dissociation, and a high degree of insurmountability for the AT1 receptor (CitationPugsley 2006). The interaction of olmesartan with the AT1 receptor is believed to occur via a two-step mechanism: the molecule first undergoes a loose “surmountable” binding followed by the formation of a tighter, “insurmountable” binding complex. The slow dissociation of olmesartan medoxomil from the AT1 receptor compares favorably with other ARBs’ dissociation, including telmisartan, and may contribute to the antihypertensive efficacy of olmesartan medoxomil in vivo (CitationPugsley 2006). The pressor response to exogenous angiotensin I is inhibited to a clinically relevant extent (>75%) by single doses of olmesartan medoxomil 10–40 mg, with substantial inhibition still apparent 24 hours after dosing (CitationBrunner and Nussberger 2001).
Thiazide diuretics such as HCTZ promote sodium excretion, leading to a reduction in plasma volume and peripheral resistance (CitationMeredith 2005). The resulting activation of the RAS means that the effect of blocking AT1 receptors and, therefore, the response to ARB therapy, is enhanced, providing a rationale for co-administration of agents from these two drug classes (CitationMeredith 2005). Other synergistic mechanisms are thought to be involved but are not clearly understood. The increased efficacy resulting from the combination of HCTZ with an ARB does not greatly compromise the good tolerability profile of the ARB. The combination of these two agents may permit the use of lower doses of HCTZ and the ARB, which may be less likely to result in adverse events typically associated with HCTZ use.
Olmesartan medoxomil is a pro-drug that is de-esterified to its active metabolite, olmesartan, during absorption from the gastrointestinal tract (CitationLaeis et al 2001). Absorption is rapid, with steady-state levels reached within 5 days (CitationLaeis et al 2001). Importantly, the long terminal elimination half-life of 10–18 hours, coupled with minimal accumulation, allows for once-daily dosing (CitationLaeis et al 2001; CitationSchwocho and Masonson 2001). Systemically available olmesartan is excreted via the kidneys and, after secretion in bile, in the feces (CitationLaeis et al 2001).
HCTZ is absorbed rapidly after oral administration and is eliminated unchanged in the urine (CitationCarter et al 2004; CitationSweetman 2005). It has an elimination half-life of 8–15 hours after repeated doses, and the pharmacodynamic response is sufficiently long to allow once-daily dosing (CitationCarter et al 2004). Clinically relevant pharmacokinetic interactions do not occur between these two agents (CitationKreutz et al 2006). Given both the pharmacokinetic and the pharmacodynamic profiles of these agents, the combination of olmesartan medoxomil with HCTZ is suitable for once-daily administration in a single tablet.
Antihypertensive efficacy of olmesartan medoxomil/HCTZ
A number of studies have provided evidence that the combination of olmesartan medoxomil/HCTZ is an effective option for antihypertensive therapy.
Comparison with monotherapy
A clinical trial using a randomized, double-blind, factorial design showed that olmesartan medoxomil/HCTZ combination therapy reduced DBP and SBP to a greater extent than monotherapy with either component (CitationChrysant et al 2004). In this study, hypertensive patients (n=502) with a seated DBP of 100–115 mmHg were randomized to one of 12 treatment groups for 8 weeks: olmesartan medoxomil (10, 20, or 40 mg/day), HCTZ monotherapy (12.5 or 25 mg/day), one of six groups of olmesartan medoxomil/HCTZ combination therapy (covering each possible dosage combination), or placebo.
A dose-dependent decrease in BP was seen across all olmesartan medoxomil/HCTZ combinations compared with the individual components, with the maximum mean reduction of 26.8/21.9 mmHg observed with the highest-dose combination, olmesartan medoxomil/HCTZ 40/25 mg/day. After 8 weeks, mean reductions in seated DBP were 13.5–21.9 mmHg with combination therapy, compared with 11.3–14.6 mmHg with olmesartan medoxomil monotherapy (10–40 mg/day), and 10.2–12.9 mmHg with HCTZ monotherapy (12.5 or 25 mg/day). All combination and monotherapy regimens reduced DBP and SBP significantly more than placebo (CitationChrysant et al 2004).
The proportion of patients achieving a BP response (defined as trough seated DBP <90 mmHg or a decrease from baseline of ≥10 mmHg) increased in a dose-dependent manner with each monotherapy and with combination therapy, although between-group statistical comparisons were not performed (CitationChrysant et al 2004). The response rate was 92.3% for olmesartan medoxomil/HCTZ 40/25 mg/day; diastolic control (trough seated DBP <90 mmHg) and systolic control (trough seated SBP <140 mmHg) were achieved in 79.5% and 87.2% of patients, respectively (CitationChrysant et al 2004).
Additional support for combination therapy with olmesartan medoxomil/HCTZ comes from a trial in hypertensive patients with a seated DBP of 100–115 mmHg, a seated SBP >150 mmHg, a 24-hour DBP ≥84 mmHg, and at least 30% DBP daytime readings >90 mmHg. Mean seated DBP remained ≥90 mmHg after 4 weeks of treatment with olmesartan medoxomil 20 mg/day; however, the addition of HCTZ 12.5 or 25 mg for 8 weeks significantly reduced mean 24-hour DBP (−1.9 mmHg for HCTZ 12.5 mg, p=0.0167; −3.7 mmHg for HCTZ 25 mg, p<0.0001) and SBP (−3.8 mmHg for HCTZ 12.5 mg, p=0.0018; −7.4 mmHg for HCTZ 25 mg, p<0.0001) compared with the addition of placebo (CitationSellin et al 2005).
Evaluation of treatment algorithm including olmesartan medoxomil/HCTZ
A stepwise treatment algorithm in which uptitration of olmesartan medoxomil is followed by the addition of increasing dosages of HCTZ is an effective option in patients with Stage 1 or Stage 2 hypertension (CitationNeutel et al 2004, Citation2006).
In this open-label, multicenter study (n=210), olmesartan medoxomil 20 mg/day was initially administered for 4 weeks. Antihypertensive drug treatment was then uptitrated at 4-week intervals until goal BP was achieved, progressing through the following steps: uptitration to olmesartan medoxomil 40 mg/day, addition of HCTZ 12.5 mg/day, uptitration of HCTZ to 25 mg/day, addition of amlodipine besylate 5 mg/day, and then uptitration of amlodipine besylate to 10 mg/day (CitationNeutel et al 2004, Citation2006). Endpoints included the percentage of patients who achieved the BP goal of ≤140/90 mmHg, and the more aggressive BP goal of ≤130/85 mmHg. Those patients who achieved the more aggressive BP goal at any time during the study exited the trial.
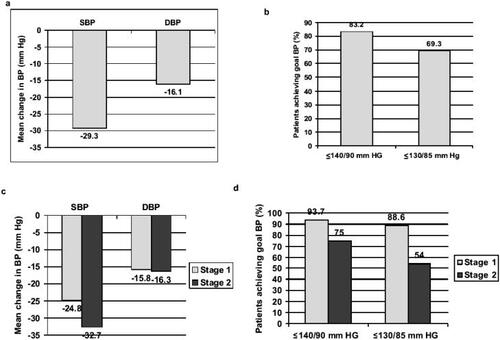
The results of this study showed that a BP goal of ≤140/90 mmHg and a more aggressive BP goal of ≤130/85 mmHg could be achieved in many patients using a combination of olmesartan medoxomil and HCTZ (CitationNeutel et al 2004, Citation2006). The goal of ≤140/90 mmHg was achieved in 83% of patients after 8 weeks of dual combination therapy with olmesartan medoxomil/HCTZ (titrated to 40/25 mg/day), while the goal of ≤130/85 mmHg was attained in 69% (CitationNeutel et al 2004). Mean BP reductions and goal attainment rates for all patients and for patients separated into those with Stage 1 or Stage 2 hypertension are shown in . Looking specifically at the SBP goal (≤140 mmHg), 96.2% of patients with Stage 1 hypertension (mean baseline SBP of 151.1 mmHg) and 78% of those with Stage 2 hypertension (mean baseline SBP 169.8 mmHg) achieved this goal (CitationNeutel et al 2006). For all patients combined after 8 weeks of treatment with olmesartan medoxomil/HCTZ (titrated to 40/25 mg/day), mean BP had decreased from baseline by 29.3/16.1 mmHg, representing an incremental mean BP reduction of approximately 11.6/5.4 mmHg beyond that achieved with monotherapy. The addition of amlodipine besylate led to further decreases in BP (CitationNeutel et al 2004).
Figure 1 Antihypertensive efficacy of olmesartan medoxomil/HCTZ in patients with Stage 1 (SBP 140–159 mmHg or DBP 90–99 mmHg) and Stage 2 (SBP ≥160 mmHg or DBP ≥100 mmHg) hypertension. Data are from an open-label study (n=201) in which a stepwise treatment algorithm was followed, with titration at 4-weekly intervals until goal BP was achieved, when patients withdrew from the study (CitationNeutel et al 2004, Citation2006). Eight weeks of monotherapy with olmesartan medoxomil 20–40 mg/day was followed by 8 weeks of combination therapy with olmesartan medoxomil/HCTZ 40/12.5–25 mg/day. Reduction in BP and rate of attainment of BP goals (≤140/90 mmHg and an aggressive goal of ≤130/85 mmHg) after 16 weeks are shown. (a) Mean BP reduction for patients in the combined Stage 1/Stage 2 population treated with olmesartan medoxomil/HCTZ 40/12.5–25 mg/day (n=123) (CitationNeutel et al 2004); (b) Cumulative goal attainment rates for the combined Stage 1/Stage 2 population (CitationNeutel et al 2004); (c) Cumulative mean BP reduction according to baseline stage of hypertension (CitationNeutel et al 2006); (d) Cumulative goal attainment rates according to baseline stage of hypertension (CitationNeutel et al 2006). Cumulative results are based on the intent-to-treat population using last observation carried forward, and include data for patients treated only with olmesartan medoxomil monotherapy as well as those treated with dual combination therapy.
Comparison with other ARB/HCTZ combinations
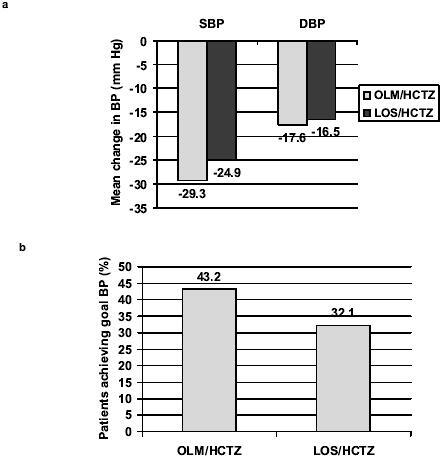
One direct comparison of olmesartan medoxomil/HCTZ with another ARB/HCTZ combination has been published recently. In this starting-dose, open-label comparison, olmesartan medoxomil/HCTZ and losartan/HCTZ were compared as initial therapy in patients with either newly diagnosed Stage 2 hypertension or a DBP of 90–100 mmHg despite treatment (CitationRump et al 2006). In this randomized, double-blind, multicenter trial, patients were treated with starting doses of each ARB (olmesartan medoxomil 20 mg/day [n=308] or losartan 50 mg/day [n=305]) plus HCTZ 12.5 mg/day for 12 weeks. Mean BP reductions and BP control rates (<140/90 mmHg) were compared after 1, 2, 4, 8, and 12 weeks of therapy; the primary endpoint was change in DBP from baseline to 12 weeks. Olmesartan medoxomil/HCTZ and losartan/HCTZ produced similar mean reductions in DBP (−17.6 and −16.5 mmHg, respectively; ). The mean reduction in SBP was 29.3 mmHg for olmesartan medoxomil/HCTZ compared with 24.9 mmHg for losartan/HCTZ (p≤0.0003). A significantly higher percentage of olmesartan medoxomil/HCTZ recipients achieved BP control (<140/90 mmHg) at week 12 compared with losartan/HCTZ recipients (43.2% vs 32.1%, p=0.002) (CitationRump et al 2006).
Figure 2 Antihypertensive efficacy of olmesartan medoxomil/HCTZ 20/12.5 mg/day (n=308) compared with losartan/HCTZ 50/12.5 mg/day (n=305) as initial therapy in patients with moderate to severe hypertension. Results of a randomized, double-blind trial of 12 weeks’ treatment in 613 patients who had either newly diagnosed hypertension with mean seated DBP 100–120 mmHg and SBP ≥160 mmHg or inadequately controlled hypertension with DBP 90–110 mmHg despite previous treatment (CitationRump et al 2006). (a) Reduction in mean trough BP (DBP = primary endpoint); (b) Proportion of patients achieving BP control (<140/90 mmHg) are shown for the intent-to-treat population. *p=0.002, **p≤0.0003.
An indirect comparison of olmesartan medoxomil/HCTZ with several other ARB/HCTZ combinations was performed in a review of randomized, double-blind, placebo-controlled factorial studies of similar design in hypertensive patients with a DBP of 95–115 mmHg (CitationRam 2004). At maximum US and European approved dosages, olmesartan medoxomil/HCTZ compared favorably with irbesartan/HCTZ, telmisartan/HCTZ, and valsartan/HCTZ, with each combination producing double-digit reductions (not placebo-adjusted) in both SBP and DBP () (CitationRam 2004). DBP response rates (DBP<90 mmHg or a ≥10 mmHg reduction from baseline) were numerically greater with olmesartan medoxomil/HCTZ than telmisartan/HCTZ or valsartan/HCTZ (eg, 92% vs 79% vs 81%; value not reported for irbesartan) (CitationRam 2004).
Table 1 Antihypertensive efficacy of angiotensin II receptor blocker/HCTZ combination therapies based on indirect comparison of factorial studies of 8 weeks' duration in hypertensive patients with a DBP of 95–115 mmHg. Results are shown for the starting and the maximum approved dosages in the United States and Europe for each combination therapy (CitationBenz et al 1998; CitationMcGill and Reilly 2001; CitationChrysant et al 2004; CitationRam 2004)
For indirect comparative purposes, data from a meta-analysis (CitationConlin et al 2000) evaluating randomized controlled trial data of several other ARB/HCTZ combinations are presented in . All trials were in hypertensive patients with a DBP of 95–115 mmHg, assessed cuff BP, and used a similar definition of response rates (DBP <90 mmHg or a decrease from baseline of ≥10 mmHg). Combination therapy with HCTZ 12.5 mg/day plus starting doses of candesartan, irbesartan, losartan, or valsartan was associated with mean reductions in BP of 16.1–20.6/9.9–13.6 mmHg and response rates of 56–70% when administered at starting combination doses. In a trial of eprosartan/HCTZ 600/12.5 mg/day which used similar criteria as above, mean BP reductions of 9.2/10.7 mmHg and a response rate (DBP<90 mmHg or within 90–100 mmHg with a decrease of ≥10 mmHg) of 73% were reported (CitationSachse et al 2002). Based on these indirect comparisons, olmesartan medoxomil/HCTZ may achieve BP response rates that compare favorably with other ARB/HCTZ combinations at starting dosages (). However, direct comparative studies are required to confirm these findings.
Table 2 Antihypertensive efficacy of other angiotensin II receptor blocker/HCTZ combinations based on indirect comparison through a meta-analysis of randomized, double-blind, controlled trials assessing cuff BP in hypertensive patients with a DBP of 95–115 mmHg. Results are shown for starting dosages in the United States or Europe for each combination (CitationConlin et al 2000). For indirect comparative purposes, data for the starting dosage of olmesartan medoxomil/HCTZ from a randomized, double-blind, controlled trial in patients with Stage 1 or Stage 2 hypertension are also tabulated (CitationChrysant et al 2004; CitationRump et al 2006)
Lastly, a study evaluating irbesartan/HCTZ in patients with Stage 1 systolic hypertension (SBP 140–159 mmHg) uncontrolled by monotherapy found that treatment with irbesartan/HCTZ 300/25 mg/day led to a mean reduction in BP of approximately 21.5/10.4 mmHg (from a mean baseline BP of 154/91 mmHg), with 69% of patients reaching the combined BP goal of <140/90 mmHg, and 77% achieving SBP goal, after 18 weeks (CitationNeutel et al 2005). In comparison, in a trial discussed earlier, olmesartan medoxomil/HCTZ 40/25 mg/day reduced mean BP by 24.8/15.8 mmHg (from a baseline of 151.1/94.7 mmHg) in patients with Stage 1 hypertension, and by 32.7/16.3 mmHg (baseline 169.8/98.6 mmHg) in those with Stage 2 hypertension; SBP goal (≤140 mmHg) was achieved in 96.2% and 78% of these subgroups, respectively (CitationNeutel et al 2006). Additional direct comparative studies are now needed to more accurately determine the comparative efficacy of olmesartan medoxomil/HCTZ and other ARB/HCTZ combinations.
Comparison with other antihypertensive combinations
Several clinical studies have compared the efficacy of olmesartan medoxomil/HCTZ with other antihypertensive combination therapies. For example, a study that compared olmesartan medoxomil/HCTZ with atenolol/HCTZ found that the antihypertensive efficacy of both combinations was statistically comparable (CitationBall et al 2001). In this double-blind, non-inferiority study, 328 patients with a mean seated DBP of 100–120 mmHg receiving HCTZ 25 mg/day were randomized to receive olmesartan medoxomil 10 mg/day or atenolol 50 mg/day in addition to the HCTZ dose for 12 weeks, with dose-doubling after 4 weeks, if necessary. Reductions in mean seated SBP and DBP (primary endpoint) were 20.4/17.3 mmHg for olmesartan medoxomil/HCTZ and 19.6/17.2 mmHg for atenolol/HCTZ. The upper limit of the one-sided confidence interval (CI) for the between-group difference for change in DBP (−0.08 mmHg; 90% CI −1.17, 1.02) was within the prespecified least squares mean limit of ≤3.5 mmHg, confirming that the efficacy of olmesartan medoxomil/HCTZ was not inferior to atenolol/HCTZ. The outcome was similar for SBP (between-group difference −0.8 mmHg, 95% CI −2.61, 1.00) (CitationBall et al 2001).
Amlodipine besylate/benazepril is a well-established, fixed-dose calcium channel blocker/ACE inhibitor combination antihypertensive therapy. A direct comparison between amlodipine besylate/benazepril and olmesartan medoxomil/HCTZ has not been performed; however, an indirect comparison of data from several factorial studies (CitationQuan et al 2006) suggests that mean reductions in seated DBP may be quantitatively greater with olmesartan medoxomil/HCTZ 40/25 mg/day than with amlodipine besylate/benazepril 5/20 mg/day (approximately 22 vs 17 mmHg), whereas reductions in seated SBP appear similar between the combinations (approximately 27 vs 27 mmHg) (CitationQuan et al 2006). The studies used similar designs and enrolled patients with a baseline DBP of 100–115 mmHg. However, a head-to-head trial is needed to properly compare these combination therapies, and inclusion of the highest available dosage of amlodipine besylate/benazepril 10/20 mg (for which factorial study data were not available) would be of interest.
Tolerability of olmesartan medoxomil/HCTZ
Looking first at the individual components, an integrated analysis of efficacy and safety demonstrated that the adverse events profile observed with olmesartan medoxomil monotherapy is similar to that seen with placebo, with dizziness the only adverse event to occur in a significantly greater number of olmesartan medoxomil patients compared with placebo (2.8% vs 0.9%, respectively, p=0.01) (CitationNeutel 2001). In comparison, HCTZ has been associated with metabolic disturbances and electrolyte imbalances, including hypokalemia and hyponatremia, particularly at higher doses (CitationSweetman 2005). Physiological processes that conserve sodium in the body, such as activation of the RAS, produce an augmentation of renal potassium excretion (CitationReyes 2002). Although clinical data are still lacking, it has been suggested that the inhibition of the RAS resulting from co-therapy with an ARB may reduce potassium loss, making hypokalemia less of a potential problem among patients receiving this combination (CitationKjeldsen et al 2005). In addition, the tendency of HCTZ to elevate blood glucose levels and promote type 2 diabetes mellitus may be offset with the use of ARBs which have been shown to reduce the incidence of new-onset diabetes as compared with β-blocker/HCTZ therapy (CitationDahlof et al 2002).
Adverse events associated with the combination of olmesartan medoxomil plus HCTZ are generally mild-to-moderate in severity (CitationBall et al 2001; CitationChrysant et al 2004; CitationNeutel et al 2004; CitationRump et al 2006). Dizziness was reported by more olmesartan medoxomil/HCTZ recipients than placebo recipients in placebo-controlled trials, and was associated with the addition of HCTZ (incidence 9% for olmesartan medoxomil/HCTZ vs 2% for placebo vs 8% for HCTZ monotherapy and 1% for olmesartan medoxomil monotherapy) (CitationDaiichi Sankyo, Inc. 2005). Upper respiratory tract infections were also more frequent with olmesartan medoxomil/HCTZ than with placebo (7% vs 0%) (CitationDaiichi Sankyo, Inc. 2005).
Discussion
Although it is generally recommended that antihypertensive therapy be initiated with a single agent, it is recognized that most patients will eventually require combination therapy to achieve recommended BP goals (CitationChobanian et al 2003; CitationEuropean Society of Hypertension 2003; CitationWhitworth 2003). Indeed, JNC 7 suggests that in patients with Stage 2 hypertension (SBP ≥160 mmHg or DBP ≥100 mmHg), treatment should be initiated with two antihypertensive agents (CitationChobanian et al 2003; CitationEuropean Society of Hypertension 2003). Combination therapy typically consists of drugs from different classes with complementary mechanisms of action, such as a thiazide diuretic plus an agent that acts on the RAS (CitationEuropean Society of Hypertension 2003; CitationChobanian et al 2003).
Olmesartan medoxomil/HCTZ is one such combination, and is available as fixed-dose, once-daily preparations indicated for the treatment of hypertension, although not indicated for first-line therapy. In clinical trials, olmesartan medoxomil/HCTZ has demonstrated greater antihypertensive efficacy than either component as monotherapy (CitationChrysant et al 2004; CitationSellin et al 2005) and appears to compare favorably with other ARB/HCTZ combinations (CitationConlin et al 2000; CitationMcGill and Reilly 2001; CitationSachse et al 2002; CitationRam 2004; CitationLacourciere et al 2005; CitationNeutel et al 2005; CitationRump et al 2006) and other fixed-dose combinations from different antihypertensive drug classes (CitationBall et al 2001; CitationQuan et al 2006). However, limited head-to-head trials suggest the need for additional comparative trials in order to determine adequately potential differences in the BP-lowering effects and BP goal attainment rates achievable with these combination products.
It is well known that hypertension outcomes can be improved in clinical practice by setting target BP goals and providing physicians with easy-to-follow treatment algorithms (CitationSinger et al 2002; CitationNeutel et al 2004). A target level of <140/90 mmHg is recommended for patients with uncomplicated hypertension and no evidence of diabetes or renal disease (CitationChobanian et al 2003; CitationEuropean Society of Hypertension 2003; CitationWhitworth 2003). Importantly, in an open-label trial, 9 out of 10 patients with Stage 1 hypertension, and more than half of patients with Stage 2 hypertension achieved an aggressive BP goal of ≤130/85 mmHg when treated with olmesartan medoxomil/HCTZ (CitationNeutel et al 2006).
It is known that systolic hypertension is a better predictor of future cardiovascular morbidity than diastolic hypertension, especially in older patients (CitationIzzo et al 2000; CitationEuropean Society of Hypertension 2003). However, SBP is often more difficult to control than DBP (CitationSwales 1999; CitationMancia et al 2002). Treatment algorithms based on olmesartan medoxomil/HCTZ have been shown to significantly reduce SBP, in addition to DBP, in patients with Stage 1 and Stage 2 hypertension, allowing the majority of patients in both groups to achieve the SBP goal of ≤140 mmHg (CitationNeutel et al 2006).
Various factors affect compliance and persistency with antihypertensive treatment. Long-term compliance has been shown to be better if initial therapy is well tolerated and the efficacy response is reasonably good (CitationCaro et al 1999). Currently available once-daily, fixed-dose combinations, such as olmesartan medoxomil/HCTZ and other ARB/HCTZ combinations, may simplify treatment regimens. Olmesartan medoxomil/HCTZ is effective and allows lower doses of component agents to be used compared with monotherapy, minimizing the likelihood of adverse events associated with higher doses of HCTZ (CitationNeutel 2001; CitationChrysant et al 2004). These features may enhance patients’ acceptance of therapy and potentially increase the likelihood that they will continue with therapy through each successive stage of the treatment algorithm.
In summary, olmesartan medoxomil/HCTZ is an effective and well-tolerated combination antihypertensive therapy. It provides greater antihypertensive efficacy than either component given as monotherapy, and may provide a useful treatment option in patients unable to achieve BP goal with monotherapy.
Acknowledgements
Support for the preparation of this manuscript was provided by Daiichi Sankyo, Inc. We would like to express our thanks to Alan J Klopp, PhD, for his editorial assistance in the preparation of this manuscript.
References
- American Heart Association and A. S. AssociationHeart disease and stroke statistics - 2006 update [online]2006 Accessed 3 March 2006. URL: http://www.americanheart.org
- BallKJWilliamsPAStumpeKORelative efficacy of an angiotensin II antagonist compared with other antihypertensive agents. Olmesartan medoxomil versus antihypertensivesJ Hypertens200119Suppl 1S4956
- BenzJRBlackHRGraffAValsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double-blind placebo controlled trial comparing combination therapy with monotherapyJ Hum Hypertens19981286169883710
- BrownCLBackhouseCIGrippatJCThe effect of perindopril and hydrochlorothiazide alone and in combination on blood pressure and on the renin-angiotensin system in hypertensive subjectsEur J Clin Pharmacol199039327322076713
- BrunnerHRNussbergerJRelevance of clinical pharmacological models for the evaluation of therapeutic dose range of an AT1-receptor antagonistJ Hypertens200119Suppl 1S1520
- BurnierMAngiotensin II type 1 receptor blockersCirculation20011039041211171802
- CaroJJSpeckmanJLSalasMEffect of initial drug choice on persistence with antihypertensive therapy: the importance of actual practice dataCMAJ19991604169934342
- CarterBLErnstMECohenJDHydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeabilityHypertension2004434914638621
- ChobanianAVBakrisGLBlackHRSeventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureHypertension20034212065214656957
- ChrysantSGAntihypertensive effectiveness of low-dose lisinopril-hydrochlorothiazide combination. A large multicenter studyArch Intern Med1994154737438147677
- ChrysantSGWeberMAWangACEvaluation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazideAm J Hypertens2004172525915001200
- ConlinPRSpenceJDWilliamsBAngiotensin II antagonists for hypertension: are there differences in efficacy?Am J Hypertens2000134182610821345
- DahlofBDevereuxRBKjeldsenSECardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenololLancet2002359995100311937178
- Daiichi Sankyo, Inc.2005 Benicar HCT Prescribing Information
- European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertensionJ Hypertens20032110115312777938
- IzzoJLLevyDJr.BlackHRClinical Advisory Statement. Importance of systolic blood pressure in older AmericansHypertension2000351021410818056
- KjeldsenSEOsIHoieggenAFixed-dose combinations in the management of hypertension: defining the place of angiotensin receptor antagonists and hydrochlorothiazideAm J Cardiovasc Drugs20055172215631534
- KocharMGuthrieRTriscariJMatrix study of irbesartan with hydrochlorothiazide in mild-to-moderate hypertensionAm J Hypertens19991279780510480473
- KoikeHSadaTTriscariJIn vitro and in vivo pharmacology of olmesartan medoxomil, an angiotensin II type AT1 receptor antagonistJ Hypertens200119SupplS314
- KreutzRBolbrinkerJHuberTPharmacokinetics of olmesartan plus hydrochlorothiazide combination in healthy subjectsClin Drug Invest2006262934
- LacourciereYNeutelJMSchumacherHComparison of fixed-dose combinations of telmisartan/hydrochlorothiazide 40/12.5 mg and 80/12.5 mg and a fixed-dose combination of losartan/hydrochlorothiazide 50/12.5 mg in mild to moderate essential hypertension: pooled analysis of two multicenter, prospective, randomized, open-label, blinded-end point (PROBE) trialsClin Ther200527179580516368450
- LaeisPPuchlerKKirchWThe pharmacokinetic and metabolic profile of olmesartan medoxomil limits the risk of clinically relevant drug interactionJ Hypertens200119SupplS2132
- ManciaGBombelliMLanzarottiASystolic vs diastolic blood pressure control in the hypertensive patients of the PAMELA population. Pressioni Arteriose Monitorate E Loro AssociazioniArch Intern Med2002162582611871927
- ManolisA JGrossmanEJelakovicBEffects of losartan and candesartan monotherapy and losartan/hydrochlorothiazide combination therapy in patients with mild to moderate hypertensionClin Ther200022118620311110230
- McGillJBReillyPATelmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group trialClin Ther2001238335011440284
- MeredithPAAngiotensin II receptor antagonists alone and combined with hydrochlorothiazide: potential benefits beyond the antihypertensive effectAm J Cardiovasc Drugs200551718315901205
- MizunoMSadaTIkedaMPharmacology of CS-866, a novel nonpeptide angiotensin II receptor antagonistEur J Pharmacol199528518188566137
- NeutelJMClinical studies of CS-866, the newest angiotensin II receptor antagonistAm J Cardiol200187Suppl37C43C
- NeutelJMSaundersEBakrisGLThe efficacy and safety of low- and high-dose fixed combinations of irbesartan/hydrochlorothiazide in patients with uncontrolled systolic blood pressure on monotherapy: the INCLUSIVE trialJ Clin Hypertens (Greenwich)200575788616227760
- NeutelJMSmithDHSilfaniTNEffects of a structured treatment algorithm on blood pressure goal rates in both stage 1 and stage 2 hypertensionJ Hum Hypertens2006202556216397514
- NeutelJSmithDWeberMAUse of olmesartan medoxomil-based treatment algorithm for hypertension controlJ Clin Hypertens (Greenwich)200461687415073470
- PugsleyMA characterization of the molecular interactions of olmesartan (OLM) and telmisartan (TEL) with human angiotensin AT1 receptors [abstract]2006American Society of Hypertension 21st Annual Scientific MeetingNew York, NY
- QuanAChavanuKMerkelJA review of the efficacy of fixed-dose combinations olmesartan medoxomil/hydrochlorothiazide and amlodipine besylate/benazepril in factorial design studiesAm J Cardiovasc Drugs200661031316555863
- RamCVAntihypertensive efficacy of angiotensin receptor blockers in combination with hydrochlorothiazide: a review of the factorial-design studiesJ Clin Hypertens (Greenwich)200465697715470286
- ReyesADiuretics in the therapy of hypertensionJ Hum Hypertens200216Suppl 1S788311986901
- RumpLCAmbrosioniEBurnierMInitial combination therapy with olmesartan/hydrochlorothiazide in moderate-to-severe hypertensionJ Hum Hypertens20062029930116452995
- SachseAVerboomCNJagerBEfficacy of eprosartan in combination with HCTZ in patients with essential hypertensionJ Hum Hypertens2002161697611896506
- SchwochoLRMasonsonHNPharmacokinetics of CS-866, a new angiotensin II receptor blocker, in healthy subjectsJ Clin Pharmacol2001415152711361048
- SellinLStegbauerJLaeisPAdding hydrochlorothiazide to olmesartan dose dependently improves 24-h blood pressure and response rates in mild-to-moderate hypertensionJ Hypertens20052320839216208152
- SingerGMIzharMBlackHRGoal-oriented hypertension management: translating clinical trials to practiceHypertension200240464912364348
- SwalesJDCurrent clinical practice in hypertension: the EISBERG (Evaluation and Interventions for Systolic Blood pressure Elevation-Regional and Global) projectAm Heart J1999138231710467218
- SweetmanSCMartindale: the complete drug reference2005LondonPharmaceutical Press
- WhitworthJA2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertensionJ Hypertens20032119839214597836